1 School of Pharmacy, Faculty of Health and Medical Sciences, Taylor’s University, 47500 Subang Jaya, Selangor, Malaysia
2 Department of Pharmaceutical Life Sciences, Faculty of Pharmacy, Universiti Malaya, 50603 Kuala Lumpur, Malaysia
3 Centre for Natural Product Research and Drug Discovery (CENAR), Universiti Malaya, 50603 Kuala Lumpur, Malaysia
4 Faculty of Pharmacy, Universiti Teknologi MARA (UiTM) Cawangan Selangor, Puncak Alam Campus, 42300 Puncak Alam, Selangor, Malaysia
5 Department of Pharmaceutical Technology & Industry, Faculty of Pharmacy, University of Cyberjaya, 63000 Cyberjaya, Selangor, Malaysia
6 Division of Pathology, School of Medicine, International Medical University, 57000 Kuala Lumpur, Malaysia
Abstract
Tinospora cordifolia has been recognized in Ayurvedic medicine for its antidiabetic properties; however, the role of T. cordifolia in diabetic wound healing remains unexplored. Thus, this study aimed to investigate the diabetic wound-healing potential of the aqueous ethanolic stem extract of T. cordifolia (AETC) in streptozotocin–nicotinamide (STZ–NAD)-induced diabetic rats. The extract was prepared using 70% aqueous ethanol via Soxhlet extraction.
A full-thickness excision wound was created on the dorsal skin of diabetic rats, followed by a 14-day oral treatment with the AETC at low (250 mg/kg) and high (500 mg/kg) doses. Key parameters were assessed, including blood glucose levels, the rate of wound contraction, and epithelization time, alongside the histopathological evaluation of wound tissues.
The AETC treatment significantly reduced blood glucose (p < 0.01), enhanced wound contraction (p < 0.05), and accelerated epithelization (p < 0.05) compared to diabetic controls. The histological analysis revealed improved epidermal regeneration, reduced inflammation, and increased granulation tissue and collagen deposition.
These findings suggest that the AETC can exert glycemic control and promote wound healing in diabetic conditions.
Keywords
- Tinospora cordifolia
- diabetic wound healing
- guduchi
- excision wound
- wound healing
Diabetes significantly impairs wound healing, often leading to complications such as chronic ulcers and amputations [1]. Its complex pathophysiology disrupts all four phases of the normal wound healing, thereby prolonging healing time and increasing the risk of infection [2]. Impaired wound healing (WH) in individuals with diabetes contributes significantly to increased risk of morbidity and mortality. This is primarily attributed to macrovascular and microvascular complications, including peripheral neuropathy, peripheral arterial disease, and ischemia resulting from progressive atherosclerosis and other contributing factors such as poor glycaemic control, mechanical foot deformities, callus formation, inadequate foot care and infections [3]. As a consequence of impaired WH, approximately 25% of patients with diabetes will develop diabetic skin wounds at some point in their life, often leading to lower limb amputation [4].
Diabetic skin wounds are a significant complication among patients with diabetes and have emerged as a major global health burden, necessitating multidisciplinary management [5]. The pathophysiology involves complex interactions between hyperglycemia, neuropathy, vascular insufficiency, and impaired immune response [3, 4, 6]. These factors contribute to prolonged inflammation, dysregulated protease activity, and tissue hypoxia, collectively impairing the wound healing process [6, 7]. Standard treatment includes a coordinated multidisciplinary approach encompassing offloading, debridement, dressing, glycemic control and antibiotic therapy [8]. In addition, several novel and innovative therapies are under investigation, including plant-based remedies, autologous skin transplants and extracorporeal shock wave therapy [8, 9].
At present, various interventions are employed to enhance the healing of chronic diabetic wounds, including debridement, wound dressings, oxygen therapy, negative pressure wound therapy and physical therapies [6, 10]. However, their effectiveness in promoting wound healing within wound management remains inadequately justified [10]. A review of the WH abilities of the existing antidiabetic medications revealed that glibenclamide is the only oral antidiabetic medication with minimal side effects that also exhibits some WH properties [11].
A growing body of research supports the therapeutic relevance of plant-based phytochemicals in accelerating and improving WH outcomes [12]. Tinospora cordifolia (TC), commonly known as Guduchi, is a known antihyperglycemic agent [13] in Ayurvedic medicine. TC is well recognized for its application in many diseases, and one of its most prominent uses is in the management of diabetes [13, 14]. In recent years, the WH ability of TC has been explored on different wound models, through both oral and topical routes of administration. These studies have shown that TC exerts a positive effect on wound repair [15, 16]. However, its effect on diabetic WH activity has not been investigated. Therefore, this study investigated the diabetic WH property of oral aqueous ethanolic extract of Tinospora cordifolia (AETC).
The stem of TC was sourced from Trivandrum district in Kerala, India, during January 2018. For botanical identification, the sample specimen was verified and deposited at the University Putra Malaysia herbarium, where a plant voucher was issued (UPMFHAS/HERBARIUM/07/22: Voucher no. H079).
The shade-dried and powdered stem of TC was extracted using Soxhlet extraction. A total of 150 g of powdered stem was defatted with 1 L of petroleum ether (Chemiz, Shah Alam, Selangor, Malaysia) (60–80 °C) using the Soxhlet apparatus (J600RDSX, PLT Scientific Sdn Bhd, Kuala Lumpur, Malaysia) for 12 hours or until the solvent ran clear. The defatted plant material (marc) was separated from the solvent and dried overnight in the fume hood. The dried marc was then placed in a cellulose thimble in the Soxhlet extraction chamber, and further extracted using 70% v/v ethanol (J.T Baker, Deventer, Overijssel, Netherlands) under reflux at 50 °C for 12–24 hours. The resulting extract was subjected to rotary evaporation using a rotary evaporator (Rotavapor R-210, Buchi, St. Gallen, Switzerland) under reduced pressure until complete dryness was attained [17, 18]. After this, it was lyophilized using a freeze dryer (ScanVac Coolsafe Touch 110-4 Freeze Dryer, Labogene, Lynge, Denmark) to obtain the dry crude AETC, which was collected and stored at 4 °C until further use.
The research was conducted in alignment with the ethical and methodological principles established by the Basic & Clinical Pharmacology & Toxicology policy for experimental and clinical studies [19]. It was conducted in accordance with the ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines [20]. A total of 75 male Wistar rats (weight 200 to 220 g; age 8 to 10 weeks) were obtained from the Laboratory Animal Facility and Management, Faculty of Pharmacy, University Teknologi MARA. The animals were housed under controlled conditions: 50–70% humidity, 20–22 °C, and a 12:12-h light: dark cycle with free access to pelleted rodent chow and filter-sterilized water. All animal care and handling were conducted following the protocol approved by the committee of Animal Research and Ethics, Universiti Teknologi Mara (UiTM CARE) (Approval number: 250/2018).
