1 School of Pharmacy, Harbin University of Commerce, 150076 Harbin, Heilongjiang, China
Abstract
This study aimed to elucidate the therapeutic efficacy and underlying mechanisms of the combining of Chuanxiong Rhizoma Hort. (CX) and Ganoderma lucidum Karst. (GL) in treating hypertension (HTN) induced by chronic oxidative stress (OS). This research provides novel insights into the development of anti-hypertensive agents within the scope of medicine and food homologues, using network pharmacology and in vivo experimental validation.
Active constituents and corresponding targets of CX and GL were respectively retrieved on the Traditional Chinese Medicine Systems Pharmacology (TCMSP) platform. Molecular docking was utilized to assess the binding efficacy between the constituents and core targets. Moreover, Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway and Gene Ontology Biological Process (GOBP) enrichment analyses were performed against the core targets. The anti-hypertensive effects of the combination were validated in the N-Nitro-L-arginine methyl ester (L-NAME)-induced hypertensive rat model; meanwhile, the potential mechanism of action was investigated through indices assay and pathological examination.
A total of 6 and 14 core active constituents of CX and GL, respectively, were identified, along with 30 and 39 potential corresponding targets. The molecular docking established prostaglandin-endoperoxide synthase 2 (PTGS2) as the target with the highest binding affinity for treating both HTN and OS. The KEGG pathway analysis revealed the presence of the “estrogen signaling” and “vascular endothelial growth factor (VEGF) signaling” pathways. Additionally, the GOBP analysis showed significant enrichment in the terms “positive regulation of nitric oxide (NO) biosynthetic process” and “negative regulation of smooth muscle contraction”. These findings highlight the shared pathways between CX and GL in relation to HTN and OS. Moreover, the in vivo experiments validated that the combined CX and GL treatment contributed to significantly decreasing systolic blood pressure (SBP) and serum Ang-Ⅱ levels, increasing aortic prostaglandin I2 (PGI2) and total antioxidant capacity (T-AOC), reducing aortic vascular cell adhesion molecule-1 (VCAM-1), reactive nitrogen species (RNS), and heart index, and improving the aortic damage in a synergistic pattern in the L-NAME-induced hypertensive rat model.
Administering the combination of CX and GL synergistically treated OS-induced HTN by improving vascular endothelial NO transduction, vasodilation, and anti-oxidative capacity, via co-regulation of the estrogen and VEGF signaling pathways. This finding provides a perspective for the development of novel therapeutic strategies in the treatment of HTN based on the dietary-medicinal properties of Chinese medicine in treatment of HTN.
Keywords
- Chuanxiong Rhizoma Hort.
- Ganoderma lucidum Karst.
- hypertension
- oxidative stress
- network pharmacology
- molecular docking
Hypertension (HTN), a condition characterized by persistently elevated blood pressure (BP) against arterial walls, arises from intricate interactions between genetic predisposition, environmental factors, and lifestyle choices [1]. Globally, HTN affects approximately one-fifth of the population, with the majority of cases occurring in adults aged 30 to 79 years old. Annually, HTN leads to about 10 million deaths, with the incidence rising all the time [2]. Furthermore, HTN is closely associated with the development of cardiovascular diseases, including stroke, ischemic heart disease, and coronary heart disease [3]. HTN represents a significant global public health challenge [4], necessitating the increase of public awareness, the improvement of management strategies, and the development of more effective and safer anti-hypertensive therapeutics.
Oxidative stress (OS) serves as a significant etiological factor in the pathogenesis of HTN. Under physiological conditions, the human body maintains a robust anti-oxidant defense system to effectively neutralize reactive oxygen species (ROS) and reactive nitrogen species (RNS) generated during cellular metabolism, thereby mitigating disease progression and senescence [5]. However, an imbalance ensues when the produced ROS and RNS surpasses the capacity of endogenous enzymatic and non-enzymatic anti-oxidants, leading to the accumulation of ROS and RNS, and ultimately, the occurrence of OS [6, 7, 8]. In this situation, excessive oxidative intermediates are generated, which induce endothelial cell damage and vascular dysfunction, and trigger inflammatory and immune responses that adversely affect the cardiovascular system. Consequently, the heart, vasculature, brain, kidneys, and other target organs are impaired, thereby promoting the development of HTN [9]. N-Nitro-L-arginine methyl ester (L-NAME), a non-specific nitric oxide synthase (NOS) inhibitor, competitively binds to and inhibits endothelial NOS to diminish NO bioavailability and synthesis. This action precipitates vasoconstriction, endothelial dysfunction, and heightened OS, ultimately culminating in HTN. As a result, L-NAME is frequently employed in the establishment of experimental models to investigate HTN induced by chronic OS [10].
Despite advancements in anti-hypertensive pharmacotherapy, effective BP control remains suboptimal, largely due to limitations in drug availability and adverse effects. Furthermore, current clinical medicine lacks sustained HTN effects, leading to frequent rebound of HTN. To address these concerns, a subset of patients seeks complementary and alternative medicine, including traditional Chinese medicine (TCM), to identify treatments with potentially enhanced efficacy and reduced adverse effects [11]. TCM, with over 2000 years of clinical application, is extensively utilized in cardiovascular disease management [12]. In recent years, the treatment of cardiovascular diseases with TCM has been vigorously discussed [13]. Featuring multi-target, multi-component, and holistic regulatory effects, TCM not only reduces BP but also ameliorates HTN-related clinical symptoms, improves quality of life, controls risk factors, and protects target organs like the heart, brain, and kidneys from damage [14]. Considering the TCM principle of “food and medicine homology”, the incorporation of food-based TCM with anti-hypertensive properties into daily diets or health supplements could potentially address the medical challenges associated with anti-hypertensive drug therapy, alleviate strain on healthcare resources, and possibly offer preventive benefits for hypertension [15, 16].
As typical TCM herbs documented in the Shennong Ben Cao Jing, Chuanxiong Rhizoma Hort. (CX) and Ganoderma lucidum Karst. (GL) have been used for thousands of years. The dried rhizome of the Apiaceae plant Ligusticum chuanxiong Hort., is traditionally recognized for its pharmacological effects of promoting blood circulation, regulating breathing, dispelling wind, and alleviating pain. Many contemporary studies have validated that CX can be used for effectively treating atherosclerosis [17, 18, 19] and hypertension [20], among other cardiovascular pathologies. Nowadays, lots of classic prescriptions containing CX have been developed into proprietary Chinese medicines. For example, Dachuanxiong Koufuye and Fufang Chuanxiong Pill can enhance blood circulation and regulate hepatic function to alleviate wind-induced conditions [21]. GL, the dried fruiting bodies of Ganoderma lucidum Karst. or Ganoderma sinense, is distinguished within the fungal kingdom for their notable medicinal properties, frequently applied in both pharmaceutical applications and dietary supplements [22]. The Compendium of Materia Medica, the first Chinese pharmacopoeia, records that in 1590 during the Ming Dynasty, GL has been attributed with therapeutic properties, such as tonifying the kidney, enhancing vital energy, strengthening cardiac function, increasing memory, and exhibiting anti-aging effects. Contemporary research indicates that GL demonstrates significant therapeutic potential in the management of HTN [23]. Since the anti-hypertensive effects of the combination are rarely explored, this study aims to investigate the synergistic anti-hypertensive effects and mechanism of the herbal pair through computational prediction and experimental validation, thereby providing a theoretical foundation for exploring its therapeutic potential in HTN management.
On the Traditional Chinese Medicine Systems Pharmacology Database and Analysis
Platform (TCMSP, https://www.tcmsp-e.com/tcmsp.php), the keywords “Chuanxiong” (CX in
Chinese) and “Lingzhi” (GL in Chinese) were input with two critical indicators
of clinical application for traditional Chinese medicine as the screening
criteria [24]: Oral bioavailability (OB)
Utilizing the databases Genecards (https://www.genecards.org/), TTD (https://db.idrblab.net/ttd/), and OMIM (https://www.omim.org/), a comprehensive search was conducted using the keywords “hypertension” and “oxidative stress” to identify all pertinent targets HTN and OS, respectively. The resultant datasets were then subjected to an intersection operation, with the redundant entries eliminated.
On the BioLadder bioinformatics platform (https://www.bioladder.cn/web/#/pro/cloud), the intersection of active components and the predicted targets for CX and GL associated with HTN or OS was determined, respectively. A Venn diagram was in turn generated. A network diagram was constructed using the software Cytoscape (version 3.10.2. Institute for Systems Biology, Seattle, WA, USA).
The identified intersection targets were subsequently imported into the STRING
12.0 platform (https://string-db.org/), with “Homo sapiens” designated as the
selected organism. The “Minimum Required Interaction Score” was set as
“
According to the degree values, the top five key targets were selected for the docking analysis, following the screening of the primary active components of CX and GL with the targets identified through PPI analysis. Initially, the 3D structures of the relevant genes were retrieved from the Uniprot and PDB (http://www.rcsb.org/) databases. Subsequently, crystallographic water molecules and other small molecules were removed from the receptor protein structures using software PyMOL (version 3.0.3. DeLano Scientific LLC, San Carlos, CA, USA), thereby establishing the receptor molecules for subsequent analysis. Subsequently, the “mol2” format of the primary constituents of CX and GL, serving as ligands, was obtained from the TCMSP database. The molecular docking was performed using software AutoDockTools (version 1.5.7. Olson Laboratory of Scripps Institute, San Diego, CA, USA), and the “optimal docking pose” was selected as the result of the molecular docking analysis. The binding energy reflects the stability of the interaction between the active component and the key target protein; a lower binding energy indicates a more stable docking conformation. The results were visualized using PyMOL software.
All the identified core targets were imported into the DAVID platform (https://davidbioinformatics.nih.gov/home.jsp). The Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses and GO biological process (GOBP) enrichment analysis of the targets were performed under a p-value threshold of less than 0.05. The resultant data were visualized to facilitate the prediction of potential functional pathways.
Male 6-week-old Sprague-Dawley rats were purchased from Changsheng Biotechnology Co., Ltd., Benxi, Liaoning, China. Feeding conditions: Ad libitum food and water intake, artificial light illumination (12 h light/dark cycle), 24 °C temperature, 40%–60% relative humidity and adaptive feeding for 7 days. Laboratory animal permission license number: SYXK(Hei)2024-012. The research protocol was approved by the Animal Research Ethics Committee of Harbin University of Commerce (Approval No.: HSDYXY-2024066).
