1 Technology Innovation Center for Exploitation of Marine Biological Resources, Third Institute of Oceanography, Ministry of Natural Resources, 361005 Xiamen, Fujian, China
Abstract
Probiotics are increasingly recognized for promoting beneficial effects on intestinal health. However, most probiotic strains have been insufficiently researched, underscoring the need for further studies to fully understand their potential health benefits, especially in metabolic conditions. Therefore, this study aimed to explore the role and possible mechanism of Lactiplantibacillus plantarum YDJ-03 (YDJ-03) and Limosilactobacillus fermentum YDJ-6 (YDJ-6) in metabolic syndrome (MetS) and hyperuricemia.
Twelve mice per group were fed a high-fat, high-fructose, high-cholesterol (HFFC) diet for 90 days. Mice in both the YDJ-03 and YDJ-6 groups were administered a dose of 1.2 × 109 colony-forming units (CFU) intragastrically per mouse for 28 days before being injected with hypoxanthine (400 mg/kg) to induce hyperuricemia. Blood lipids (triglycerides (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C)), liver injury markers (aspartate aminotransferase (AST) and alanine aminotransferase (ALT)), oxidative stress indicators (malondialdehyde (MDA) and superoxide dismutase (SOD)), and renal injury markers (uric acid (UA) and creatinine (CREA)) levels were analyzed after the conclusion of the study.
In contrast to the model group, the YDJ-03 group exhibited a marked decrease in liver TGs (p = 0.033), MDA (p = 0.0041), serum UA (p = 0.0071) and CREA (p = 0.0072). The mRNA levels of renal toll-like receptor 2 (Tlr2) (p = 0.0018), tumor necrosis factor receptor-associated factor 6 (Traf6) (p = 0.0013), and nuclear factor kappa B subunit 1 (Nfkb1) (p = 0.032) were downregulated, accompanied by marked attenuation of inflammatory cell infiltration in renal tissues and alleviation of glomerular epithelial cell swelling. Furthermore, YDJ-6 treatment promoted significant downward adjustments in hepatic TG (p = 0.0055), serum TG (p = 0.0082), and LDL-C (p = 0.0233) levels. YDJ-6 treatment also decreased serum ALT (p = 0.0458) and AST (p = 0.029) concentrations, downregulated the gene expression levels of inflammation-related adhesion G protein-coupled receptor E1 (Adgre1) (p = 0.033) and prostaglandin-endoperoxide synthase 2 (Ptgs2) (p = 0.0077), and effectively ameliorated hepatocellular lipid deposition and ballooning degeneration with hepatocyte necrosis.
YDJ-03 may exert nephroprotective effects by regulating the TLR2-mediated NF-κB pathway, and YDJ-6 can effectively reduce hepatic fat deposition and inflammation to alleviate liver injury.
Keywords
- hyperuricemia
- hyperlipidemias
- hyperglycemia
- hypercholesterolemia
- metabolic syndrome
- probiotics
The metabolic syndrome (MetS) is a chronic disorder of glucose and lipid metabolism, including abdominal obesity, hyperglycemia, hypercholesterolemia and hyperlipidemias [1]. With changes in lifestyle and diet, MetS has emerged as a worldwide issue in public health [2]. Approximately, 25% of the global populace is afflicted with MetS [3]. Obesity is considered to be a key factor in MetS [4], it stimulates the attraction of macrophages to adipose tissue and plays an important role in the course of metabolic abnormalities and diseases associated with obesity in these patients [5, 6]. Current therapies (e.g., lifestyle interventions and anti-obesity drugs) face limitations due to side effects, necessitating safer alternatives.
Modern dietary shifts, particularly increased intake of high-purine foods, alcohol, and fructose-sweetened beverages, elevate serum uric acid (UA) levels, driving hyperuricemia, a condition linked to cardiometabolic disorders (e.g., hypertension, obesity-related complications, insulin resistance) [7, 8, 9] and multi-organ damage including chronic kidney disease [9]. Although not included in World Health Organization (WHO) MetS criteria, hyperuricemia serves as a clinical marker of MetS [10]. In MetS patients, systemic inflammation and renal microcirculation dysfunction exacerbate renal impairment, evidenced by declining glomerular filtration, albuminuria, and accelerated progression to end-stage disease. Mechanistically, fructose metabolism depletes hepatic ATP via fructokinase activation, promoting purine catabolism and UA overproduction, thereby sustaining hyperuricemia [11].
Lactiplantibacillus plantarum (formerly known as Lactobacillus plantarum) YDJ-03 (YDJ-03) is derived from kimchi, and is highly adaptable in high salt, acidic and low temperature environments, secreting natural antibiotics—bacteriocins, which can hinder the proliferation of detrimental microorganisms. This strain also has good intestinal cell adhesion, which helps it to colonise the intestines and maintain the balance of intestinal microorganisms, as well as synthesizing an array of digestive enzymes, facilitating improved nutrient uptake from dietary sources [12, 13, 14]. Limosilactobacillus fermentum (formerly known as Lactobacillus fermentum) YDJ-6 (YDJ-6) originates from the healthy human intestinal tract and is a common intestinal probiotic with good acid and bile salt resistance, capable of surviving in the environment of gastric acid and bile. It can generate a range of beneficial metabolites, such as organic acids, vitamins and antioxidants, which can improve the intestinal environment, strengthen the functionality of the intestinal barrier, thereby impeding the entry of detrimental substances and pathogens [15].
Although there are a wide variety of probiotics used in the food industry, the functions of most probiotic products lack sufficient scientific support. Consequently, this study investigated the effects of orally administered probiotics YDJ-03 and YDJ-6 in mice with MetS to determine a scientific rationale for the clinical application of probiotic products.