On the day of induction, rats were fasted for 6–8 hours, with water provided ad libitum. The rats were induced with a single intravenous injection of a freshly prepared streptozotocin (STZ) solution (Sigma Life Sciences, St Louis, MO, USA) at a dose of 65 mg/kg (2 mL/kg) in 0.1 M citrate buffer (pH 4.5) (R&M Chemicals, Madhya Pradesh, India), administered 15 min after an intraperitoneal injection of nicotinamide (NAD) at 120 mg/kg (Sigma Life Sciences, St Louis, MO, USA) [20]. The normal control rats received an equal volume of vehicle, i.e., citrate buffer, pH 4.5 via intravenous injection. Ten days post-induction, blood glucose levels were measured using tail-vein blood with a glucometer (ONETOUCH®, LifeScan, Zug, Switzerland). Rats with fasting blood glucose levels exceeding 150 mg/dL (i.e., 8.3 mmol/L) were classified as diabetic and included in the study [21, 22].
On the first day of treatment, both the diabetic and control rats were subjected to excision wounds. Rats were anesthetized using an intraperitoneal injection of Ketamine-Xylazine (Troy Laboratories Pty. Limited, Glendenning, New South Wales, Australia) cocktail at a dose of 0.1 mL/100 g body weight. The cocktail was prepared by combining ketamine (10 mL; 100 mg/mL) with xylazine (1 mL; 100 mg/mL). The dorsal surface hair was shaved using a razor blade to expose the skin. To create the excision wound, the dorsal skin was gently folded and raised cranially and caudally at the midline using the index finger and thumb to form a sandwiched skinfold. The animal was placed in a lateral position, and an 8 mm sterile biopsy punch (Premier® Medical Products, Plymouth Meeting, PA, USA) was used to remove both layers of skin, producing symmetrical full-thickness excisional wounds [23]. A total of four full-thickness wounds were created on each rat using the biopsy punches with two wounds on each side of the midline. Following full recovery from anesthesia, the rats were housed individually to maintain wound dressing integrity and prevent wound interference [23]. An occlusive dressing, Tegaderm (3M Health Care, St. Paul, MN, USA), was applied to the excision wound for all groups to minimize the risk of infection.
The rats were treated once daily with their respective treatment for 14 consecutive days. Each group consisted of 15 rats. Group 1 consisted of control rats, which were induced with vehicle (citrate buffer) and were treated with 0.3% carboxymethylate cellulose (CMC) (Chemiz, Shah Alam, Selangor, Malaysia). The diabetic rats were randomly assigned to Group 2–5 as the following: Group 1: control rats with oral 0.3% CMC; Group 2: STZ-NAD induced rats with 0.3% CMC; Group 3: STZ-NAD induced rats with oral 250 mg/kg AETC; Group 4: STZ-NAD induced rats with oral 500 mg/kg AETC; Group 5: STZ-NAD induced rats with oral 600 µg/kg glibenclamide.
Random blood glucose for all the rats was measured on treatment days 0, 3, 7, 11 and 14, in the morning from the tail vein of the rats using a glucometer.
Wounds were monitored throughout the 14-day treatment period, and wound area measurements were recorded. Digital images of the wounds were captured on days 3, 5, 7, 11 and 14. The wound area was quantified using ImageJ software (Version 1.53.c, Research Services Branch, NIMH, Bethesda, MD, USA). Based on the measurements of wound area, the rate of contraction of the wound area was determined using the following formula [23].
The epithelization time, i.e., the number of days required for the sloughing of dead tissue remnants with complete wound closure and absence of any residual raw wound and the scar area, was recorded.
Three rats were sacrificed on days 3, 5, 7 and 14 from each group respectively, using a chemical overdose method with intraperitoneal injection of 100 mg/kg pentobarbital (100 mg/mL), in accordance with the guidelines of the American Veterinary Medical Association [24]. Granulation tissues were harvested and sent to the Veterinary Histopathology Laboratory of University Putra Malaysia for staining with hematoxylin and eosin (H&E) for general morphological observations and Masson-trichrome to visualize collagen fibres. The stained slides were examined qualitatively under an upright light microscope (Nikon Eclipse Ni upright microscope, Nikon Corporation, Chiyoda-ku, Japan) to evaluate collagen deposition, fibroblast proliferation, angiogenesis, and granulation tissue formation [25]. Each specimen was analyzed across two sections, with a minimum of five fields per section examined for both staining protocols.
Data were presented as mean
The blood glucose-lowering effect of AETC is depicted in Fig. 1. Beginning on day 7, both treatment groups (250 mg/kg and 500 mg/kg) exhibited significantly reduced blood glucose levels compared to the diabetic control group (p
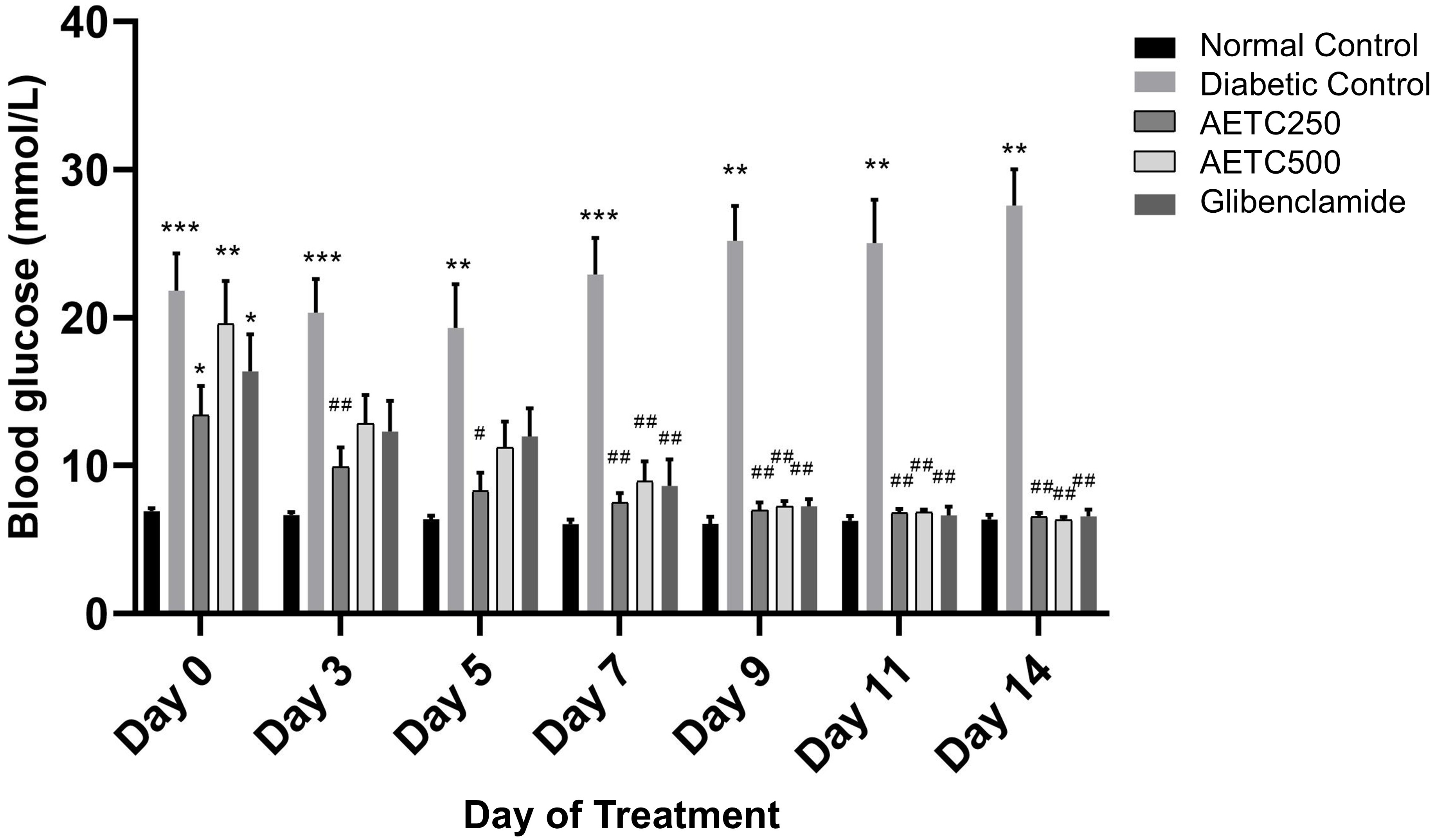 Fig. 1.
