1 Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education), Department of Pharmacy, Peking University Cancer Hospital and Institute, 100142 Beijing, China
Abstract
This study aimed to elucidate correlations between obesity-related indicators and vitamin D (VD) status in a nationally representative sample of U.S. adults.
We analysed data from 9168 adults aged 20–59 years obtained from the 2011–2018 National Health and Nutrition Examination Survey. Serum 25 hydroxyvitamin D [25(OH)D] levels were measured and categorised into quartiles. Anthropometric measurements, including weight, waist circumference, and fat mass in various body regions quantified through dual-energy X-ray absorptiometry, were collected. Multiple imputation was employed to replace missing data. The importance of obesity-related indicators and serum 25(OH)D concentration was explored using multiple linear regression adjusted for demographics, lifestyle factors, dietary intake, and clinical biomarkers, and stepwise regression.
Weight, waist circumference, and fat mass across all body regions were inversely associated with serum 25(OH)D levels (all p < 0.001). Notable differences were observed between men and women. Stepwise regression revealed a strong inverse correlation between visceral adipose tissue and serum 25(OH)D concentration in men [β 95% CI: –13.04 (–18.10, –7.99), p < 0.001], whereas in women, only weight was significantly correlated with serum 25(OH)D concentration [β 95% CI: –0.20 (–0.28, –0.12), p < 0.001]. Demographic attributes, seasonal sunlight exposure, dietary VD, calcium, phosphorus, and magnesium intake, and biomarkers including alkaline phosphatase and creatinine also emerged as significant predictors.
Besides conventional obesity measures, abdominal fat metrics exhibit robust associations with VD deficiency, especially in men. Public health initiatives and clinical management strategies for hypovitaminosis D in obese populations should consider nuanced aspects of adiposity distribution alongside other demographic, lifestyle, and dietary factors influencing VD.
Keywords
- vitamin D
- 25-hydroxyvitamin D
- obesity
- NHANES
- adult
Obesity represents a critical public health challenge, with links to a range of morbidities including type 2 diabetes, cardiovascular diseases, chronic kidney diseases, and various cancers [1, 2, 3, 4]. These conditions drastically diminish an individual’s quality of life and considerably shorten life expectancy. The prevalence of adult obesity in the United States has surged from 14% in 1980 to 42% in 2018 [5]. By 2030, nearly half of the U.S. adult population is projected to be contending with obesity [6].
A notable concurrent issue is the frequent detection of vitamin D (VD) deficiency in individuals with obesity. Evidence supporting an inverse correlation between obesity and VD levels is growing [7, 8, 9, 10]. Although the underpinnings of this relationship are complex, factors such as limited sunlight exposure, sedentary lifestyles, and the sequestration of VD in adipose tissue are likely significant contributors [11]. Considering the pivotal role adipocytes play in VD metabolism, VD deficiency might accelerate adipogenesis, thereby exacerbating the challenge of obesity [11].
Although body mass index (BMI) is a standard metric for assessing adiposity [12, 13], it does not fully capture the nuances of body fat distribution. Emerging evidence highlights the prognostic importance of abdominal obesity for cardiovascular and metabolic disorders, often surpassing the implications of BMI [14, 15]. Alternative metrics such as waist circumference (WC), waist-to-hip ratio, and waist-to-height ratio (WHtR) provide insights into abdominal obesity [16]. Notably, adipose tissue serves as a primary reservoir for VD, which, in turn, critically influences adipocyte physiology [17, 18, 19, 20]. Moreover, studies indicate that reducing body fat can elevate serum 25(OH)D concentrations [21, 22, 23]. In light of the escalating obesity crisis and the critical role of VD, exploring their interrelationship is imperative.
This study aimed to elucidate this correlation by comparing weight, WC, and detailed indicators of body fat distribution with serum 25(OH)D levels in a comprehensive sample of adults in the United States. We hypothesised that the primary cause of lower serum VD concentrations is the accumulation of visceral fat. Our findings aim to facilitate tailored clinical and public health strategies for mitigating obesity and addressing VD deficiency.
This study utilised data from the 2011–2018 National Health and Nutrition Examination Survey (NHANES), which employed a stratified, multistage, probability sampling design to obtain representative samples of the U.S. population. Detailed survey operations and procedures have been described online (https://www.cdc.gov/nchs/nhanes). Briefly, NHANES surveys included an in-home interview and a subsequent health examination at a mobile examination centre (MEC). Information on demographics, lifestyle behaviours, and health conditions was collected through interviews. Physical examinations, body measurements, and laboratory tests were performed at the MEC. Dietary related information was obtained through two follow-up telephone interviews to obtain a 24-hour dietary recall.