2.2.2.1 Experimental Devices
Non-invasive blood pressure measurement system for rats (Equipment No. KW2022112427, Calvin Biotechnology Co., Ltd., Nanjing, Jiangsu, China); ST-360 microplate reader (Equipment No. 20212220416, Kehua Experimental System Co., Ltd., Shanghai, China); LE204E/02 electronic balance (Equipment No. BSA224S, Sartorius Instrument System Engineering Co., Ltd., Beijing, China); 2135 rotary microtome (LEICA Biosystem, Nussloch, Germany).
2.2.2.2 The Herbal Products and Preparation
The raw Chuanxiong Rhizoma Hort. (CX) and Ganoderma lucidum Karst. (GL) were purchased from Nanjing Tongrentang, the specialty store for Chinese herbal medicinal materials (both harvested at Bozhou in Anhui Province, China, in August. Lot No.: 6124442351 and 5917441916).
The quality control process was carried out referring to the Chinese Pharmacopoeia (Edition 2020) [21], The products were both authenticated as the genuine products in the premier quality by Prof. Qu Zhongyuan from school of Pharmacy, Harbin University of Commerce. The quantitative analysis revealed that the ferulic acid content in CX is 0.13% in average, which is higher than the criterion 0.10%; the total content of triterpenoid and sterols in GL was measured as 0.97% in average, which is higher than the criterion 0.50%. Both of the herbal products comply with the criterion outlined in the 2020 Chinese Pharmacopoeia (see Supplementary Material 1 for details).
The authenticated products were grinded into ultrafine powder, dissolved in distilled water and thoroughly mixed right before the administration. The gavage volume for the mixed solution of the products was 1.0 mL/100 g (body weight).
2.2.2.3 Experimental Reagents
L-NAME (Dalian Meilun Biotechnology Co., purity
HE (hematoxylin-eosin staining) reagents: 10% neutral buffered formalin (NBF), Harris hematoxylin, alcohol-soluble eosin Y, 1% acid alcohol, 95% ethanol, xylene, Scott’s tap water substitute, neutral balsam.
Reagent kits: rat Angiotensin II (AngII) ELISA research kit (JM-01618R1. Jingmei Biotechnology Co., Ltd., Yancheng, Jiangsu, China), rat prostaglandin I2 (PGI2) ELISA research kit (JM-02114R2. Jingmei Biotechnology Co., Ltd., Yancheng, Jiangsu, China), rat vascular cell adhesion molecule-1 (VCAM-1) ELISA research kit (JM-10755R2. Jingmei Biotechnology Co., Ltd., Yancheng, Jiangsu, China), total antioxidant capacity (T-AOC) assay research kit (FRAP method) (A015-3-1. Jiancheng Bioengineering Institute, Nanjing, Jiangsu, China), and reactive nitrogen species (RNS) assay research kit (Microwell plate method) (A013-2-1. Jiancheng Bioengineering Institute, Nanjing, Jiangsu, China).
SD rats were randomly divided into the blank control group and model induction group. The model induction group was given 6 mg/kg L-NAME dissolved in distilled water by gavage every day, while the blank control group was given the same volume of distilled water. Four weeks later, rats with a systolic blood pressure (SBP) increase of more than 20 mmHg were selected as models for the next experiments.
2.2.4.1 Effective Dosage of CX and GL Determination
The modelled hypertensive rats were randomly divided into the following administration groups: CX lower-, medium- and higher- dosage groups (CX-L: 50 mg/kg, CX-M: 100 mg/kg, CX-H: 300 mg/kg) and GL lower-, medium- and higher- dosage groups (GL-L: 50 mg/kg, GL-M: 100 mg/kg, GL-H: 300 mg/kg), while the blank group and model group were given an equal volume of solvent by gavage for 3 weeks. The administration of intragastric L-NAME was continued during that of the drugs, with a 12-hour interval. SBP of each group was measured once a week during the administration.
2.2.4.2 Combination of CX and GL Administration
The modelled hypertensive rats were randomly divided into the following administration groups: CX group (CX, 100 mg/kg), GL group (GL, 100 mg/kg), CX and GL combination group (Combo, 100 mg/kg for each), Captopril group (Capt, 10 mg/kg), and the blank group and model group were given an equal volume of solvent by gavage for 3 weeks. The administration of intragastric L-NAME was continued during that of the drugs, with a 12-hour interval. SBP of each group was measured once a week during the administration, and the combination index (CI) value [25] was calculated by the Chou-Talalay method [26] to analyze the synergistic effect.
The rats were weighed and i.p. anesthetized with 42 mg/kg 3% sodium pentobarbital after 16 h fasting following the last administration. The dosage was strictly calculated based on the animal’s body weight, with the volume of 1.5 mL/kg. During the administration, the warmth was maintained. Death was confirmed by absent respiration and no toe-pinch response. The serum, heart and thoracic aorta were then carefully collected. The concentration of serum AngⅡ and aortic PGI2, VCAM-1, RNS, T-AOC were detected using the ELISA kits. The heart was weighed to calculate the heart index.
The same part of the thoracic aortas for each individual was prepared for the HE
staining, following a regular procedure: 10% NBF fixation for over 24 hours
All data were analyzed and processed using the software IBM SPSS Statistics
(version 26.0. Chicago, IL, USA). The results were expressed as “mean
3.1.1.1 Screening of Active Constituents for CX and GL
On the TCMSP platform, 189 active components of CX and 242 active components of
GL were retrieved. Using “OB
3.1.1.2 Predicted Targets of CX and GL
On the TCMSP platform, 30 and 39 potential targets were identified associated with the active components of CX and GL (see Supplementary Material 2-Tables 3,4), respectively. 57 related targets for the combined use of CX and GL were obtained (see Supplementary Material 2-Table 5).
3.1.1.3 Analysis of Targets Related to HTN and OS
Adopting the median value once in the GeneCards database, 6840 and 7013 protein targets were identified associated with HTN and OS, respectively. 100 HTN-related and 2 OS-related protein targets were yielded in the TTD database. 812 HTN-related and 811 OS-related protein targets were retrieved from the OMIM database. 73 HTN-related and 210 OS-related protein targets were identified in the Gene Map database. By summary of the protein targets obtained from each database and the redundancies elimination, a total of 7331 unique HTN-related protein targets (see Fig. 1A) and 7519 unique OS-related protein targets (see Fig. 1B) were obtained presented by the Venn diagram.
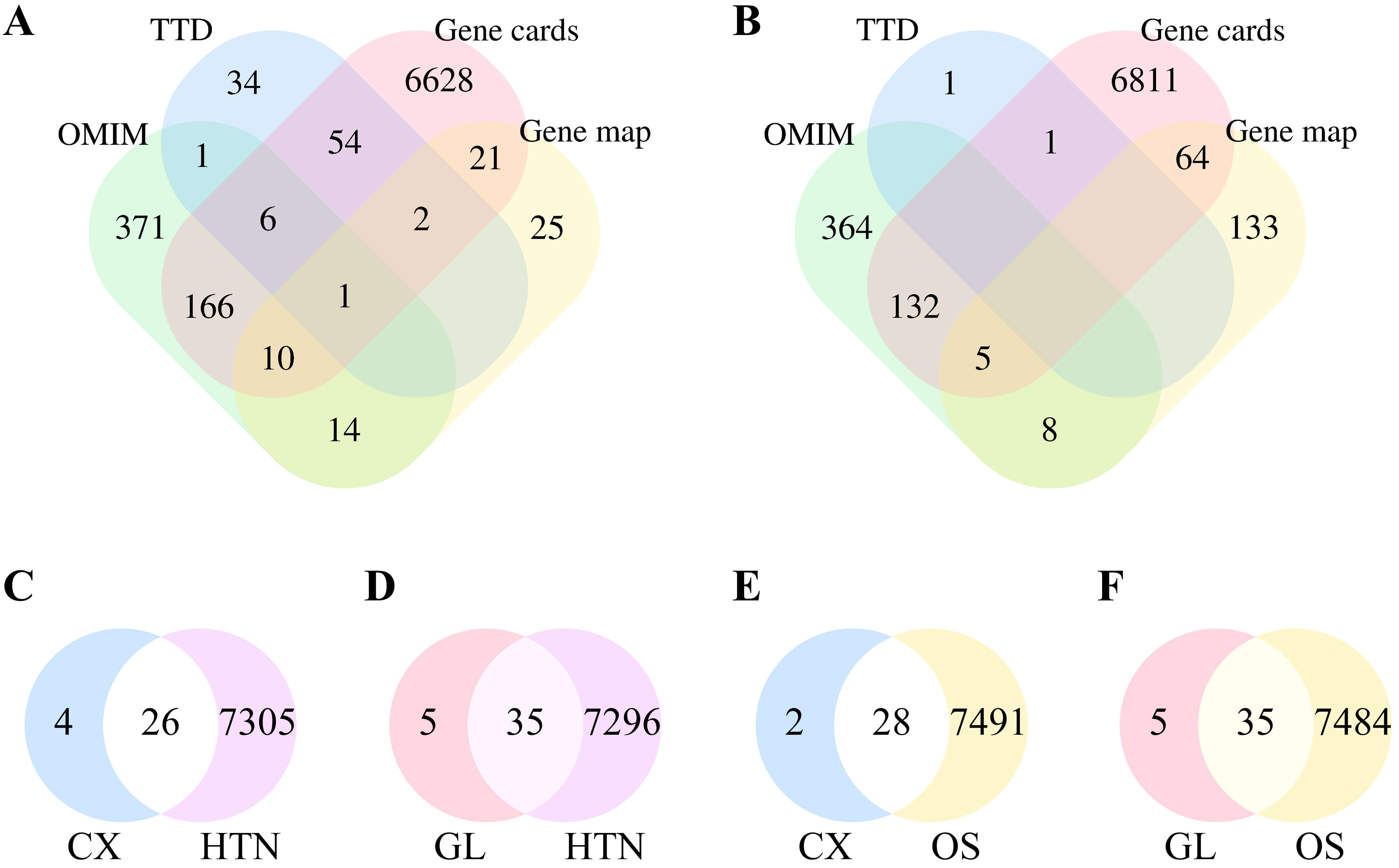 Fig. 1.
Fig. 1.