Fig. 1. Random blood glucose levels post-treatment in streptozotocin-nicotinamide induced diabetic rats. Data represent mean values (
Based on the random blood glucose from day 0 to 14, it was noted that there is a significant decrease in the mean blood glucose level on day 14 in AETC-treated, and glibenclamide-treated rats in comparison to diabetic controls. Both AETC-treated groups (250 mg/kg and 500 mg/kg) showed a significant decrease in mean blood glucose levels (Fig. 1).
Photographic representations of the wound contraction on different post-excision days for each group are shown in Fig. 2. Macroscopic wound closure was quantified as the percentage change in the initial wound area at selected time points (Fig. 3). Although all the animals achieved 100% wound closure by 14 days, the diabetic control group demonstrated a lower percentage of wound contraction at day 3, 5, 7, 9 and 11 compared to normal control, AETC-treated rats and glibenclamide-treated groups (p
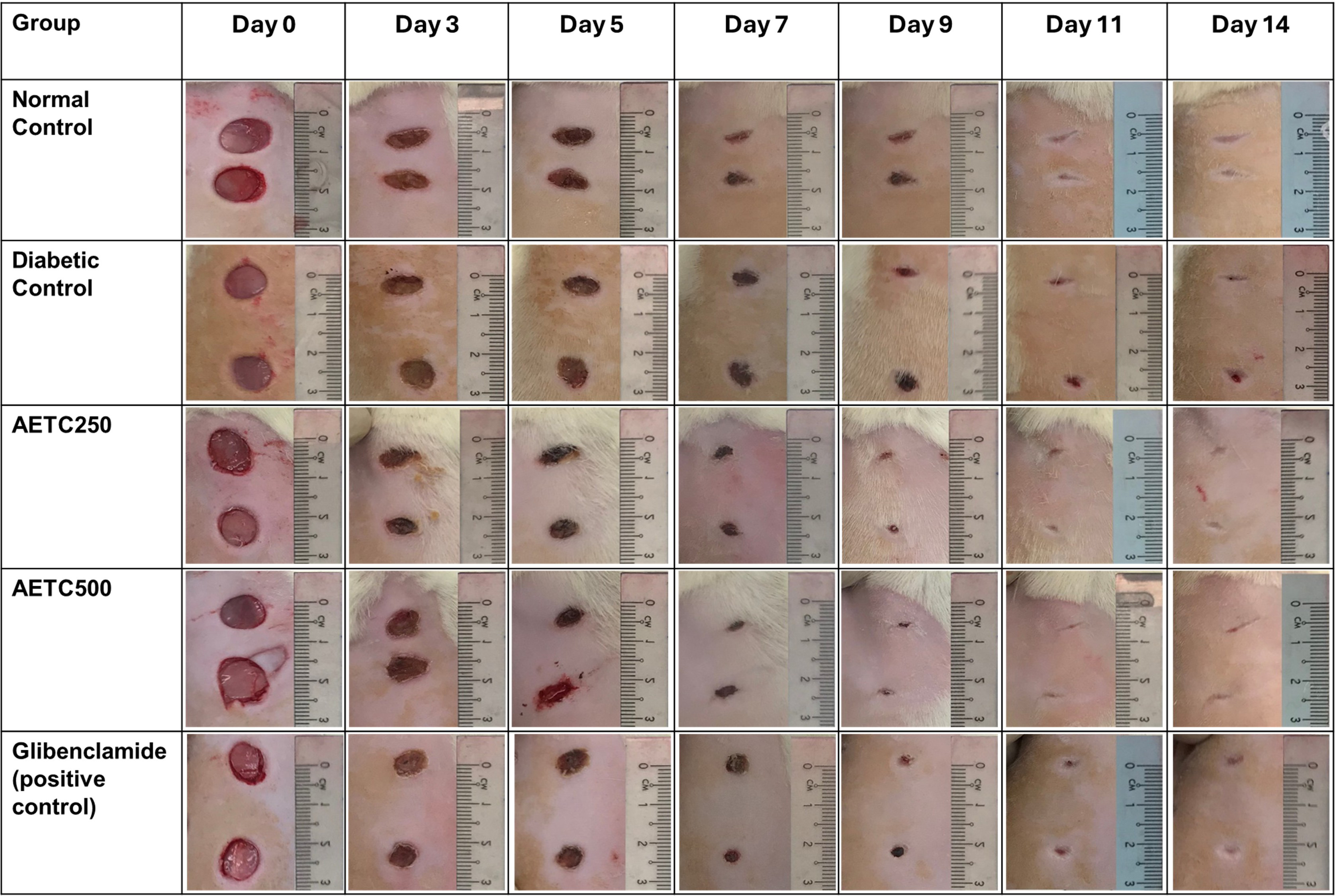 Fig. 2.
Fig. 2. Macroscopic representation of the wound contraction on different post-excision days. AETC250, Aqueous ethanolic stem extract of Tinospora Cordifolia 250 mg/kg; AETC500, Aqueous ethanolic stem extract of Tinospora Cordifolia 500 mg/kg.