We included people who participated in NHANES from 2011 to 2018, and the exclusion criteria were as follows: we excluded patients who were only interviewed, considering the correlation between 25 (OH)D concentration and sun exposure. We included a population aged 20–59 years who participated in the sun exposure and sun protective behaviour interviews. Moreover, dietary intake and supplements can also affect 25 (OH)D levels; hence, individuals who did not participate in dietary follow-up were excluded from the analysis. To ensure the accuracy of the dual-energy X-ray absorptiometry (DXA) scan data, only complete and valid whole-body scans were included in the study. Furthermore, participants were excluded if they wore clothes or carried medical equipment during weight measurements. Participants who did not maintain an upright posture during height measurement were also excluded. Finally, any individuals with missing data on obesity-related indicators was also excluded from the analysis. The detailed screening process is shown in Fig. 1.
 Fig. 1.
Fig. 1. Flowchart of the study population. Abbreviations: 25(OH)D, 25-hydroxyvitamin D; DXA, dual-energy X-ray absorptiometry; WC, waist circumference; BMC, bone mineral content; VD, vitamin D.
Serum concentrations of 25(OH)D2, 25(OH)D3, and C3-epimer-25(OH)D3 were measured using a fully validated, standardised, high-performance liquid chromatography-tandem mass spectrometry method (Thermo Vantage mass spectrometer and Thermo Accela ultra-high-performance liquid chromatography system, ThermoElectron Corp). The total 25(OH)D concentration was defined as the sum of 25(OH)D2 and 25(OH)D3, excluding C3-epimer-25(OH)D3. C3-epimer-25(OH)D3 is an epimer of 25(OH)D3 and was excluded to avoid overestimating VD levels. The detection limits were constant for all analytes. It should be noted that 25(OH)D2 detection results may be lower than the limit of detection (LOD). For analytes with results below the LOD, an imputed value of 1.45 nmol/L (the LOD divided by the square root of 2) was placed in the analyte results field.
BMI is an internationally recognised classification indicator for overweight and obesity, calculated by dividing weight by the square of height [24]. WC is frequently used as an indicator for evaluating abdominal obesity. Therefore, in this study, we used height, weight, and WC, along with the distribution of body fat, to evaluate the correlation between obesity and 25 (OH)D serum concentration. To ensure the accuracy of our results, we excluded individuals from the NHANES data who were wearing clothes during weight measurement, carrying medical equipment, or those who failed to maintain an upright posture during height measurement, as these factors could potentially affect the results. For the measurement of human body fat, DXA is the most widely accepted method for measuring body composition due in part to its speed, ease of use, and low radiation exposure. Only data obtained from scans covering the entire body and subsequently validated were included in our analysis.
Demographic variables included age, sex, ethnicity, education level, family income-to-poverty ratio, and examination period. Lifestyle factors comprised smoking status, alcohol consumption, time spent outdoors on weekdays and weekends, and physical activity level. Dietary intake of VD, calcium, phosphorus, and magnesium from foods and supplements were also considered as potential confounders. Additionally, biomarkers included serum calcium and phosphorus, alkaline phosphatase (ALP), alanine aminotransferase (ALT), and creatinine. Given the classic role of VD in regulating calcium and phosphorus metabolism and promoting bone development [25, 26], we included dietary intake and supplementation of calcium and phosphorus, along with serum calcium and phosphorus levels, as covariates. Since ALP indicates bone formation and is involved in generating phosphorus ions and bone salt crystals [27], it was also included in the analysis as a covariate. Given that VD is metabolised in the liver and kidneys [28], serum creatinine reflects renal function [29], and ALT reflects liver function, creatinine and ALT were included as covariates.
Serum 25(OH)D concentrations were categorised into quartiles (
We used multiple imputation to fill in missing data. Predictive mean matching was used to impute numeric features, logistic regression to impute binary variables, and Bayesian polytomous regression to impute factor features. Simultaneously, we performed statistical analysis on both imputed and non-imputed data to evaluate the stability of the model.
We incorporated all previously mentioned covariates into the multivariable linear regression models. The models were generated to examine the associations of weight, WC, head fat, arm fat, leg fat, subcutaneous fat, and visceral adipose tissue with serum 25(OH)D levels separately. Due to the inconsistency of evaluation index units, we standardised the data. Model 1 was adjusted for demographic characteristics, including age, sex, height, ethnicity, education level, examination period, and family income. Model 2 was further adjusted for lifestyle factors, including smoking history, alcohol consumption, sunlight exposure, and physical activity. Model 3 incorporated dietary intake and supplements (VD, calcium, magnesium, and phosphorus), bone mineral content (BMC), and biomarkers, including serum calcium and phosphorus, ALP, ALT, and creatinine. Simultaneously, we used bidirectional stepwise regression to explore which component of fat plays a key role in serum 25(OH)D levels. Stratified analysis based on sex was also performed.