Compared to prior probiotic studies using a disease model which predominantly focused on a single metabolic abnormality, such as obesity and diabetes, we used a combination of chronic metabolic disorders and acute hyperuricemia to establish a dual metabolic stress model in mice, which more closely resembles the development of MetS in humans, where chronic metabolic disorders are coupled with an acute causative factor. We also conducted combined histopathological evaluation of both the liver and kidneys, revealing the systemic damage inherent to MetS. We validated and compared the differential therapeutic effects of two ecologically distinct probiotics in this composite model. By elucidating niche-specific regulatory mechanisms, our findings provide direct evidence for precise strain functional profiling and personalized clinical applications targeting multifactorial metabolic dyshomeostasis.
Packaged live YDJ-03 (preserved in China General Microbiological Culture
Collection Center (CGMCC), No.28037) and YDJ-6 (CGMCC No.28036) powder were
provided by Hangzhou Key Points Food Technology Co., Ltd., China. Male C57BL/6
mice (18–22 g) purchased from Shanghai Shilaike Experimental Animal Co., Ltd.
were divided into 4 groups including normal (n = 11), model (n = 12), YDJ-03 (n =
12) and YDJ-6 (n = 12). The normal group was given a control diet (10% kcal from
fat, D09100304, Research Diets, New Brunswick, NJ, USA), and the other 3 groups were fed a high-fat,
high-fructose, high-cholesterol (HFFC) diet (40% kcal from fat, D09100310,
Research Diets, USA) for 90 days [16, 17, 18, 19]. Mice in the YDJ-03 and YDJ-6 groups
were administered an intragastric dose of 1.2
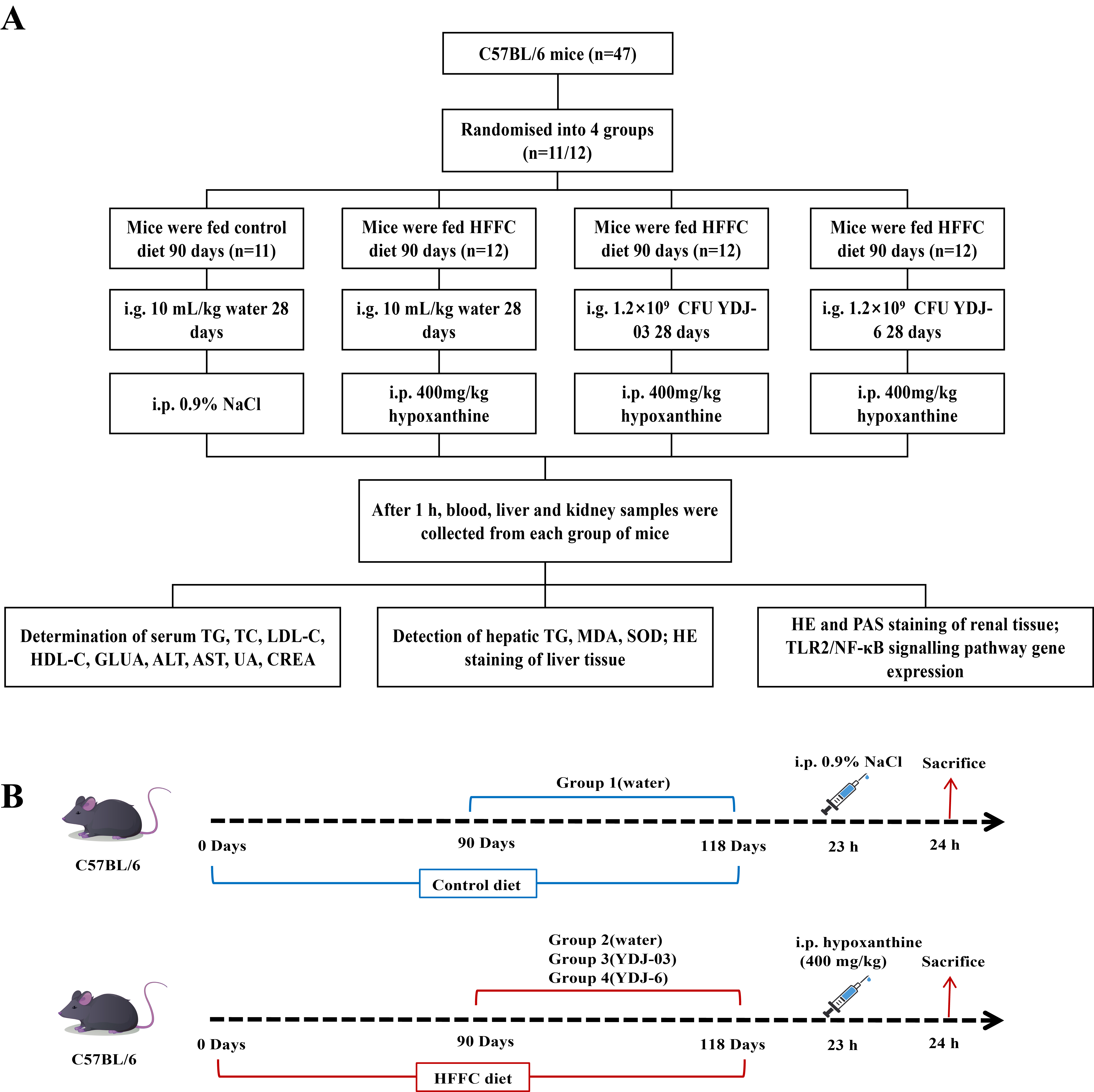 Fig. 1.
Fig. 1.
Implementation design flowchart. (A) Flowchart for study design. (B) Chronological workflow of HFFC diet-induced MetS mouse model with probiotic intervention. YDJ-03, Lactiplantibacillus plantarum YDJ-03 supplementation; YDJ-6, Limosilactobacillus fermentum YDJ-6 supplementation; HFFC, high-fat, high-fructose, high-cholesterol; CFU, colony forming units; TG, triglycerides; TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; GLUA, glucuronic acid; ALT, alanine aminotransferase; AST, aspartate aminotransferase; UA, uric acid; CREA, creatinine; MDA, malondialdehyde; SOD, superoxide dismutase; HE, hematoxylin-eosin; PAS, periodic acid-Schiff; MetS, metabolic syndrome; TLR2, toll-like receptor 2.