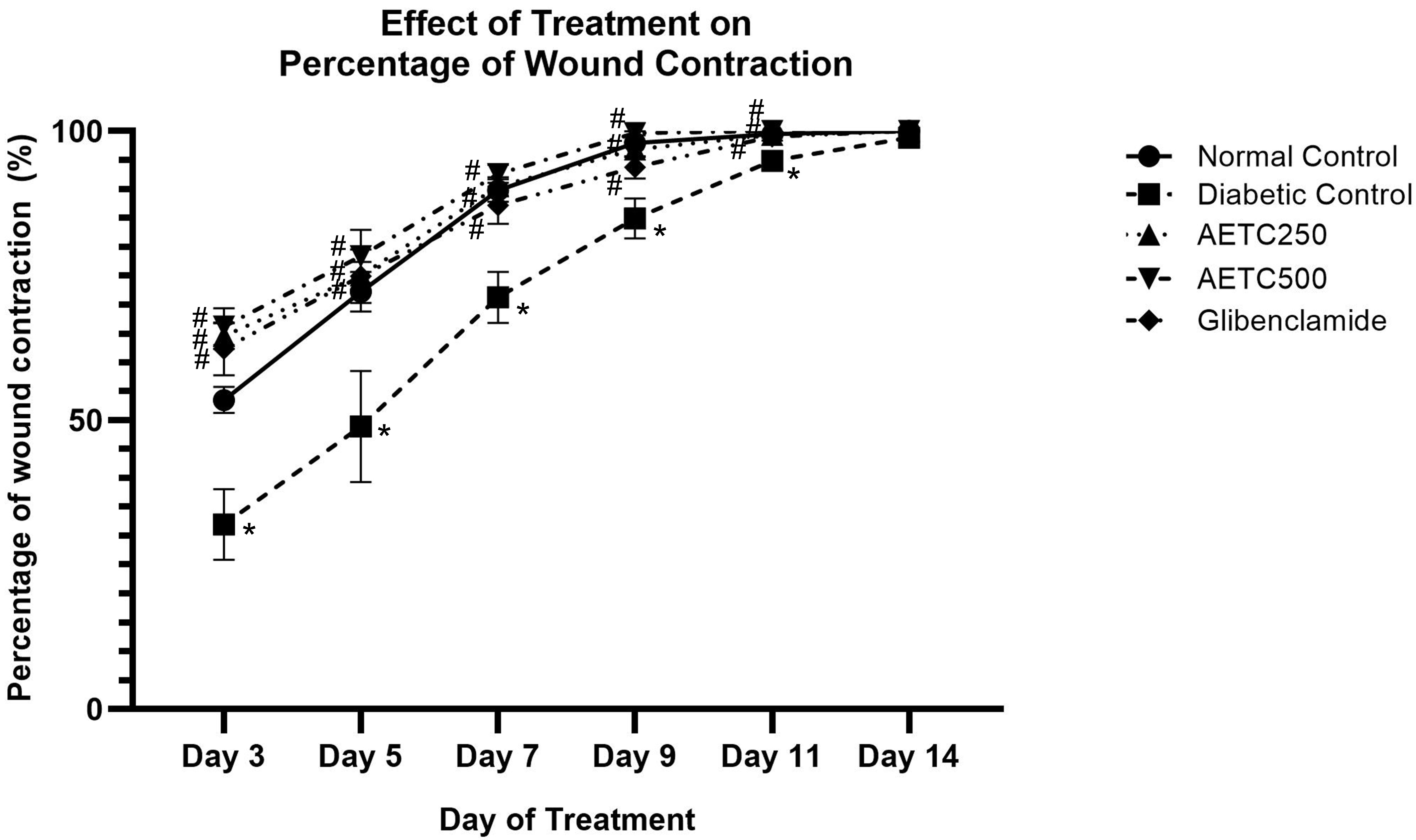 Fig. 3.
Fig. 3. Percentage of wound contraction throughout 14-day treatment in streptozotocin-nicotinamide induced diabetic rats. Data represent mean values (
A one-way ANOVA test was performed to assess differences in the mean epithelization time among control (normal and diabetic), extract-treated (250 mg/kg and 500 mg/kg) and standard-treated groups (Fig. 4). There was a significantly longer epithelization time in diabetic control rats (13.0
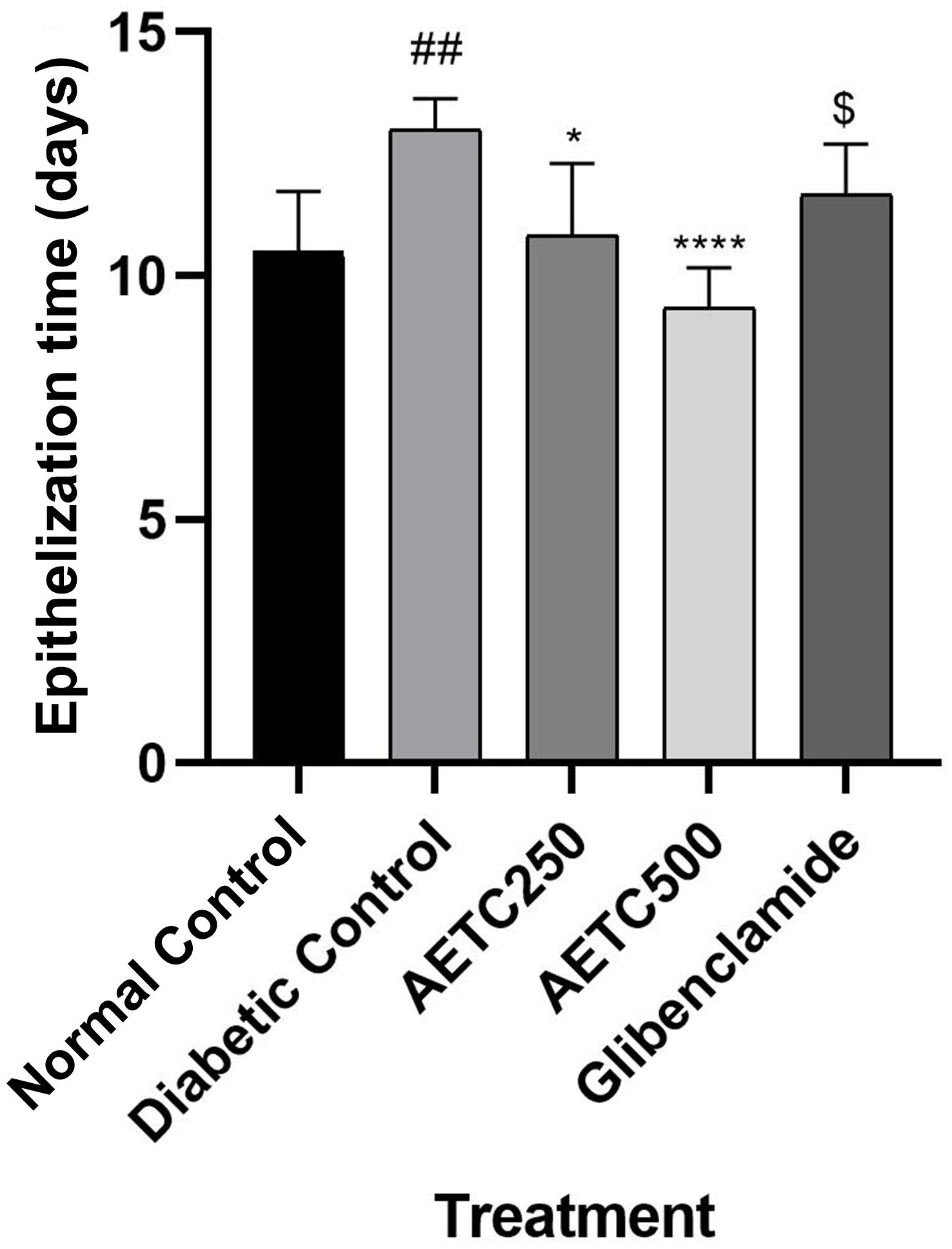 Fig. 4.