All statistical analyses accounted for the complex survey design and sample weights in NHANES. Two-sided p
Using imputed data from 9168 participants categorised by serum 25(OH)D levels, we stratified them into quartiles: quartile 1 (
| Characteristics | Overall | Serum 25(OH)D concentration (nmol/L) | p-value | ||||
| Quartile 1 ( | Quartile 2 (49.0–64.2) | Quartile 3 (64.3–80.3) | Quartile 4 ( | ||||
| Number of participants | 9168 (100.0%) | 3161 (25.0%) | 2397 (25.0%) | 1927 (25.0%) | 1683 (25.0%) | ||
| Age (years) | 38.7 (11.8) | 36.2 (11.3) | 37.5 (11.6) | 39.4 (11.7) | 41.9 (11.9) | ||
| Male | 4651 (51.7%) | 1656 (54.2%) | 1273 (55.6%) | 1009 (54.2%) | 713 (42.9%) | ||
| Ethnicity | |||||||
| Mexican American | 1402 (11.6%) | 581 (19.3%) | 462 (15.3%) | 258 (8.5%) | 101 (3.3%) | ||
| Other Hispanic | 967 (7.4%) | 283 (8.7%) | 321 (9.3%) | 230 (7.3%) | 133 (4.3%) | ||
| Non-Hispanic White | 3240 (59.7%) | 484 (30.1%) | 808 (56.7%) | 907 (69.9%) | 1041 (82.7%) | ||
| Non-Hispanic Black | 1841 (10.7%) | 1139 (26.9%) | 360 (8.3%) | 198 (4.4%) | 144 (3.1%) | ||
| Other Ethnicity | 1718 (10.5%) | 674 (15.0%) | 446 (10.4%) | 334 (9.8%) | 264 (6.7%) | ||
| Education level | |||||||
| 3626 (34.3%) | 1380 (42.7%) | 972 (34.9%) | 716 (31.7%) | 558 (28.1%) | |||
| Above high school | 5540 (65.6%) | 1780 (57.2%) | 1425 (65.1%) | 1210 (68.3%) | 1125 (71.9%) | ||
| Unknown | 2 (0.0%) | 1 (0.1%) | 0 (0.0%) | 1 (0.0%) | 0 (0.0%) | ||
| Examination period | |||||||
| November–April | 4545 (45.3%) | 1906 (62.2%) | 1144 (45.5%) | 841 (40.1%) | 654 (33.2%) | ||
| May–October | 4623 (54.7%) | 1255 (37.8%) | 1253 (54.5%) | 1086 (59.9%) | 1029 (66.8%) | ||
| Family income-to-poverty ratio | 2.9 (1.7) | 2.4 (1.6) | 2.8 (1.7) | 3.1 (1.7) | 3.4 (1.6) | ||
| Drinking | 7685 (87.6%) | 2574 (84.7%) | 2010 (86.7%) | 1636 (89.0%) | 1465 (90.1%) | 0.009 | |
| Smoking | 3571 (40.6%) | 1160 (38.1%) | 907 (38.6%) | 798 (43.8%) | 706 (41.8%) | 0.200 | |
| Stay in the shade | 0.028 | ||||||
| Always, most of the time, or sometimes | 6904 (73.2%) | 2468 (77.0%) | 1826 (74.0%) | 1395 (71.5%) | 1215 (70.2%) | ||
| Rarely or never | 2261 (26.8%) | 691 (23.0%) | 570 (25.9%) | 532 (28.5%) | 468 (29.8%) | ||
| Unknown | 3 (0.0%) | 2 (0.1%) | 1 (0.0%) | 0 (0.0%) | 0 (0.0%) | ||
| Outdoors work day (minutes) | 104.1 (132.3) | 91.8 (123.8) | 100.2 (132.1) | 118.3 (141.5) | 106.3 (130.0) | ||
| Outdoors, not work day (minutes) | 155.5 (127.5) | 126.3 (119.6) | 147.3 (123.3) | 177.9 (131.7) | 170.8 (128.6) | ||
| Physical activity (kcal/day) | 1639.0 (4536.5) | 1630.9 (3230.1) | 1661.8 (3075.1) | 1644.8 (3389.2) | 1618.3 (7144.5) | 0.046 | |
| Height (cm) | 169.0 (9.5) | 168.1 (9.7) | 168.8 (9.5) | 170.0 (9.7) | 169.3 (9.2) | 0.001 | |
| Weight (kg) | 81.6 (19.8) | 84.8 (22.3) | 82.5 (19.1) | 81.9 (19.2) | 77.0 (17.5) | ||
| BMI (kg/m2) | 28.5 (6.3) | 30.0 (7.3) | 28.9 (6.1) | 28.3 (6.1) | 26.8 (5.4) | ||
| WC (cm) | 96.9 (15.5) | 99.7 (17.4) | 97.9 (15.1) | 96.8 (14.9) | 93.3 (13.8) | ||
