1 Department of Cardiology CCU, Kashi Prefecture Second People’s Hospital, 844000 Kashi, Xinjiang, China
Abstract
Drug-eluting stents (DES) have become a crucial strategy for improving the outcomes of patients with coronary artery disease (CAD). This work aimed to evaluates the therapeutic efficacy of metoprolol-loaded poly (lactic-co-glycolic acid)–poly(trimethylene carbonate)–poly (glycolic acid) (PLGA–PTMC–PGA) DES (Meto-PLGA/PTMC DES) in a rabbit model of CAD.
A total of 30 New Zealand white rabbits (male, weighing 4–6 kg) were randomly divided into three groups: Sham group (chest suturing without intervention), Model group (CAD animal model without DES), and stent group (treatment with the Meto-PLGA/PTMC DES). The blood samples were collected at regular intervals to assess the serum inflammatory markers, cardiac function parameters, hemodynamic parameters and coronary remodeling. Statistical analysis was performed using One-way analysis of variance (ANOVA), followed by a post hoc Tukey’s test to compare the differences between groups (p < 0.05 indicated statistically significance).
The Meto-PLGA/PTMC DES, through its unique co-polymer structure, achieved stable Meto loading in vivo. This stent enabled the gradual release of Meto, thus maintaining sustained drug concentrations and optimizing CAD treatment. The animal experiment results indicated that the sent group (Meto-loaded DES) exhibited markedly lower levels of interleukin-8 (IL-8), tumor necrosis factor-alpha (TNF-α), vascular cell adhesion molecule-1 (VCAM-1), and intercellular adhesion molecule-1 (ICAM-1) relative to the model group (p < 0.05). In terms of cardiac function, compared with the model group, the stent group exhibited significantly elevated left ventricular systolic pressure (LVSP), ±dp/dtmax, arterial systolic blood pressure (BPs), and diastolic blood pressure (BPd), along with a marked reduction in left ventricular end-diastolic pressure (LVEDP) compared with the model group (p < 0.05). The coronary tissue morphology in the stent group revealed notably reduced intimal thickness, intimal area and degree of stenosis versus the Model group, with a prominent increase in lumen area (p < 0.05).
The Meto-PLGA/PTMC DES not only effectively provides sustained drug release and exhibits superior mechanical properties and excellent blood compatibility. Animal experiments further validated the crucial role of the Meto-PLGA/PTMC DES in reducing inflammatory responses, improving cardiac function and alleviating coronary artery stenosis.
Keywords
- Metoprolol
- drug-eluting stent
- cardiovascular diseases
- sustained-release medication
- inflammation
Coronary artery disease (CAD) is a leading cause of mortality and disease burden [1]. The CAD results in myocardial ischemia and cardiac functional impairment, posing a considerable threat to life and health [2]. Although percutaneous coronary intervention with stenting is widely adopted due to its minimal invasiveness and rapid recovery, conventional bare-metal stents are prone to restenosis and thrombotic complications [3, 4]. Consequently, drug-eluting stents (DES) have increasingly become a focal point of research in recent years [5, 6, 7].
The DES have been applied for treating cardiovascular diseases, particularly demonstrating marked efficacy in preventing coronary artery restenosis [8]. Conventional DES typically loads antiproliferative drugs, such as sirolimus or paclitaxel, to inhibit neointimal hyperplasia [9]. However, the potential of
To address these issues, this work developed a Meto-PLGA/PTMC DES using PLGA-PTMC-PGA as the material. This novel DES controls the slow release of the drug, maintaining stable drug concentrations over extended periods and thereby enhancing therapeutic efficacy. Additionally, the Meto-PLGA/PTMC DES exhibited excellent biocompatibility and degradability, gradually breaking down into non-toxic byproducts that are fully absorbed and metabolized by the body. This work aimed to explore the adoption potential of this DES in the treatment of cardiovascular diseases and to evaluate its impact on the efficacy and safety of Meto.
The study was conducted at Kashi Prefecture Second People’s Hospital (Xinjiang, China) from May, 2023 to March, 2024.
The L-lactic acid (Catalog No. L0165, TCI America, Portland, OR, USA) was employed as the starting material to synthesize lactide oligomers via a dehydration polycondensation methodology. The lactide oligomers were then depolymerized into L-lactide (LLA) under high-temperature vacuum conditions at 50–130 °C. Following crystallization through washing with anhydrous ether and vacuum drying at 35 °C to constant weight, poly LLA (PLLA) was obtained. Using diethyl carbonate (Catalog No. 436131000, Thermo Scientific Chemicals, Waltham, MA, USA) and 1,3-propanediol as raw materials, PTMC oligomers were synthesized through polycondensation under high-temperature vacuum conditions at 180 °C after ethanol removal. The crude product was dissolved in ether and acetone mixture, repeatedly crystallized five times in an ice water bath, washed with anhydrous ether and then vacuum filtered and dried at 25 °C to constant weight, yielding PTMC. Using carboxylic acid as the raw material, the mixture was heated to 140 °C in oil. After dehydration between hydroxy acetic acid molecules, stannous octoate was added under vacuum conditions at 200 °C to yield pale yellow crystals. The product was recrystallized five times using ethyl acetate, washed once with anhydrous ether and vacuum-dried at 35 °C to constant weight, resulting in poly (glycolic acid) (PGA). Under nitrogen protection, PLLA, PTMC, and PGA were melted and mixed uniformly (the mass ratios of PLLA:PTMC:PGA were 1:1:1, 1:1:2 and 1:1:3, respectively), then cooled and solidified in an ice-water bath. The mixture was subjected to vacuum treatment for 3 hrs and reacted at 130 °C for 72 hrs. The copolymer was dissolved in a Dichloromethane solution (Catalog No. D807825, Shanghai Macklin Biochemical Technology Co., Ltd., Shanghai, China), precipitated with ethanol and vacuum-dried at 60 °C to constant weight, yielding the PLLA-PTMC-PGA composite.