Fig. 4. Effect of treatments on epithelization time in streptozotocin-nicotinamide induced diabetic rats. There was a significant difference between normal control and diabetic control (##p
Fig. 5 depicts representative micrographs of H&E-stained sections illustrating granulation or healing tissue across the five experimental groups on days 3, 5, 7 and 14. Notably, by day 14, all wounds exhibited thorough healing. Specifically, the wound healing observed in AETC500-treated diabetic rats closely resembled that of the normal control group. Furthermore, it’s worth noting that the AETC500-treated group exhibited better wound healing compared to the positive control group.
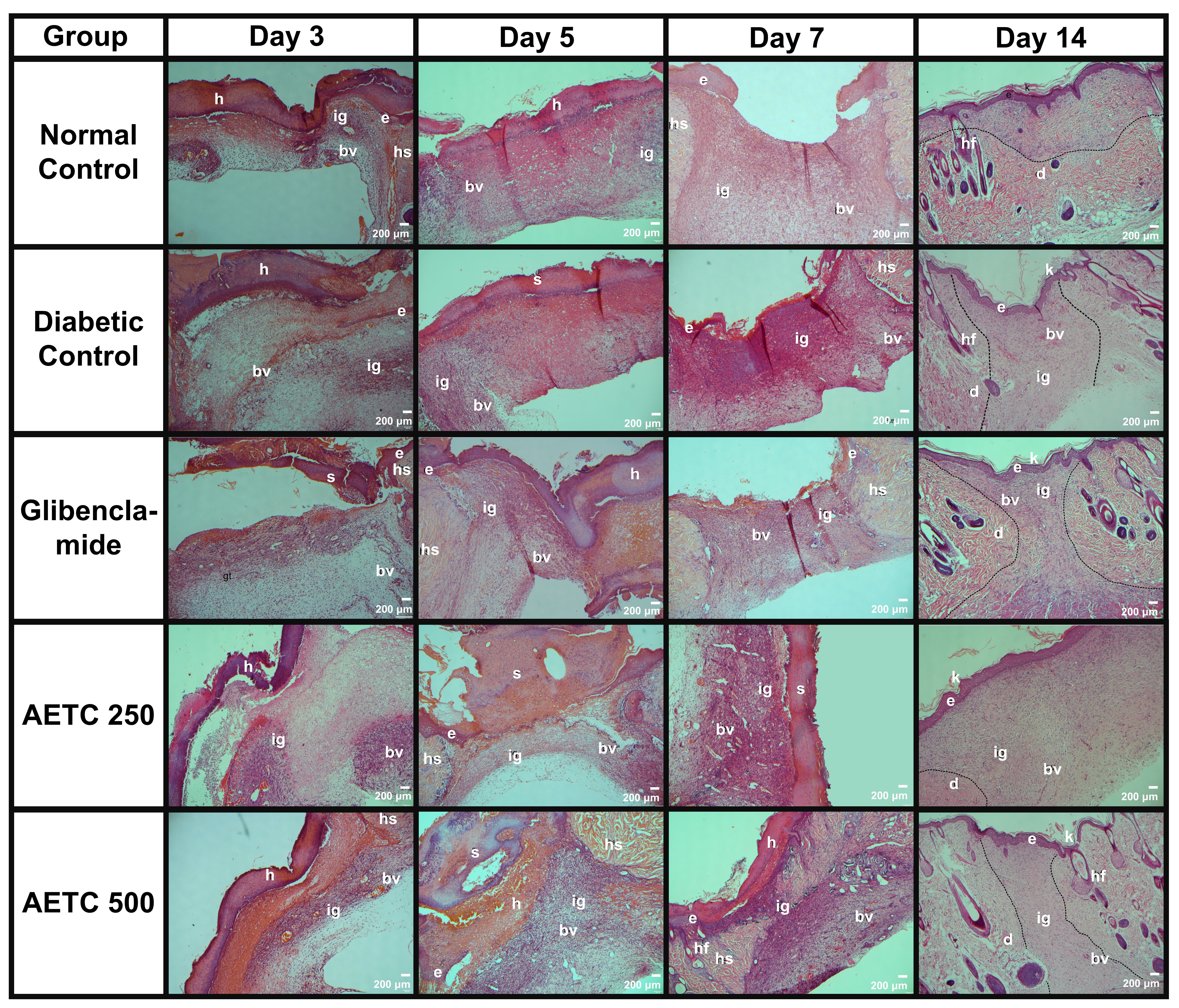 Fig. 5.
Fig. 5. Overview of the histopathological evaluation of hematoxylin-eosin-stained samples in relation to wound healing in diabetic rats treated with aqueous ethanolic extract of Tinospora cordifolia (AETC) stem on days 3, 5, 7 and 14. Rats were orally treated with AETC (250 mg/kg or 500 mg/kg) for 14 consecutive days. Skin tissue was obtained from the excision wound site and stained with hematoxylin and eosin stain. The micrograph panels visualize intersection areas between normal and wounded skin at 4
On day 3, all wounds were observed to be in the inflammation and early proliferation stage (Fig. 6). This was evidenced by the presence of inflammatory cells and marked edema that were clearly visible in the microscopy slides. Edema was present across all photomicrographs, with the most pronounced swelling observed in the AETC250-treated group, followed by the diabetic control, AETC500-treated, normal control, and glibenclamide-treated groups. Furthermore, signs of proliferation were evident, as indicated by the formation of granulation tissue. This feature was most prominent in the AETC500-treated group, followed by the AETC250-treated, glibenclamide-treated, normal control, and diabetic control groups.