| WHtR | 0.57 (0.09) | 0.59 (0.10) | 0.58 (0.09) | 0.57 (0.09) | 0.55 (0.08) | ||
| Total BMC (g) | 2360.2 (441.2) | 2361.8 (444.3) | 2341.7 (413.8) | 2404.7 (456.9) | 2333.0 (445.5) | 0.007 | |
| Head fat (g) | 1164.9 (164.8) | 1201.3 (170.7) | 1178.3 (162.2) | 1166.1 (165.7) | 1113.4 (146.9) | ||
| Arms fat (g) | 3303.8 (1523.9) | 3588.6 (1757.2) | 3350.6 (1476.6) | 3228.7 (1446.4) | 3044.2 (1329.5) | ||
| Legs fat (g) | 9611.3 (4071.3) | 10,278.2 (4592.0) | 9602.9 (4000.6) | 9426.7 (3889.5) | 9130.9 (3653.4) | ||
| SAT (g) | 1585.6 (776.8) | 1738.4 (877.1) | 1625.1 (759.9) | 1539.3 (746.4) | 1438.1 (678.0) | ||
| VAT (g) | 489.9 (272.3) | 517.3 (282.6) | 511.0 (263.0) | 488.5 (268.6) | 442.6 (268.5) | ||
| Dietary intake | |||||||
| Vitamin D (µg/day) | 4.5 (4.6) | 3.8 (3.9) | 4.5 (4.7) | 4.9 (4.6) | 4.7 (4.8) | ||
| Calcium (mg/day) | 994.5 (531.8) | 906.9 (526.0) | 970.7 (501.7) | 1072.0 (551.6) | 1029.7 (532.5) | ||
| Phosphorus (mg/day) | 1433.4 (617.6) | 1372.8 (620.1) | 1420.9 (609.4) | 1504.1 (636.1) | 1436.7 (597.5) | ||
| Magnesium (mg/day) | 310.6 (139.7) | 283.7 (130.5) | 303.9 (137.2) | 329.0 (146.8) | 326.1 (139.4) | ||
| Dietary supplements | |||||||
| Vitamin D (µg/day) | 9.7 (41.6) | 1.8 (13.2) | 4.3 (19.2) | 8.7 (32.4) | 24.2 (71.1) | ||
| Calcium (mg/day) | 92.7 (228.4) | 31.8 (126.3) | 61.0 (161.4) | 99.4 (211.8) | 179.3 (331.5) | ||
| Phosphorus (mg/day) | 4.3 (27.3) | 1.0 (9.0) | 3.2 (19.6) | 4.4 (27.7) | 8.5 (41.5) | ||
| Magnesium (mg/day) | 21.0 (70.4) | 7.2 (44.2) | 13.5 (46.8) | 23.9 (68.0) | 39.5 (102.5) | ||
| Biomarkers | |||||||
| Alkaline phosphatase (U/L) | 66.5 (21.5) | 69.6 (23.1) | 67.4 (22.9) | 65.2 (19.2) | 63.6 (20.1) | ||
| Alanine aminotransferase (U/L) | 26.3 (20.2) | 28.0 (22.6) | 26.8 (19.8) | 25.6 (20.8) | 24.9 (17.3) | 0.048 | |
| Creatinine (µmol/L) | 76.0 (26.4) | 74.7 (28.4) | 74.5 (17.7) | 75.8 (16.5) | 78.8 (37.2) | ||
| Serum calcium (mmol/L) | 2.35 (0.08) | 2.34 (0.09) | 2.34 (0.09) | 2.35 (0.08) | 2.35 (0.08) | ||
| Serum phosphorus (mmol/L) | 1.21 (0.18) | 1.20 (0.18) | 1.21 (0.18) | 1.21 (0.18) | 1.22 (0.18) | 0.017 | |
Abbreviations: 25(OH)D, 25-hydroxyvitamin D; BMI, body mass index; WC, waist circumference; WHtR, waist-to-height ratio; BMC, Bone Mineral Content; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
Note: Continuous variables are described as means with standard deviations. Categorical variables are presented as unweighted sample sizes and weighted proportions. All estimates were adjusted to account for the complex survey design by incorporating dietary weight. Non-normally distributed data were analysed using the Kruskal-Wallis test, whereas normally distributed data were tested using the analysis of variance. Categorical variables were analysed using the Chi-square test. Family income-to-poverty ratio was calculated by dividing family (or individual) income by the poverty guidelines specific to the survey year. Bold p-values indicate statistical differences.