Results of targets analysis presented. (A) Number of targets associated with HTN. (B) Number of targets associated with OS. (C) Intersection of targets between CX and HTN. (D) Intersection of targets between GL and HTN. (E) Intersection of targets between CX and OS. (F) Intersection of targets between GL and OS. HTN, hypertension; CX, Chuanxiong Rhizoma Hort.; GL, Ganoderma lucidum Karst.; OS, oxidative stress.
3.1.2.1 Network Construction
The intersection of the action targets of CX and GL combined with HTN and OS disease was retrieved based on the results above (see Supplementary Material 2-Table 6). 26 mutual targets were collected between the main active components of CX and HTN (see Fig. 1C), 35 ones were collected between the main active components of GL and HTN (Fig. 1D), 28 ones were collected between the main active components of CX and OS (Fig. 1E), 35 ones were collected between the main active components of GL and OS (Fig. 1F).
The “active components–targets” network was shown in Fig. 2. The left side illustrated the six active components of CX and their corresponding target genes; the right side illustrated the five active components of GL and their corresponding target genes; the middle section represented the 12 common target genes of the active components of both.
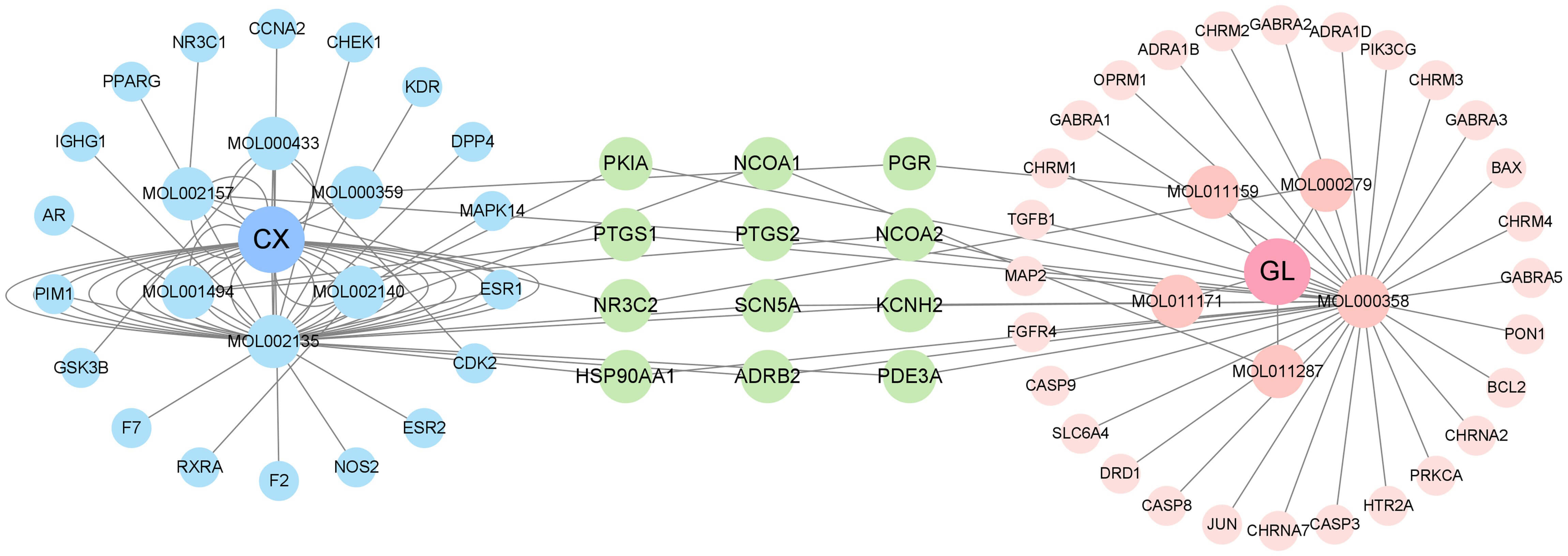 Fig. 2.
Fig. 2.
The active components - targets network in combination of CX and GL. The middle section represented the 12 common target genes of the active components of both (see Supplementary Material 2-Tables 1–5 for more details).
3.1.2.2 Core Targets Identification
PPI networks were constructed to identify core targets of CX and GL, as well as their intersections with HTN and OS (see Fig. 3A–D).
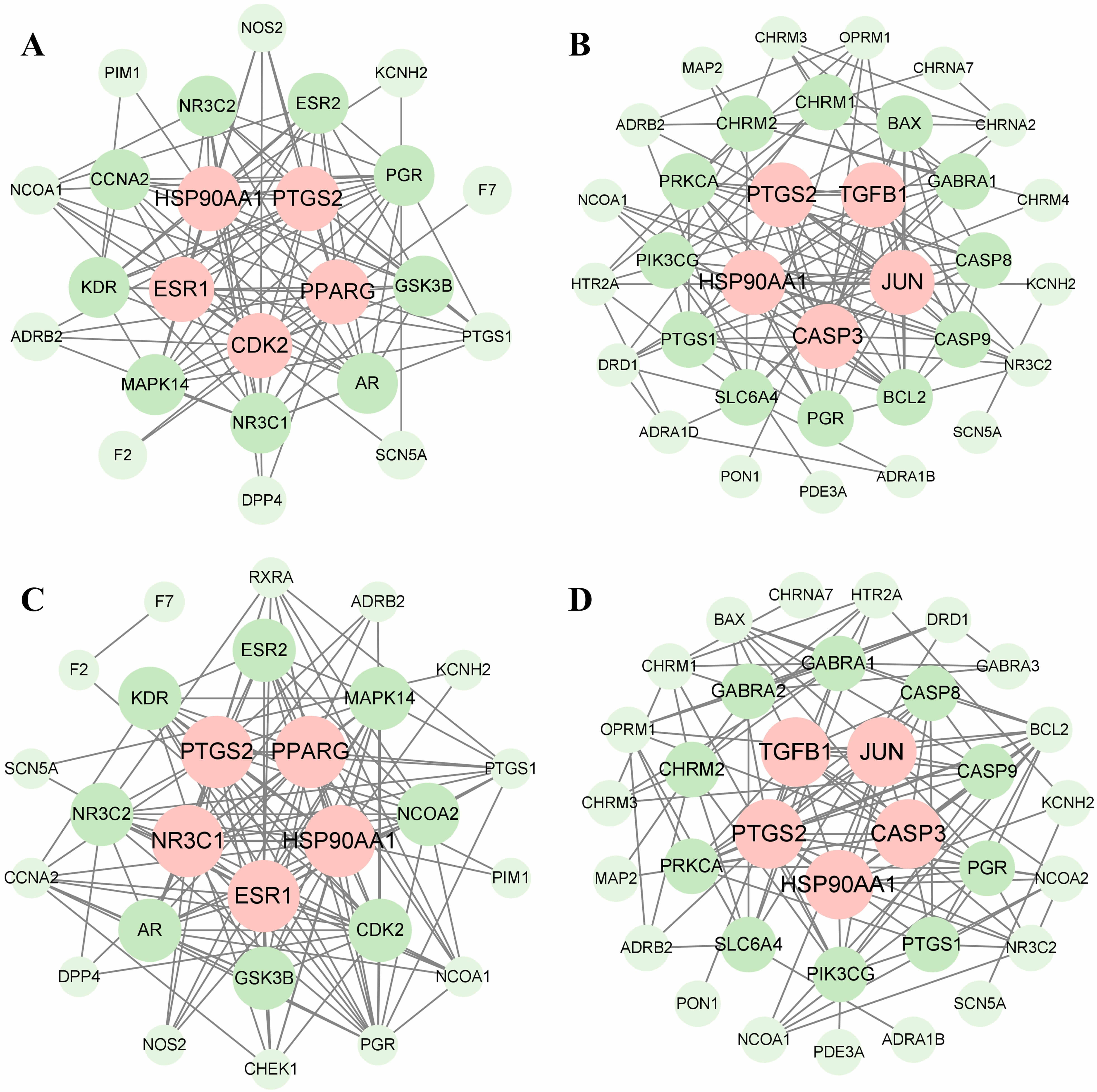 Fig. 3.
Fig. 3.
Results of core targets identification. (A) PPI network of CX and HTN-related proteins. (B) PPI network of GL and HTN-related proteins. (C) PPI network of CX and OS-related proteins. (D) PPI network of GL and OS-related proteins. The core targets are aligned in the center and marked as pink. PPI, protein–protein interaction.
Molecular docking was performed for the top 5 key targets and active components selected from the PPI networks based on degree values (see Supplementary Material 2-Table 7). A total of 100 ligand-receptor docking results were obtained (see Fig. 4A,B). The pairs perlolyrine (from CX) – prostaglandin-endoperoxide synthase 2 (PTGS2) and lucidone A (from GL) – PTGS2 were docked with the lowest binding energy (–9.8 kcal/mol and –9.2 kcal/mol) respectively, for both HTN and OS (see Fig. 4A,B and Fig. 5A,B).