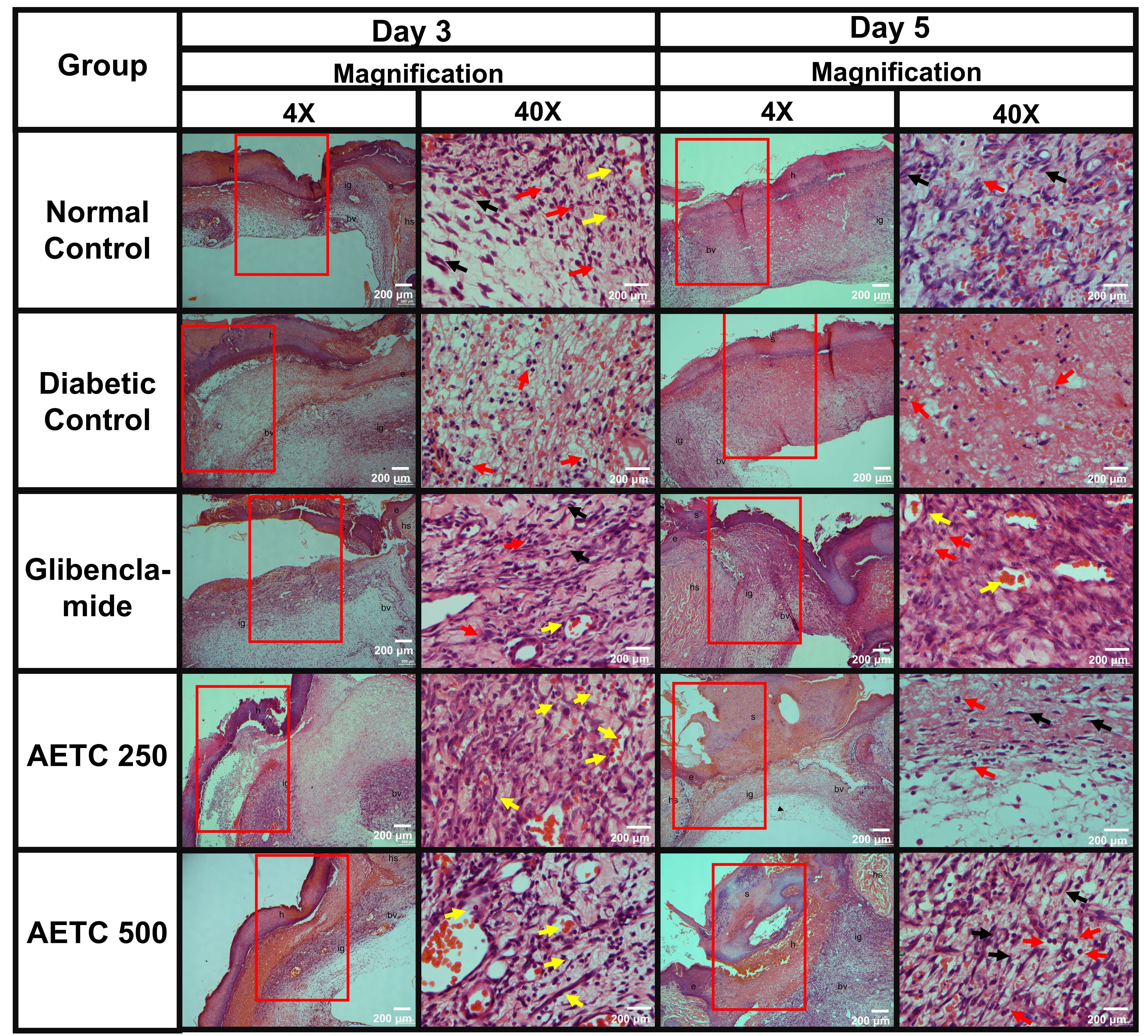 Fig. 6.
Fig. 6. Histopathological evaluation of hematoxylin-eosin-stained samples in relation to the wound healing in diabetic rats treated with aqueous ethanolic extract of Tinospora cordifolia (AETC) stem on days 3 and day 5. Skin tissue was obtained from the excision wound site and stained with hematoxylin and eosin stain. The micrograph panels visualize intersection areas between normal and wounded skin at 4
Samples from day 5 showed minimal differences compared to day 3 (Fig. 5). Inflammation remained evident across all groups, characterized by the presence of inflammatory cells and edema. Among the groups, the diabetic control exhibited the most pronounced edema, followed by the normal control, glibenclamide-treated, AETC500-treated, and AETC250-treated groups. In terms of granulation tissue proliferation, the AETC500-treated group displayed the densest granulation tissue, followed by the glibenclamide-treated, normal control, diabetic control, and AETC250-treated groups. This observation was further supported by the relative abundance of fibroblasts, which was highest in the AETC500-treated group, followed by the glibenclamide-treated, normal control, diabetic control, and AETC250-treated groups.
On day 7, all the groups remained in the proliferation stage. Complete granulation tissue was observed at the wound sites of all treated groups. Inflammation persisted only in the diabetic control group, as indicated by the presence of edema. A notable finding on day 7 was the emergence of immature epidermis in all groups except the AETC250-treated group. Additionally, substantial angiogenesis was evident across all groups.
By day 14, all photomicrographs revealed an intact and fully formed epithelial layer. The relative wound sizes, in ascending order, were: AETC250-treated, diabetic control, glibenclamide-treated, AETC500-treated, and normal control groups. The AETC250-treated, diabetic control, AETC500-treated, and glibenclamide-treated groups remained in the proliferation phase, as indicated by prominent angiogenesis and the absence of glands or hair follicles at the wound sites. Although both AETC500-treated and glibenclamide-treated groups exhibited histological features similar to the diabetic control group, the glibenclamide-treated group showed a greater density of blood vessels. Furthermore, the wound site in the AETC500-treated group appeared smaller than that of the diabetic control group and comparable in size to the glibenclamide-treated group. Notably, the epidermis at the wound site of the AETC500-treated group appeared thicker than in the other groups. In contrast, only the normal control group exhibited signs of the maturation phase, as evidenced by the presence of glands and hair follicles at the wound site.
Fig. 7 depicts representative photomicrographs of Masson’s trichrome-stained sections from the granulation or healing tissue of all five experimental groups of rats (at 4
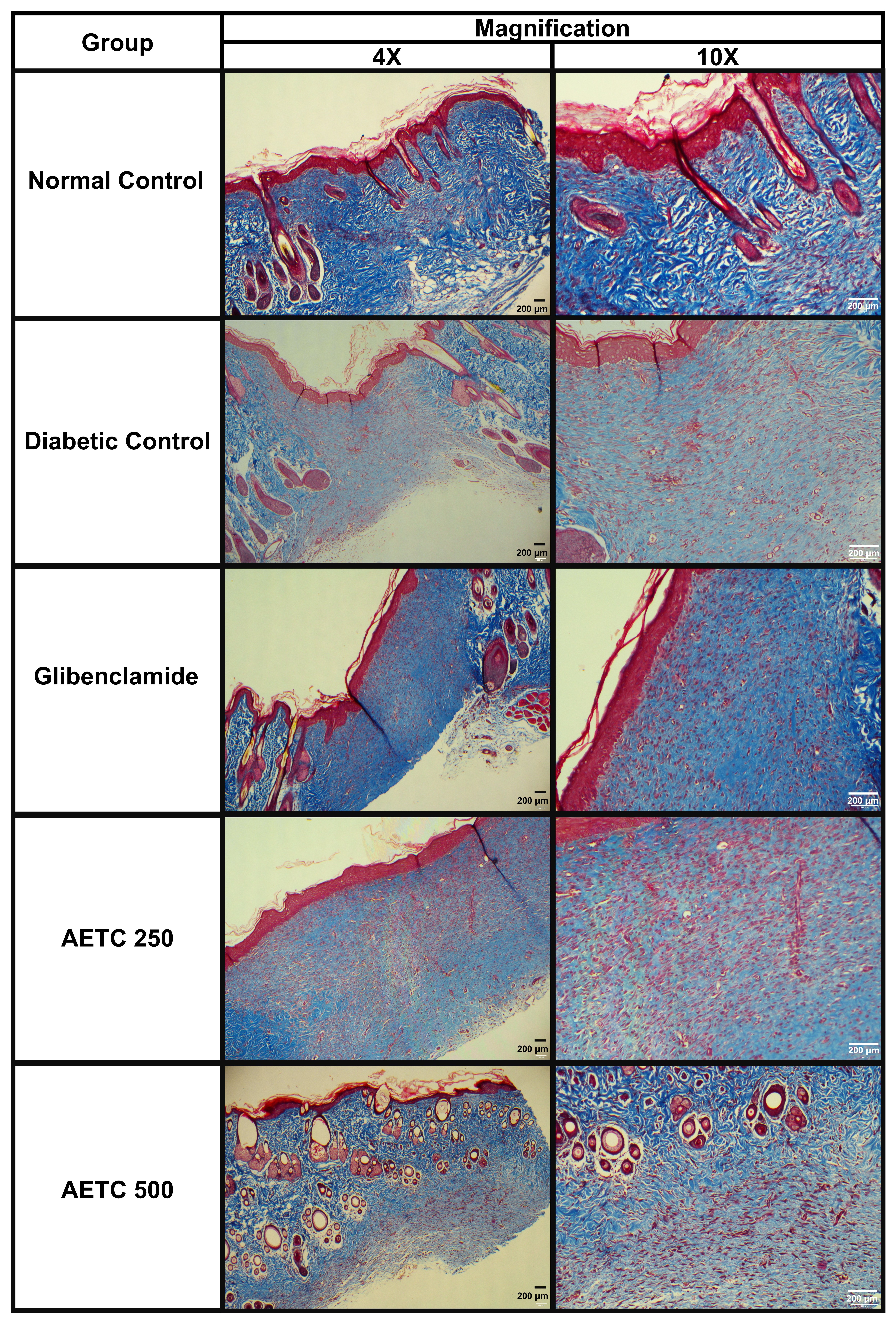 Fig. 7.