| Characteristics | Overall | Serum 25(OH)D concentration (nmol/L) | p-value | |||
| Quartile 1 ( | Quartile 2 (49.0–64.2) | Quartile 3 (64.3–80.3) | Quartile 4 ( | |||
| Family income-to-poverty ratio (n = 8480) | 2.9 (1.7) | 2.4 (1.6) | 2.8 (1.7) | 3.2 (1.7) | 3.4 (1.6) | |
| Outdoors work day (minutes) (n = 9152) | 104.1 (132.3) | 91.7 (123.6) | 100.2 (132.1) | 118.2 (141.5) | 106.4 (130.0) | |
| Outdoors, not work day (minutes) (n = 9144) | 155.5 (127.5) | 126.3 (119.5) | 147.4 (123.3) | 177.9 (131.7) | 171.0 (128.6) | |
| Physical activity (kcal/day) (n = 9135) | 1633.8 (4539.7) | 1630.9 (3233.1) | 1652.2 (3066.9) | 1636.5 (3388.7) | 1615.6 (7154.8) | 0.059 |
| Alkaline phosphatase (U/L) (n = 9093) | 66.5 (21.5) | 69.6 (23.1) | 67.5 (23.0) | 65.2 (19.2) | 63.6 (20.1) | |
| Alanine aminotransferase (U/L) (n = 9092) | 26.3 (20.2) | 28.0 (22.6) | 26.8 (19.8) | 25.6 (20.8) | 24.9 (17.3) | 0.050 |
| Creatinine (µmol/L) (n = 9095) | 76.0 (26.4) | 74.8 (28.5) | 74.5 (17.7) | 75.9 (16.5) | 78.9 (37.2) | |
| Calcium (mmol/L) (n = 9076) | 2.35 (0.08) | 2.34 (0.09) | 2.34 (0.09) | 2.35 (0.08) | 2.35 (0.08) | |
| Phosphorus (mmol/L) (n = 9094) | 1.21 (0.18) | 1.20 (0.18) | 1.21 (0.18) | 1.21 (0.18) | 1.22 (0.18) | 0.018 |
Abbreviation: 25(OH)D, 25-hydroxyvitamin D.
Note: Data that did not need to be imputed is not displayed again. Continuous variables are described as means with standard deviations. All the estimates were adjusted to account for the complex survey design by incorporating dietary weight. Non-normally distributed data were analysed using the Kruskal-Wallis test, whereas normally distributed data were analysed using analysis of variance. Family income-to-poverty ratio was calculated by dividing family (or individual) income by the poverty guidelines specific to the survey year. Bold p-values indicate statistical differences.
Fig. 2 delineates the outcomes of multiple linear regression analyses in the general population, and the male and female subsets, which explored the correlation between distinct metrics—namely weight, WC, and fat in different parts of the body—and serum 25(OH)D levels. Adjustments were made and all indicators of the adjusted model showed statistical significance (all p
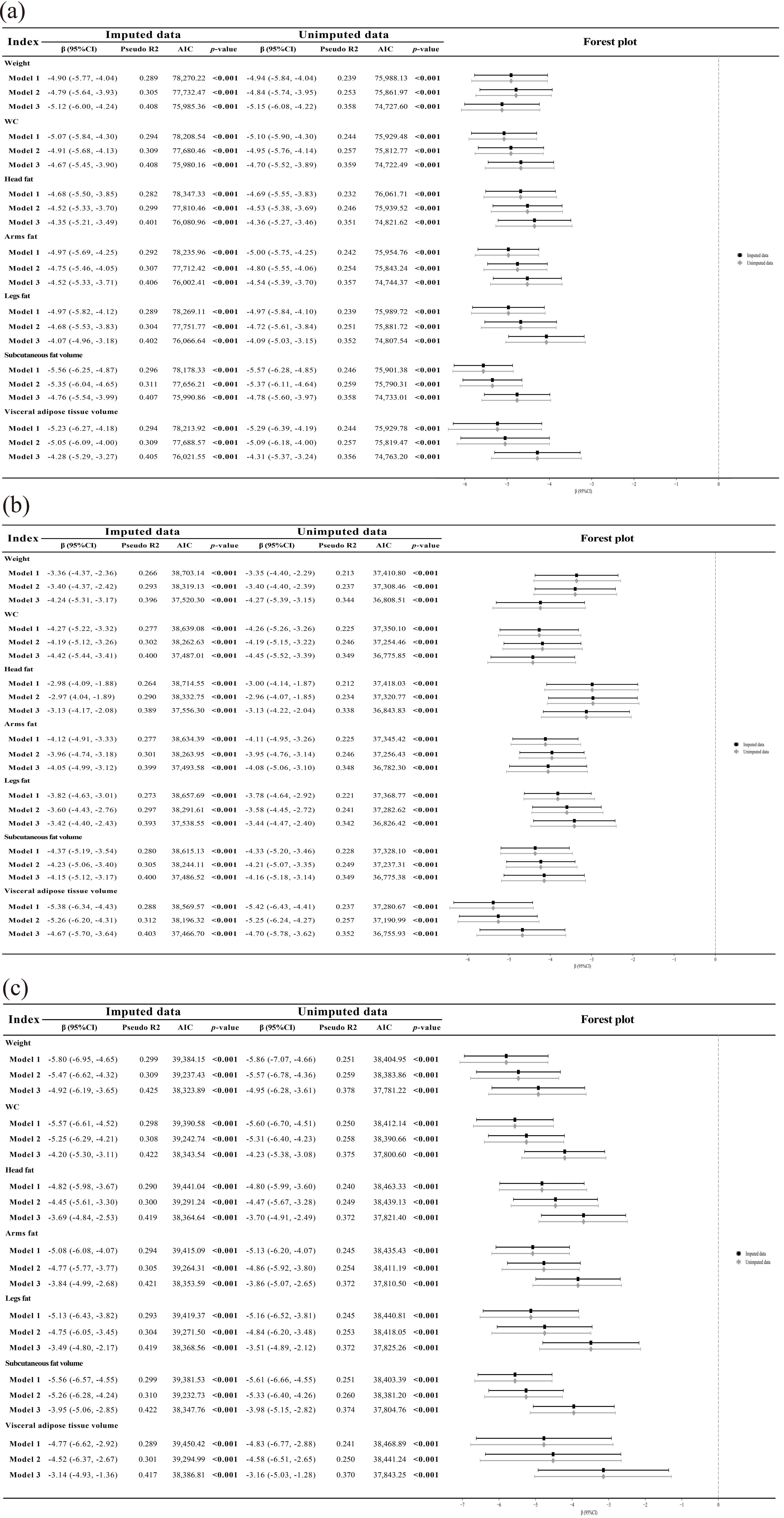 Fig. 2.
Fig. 2. Association between obesity indicators and serum 25(OH)D in total population (a), and male (b), and female subpopulations (c): Forest plot from multiple linear regression. Model 1 adjusted for demographic characteristics, including age, sex, height, ethnicity, education level, examination period and family income. Model 2 further adjusted for lifestyle factors, including smoking history, alcohol consumption, sunlight exposure, and physical activity. Model 3 incorporated dietary intake and supplements (VD, calcium, magnesium, and phosphorus), bone mineral content, and biomarkers including serum calcium and phosphorus, alkaline phosphatase, alanine aminotransferase, and creatinine.