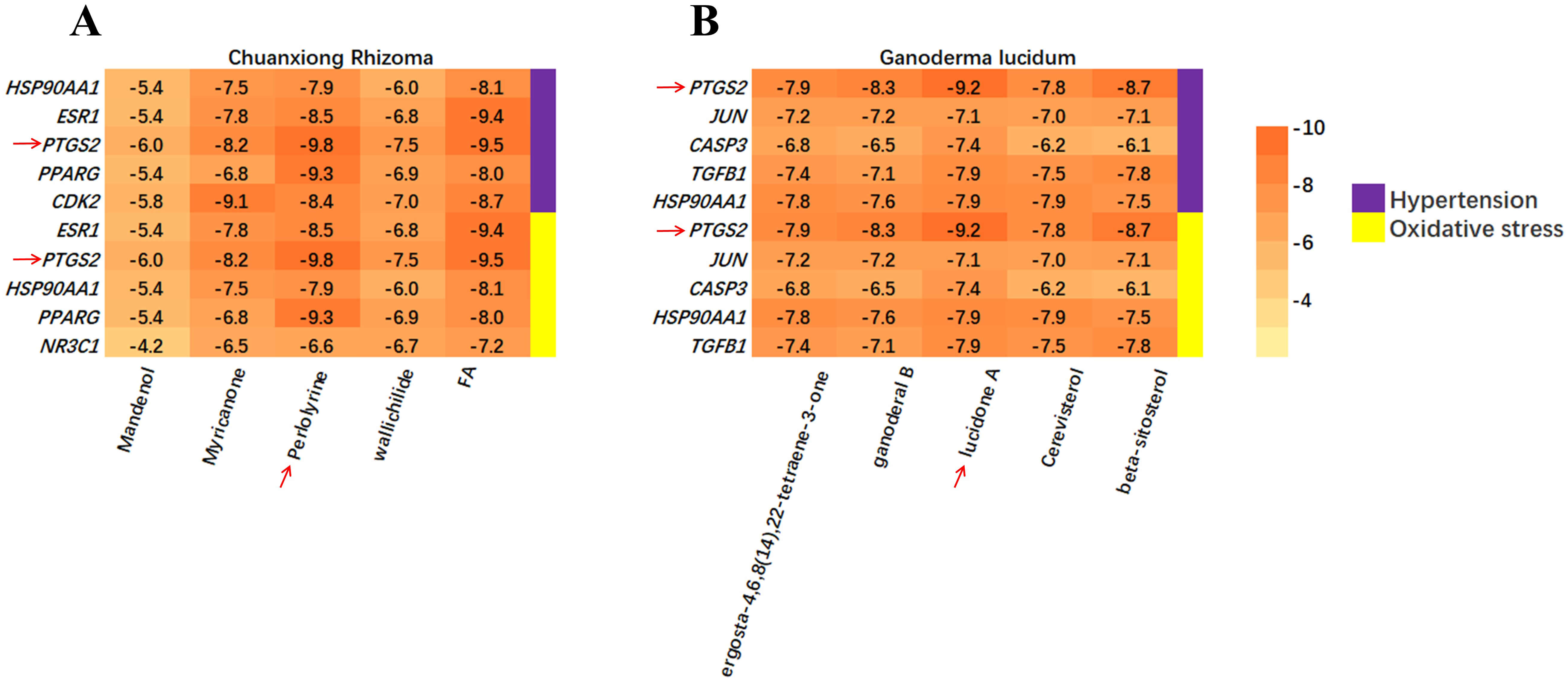 Fig. 4.
Fig. 4.
Calculation of the binding energy. (A) Binding energy between core targets and constituents of CX. The red arrows indicate PTGS2 and perlolyrine. (B) Binding energy between core targets and constituents of GL. The red arrows indicate PTGS2 and lucidone A. PTGS2, prostaglandin-endoperoxide synthase 2.
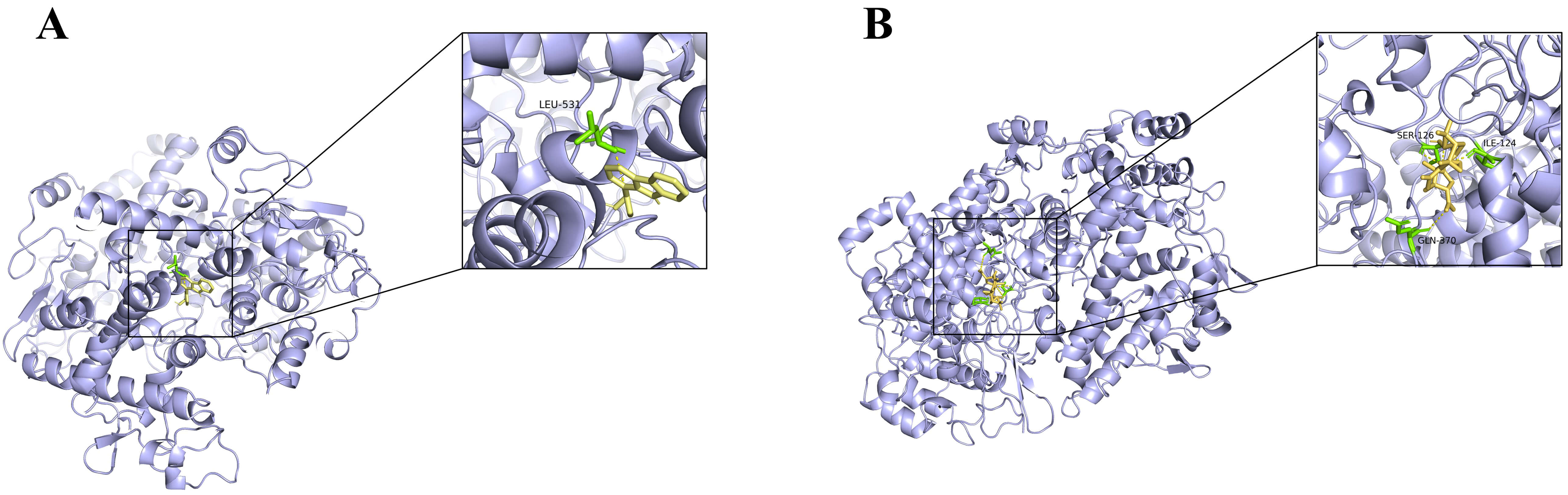 Fig. 5.
Fig. 5.
Analog images of docking. (A) Analog image of docking between Perlolyrine (from CX) and PTGS2. (B) Analog image of docking between Lucidone A (from GL) and PTGS2.
The KEGG enrichment analysis was performed on the identified core targets of CX and GL, respectively, associated with HTN or OS. The top 30 signaling pathways were selected for each by sorting the p values in ascending order, to form the bubble charts (see Fig. 6A–D). The mutual KEGG pathways “Estrogen signaling pathway” and “VEGF (vascular endothelial growth factor) signaling pathway” triggered by CX and GL, which were highly associated with HTN and in endothelial Nitric Oxide Synthase (eNOS) was involved in, were screened out of the 12 mutual ones between 12 HTN - related and 15 OS - related terms (see Supplementary Material 2-Tables 8,9); the core targets and corresponding biological functions involved in the pathway were illustrated as in Fig. 7.
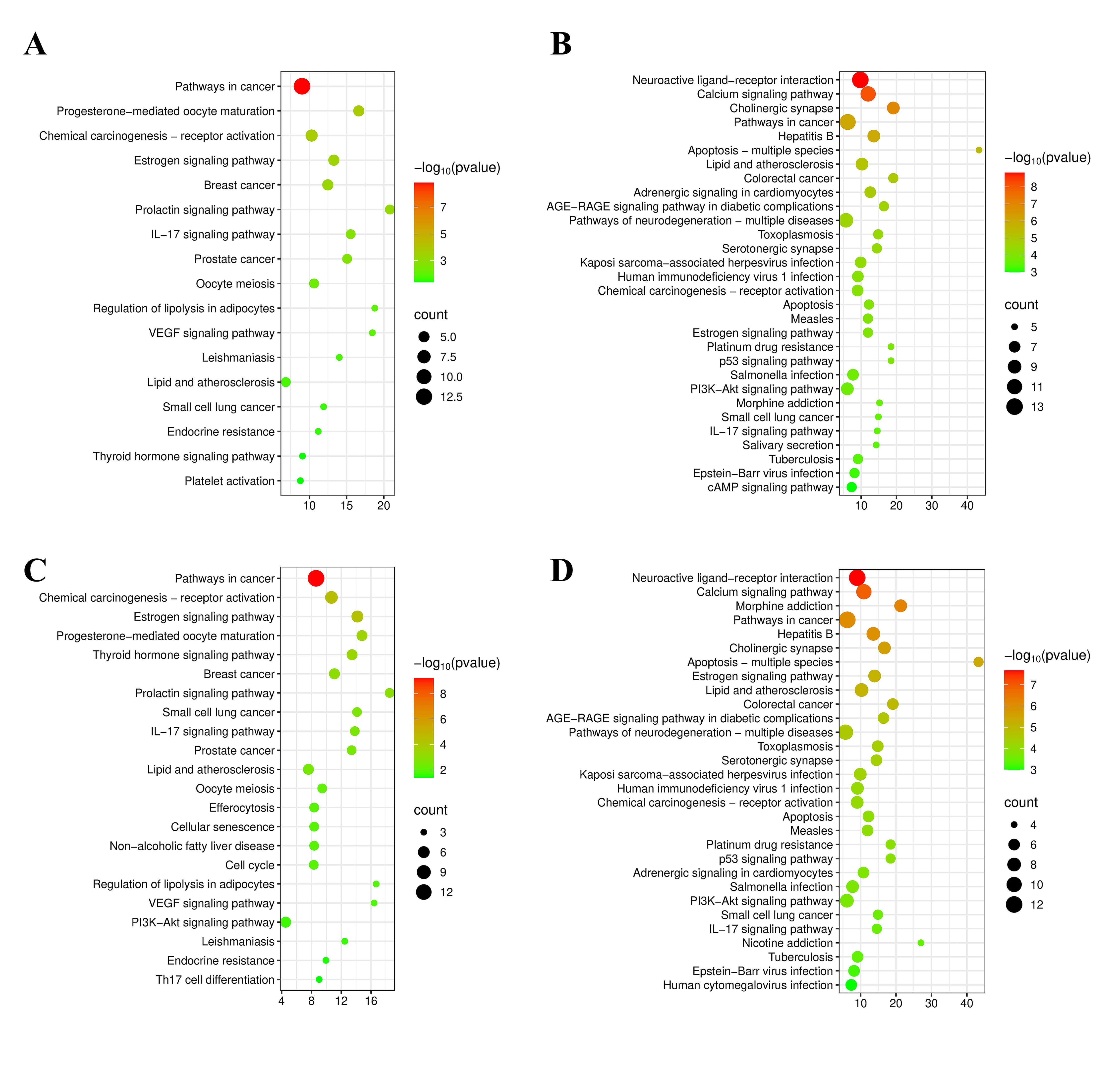 Fig. 6.
Fig. 6.
Enrichment analysis results. (A) Enriched KEGG pathways for CX associated with HTN. (B) Enriched KEGG pathways for GL associated with HTN. (C) Enriched KEGG pathways for CX associated with OS. (D) Enriched KEGG pathways for GL associated with OS. KEGG, Kyoto Encyclopedia of Genes and Genomes.