The liver or kidney tissues were fixed by immersion in 10% formaldehyde solution (311010015, Wexis Biological Technology Co., Ltd., Guangzhou, China), followed by embedding in paraffin wax. The tissue sections were sliced and stained with hematoxylin-eosin (HE) (G1076, Servicebio Biological Technology Co., Ltd., Wuhan, China). Kidney sections underwent additional staining with periodic acid-Schiff (PAS) reagent (G1008, Servicebio Biological Technology Co., Ltd., Wuhan, China).
The triglycerides (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), glucose (GLU), aspartate aminotransferase (AST), alanine aminotransferase (ALT), uric acid (UA), creatinine (CREA) in the serum were measured with a veterinary automatic biochemical analyzer (BS-240VET, Mindray Biomedical Electronics Co., Shenzhen, China).
In addition, 0.1 g of liver tissue and 0.9 mL of normal saline were homogenized for 2 min with a homogenizer (KZ-Ⅲ-F, Servicebio, Wuhan, China), and then centrifuged for 10 min at 4000 rpm to obtain the supernatant for the determination of hepatic TG (A110-1-1, Nanjing Jiancheng Bioengineering Institute, Nanjing, China), malondialdehyde (MDA) (A003-1-2, Nanjing Jiancheng Bioengineering Institute) and superoxide dismutase (SOD) with commercial kits (A001-3-2, Nanjing Jiancheng Bioengineering Institute).
The hepatic or renal tissue was lysed with Trizol reagent (15596026CN, Thermo Fisher Scientific Co., Ltd., WLM, MA, USA) and the total mRNA was extracted as per the manufacturer’s instructions. The cDNA was synthesized with HiScript III All-in-one RT SuperMix Perfect for qPCR (R333, Vazyme Biotech, Shanghai, China). Quantitative reverse transcription polymerase chain reaction (RT-qPCR) was conducted on a Light Cycler 96 system (Roche, Basel, Switzerland), employing Taq Pro Universal SYBR qPCR Master Mix (Q712-02, Vazyme Biotech, Nanjing, China). The expression of genes were calculated by the 2-ΔΔCt method. GAPDH served as the internal control. The primers used are listed in Table 1.
| Genes | Forward primers | Reverse primers |
| Ptgs2 | TGAGTACCGCAAACGCTTCT | CAGCCATTTCCTTCTCTCCTGT |
| Adgre1 | TGTGTCGTGCTGTTCAGAACC | AGGAATCCCGCAATGATGG |
| Tlr2 | GGAGCATCCGAATTGCATCA | CCAAAGAGCTCGTAGCATCC |
| Tlr4 | GCCTGACACCAGGAAGCTTGA | TCTTCAAGGGGTTGAAGCTCAGA |