To comprehensively consider the effects of body fat composition on serum 25(OH)D levels, we conducted a rigorous bidirectional stepwise regression analysis on the entire population, and the male and female subpopulations. Head fat, visceral adipose tissue, and subcutaneous fat were independent determinants in the entire population [head fat:
| Characteristics | Overall | Male | Female | ||||
| p-value | p-value | p-value | |||||
| Age (year) | 0.32 (0.22, 0.42) | 0.40 (0.29, 0.51) | 0.26 (0.14, 0.38) | ||||
| Ethnicity | |||||||
| Mexican American | Reference | ||||||
| Other Hispanic | 4.07 (1.40, 6.74) | 0.003 | 3.10 (–0.11, 6.30) | 0.058 | 5.28 (1.58, 8.98) | 0.005 | |
| Non-Hispanic White | 11.08 (9.01, 13.16) | 9.76 (7.24, 12.27) | 13.22 (10.25, 16.20) | ||||
| Non-Hispanic Black | –12.58 (–15.03, –10.13) | –16.13 (–19.03, –13.23) | –8.44 (–11.76, –5.12) | ||||
| Other Race | –0.34 (–2.89, 2.21) | 0.792 | –1.41 (–4.49, 1.67) | 0.369 | 1.26 (–2.02, 4.55) | 0.451 | |
| Examination period | |||||||
| November–April | Reference | ||||||
| May–October | 6.58 (4.70, 8.45) | 5.85 (3.86, 7.84) | 7.22 (4.71, 9.73) | ||||
| Family income-to-poverty ratio | 0.95 (0.49, 1.42) | 0.59 (0.07, 1.11) | 0.026 | 1.40 (0.60, 2.20) | 0.001 | ||
| Outdoors work day (seconds) | 0.83 (0.56, 1.11) | 0.76 (0.49, 1.04) | 0.82 (0.15, 1.49) | 0.016 | |||
| Outdoors not work day (seconds) | 0.81 (0.50, 1.11) | 0.71 (0.39, 1.03) | 0.91 (0.34, 1.49) | 0.002 | |||
| Vitamin D intake (µg/d) | 0.33 (0.12, 0.53) | 0.002 | 0.41 (0.19, 0.63) | 0.25 (–0.13, 0.62) | 0.196 | ||
| Magnesium intake (µg/d) | 17.30 (8.71, 25.89) | 11.99 (4.13, 19.85) | 0.003 | 22.89 (7.73, 38.06) | 0.003 | ||
| Phosphorus intake (µg/d) | –3.95 (–6.09, –1.81) | –3.06 (–5.33, –0.80) | 0.008 | –4.56 (–9.93, 0.81) | 0.096 | ||
| Vitamin D supplement intake (µg/d) | 0.13 (0.08, 0.19) | 0.21 (0.09, 0.32) | 0.001 | 0.11 (0.06, 0.17) | |||
| Calcium supplement intake (µg/d) | 17.21 (11.99, 22.42) | 4.51 (–3.66, 12.68) | 0.279 | 19.84 (13.57, 26.11) | |||
| Magnesium supplement intake (µg/d) | 5.07 (–9.81, 19.96) | 0.504 | 19.23 (–13.16, 51.62) | 0.244 | 4.20 (–13.96, 22.35) | 0.651 | |
| Creatinine (µmol/L) | 0.09 (0.04, 0.14) | 0.08 (0.03, 0.13) | 0.002 | 0.14 (0.04, 0.23) | 0.005 | ||
| Serum Calcium (mmol/L) | 20.74 (10.55, 30.93) | 23.18 (13.00, 33.36) | 19.36 (2.77, 35.95) | 0.022 | |||
| Total BMC (mg) | 5.23 (2.61, 7.84) | 4.84 (2.25, 7.43) | 3.30 (–1.77, 8.37) | 0.202 | |||
| Head fat (mg) | –9.24 (–16.46, –2.03) | 0.012 | –4.91 (–16.28, 6.46) | 0.397 | –11.02 (–23.48, 1.45) | 0.083 | |