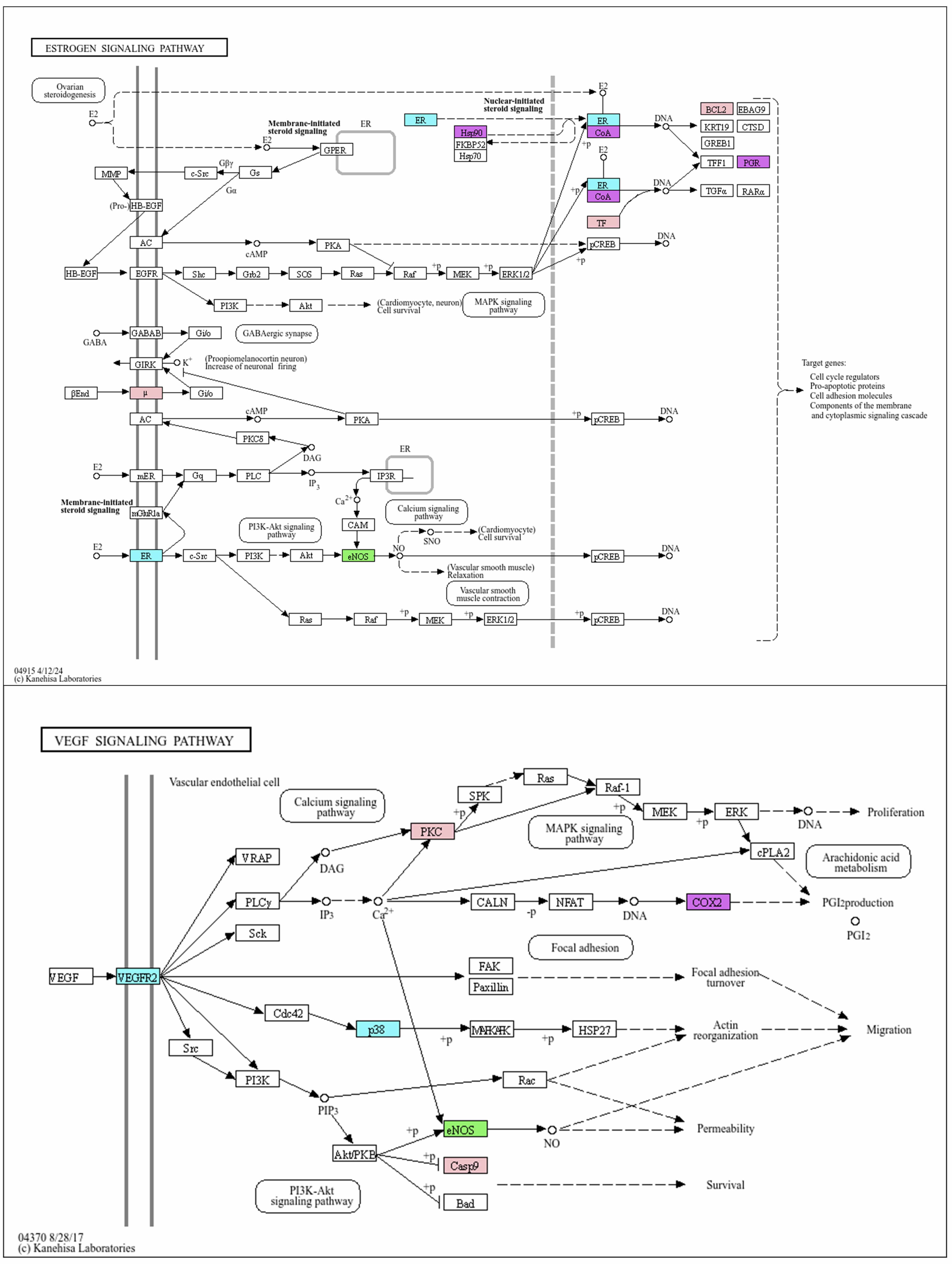 Fig. 7.
Fig. 7.
Mutual enriched KEGG pathways triggered by CX and GL: Estrogen signaling pathway and VEGF signaling pathway. Target of L-NAME (green): eNOS; targets of CX (blue): ESR (ESR1 and ESR2), VEGFR2 (or KDR), p38 (or MAPK14); targets of GL (pink): µ (OPRM1), TF (transcription factor: JUN), BCL2, PKC (or PRKCA), Casp9 (CASP9); mutual target of CX and GL (purple): CoA (NCOA1), Hsp90 (HSP90AA1), PGR, COX2 (or PTGS2). VEGF, vascular endothelial growth factor; L-NAME, N-Nitro-L-arginine methyl ester; eNOS, endothelial Nitric Oxide Synthase; VEGFR2 (KDR), Vascular Endothelial Growth Factor Receptor 2; p38 (or MAPK14), mitogen-activated protein kinase; µ (OPRM1), mu-1 opioid receptor; TF (JUN), Jun Proto-Oncogene; BCL2, Apoptosis regulator Bcl-2; PKC (PRKCA), Protein kinase C alpha type; Casp9 (CASP9), Caspase-9; CoA (NCOA1), Nuclear receptor coactivator 1; Hsp90 (HSP90AA1), Heat shock protein HSP 90-alpha; PGR, Progesterone receptor; COX2, Cyclooxygenase-2.
The GOBP enrichment analysis was performed on the targets as well. The number of significantly enriched GOBPs was shown in Fig. 8A,B. The mutual significantly enriched GOBP terms between CX and GL “positive regulation of nitric oxide (NO) biosynthetic process” and “negative regulation of smooth muscle contraction”, which were the most highly related to hypertensive pathology given prior knowledge, were screened out of the 9 mutual ones between 19 HTN-related and 10 OS-related terms (see Supplementary Material 2-Tables 10,11).
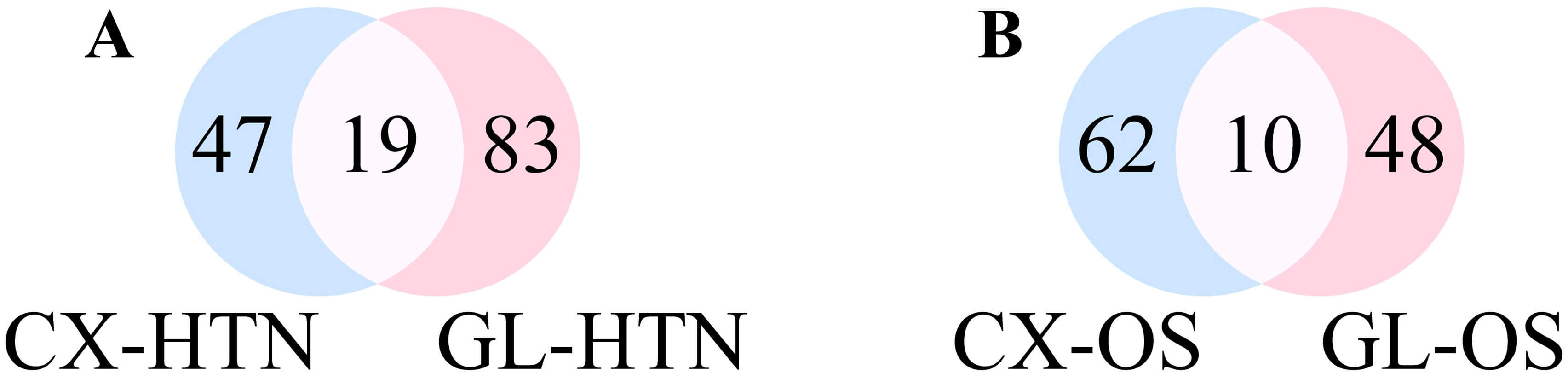 Fig. 8.
Fig. 8.
Number of the enriched GOBP terms. (A) Number of enriched GOBPs for core targets of CX and GL associated with HTN. (B) Number of enriched GOBPs for core targets of CX and GL associated with OS. GOBP, Gene Ontology Biological Process.
The constructed network of components - targets - pathways and biological processes indicated that the combination of CX and GL could synergistically improve the HTN induced by NO depletion, by regulating the vascular NO signaling conduction and smooth muscle contraction via the Estrogen and VEGF signaling pathway in common.
The SBP of both CX and GL groups significantly decreased compared with that of the model group after three-week administration (see Fig. 9), indicating that the dosing range of 50–300 mg/kg exerted a significant anti-hypertensive effect on the L-NAME induced hypertensive rat model. The medium dosage (100 mg/kg) for both was applied in the experiments on the combination.
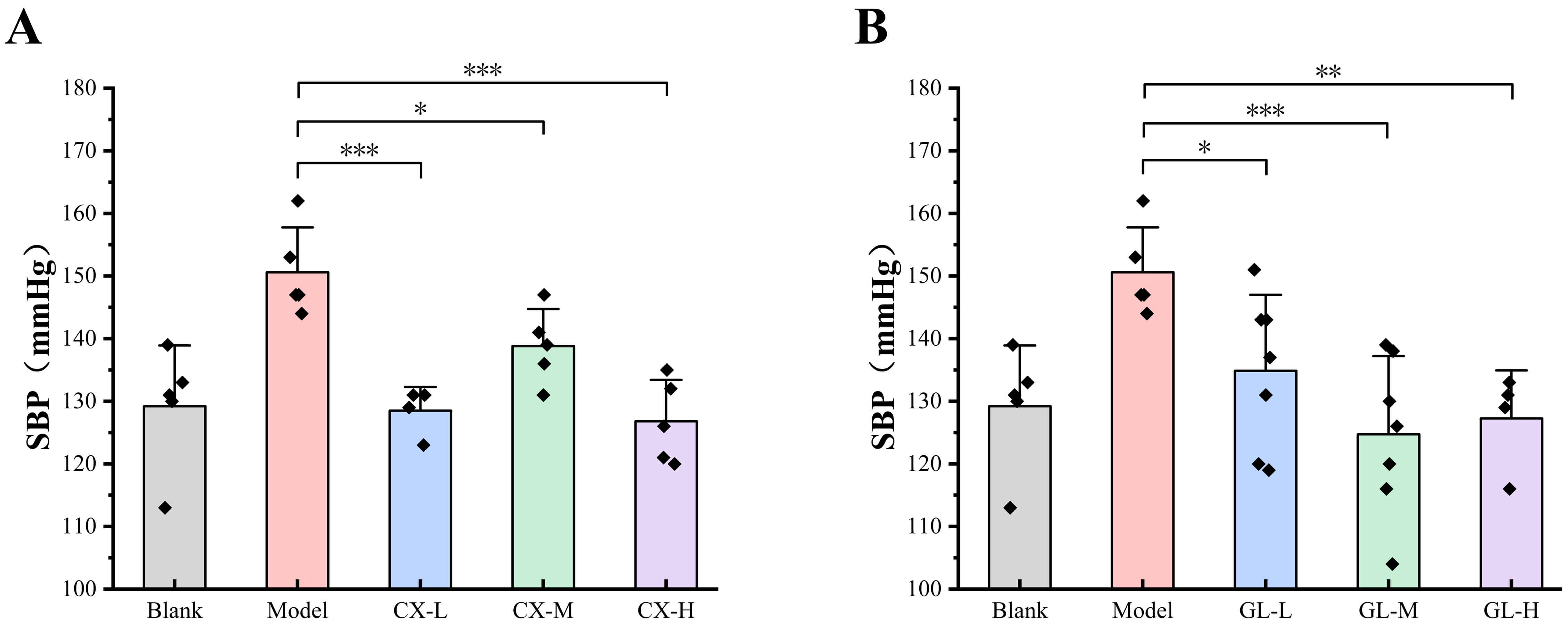 Fig. 9.