Fig. 7. Histopathological evaluation of the Masson’s Trichrome stained samples in relation to the wound healing in diabetic rats treated with aqueous ethanolic extract of Tinospora cordifolia stem on day 14. Rats were orally treated with AETC (250 or 500 mg/kg) for 14 consecutive days. On day 14, skin tissue was obtained from the excision wound site and stained with Masson Trichrome stain. Left photomicrograph panels were visualized intersection areas between normal and wounded skin at 4
TC has been well studied for its antidiabetic effect. This is congruent with previous studies, both dosages of AETC (250 and 500 mg/kg) significantly reduced the elevated random blood glucose levels in STZ-NAD induced diabetic rats [13, 26]. Previous studies utilized extracts of the whole plant or specific parts such as root, leaves and stem, employing various solvents including aqueous, alcoholic, chloroform, ethyl acetate and hexane, with dose ranges typically between 100 and 500 mg/kg [13]. The dosages selected for AETC in this study were based on a previous literature review, which identified 500 mg/kg as the highest dose used for stem extracts and 100 mg/kg as the lowest [13]. Accordingly, the highest dose and its half were chosen to evaluate the diabetic wound healing potential of AETC. The study presented the novelty of investigating the oral therapeutic efficacy of AETC in diabetic wound healing. Over the past decade, researchers have studied the wound healing activity of TC extracts, which are mainly administered topically, and its oral therapeutic effect using aqueous ethanolic extract has not been explored [16]. Furthermore, it was also noted that the blood glucose-lowering effect of AETC was comparable to that of glibenclamide. Studies have reported that the mechanisms underlying the antidiabetic effect of TC were associated with its ability to mitigate oxidative stress, promote insulin secretion, inhibit gluconeogenesis and glycogenesis, and enhance blood glucose transport to peripheral tissue for glycemic regulation [13, 26, 27].
This study employed a 14-day window to observe and treat the rodent after the wound creation because it aligns with key phases of the wound healing process from inflammation to the repair phase involving epithelialization [28]. Epithelialization, which begins within hours of injury, is a critical component of wound healing, and the presence of abundant epithelializing tissue is commonly indicative of successful recovery [29]. Wound contraction also plays a vital role, contributing to up to a 40% reduction in wound size and accelerating healing by limiting the oversynthesis of extracellular matrix (ECM) proteins [29, 30]. In the present study, oral administration of AETC significantly accelerated the rate of wound contraction. A marked difference in contraction was observed between treated groups and the diabetic control group as early as day 3. Moreover, AETC-treated rats exhibited a significantly higher rate of wound contraction compared to glibenclamide-treated rats. Both AETC doses (250 mg/kg and 500 mg/kg) also resulted in a significant reduction in epithelialization time relative to the diabetic control group. Notably, epithelialization time in the AETC 500 mg/kg group was significantly shorter than in the glibenclamide-treated group. These findings are consistent with previous studies on acute excision wound models [18, 31, 32, 33]. These studies collectively demonstrated that oral TC treatment exhibited early reepithelization and fast wound closure, despite variations in plant parts and solvents used [32, 33]. In the acute wound model, the ethanol extract-treated group achieved the highest percentage of wound closure [33]. The early re-epithelialization and faster wound closure in TC-treated wounds may be attributed to increased keratinocyte proliferation and migration across the wound surface [34].
Wounds with impaired healing, including chronic wounds, often fail to progress through the normal stages of tissue repair. These wounds generally enter a state of pathological inflammation due to delayed, incomplete, or uncoordinated healing processes. While inflammation is a natural and essential phase of wound healing, prolonged inflammation can lead to elevated levels of matrix metalloproteinases, a family of proteases that degrade the ECM [35]. In diabetes, increased reactive oxidative species stimulate the secretion of pro-inflammatory cytokines, thereby exacerbating inflammation [36]. In the present study, histological evaluations of H&E-stained samples revealed that by day 7, inflammation had subsided in all groups, except for diabetic control, where edema remained evident. On day 5, it was noted that the AETC-treated groups exhibited reduced inflammation compared to the other groups. This may be attributed to the anti-inflammatory properties of TC. A previous study reported that aqueous extract of TC significantly reduced nitric oxide production and down-regulated the gene expression of tumor necrosis factor-alpha and cyclooxygenase-2 [37]. Besides this, this extract also inhibited the gene expression of toll-like receptor 4, an important regulator of wound inflammation [37]. Another study demonstrated that TC significantly enhanced the expression of vascular endothelial growth factor (VEGF) while markedly reducing levels of pro-inflammatory mediators such as cyclooxygenases and cell adhesion molecules, including vascular cell adhesion molecules [38].
Histological evaluation of the H&E-stained samples revealed that on day 3, granulation tissue was most prominent in AETC-treated diabetic rats, followed by the positive control, normal control and diabetic control groups. By day 5, the densest granulation tissue was observed in the AETC500-treated group, followed by the positive control, normal control, diabetic-control and AETC250-treated groups. As the replacement of clots by granulation tissue occurs in the proliferative stage, it was deduced that AETC500-treated diabetic rats progressed into the proliferation stage faster than the other groups [39]. Hyperglycemia is known to impair the formation of granulation tissue by promoting the accumulation of abnormal fibroblasts with impaired migration and proliferation, ultimately leading to malformed ECM [40, 41]. Therefore, it can be hypothesized that improved glycemic control in AETC-treated rats contributed to the earlier and denser formation of granulation tissue. This observation aligns with previous studies demonstrating enhanced granulation tissue development in acute wound models, particularly the dead space wound model [18, 33].