| Irak4 | GCTCAGGGGACAGCAAATGG | CGTGCAAGCCCAAAGTCAGA |
| Traf6 | CCTGACGGTAAAGTGCCCAAA | ACGTTGGCACTGGGGACAAT |
| Myd88 | TGCTAGAGCTGCTGGCCTTG | GCTTTCCACTCTGGCCACCT |
| Nfkb1 | AGCTTATGCCGAACTTCTCG | GACTCCGGGATGGAATGTAA |
| Gapdh | GGTGAAGGTCGGTGTGAACG | CTCGCTCCTGGAAGATGGTG |
RT-qPCR, quantitative reverse transcription polymerase chain reaction; Ptgs2, prostaglandin-endoperoxide synthase 2; Adgre1, adhesion G protein-coupled receptor E1; Tlr2, toll-like receptor 2; Tlr4, toll-like receptor 4; Irak4, interleukin 1 receptor-associated kinase 4; Traf6, tumor necrosis factor receptor-associated factor 6; Myd88, MYD88 innate immune signal transduction adaptor; Nfkb1, nuclear factor kappa B subunit 1; Gapdh, glyceraldehyde-3-phosphate dehydrogenase.
All histograms presented in this study were generated using Prism 8 software
(GraphPad Software, Inc., San Diego, CA, USA). For statistical analysis, all
multiple group comparisons associated with histograms were exclusively performed
by one-way ANOVA, followed by Tukey’s post hoc tests to identify specific
differences between groups (statistical significance defined as p
The HFFC diet resulted in a significant increase in body weight gain. However,
none of the YDJ-03 or YDJ-6 groups had reduced body weight (p
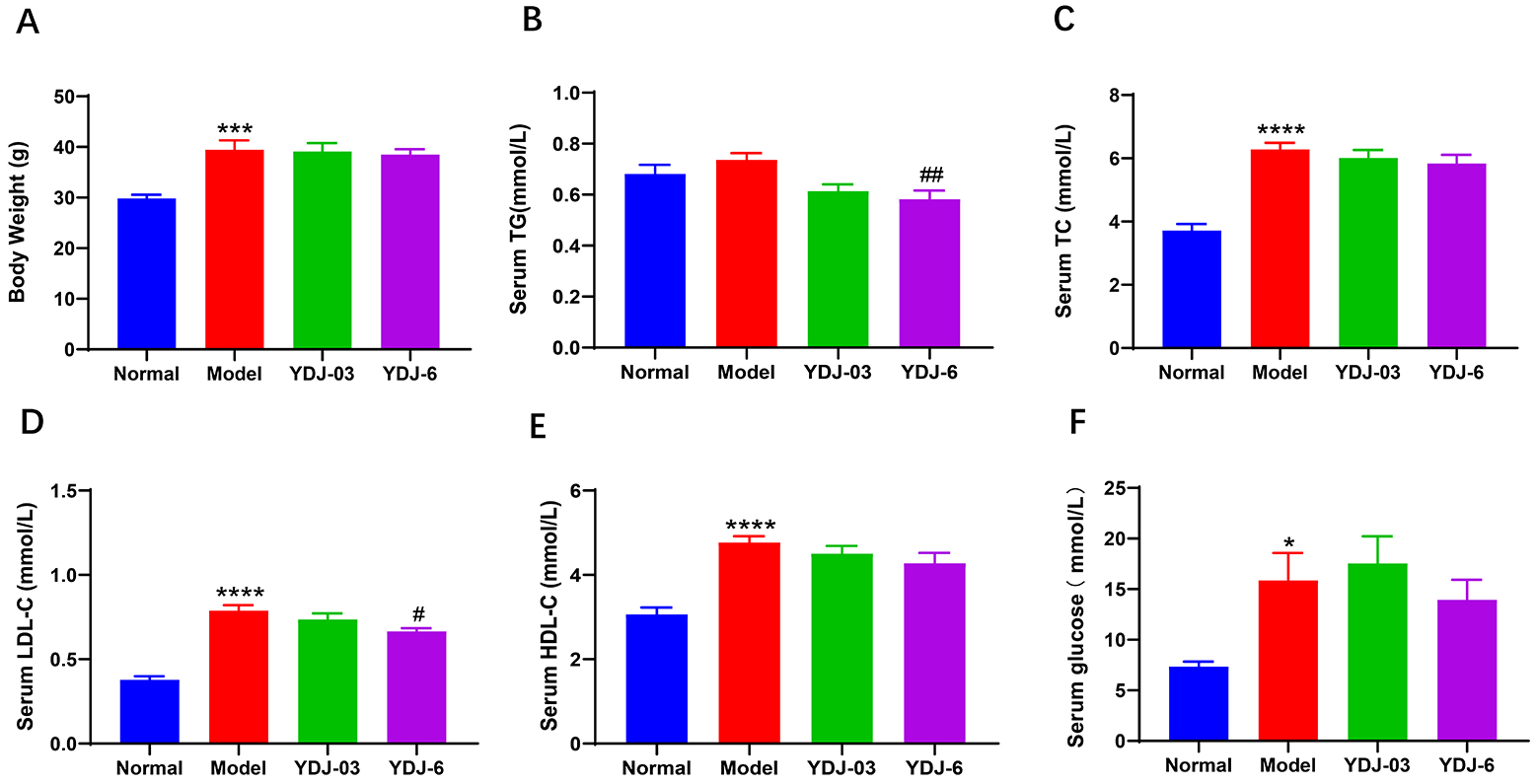 Fig. 2.
Fig. 2.
YDJ-6 attenuated hyperlipidemia in HFFC diet-fed mice. (A) Body
weight, serum, (B) TG, (C) TC, (D) LDL-C, (E) HDL-C and (F) glucose were shown.
There were 11 mice in the normal group and 12 mice in each of the other three
groups. Data are expressed as mean
HE staining of the liver showed that in the model group there were predominantly
ballooned hepatocytes and macrovesicular steatosis, while YDJ-03 slightly reduced
the fat deposition. YDJ-6 greatly increased fat accumulation and balloon
degeneration of hepatocytes (Fig. 3A). The highly elevated hepatic TG was also
significantly reduced by both YDJ-03 (p = 0.033) and YDJ-6 (p =
0.0055) (Fig. 3B), especially YDJ-6, which also significantly lowered serum ALT
(p = 0.0458) and AST (p = 0.029) (Fig. 3C,D). In addition, both
YDJ-03 and YDJ-6 greatly reduced hepatic MDA levels (p = 0.0041 and
0.0013, respectively) , while their modest increases in SOD activity did not
reach statistical significance (p
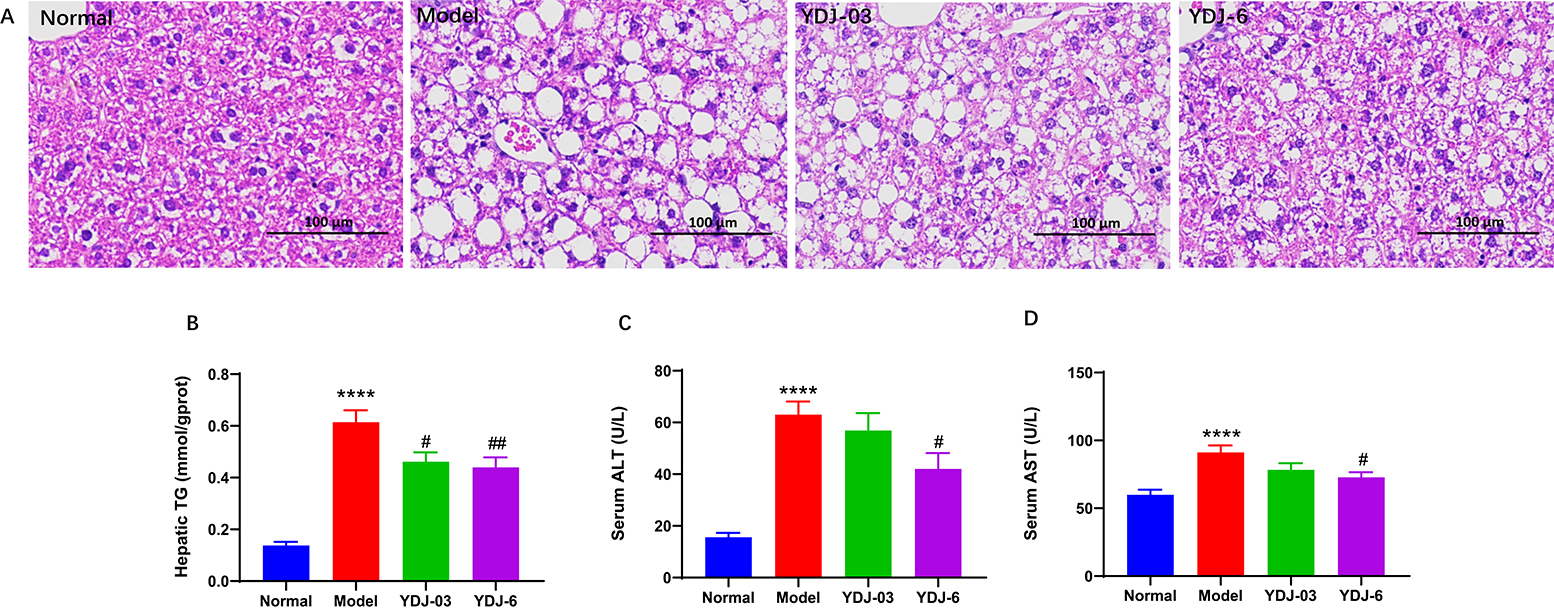 Fig. 3.