Fig. 9.
Effective dosage of single drug administration. (A) SBP of CX
groups after three-week administration. (B) SBP of GL groups after three-week
administration. Lower dosage (L): 50 mg/kg; Medium dosage (M): 100 mg/kg; Higher
dosage (H): 300 mg/kg. Compared with the model group, *p
The SBP of the Combo group exhibited constant significant decrease compared with
that of the model group during the three-week administration (see Fig. 10A). The
CI values were all less than 1 at different time points (0.506 for week 1, 0.641
for week 2, 0.880 for week 3), demonstrating synergistic anti-hypertensive
effects between CX and GL. The concentration of serum AngII of all administration
groups was significantly lower than that of the model group (p
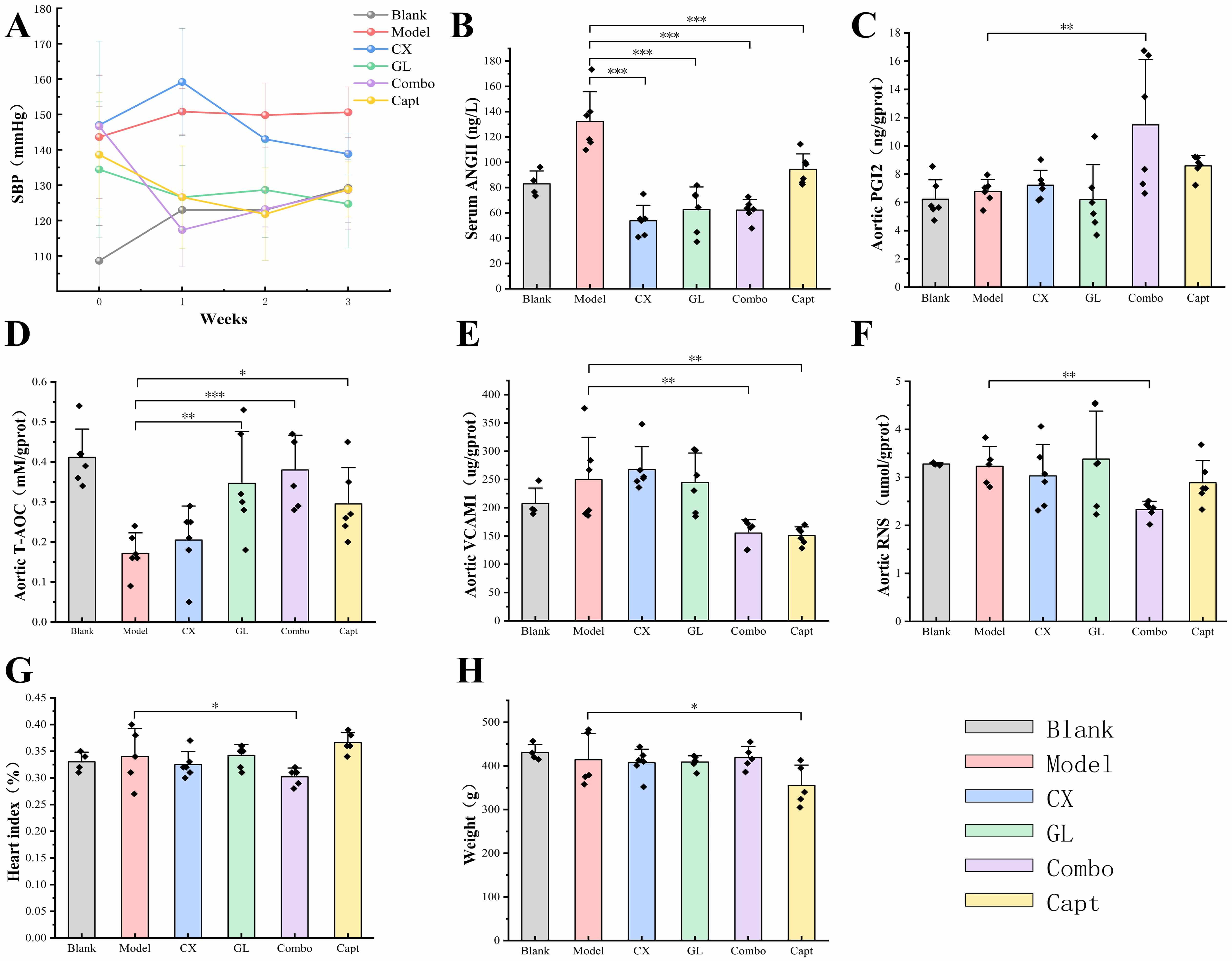 Fig. 10.
Fig. 10.
Results of indicators determination. (A) SBP tendency during
three-week administration. CI: week1 = 0.506, week2 = 0.641, week3 = 0.880. (B)
Serum AngII concentration. (C) Aortic PGI2 expression. (D) Aortic T-AOC
expression. (E) Aortic VCAM-1 expression. (F) Aortic RNS expression. (G) Cardiac
index. (H) Body weight. Compared with the model group *p
The index measurements revealed that, compared with the model group, the aortic
expressions of PGI2, T-AOC, VCAM-1 and RNS and the value of heart index were all
significantly different for the Combo group (see Fig. 10C–G). By contrast, those
indices of the Combo group all performed more remarkably than those of the CX, GL
or Capt group. Specifically, the aortic expressions of PGI2, VCAM-1 and RNS and
the heart index showed no significant difference with those of the model when CX
or GL was administered alone. In addition, the body weight of the Capt group
significantly decreased compared with that of the model group (p
Fig. 11 presents the results of the HE staining of the thoracic aorta. The intact endothelial structure and vessel tissue were constantly observed in the blank group, with normal morphology and clear boundaries of smooth muscle cells in the tunica media, and abundant, regularly arranged collagen fibers in the outer layer. In the model group, extensive loss of endothelial structure (black arrows) was identified in the vessel tissue, while hydropic degeneration of smooth muscle cells in the tunica media was evident, with cell swelling and pale staining of the cytoplasm (red arrows). Both the CX group and the GL group showed a small amount of endothelial structure loss in the vessel tissue, and a small amount of hydropic degeneration of smooth muscle cells in the tunica media, with pale staining of the cytoplasm. In the Combo group, the vascular endothelial structure exhibited minor focal disruptions. Vascular smooth muscle cells maintained normal morphology, with better overall structural integrity compared with the model group. No significant infiltration of inflammatory cells, edema, or aberrant proliferation of fibrous tissue was observed in the Combo group, indicating a preserved vascular architecture. The results of the Capt were similar to those of the Combo group.
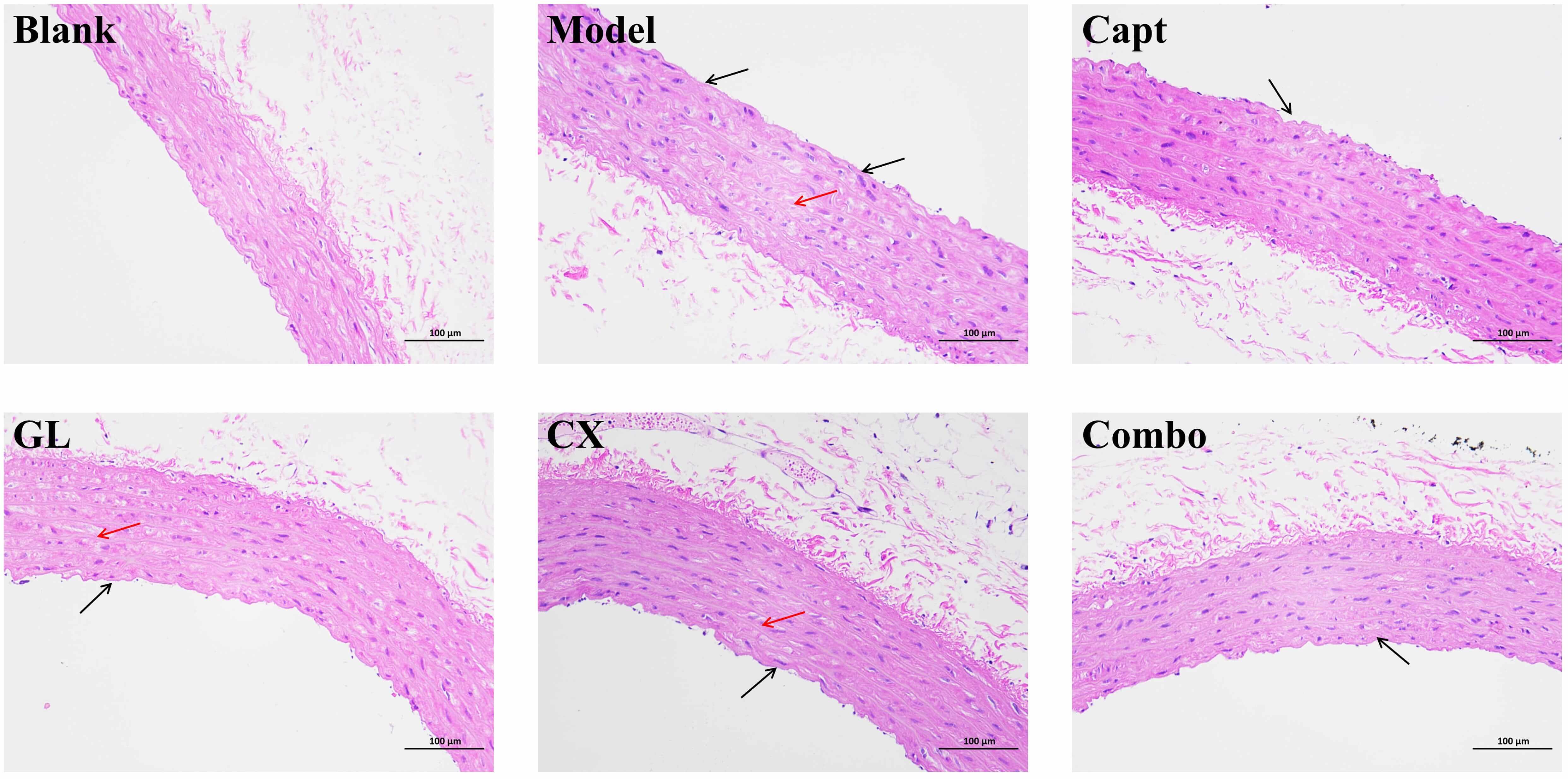 Fig. 11.