| Sex | |||||||
| Male | Reference | ||||||
| Female | 10.54 (7.66, 13.41) | NG | NG | ||||
| Physicail activity (cal/d) | 0.09 (–0.04, 0.23) | 0.179 | 0.14 (0.04, 0.23) | 0.006 | NG | ||
| Subcutaneous fat (mg) | –3.40 (–4.93, –1.88) | –4.69 (–8.76, –0.61) | 0.024 | NG | |||
| Visceral adipose tissue (mg) | –6.74 (–12.66, –0.81) | 0.026 | –13.04 (–18.10, –7.99) | NG | |||
| Stay in the shade | |||||||
| Always, most of the time or sometimes | Reference | ||||||
| Rarely or never | 1.32 (–0.43, 3.07) | 0.139 | NG | 2.88 (0.13, 5.63) | 0.040 | ||
| Unknown | –2.57 (–5.83, 0.69) | 0.122 | NG | –1.18 (–6.20, 3.84) | 0.646 | ||
| Calcium intake (µg/d) | 1.53 (–0.99, 4.05) | 0.235 | NG | 3.04 (–1.82, 7.90) | 0.220 | ||
| Alkaline phosphatase (IU/L) | –0.06 (–0.09, –0.02) | 0.001 | NG | –0.12 (–0.17, –0.07) | |||
| Height (cm) | NG | –0.13 (–0.36, 0.09) | 0.246 | 0.24 (0.00, 0.48) | 0.055 | ||
| Weight (kg) | NG | 0.20 (–0.02, 0.42) | 0.068 | –0.20 (–0.28, –0.12) | |||
| Arms fat (mg) | NG | –1.36 (–3.26, 0.54) | 0.159 | ||||
| Phosphorus (mmol/L) | NG | –2.84 (–7.17, 1.49) | 0.198 | ||||
| Education level | |||||||
| Less than or equal to high school | Reference | ||||||
| Above high school | NG | NG | –1.88 (–4.69, 0.92) | 0.188 | |||
| Unknown | NG | NG | 6.89 (2.90, 10.87) | 0.001 | |||
Abbreviations: BMC, bone mineral content; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue; NG, not given.
Note: Family income-to-poverty ratio was calculated by dividing family (or individual) income by the poverty guidelines specific to the survey year. Bold p-values indicate statistical differences.
Our analysis of a nationally representative, contemporary sample of adults in the United States affirms an inverse association between body fat and serum 25(OH)D levels. While previous studies have highlighted the relationship between obesity and 25 (OH)D concentration using BMI [30, 31], it is acknowledged that BMI does not capture nuances in fat distribution. This study expands this correlation to distinct facets of adiposity distribution. In the linear regression model adjusting for demographics, lifestyle, diet, biomarkers, and BMC, weight, WC, and regional fat depots (arms, legs, head, subcutaneous, and visceral) all demonstrate robust inverse relationships with 25(OH)D, aligning with previous evidence [32, 33]. In the stepwise regression analysis, subcutaneous and visceral fat emerged as significant predictors of serum 25(OH)D levels, particularly in the male subpopulation, while weight was significantly correlated with serum 25(OH)D levels in women. These results suggest that both sex and body fat distribution play critical roles in determining the VD status.