Fig. 3.
YDJ-03 and YDJ-6 attenuated fatty liver in HFFC diet-fed mice.
(A) HE staining of liver tissue, scale bar = 100 µm, (B) hepatic TG, serum
(C) ALT and (D) AST were shown. There were 11 mice in the normal group and 12
mice in each of the other three groups. Data are expressed as mean
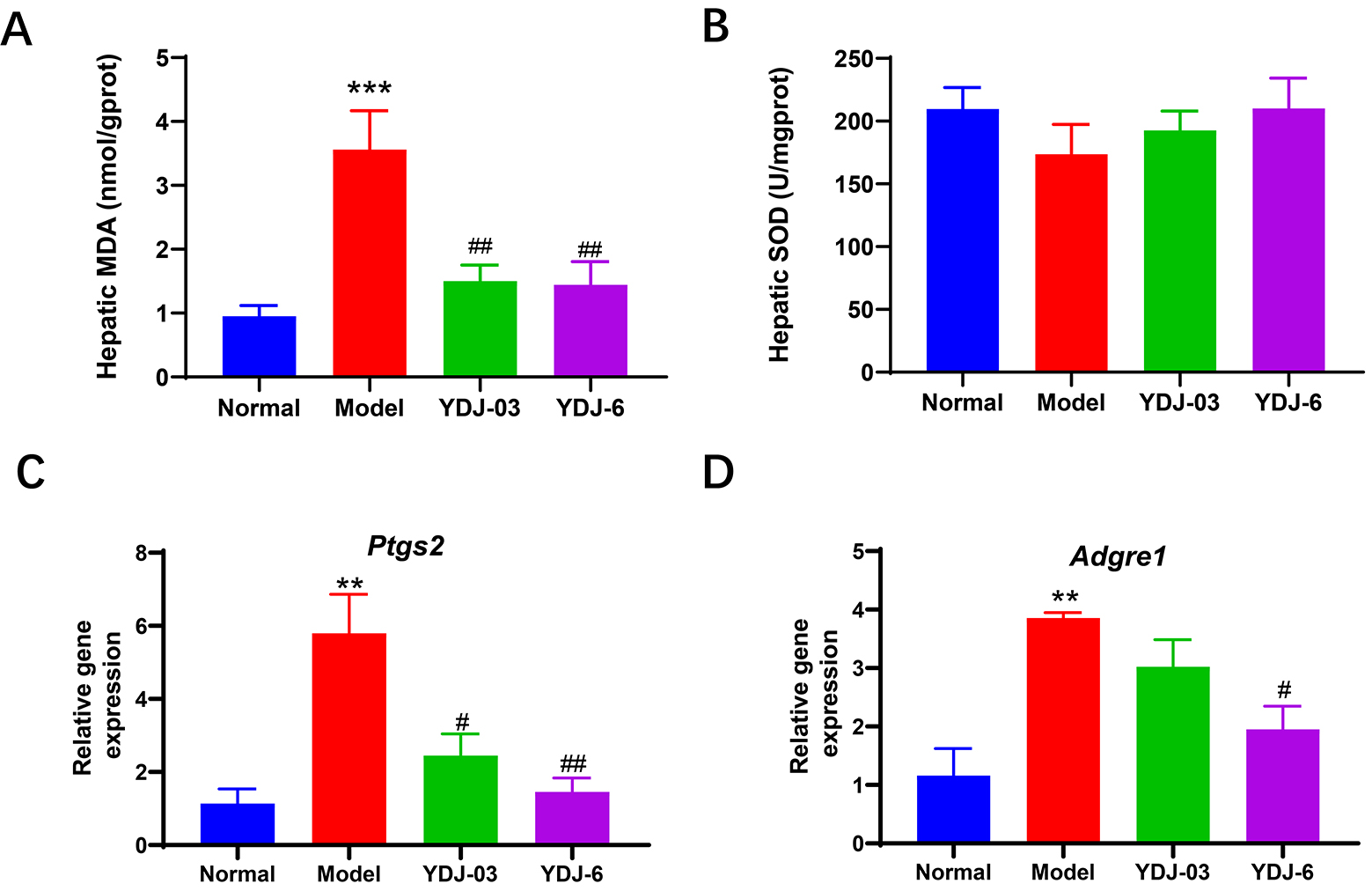 Fig. 4.
Fig. 4.
YDJ-03 and YDJ-6 altered liver lipid peroxidation and
inflammation-related genes. (A) Hepatic MDA and (B) SOD, and mRNA levels of (C)
Ptgs2 and (D) Adgre1 were shown. There were 11 mice in the
normal group and 12 mice in each of the other three groups. Data are expressed as
mean
As shown by HE staining (Fig. 5A), in the normal group, the renal tubular cells were closely arranged, with clear tubular outlines, and no swelling cells were observed. Regarding the model group, the epithelial cells of the renal tubules exhibited severe swelling or granular vacuolar degeneration, significant dilation of the lumen, and infiltration of large numbers of inflammatory cells. In the YDJ-03 group, the renal tubular epithelial cells showed mild to moderate swelling and reduced infiltration of inflammatory cells. PAS staining (Fig. 5B) showed that compared with the normal group, the basement membrane of the glomerulus in the model group was thickened, had mesangial cell proliferation, and increased extracellular matrix. Treatment with YDJ-03 significantly improved these pathological conditions, whereas YDJ-6 exhibited slight improvement. In addition, Fig. 5C,D show that versus the control group, the content of serum UA and CREA in the model group significantly increased, and YDJ-03 significantly reduced the serum levels of UA (p = 0.0071) and CREA (p = 0.0072).