Angiogenesis plays a critical role in wound healing by not only supplying essential nutrients to the regenerating tissue, but also contributing to structural repair through the formation of granulation tissue [1]. In the absence of angiogenesis, granulation tissue formation is impaired, resulting in delayed wound healing [39]. Histopathological findings from the present study revealed increased blood vessel formation within the granulation tissue across all groups on day 3, which persisted until day 7. By day 14, most blood vessels had regressed in the normal control, glibenclamide, and AETC-500 treated group, while they remained evident in the diabetic control and AETC250-treated groups. It is normal for blood vessels to disappear in the maturation/remodeling phase through emigration processes, apoptosis, or other unknown mechanisms of cell death [42]. The angiogenic potential of TC remains controversial. Through its immunomodulatory effects, TC has been shown to increase granulocyte-monocyte colony-stimulating factor, which subsequently enhances the expression and activity of platelet- and neutrophil-derived growth factors that promote angiogenesis [43]. Conversely, TC has also been reported to exert anti-angiogenic effects by downregulating VEGF and elevating levels of the anti-angiogenic cytokine interleukin-2 [17, 27]. A previous study reported that enhanced angiogenesis in AETC-treated non-diabetic rats was associated with earlier and more effective wound healing [32]. Therefore, the role of TC in modulating angiogenesis remains inconclusive, warranting further investigation to elucidate the underlying mechanisms behind these conflicting observations.
Another key player in wound healing is the fibroblast. Fibroblasts are responsible for the synthesis, deposition, and remodeling of the ECM. Upon migrating into the wounds, they initiate the production of elastin and collagen to form the new ECM, which is essential for vascular support and granulation tissue formation [44]. Histological analysis showed enhanced fibroblast proliferation and accelerated reepithelialization, and faster wound closure in AETC-treated diabetic rats. This observation aligns with the results obtained on the rate of wound contraction in AETC-treated diabetic rats. As aforementioned, fibroblasts differentiate into myofibroblasts, which are responsible for pulling the wound edges toward the wound centre, thereby gradually reducing the wound area [29]. Aside from this, previous studies using acute wound models have similarly reported increased fibroblast proliferation in TC-treated wounds, further supporting these findings [32, 33].
Following migration into the wound site during the proliferative phase, fibroblasts initiate the synthesis of collagen, a major structural protein of the ECM that ultimately contributes to wound strength [29]. In the proliferation phase, fibroblasts synthesise type 3 collagen, which is thinner and less organized than the mature type 1 collagen found in healthy skin [39]. The maturation or remodeling phase is characterized by collagen rearrangement and wound contraction. This phase begins when the rates of collagen synthesis and degradation reach equilibrium [29]. During remodeling, type 3 collagen in the granulation tissue is gradually replaced by type 1 collagen, leading to scar formation. The increase in type 1 collagen, which undergoes rearrangement, cross-linking, and alignment along lines of mechanical tension, correlates with enhanced wound strength [29, 39]. Masson’s trichrome-stained samples revealed increased collagen deposition across all groups. Notably, the AETC-treated groups exhibited greater collagen deposition than the diabetic control group, although the collagen was less organized and compact compared to that observed in the normal control and glibenclamide-treated groups. The disorganized nature of collagen in diabetic control and AETC-250 could be explained by the presence of blood vessels in the sample, which indicates that these samples were still in the proliferation phase, where collagen deposited is typically less structured [29, 39]. Previous studies demonstrated that treatment with TC extract in an acute wound model (without diabetes) markedly increases the collagen content in comparison to the control groups via both topical [32] and oral administration routes [17, 33, 45]. The findings from this study highlighted the role of oral AETC in promoting collagen formation in diabetic wounds.
This study contributes to the existing literature by addressing the limited information on the diabetic wound healing properties of orally administered TC. The outcome of this study revealed that previous findings of the wound healing abilities of TC are relevant and applicable in the context of diabetic wounds. Although diabetic foot ulcer models offer strong clinical relevance, the dorsal skin wound model in rats was selected due to its practical advantages: ease of wound creation, consistent monitoring, lower risk of infection, and reduced interference from the animal [46]. This model reliably reflects impaired healing under diabetic conditions while circumventing the technical and ethical complexities associated with footpad wounds. However, anatomical and physiological differences between rodent dorsal skin and human foot skin limit the model’s direct applicability to diabetic foot ulcers. Despite this, its widespread use supports methodological consistency and enables cross-study comparisons with translational relevance.
In addition, this study did not include a quantitative phytochemical analysis, which can be explored in the future. Future research exploring the extract fractionation, and identification of the compounds present in the extract, followed by a quantitative phytochemical profiling, may help in determining the phytochemical constituents responsible for the wound healing ability of the extracts. The underlying mechanisms of AETC in modulating the diabetic wound healing process remain unclear. Further investigations are warranted to unravel its role in diabetic wound management.
Based on the findings, it is theorized that the pro-healing response observed in the oral AETC-treated wounds could be attributed to reduced inflammation, scab formation and the subsequent early initiation of fibroplasia, collagen fiber synthesis, maturation and organization, angiogenesis, epithelialization, and keratinization. This, therefore, establishes the diabetic wound healing potential of AETC in the STZ-NAD induced diabetes rat model. The findings of this study also reaffirm the antidiabetic properties of AETC in this model.
AETC, Aqueous ethanolic stem extract of Tinospora cordifolia; AETC250, Aqueous ethanolic stem extract of Tinospora Cordifolia 250 mg/kg; AETC500, Aqueous ethanolic stem extract of Tinospora Cordifolia 500 mg/kg; ANOVA, analysis of variance; CMC, carboxymethylate cellulose; DFU, Diabetic foot ulcers; ECM, Extracellular matrix; H&E, Hematoxylin and Eosin; NAD, Nicotinamide; S.E.M, Standard error of mean; STZ, Streptozotocin; TC, Tinospora cordifolia; VEGF, Vascular endothelial growth factor; WH, Wound healing.
All data analyzed in this study are included within this article and no additional data are required to reproduce the findings. Data are available from the corresponding or first author upon reasonable request.
Conceptualization, MF, HYY, NKHS and AAA; methodology, MF and HYY; formal analysis, MF, HYY, NM and PK; investigation, MF; resources, HYY; data curation, MF, HYY, NM and PK; writing—original draft preparation, MF; writing—review and editing, MF, HYY, NKHS, AAA, NM and PK; visualization, MF; supervision, HYY, NKHS and AAA; project administration, HYY; funding acquisition, HYY. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
This study has obtained ethical approval from committee of Animal Research and Ethics, Universiti Teknologi Mara (UiTM CARE) (Approval number: 250/2018). It was conducted in accordance with the ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines. The plant specimen was verified and deposited at the University Putra Malaysia herbarium, and plant voucher was obtained (UPMFHAS/HERBARIUM/07/22: Voucher no. H079).
Not applicable.
This study was sponsored by Taylor’s University Grant (TRGS/ERFS/1/2018/SOP/033).
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.