The mechanistic basis underlying the relationship between fat and hypovitaminosis D warrants discussion. Adipose tissue serves as a major reservoir for VD and its metabolites, as evidenced by an animal study showing 75% of supplemented VD distributed to fat in pigs, with 35% as 25(OH)D, 30% in serum, 20% in muscle, and 15% elsewhere [18]. Furthermore, clinical trials have demonstrated that vitamin D3 supplementation increases 25(OH)D3 levels in both serum and subcutaneous fat [19, 20]. The sequestration of VD in fat likely contributes to the comorbidity of VD deficiency and obesity [7]. However, the differential association of specific fat depots with VD remains unclear and merits further interrogation.
While adiposity loss increases 25(OH)D [21, 22, 23], whether greater fat accretion reciprocally lowers 25(OH)D remains uncertain. VD slowly mobilises from fat into circulation [34]; however, the precise mechanisms are incompletely elucidated. Notably, all VD hydroxylation enzymes are expressed in adipocytes and adipose tissue, implying potential tissue-specific regulation [17, 35]. The differential expression and activity of metabolic enzymes across fat depots may thus drive the divergent associations observed here with VD. In obesity, adipose dysfunction occurs, characterised by hypertrophied adipocytes, inflammation, hypoxia, and reduced angiogenesis [36]. The nuclear VD receptor, abundant in adipocytes, mediates VD activities, influencing adipokines, energy metabolism, inflammation, oxidative stress, differentiation, and apoptosis [17]. VD deficiency could thereby disrupt adipocyte function [17, 36, 37]. Therefore, it is important to pay attention to VD levels in people with obesity. Our findings highlight the importance of visceral adipose tissue and subcutaneous fat, especially in men.
Beyond adiposity, our analysis spotlights diverse facets intricately linked to 25(OH)D status, including demographic attributes, seasonal/light exposure, dietary patterns, and select biomarkers, which demand tailored public health and clinical considerations. Unsurprisingly, reduced outdoor exposure on weekdays and weekends was linked to lower 25(OH)D levels in our study. Surprisingly, physical activity did not emerge as an independent predictor in our stepwise regression model. Sedentary lifestyles probably overlap with limited sun exposure. Promoting outdoor activities could offer synergistic benefits by boosting activity levels, light exposure, and VD status. Moreover, dietary and supplementary intakes of VD, calcium, phosphorus, and magnesium positively influenced serum 25(OH)D. Clinicians should carefully assess intakes of VD, calcium, magnesium, and phosphorus and recommend balanced nutritional support alongside targeted supplementation.
Our data were derived from the specific national representativeness of the NHANES in the United States, imparting a degree of generalisability to the study results. At the same time, we included some obesity-related indicators to provide refined insights. Confounder adjustment using multivariable regression lent validity to the observed associations. Sensitivity analyses performed after multiple data imputation verified the robustness of outcomes. However, the cross-sectional nature of the study limits causal interpretations. Further longitudinal and mechanistic studies can build on these data, offering nuanced insights for the development of clinical and public health strategies against obesity and VD deficiency. It is crucial to acknowledge that the study focused exclusively on adults (aged 20–59 years), who participated in DXA testing and have data on body fat distribution. Our findings, viz. such as the observed increase in vitamin D concentration with age, may differ from those of studies that include broader or different populations. These differences underscore the potential variability in results due to the unique characteristics of our study cohort. The findings from the study warrant corroboration in diverse demographic groups. Besides, there are still other potential factors that affect VD that were not included in the study.
This nationally representative study offers unequivocal evidence affirming the association between fat and hypovitaminosis D among adults. Abdominal fat emerged as predictors of 25(OH)D, emphasising the importance of assessing fat distribution rather than simply replacing it with BMI or WHtR. Besides anthropometric indicators, demographic characteristics, lifestyle factors, and dietary habits also have complex effects on VD. Personalising clinical decisions and public health interventions based on these parameters could help mitigate the heavy burden of obesity and hypovitaminosis D.
The data for this study were sourced from the Centers for Disease Control and Prevention’s National Health and Nutrition Examination Survey, which can be obtained through the official website (https://www.cdc.gov/nchs/nhanes/about nhanes.htm).
YB contributed to editorial changes in the manuscript. YB read and approved the final manuscript. YB have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
The study protocol was reviewed and approved by the National Center for Health Statistics ethics review board. The protocol numbers are Continuation of Protocol #2011-17 (NHANES 2011–2017) and Protocol #2018-01 (NHANES 2017–2018). Detailed information can be found on the NHANES website. Written informed consent was obtained from all participants.
I would like to thank Editage (https://www.editage.cn/) for English language editing.
This research received no external funding.
The author declares no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.