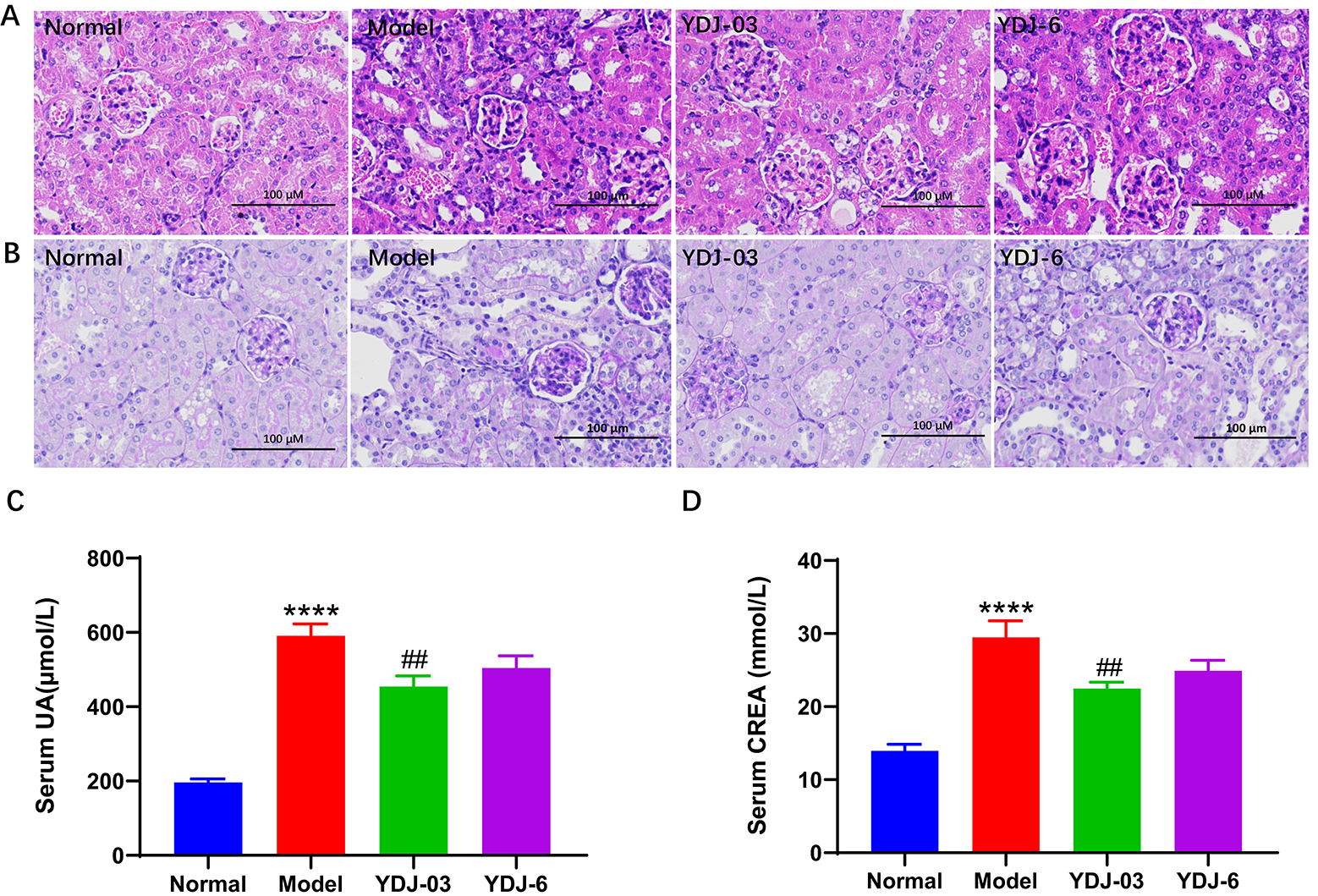 Fig. 5.
Fig. 5.
The effect of YDJ-03 and YDJ-6 on renal damage. (A) HE and (B)
PAS staining of renal tissue, scale bar = 100 µm, and serum (C) UA and (D)
CREA levels were shown. There were 11 mice in the normal group and 12 mice in
each of the other three groups. Data are expressed as mean
As shown in Fig. 6, toll-like receptor 4 (Tlr2), Tlr4,
interleukin 1 receptor-associated kinase 4 (Irak4), tumor necrosis
factor receptor-associated factor 6 (Traf6), MYD88 innate immune signal
transduction adaptor (Myd88) and nuclear factor kappa B subunit
1 (Nfkb1) all exhibited a marked elevation in the model group,
suggesting activation of the TLR2/NF-
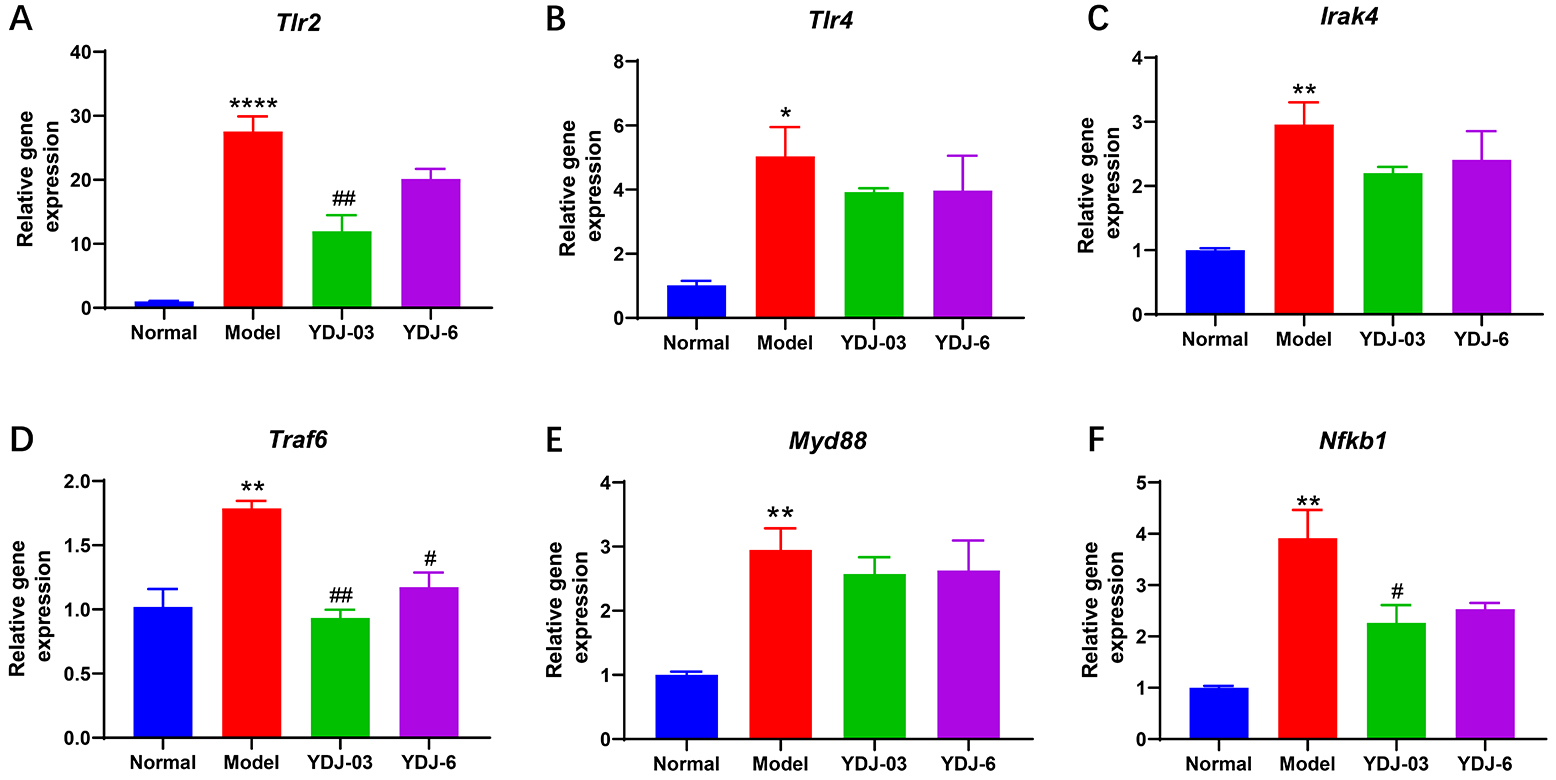 Fig. 6.
Fig. 6.
The effect of YDJ-03 and YDJ-6 on renal gene expressions. (A)
Tlr2, (B) Tlr4, (C) Irak4, (D) Traf6, (E)
Myd88 and (F) Nfkb1. There were 11 mice in the normal group and
12 mice in each of the other three groups. Data are expressed as mean
Over the past two decades, study has shown that the intestinal microbiota is a significant factor in the onset of obesity and associated metabolic disorders [22]. It has been demonstrated that supplementation with the probiotics Lactobacillus and Bifidobacterium improves anthropometric parameters in mice and rats fed high fat diet (HFD) [23, 24]. For instance, the provision of Lactobacillus plantarum K21 notably decreased the accumulation of epididymal fat and liver mass in mice that were on a 8-week high-fat diet [25]. Among probiotics, the Lactobacillus species and Bifidobacterium species play an important role in the management of MetS, by regulating body weight gain, improving glycemic and lipid metabolism, and demonstrate significant anti-obesity properties in human study, while also lowering insulin resistance [26]. The favorable impacts of these probiotics may be attributed to various mechanisms, such as enhanced synthesis of short-chain fatty acids, alteration of bile acid metabolism, safeguarding the host against metabolic endotoxemia, and the orchestration of the composition of the intestinal microbiota [27]. The number of studies on Lactobacillus plantarum with probiotic health benefits is increasing. In accordance with the change in the international taxonomic status of the strains, Lactobacillus plantarum and Lactobacillus fermentum were renamed in 2022 as Lactiplantibacillus plantarum and Limosilactobacillus fermentum.