Fig. 11.
HE staining of thoracic aorta (200
The workflow of the project is shown in Fig. 12. Using network pharmacology dependent on the platforms specifically for the TCM, 6 and 14 core active constituents of CX and GL were identified along with 30 and 39 potential corresponding targets, respectively. The molecular docking established the PTGS2 (prostaglandin-endoperoxide synthase 2, or Cyclooxygenase-2 (COX2)) as the target with the highest binding affinity involved in both HTN and OS for the active constituents of both CX and GL. “Estrogen signaling pathway” and “VEGF (vascular endothelial growth factor) signaling pathway” under KEGG, as well as “positive regulation of nitric oxide (NO) biosynthetic process” and “negative regulation of smooth muscle contraction” under GOBP, were significantly enriched and screened out as the mutual ones for CX and GL between the term HTN and OS, which indicated that the combination could synergistically improve vascular NO signaling conduction, smooth muscle contraction and OS induced by NO depletion involved in HTN (see Fig. 13). The following in vivo experimental validation suggested that, in the L-NAME induced hypertensive rat model, the combination of CX and GL contributed to significantly decreased SBP and serum ANGII, increased aortic PGI2 (the product catalyzed by PTGS2) and T-AOC, reduced aortic VCAM-1, RNS and heart index, and improved aortic damage in a synergistic way.
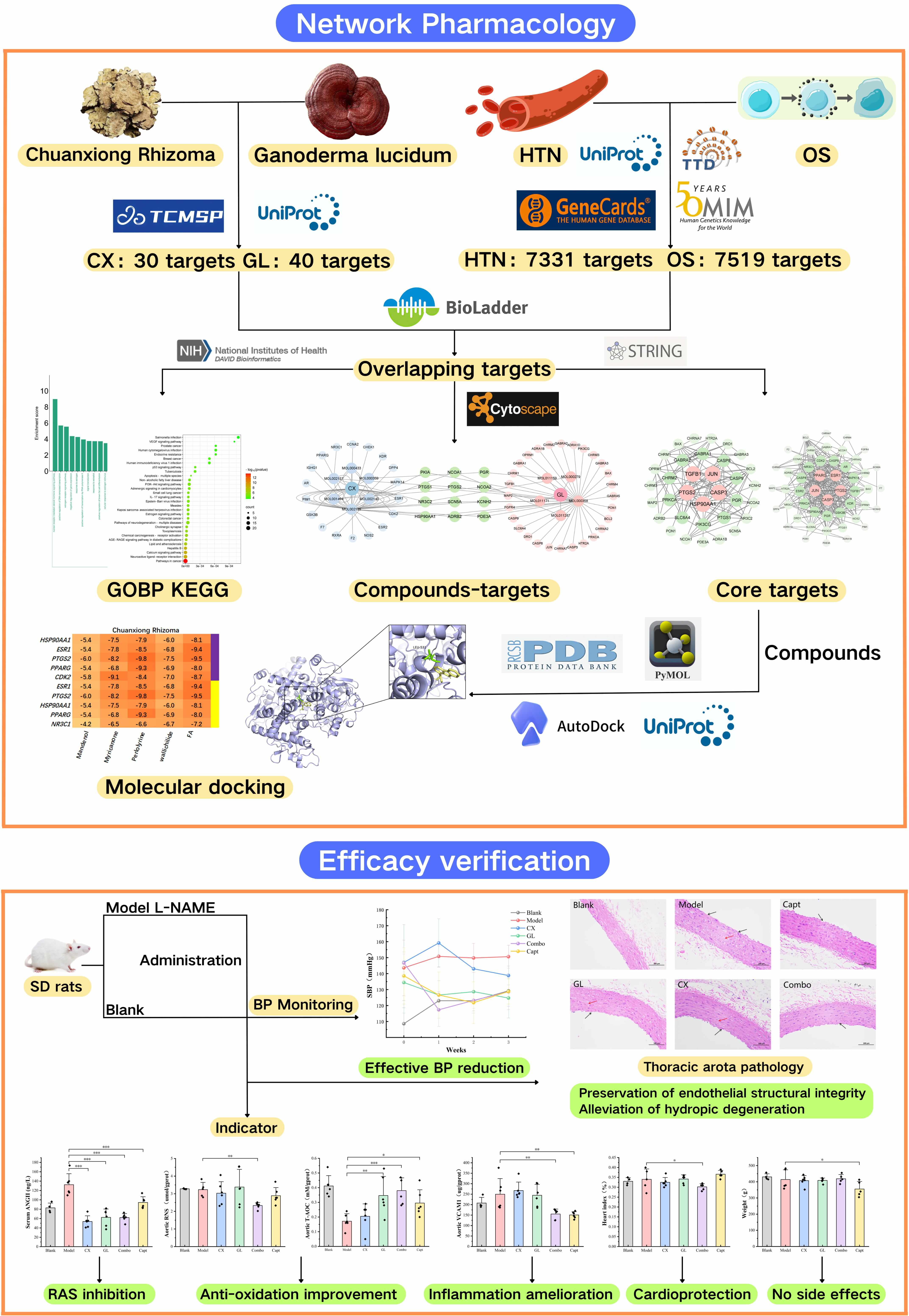 Fig. 12.
Fig. 12.
Workflow and major findings of the project. This figure was created using the online design tool Canva.cn.
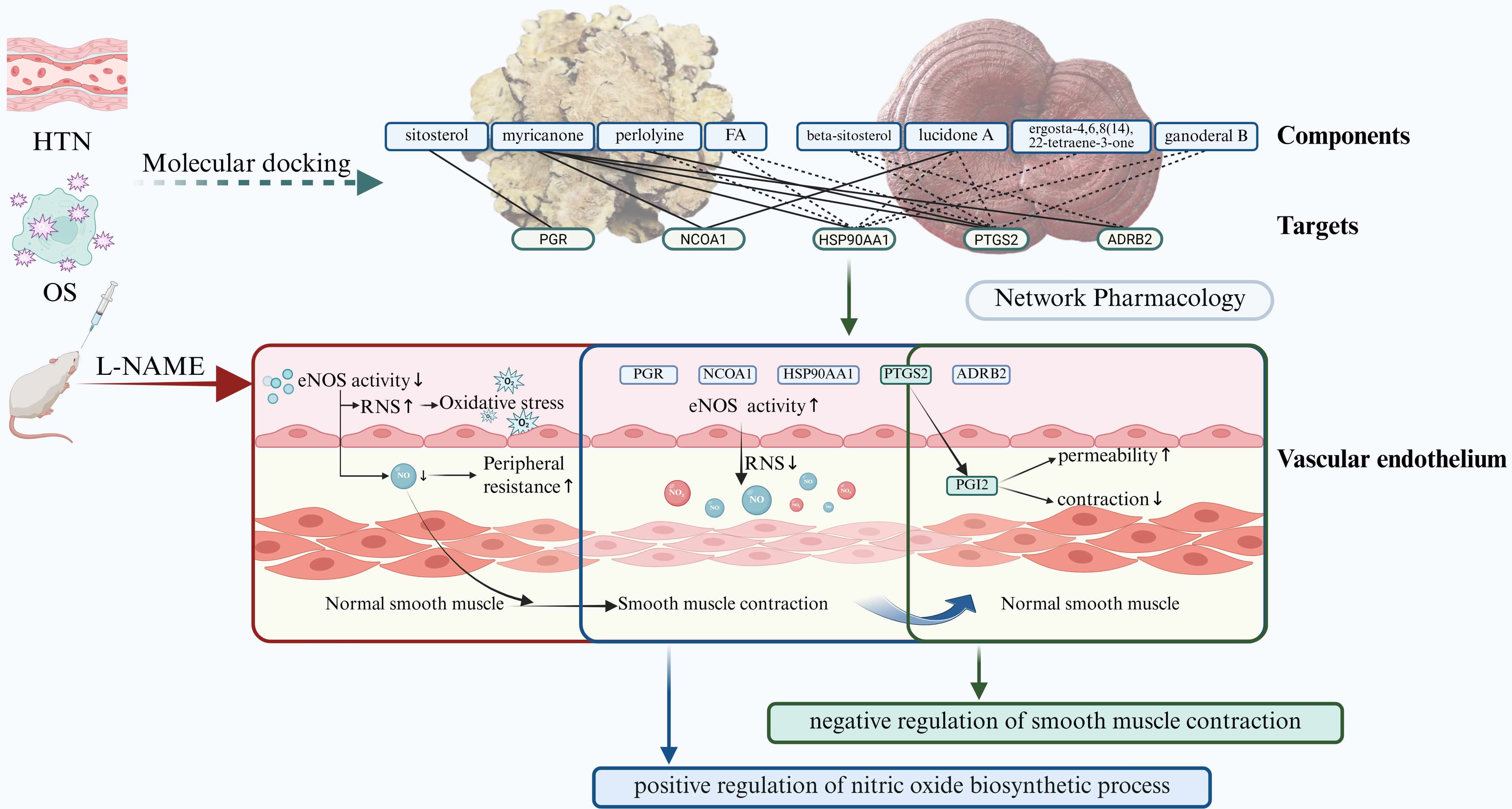 Fig. 13.
Fig. 13.