This study focuses on the role of Lactiplantibacillus plantarum YDJ-03 and Limosilactobacillus fermentum YDJ-6 in the HFFC diet-induced MetS model in mice. A high-fructose diet leads to elevated fasting blood glucose levels, which are considered to be a key marker in the diagnosis of MetS [28]. Probiotics reduce HFD-induced hyperglycemia, which is consistent with prior research indicating a decrease in blood glucose levels in rats fed a high-fructose diet following an 8-week period of oral treatment with probiotic dahi enriched with Lactobacillus acidophilus and Lactobacillus casei [29]. However, in our experiments we observed no marked decrease in body weight and blood glucose in mice consuming YDJ-03 and YDJ-6 for 28 days compared to HFFC diet-fed mice. One possible reason for this is our probiotics intervention of 28 days was too short compared to the 8 weeks of probiotic intervention in their study. In addition, hypertriglyceridemia is a key component of MetS and is closely related to insulin resistance [30]. Therefore, we examined the impact of YDJ-03 and YDJ-6 on hyperlipidemia and hypercholesterolemia, and found that the probiotic preparations reduced serum TC levels, but not significantly, while YDJ-6 significantly down-regulated serum TG, and fasting LDL-C levels, another MetS factor. This is consistent with study reporting that the combined use of Lactobacillus plantarum KY1032 and Lactobacillus curvatus HY7601 reduces triglyceride levels and LDL levels in a high-fat diet-induced hypertriglyceridemia rat model [31]. This suggests that a longer treatment period with probiotics might be required to have a significant effect on hyperglycemia and obesity.
Hepatic biomarkers of liver function, specifically ALT and AST, were also evaluated. Animals that received probiotic supplementation exhibited marked reductions in liver function markers, suggesting a reduction in liver damage and a return to normal liver function [32]. Consistent with previous study [33], supplementation with YDJ-03 and YDJ-6 both reduced hepatic ALT and AST levels in HFFC diet-fed mice, along with reduced hepatocellular fat deposition and globular degeneration with YDJ-6.
In individuals with MetS, there is often an increase in oxidative stress and inflammation, which can lead to lipid peroxidation in the liver [8]. Increasing evidence indicates that oxidative stress acts as a catalyst triggering a low-grade inflammatory reaction, which plays an important role in the onset of obesity and MetS [34, 35]. MDA, a key indicator of oxidative stress, represents the terminal byproduct of free radical-induced lipid peroxidation. In contrast, the antioxidant enzymes SOD and GSH function to neutralize superoxide radicals and eliminate reactive oxygen species. The ptgs2 gene has been associated with the pathogenesis of steatosis and MetS [36]. Study has shown that increased expression of Ptgs2 gene in the liver is linked to inflammation and oxidative stress, which are crucial elements in the progression of fatty livers [37]. In contrast, F4/80 is a cell surface glycoprotein that is commonly used as a marker for macrophages, such as kupffer cells in the liver. In conditions such as MetS, the upregulation of Adgre1 (F4/80 gene) in the liver may suggest immune cell infiltration [38]. Animals administered with probiotics exhibit elevated antioxidant levels and reduced oxidative agents, which may suppress the generation of reactive oxygen species, including peroxyl radicals, superoxide anions, and hydroxyl radicals, thus diminishing inflammatory responses [39]. Therefore, we measured hepatic MDA and SOD, as well as Ptgs2 gene expression and found that oral administration of both YDJ-03 and YDJ-6 significantly decreased hepatic MDA levels and Ptgs2 expression and slightly increased SOD levels, suggesting an improvement in hepatic oxidative stress. This data suggests that inhibition of Ptgs2 and Adgre1 by YDJ-03 and YDJ-6 might be one of the mechanisms to reduce hepatic inflammation and attenuate liver damage in the HFFC diet-induced MetS model.
The preservation of a standard serum uric acid level is crucial in clinical practice, since it not only averts hyperuricemia and gout but also serves as a preventive measure against the onset of type 2 diabetes, as well as renal, cerebrovascular, and cardiovascular pathologies [40, 41]. The HFFC diet also mimics the high fructose diet structure of humans. Serum UA levels in mice fed the HFFC diet for 90 days were approximately 3-fold higher than those in mice fed the regular diet. Our results showed that supplementation with YDJ-03 and YDJ-6 effectively reduced serum uric acid levels in MetS mice, especially by YDJ-03. We also evaluated the protective effects of YDJ-03 and YDJ-6 on the functional integrity of the kidneys, since MetS and hyperuricemia may affect kidney function through common physiological and metabolic pathways, such as persistent inflammation, oxidative stress, and endothelial dysfunction. The results showed that YDJ-03 significantly reduced renal inflammatory cell infiltration as well as the expression level of the immune cell infiltration-related gene Adgre1 in HFFC diet-fed mice and restored the structural integrity of renal tubular cells, in addition to a marked decrease in the concentrations of serum CREA and UA, both of which are biomarkers of renal function. This demonstrated that YDJ-03 had a significant nephroprotective effect in HFFC diet-induced MetS mice.
A study reported that oral intake of the probiotic strain
Bifidobacterium longum 51A (BL) mitigated inflammation triggered by
monosodium urate crystals (MSU) in a murine model of gout [42]. We examined the
expression levels of genes associated with the activated renal
TLR2/NF-
In this study we combined a HFFC diet with intraperitoneal hypoxanthine injections to induce both MetS and acute hyperuricemia. This dual metabolic stress model effectively simulates the interplay between chronic metabolic dysfunction and acute triggers observed in human disease progression, thereby enhancing its translational relevance. Furthermore, we systematically compared the organ-specific protective effects of two functionally distinct probiotics. Notably, YDJ-03 exhibited superior renoprotective efficacy, while YDJ-6 showed stronger hepatoprotective activity, suggesting strain- and tissue-specific therapeutic potentials. However, several limitations should be acknowledged in this study. First, additional studies are necessary to elucidate the precise mechanism of action behind these observed effects. Second, extended study periods are necessary to evaluate whether probiotic effects on metabolic markers are sustained and can be translatable to chronic human disease management. Third, it is also necessary to investigate the impact of probiotics on daily blood pressure in mice with MetS.
In this study, an animal model of concurrent hyperuricemia, hyperlipidemia and hyperglycemia was successfully established by combining an HFFC fed diet and intraperitoneal injection of hypoxanthine. The model innovatively linked high uric acid levels to MetS simulating elevated uric acid level and liver and kidney damage in MetS patients. The results of this study demonstrate the promising capabilities of the two bacilli from the Lactobacillaceae family, YDJ-03 and YDJ-6, in modulating key metabolic parameters in MetS as well as hyperuricemia. Both YDJ-03 and YDJ-6 alleviated the development of MetS and hyperuricaemia. YDJ-03 was more advantageous in kidney protection, while YDJ-6 was more capable of protecting the liver. Taken together, this study provides solid evidence for the potential of probiotics as a biotherapeutic approach to modulate MetS.
MetS, metabolic syndrome; HFFC, high-fat, high-fructose, high-cholesterol; TG, triglycerides; TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; GLU, glucose; AST, aspartate aminotransferase; ALT, alanine aminotransferase; UA, uric acid; CREA, creatinine.
All core datasets supporting the findings of the study have been presented in the text as tables and figures.
JH designed the study. SC, MS, XC and QL conducted the research and investigation process. SC analyzed the data and drafted the manuscript. JH revised the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
All animals received humane care according to Guide for the Care and Use of Laboratory Animals (National Research Council, USA). The protocols were approved by the Institutional Committee on the Care and Use of Animals of the Third Institute of Oceanography, Ministry of Natural Resources (TIO-IACUC-04-2022-03-01).
The authors would like to acknowledge all colleagues for their help during the animal procedures and all the peer reviewers for their constructive feedback.
This research was funded by the Scientific Research Foundation of Third Institute of Oceanography, Ministry of Natural Resources, grant number 2023009; Xiamen Natural Science Foundation, grant number 3502Z20227247.
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.