Summary of mechanism in synergistic effect of CX and GL on
L-NAME induced hypertension. Solid line between a certain target and component:
experimentally validated interaction; dotted line between a certain target and
component: predicted interaction.
TCM materials and formulas have been widely applied clinically for HTN. They not only reduce blood pressure but also broadly ameliorate pathological symptoms such as dysfunction of sympathetic system, renin-angiotensin-aldosterone system, vascular endothelium, and HTN-related risk factors, exhibiting overall regulatory characteristics [14]. As the therapeutic effects of a TCM formula derive from the interactions among multiple components and targets rather than a single one, the mechanisms of action of most TCM formulas are not easily elucidated. In our project, network pharmacology and molecular docking were employed to analyze the key components of the CX and GL combination and their corresponding mutual targets, which were then confirmed in the in vivo experiments, and the synergistic mechanism of the combination was effectively interpreted via the mutual signaling pathway.
Given the results of molecular docking in association with HTN and OS, respectively, the mandenol, myricanone, perlolyrine, wallichilide, and FA (ferulic acid) were concluded as the core effective components for CX; the ergosta-4,6,8(14),22-tetraene-3-one, ganoderal B, lucidone A, cerevisterol and beta-sitosterol were identified as the core effective components for GL. Previous research has demonstrated that mandenol (or ethyl-linoleate) effectively reduces serum cholesterol and alleviates atherosclerotic changes in rabbits through a high-cholesterol diet [27]. Myricanone has exhibited the ability to resist oxidative damage and repair heart damage [28]. Perlolyrine has been proven to alleviate pain, promote blood circulation, and exert anti-thrombotic effects [29]. Wallichilide has been found to reverse blood stasis, improve micro-circulation, and prevent focal cerebral ischemia-reperfusion injury in rats, exhibiting anti-inflammatory and anti-oxidant effects [30, 31]. FA has been reported with potent anti-oxidant, anti-inflammatory, antibacterial, and anticancer properties [32, 33]. Lucidone A has been identified with anti-oxidative and anti-inflammatory properties, preventing skin damage caused by free radicals [34]. Cerevisterol exerts anti-inflammatory effects by inhibiting inflammatory mediators and their related genes [35]. Beta-sitosterol alleviates pulmonary HTN by altering smooth muscle cell phenotype and DNA damage/cGAS/STING signaling, and effectively restores basic liver and kidney function in hypertensive rats [36, 37]. Those findings suggest that the predicted active components in CX and GL demonstrate significant effects in reducing serum cholesterol for anti-oxidation, inhibiting inflammatory mediators, and improving micro-circulation and anti-thrombosis. Those effective materials may exert a synergistic anti-hypertensive effect through multiple targets and pathways, contributing to the overall improvement of HTN. However, as the binding of the components to the targets was predicted by computational methods, their molecular interactions need to be further experimentally confirmed via Surface Plasmon Resonance (SPR), Isothermal Titration Calorimetry (ITC) or MicroScale Thermophoresis (MST), so as to clarify the mechanisms behind anti-hypertensive and vascular protective effects of CX and GL [38].
Our analysis of the core targets of active components, along with their
corresponding signaling pathways and biological functions, demonstrated that the
combination of CX and GL exerts multi-target effects to co-regulate multiple
downstream pathways related to both HTN and OS. The mutual pathways highly
associated with both HTN and OS included the “Estrogen signaling pathway” and
“VEGF signaling pathway”. Studies have shown that, estrogen could regulate a
plethora of physiological processes in mammals, including reproduction,
cardiovascular protection, bone integrity, cellular homeostasis and behavior
[39]. In the Estrogen signaling pathway for the treatment of HTN and OS, upon the
activation of the estrogen receptors ESR1 and ESR2 targeted by myricanone from CX
as well as OPRM1 (µ1 opioid receptor, or mu 1), JUN (Jun oncogene) and BCL2
(Apoptosis regulator Bcl-2) targeted by beta-sitosterol from GL, the downstream
PGR (progesterone receptor), NCOA1 (nuclear receptor coactivator 1) and HSP90AA1
(heat shock protein 90 alpha family class A member 1) were synergistically
activated through the “Nuclear-initiated steroid signaling pathway”. In turn,
the triggered regulators then promoted the eNOS activity and vascular smooth
muscle relaxation [40, 41], involved in the biological functions “positive
regulation of NO biosynthesis” and “negative regulation of smooth muscle
contraction”; typically, HTN could be regulated through the VEGF signaling
pathway by promoting angiogenesis and vascular endothelial repair to improve
vascular function [42, 43]. In the VEGF signaling pathway related to HTN and OS,
upon the activation of the VEGF receptor VEGFR2 (or KDR) and p38 (or MAPK14)
targeted by myricanone from CX as well as PKC (or PRKCA) and Casp9 targeted by
beta-sitosterol from GL, the downstream COX2 (or PTGS2) and ADRB2 (beta-2
adrenergic receptor) were synergistically activated. NF-
The following in vivo experimental results demonstrated that, the
combined administration of CX and GL exhibited synergistic anti-hypertensive and
anti-oxidative effects in the L-NAME induced HTN and OS model, significantly
surpassing the effects of monotherapy. These effects included sustained blood
pressure reduction, elevated aortic PGI2 and T-AOC, reduced aortic VCAM-1 and
RNS, reversal of cardiac index impairment, and vascular endothelial protection.
It is noteworthy that the monotherapy of CX failed to significantly improve
aortic T-AOC, whereas the combination of CX and GL exhibited a significant
improvement, indicating that the vascular anti-oxidative effect was primarily
mediated by GL. This finding aligns with the core therapeutic efficacy of GL as
applied in TCM [48]. The results demonstrate the complementary pharmacological
effects of CX and GL in the treatment of chronic OS-induced hypertension, where
CX plays a dominant role in the antihypertensive effect. In addition, only
captopril significantly reduced the body weight of participants in the model
group after three-week administration (p
This study elucidated how CX and GL exert anti-hypertensive and vascular protective effects through a network of multi-components, multi-targets and multi-pathways. The findings provide a theoretical foundation and reference for the application of CX and GL in HTN treatment. Besides, the experimentally validated active components of CX and GL involved in HTN and OS, namely, perlolyrine and FA of CX and beta-sitosterol, lucidone A, ergosta-4,6,8(14),22-tetraene-3-one and ganoderal B of GL, were predicted as the novel compounds by molecular docking in this study. A more in-depth pharmacological investigation, with particular focus on vascular endothelial effects, warrants execution for the predicted novel compounds. Such analysis would serve to more thoroughly validate the concluded mechanisms and further elucidate the medicinal value of CX and GL.
Leveraging the integrated approach of network pharmacology and experimental validation, this study reveals that the combined administration of Chuanxiong Rhizoma Hort. and Ganoderma lucidum Karst. synergistically alleviates L-NAME-induced hypertension. Mechanism investigations demonstrate that this herb pair co-regulates the Estrogen and VEGF signaling pathways, thereby augmenting vascular NO bioavailability, promoting vasodilation, and enhancing antioxidative defense. Those findings provide robust evidence for the therapeutic potential of this herb pair as a novel dietary-medicinal therapy for hypertension management.
L-NAME, N-Nitro-L-arginine methyl ester; CX, Chuanxiong Rhizoma Hort.; GL, Ganoderma lucidum Karst.; HTN, hypertension; OS, oxidative stress; PPI, protein–protein interaction networks; KEGG, Kyoto Encyclopedia of Genes and Genomes; GOBP, Gene Ontology biological process; SBP, systolic blood pressure; COX2, Cyclooxygenase-2; VEGF, vascular endothelial growth factor; NO, nitric oxide; ANGII, Angiotensin II human; PGI2, prostaglandin I2; PTGS2, prostaglandin-endoperoxide synthase 2; T-AOC, total antioxidant capacity; VCAM-1, Vascular Cell Adhesion Molecule-1; RNS, reactive nitrogen species; BP, blood pressure; ROS, reactive oxygen species; NOS, Nitricoxide synthase; TCM, Traditional Chinese medicine; OB, Oral bioavailability; DL, drug-likeness; GO, Gene Ontology; Combo, combination of CX and GL; Capt, captopril; CI, combination index; HE, hematoxylin-eosin staining; NBF, Neutral buffered Formalin; eNOS, endothelial Nitric Oxide Synthase; ESR/1/2, Estrogen receptor/1/2; VEGFR2 (KDR), Vascular Endothelial Growth Factor Receptor 2; P38 (MAPK14), mitogen-activated protein kinase; µ (OPRM1), mu-1 opioid receptor; TF (JUN), Jun Proto-Oncogene; BCL2, Apoptosis regulator Bcl-2; PKC (PRKCA), Protein kinase C alpha type; Casp9 (CASP9), Caspase-9; CoA (NCOA1), Nuclear receptor coactivator 1; Hsp90 (HSP90AA1), Heat shock protein HSP 90-alpha; PGR, Progesterone receptor; VCAM-1, vascular cell adhesion molecule-1; FA, ferulic acid; MDA, Malondialdehyde; SOD, Superoxide dismutase; GSH, Glutathione; DNA, DeoxyriboNucleic Acid; cGAS, Cyclic GMP-AMP synthase; STING, Stimulator of interferon genes; ADRB2, Beta-2 adrenergic receptor.
All the data generated and analyzed during this study are available from the corresponding author.
ML: Writing original draft, Validation, Data curation; RW, JW, TL and JT: Validation; HL, CL, BY: Data curation, FW and JZ: Project administration, Writing original draft, Data curation, Funding acquisition. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
The animal experiments strictly followed the 3Rs principle (Replacement, Reduction, Refinement) and the International Guiding Principles for Biomedical Research Involving Animals (CIOMS/ICLAS). All procedures complied with ethical requirements and were approved by the Experimental Animal Ethics Committee at Harbin University of Commerce (Approval No.: HSDYXY-2024066).
Not applicable.
The work was supported by 2024 Fundamental Research Funds in Universities of Heilongjiang Province (2024-KYYWF-1021); 2022 Harbin University of Commerce Doctoral Research Support Program (22BQ59); 2024 Heilongjiang Provincial College Student Innovation and Entrepreneurship Training Program (S202410240048).
The authors declare no conflict of interest.
Supplementary material associated with this article can be found, in the online version, at https://doi.org/10.31083/IJP46239.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.