A 5% (w/v) solution of PLLA-PTMC-PGA was dissolved in 1,4-dioxane (Catalog No. D116356, Aladdin Industrial Corporation, Shanghai, China) and stirred for 3 hrs using a magnetic stirrer (Catalog No. 85-2, Shanghai Sile Instrument Co., Ltd., Shanghai, China). Deionized water (prepared using a Milli-Q water purification system, Catalog No. Direct-Q 3, Merck KGaA, Darmstadt, Germany) was then applied and stirring continued for an additional 2 hrs. Metoprolol (Meto, Catalog No. M1013, Sigma-Aldrich, St. Louis, MO, USA) was incorporated and stirred for 1 hr to ensure its uniform distribution within the stent material. The temperature was maintained at approximately 5 °C above the cloud point using a constant temperature water bath (Catalog No. HH-S2, Jintan Medical Instrument Factory, Jintan, China) and then slowly decreased to the gel point for 2 hrs. The clear solution was quickly transferred to liquid nitrogen (Nanjing Special Gas Factory Co., Ltd., Nanjing, China) for 1 hr to freeze rapidly, ensuring stable binding of Meto with the material and preventing separation or drug precipitation.
The resulting Meto-PLGA/PTMC DES was obtained through freeze-drying for 24 hrs using a freeze dryer (Catalog No. FD-1A-50, Beijing Boyikang Experimental Instrument Co., Ltd., Beijing, China). The stent material was placed in 10 mL of Dimethyl Sulfoxide (DMSO, Catalog No. D8371, Sigma-Aldrich, St. Louis, MO, USA) and stirred at 37 °C for 1, 3, 6, 12, 24, 48 and 96 hrs using a thermostatic shaker (Catalog No. THZ-320, Shanghai Jinghong Laboratory Equipment Co., Ltd., Shanghai, China). At each time, 2 mL of release solution was sampled (buffer replenished) and analyzed for Meto release using a UV-1901 ultraviolet Spectrophotometer (Shanghai Lengguang Technology Co., Ltd., Shanghai, China).
The Meto-PLGA/PTMC DES samples were individually dissolved in dichloromethane (Catalog No. D8418, Sigma-Aldrich, St. Louis, MO, USA) to prepare solutions at 10 ng/mL. As 300 µL aliquot of each sample was applied to clean glass slides (Catalog No. 7101, Thermo Fisher Scientific, Waltham, MA, USA), vacuum-dried to constant weight using a vacuum dryer (Catalog No. DZF-6050, Shanghai Jinghong Laboratory Equipment Co., Ltd., Shanghai, China) and then UV-sterilized for 30 min using a UV sterilizer (Catalog No. SW-CJ-2FD, Suzhou Antai Air Technology Co., Ltd., Suzhou, China). The samples were extracted using either physiological saline (Catalog No. P1400, Solarbio Science & Technology Co., Ltd., Beijing, China) or DMEM (Catalog No. 12100046, Thermo Fisher Scientific, Waltham, MA, USA) at a liquid-to-membrane ratio of 1 mL/6 cm2 for 72 hrs at 37 °C under sterile conditions in a CO2 incubator (Catalog No. BB15, Thermo Fisher Scientific, Waltham, MA, USA), filtrated through a 0.22 µm pore filter (Catalog No. SLGP033RB, Merck Millipore, Darmstadt, Germany). Rabbit cardiac fibroblasts (Catalog No. KL-C1025, Shanghai Kanglang Bio-Tech Co., Ltd., Shanghai, China) were cultured under standard conditions and prepared as a cell suspension at 1
Rabbit blood (Guangzhou Ruite Biotechnology Co. Ltd., China) was anticoagulated and mixed with physiological saline (Catalog No. P1400, Solarbio Science & Technology Co., Ltd., Beijing, China) at a 4:5 ratio. The mixture was centrifuged at 1500 rpm for 10 min using a centrifuge (Catalog No. 5810R, Eppendorf AG, Hamburg, Germany) to collect supernatant. A 10 mL aliquot of the Meto-PLGA/PTMC DES sample extract was incubated at 37 °C for 30 min in a water bath (Catalog No. HH-S4, Jintan Medical Instrument Factory, Jintan, China), mixed with 200 µL of fresh anticoagulated blood and incubated for an additional hour. The mixture was centrifuged at 3000 rpm for 5 min using a centrifuge (Catalog No. 5810R, Eppendorf AG, Hamburg, Germany) and the supernatant’s absorbance was measured at 540 nm employing a microplate reader (Catalog No. Multiskan FC, Thermo Fisher Scientific, Waltham, MA, USA).
The hemolytic ratio was calculated using [7]:
The Meto-PLGA/PTMC DES samples were dissolved in dichloromethane (Catalog No. D8418, Sigma-Aldrich, St. Louis, MO, USA) and evenly coated on the concave center of a glass surface dish (Catalog No. xy085, Shanghai Yubo Biotechnology Co., Ltd., Shanghai, China), then allowed to evaporate at 25 °C. Following this, 200 mL of anticoagulated blood was applied. At 0, 0.25, 0.5, 1 and 2 hrs, the glass surface was gently washed with 100 µL of ultrapure water (prepared using a Milli-Q water purification system, Catalog No. Direct-Q 5, Merck KGaA, Darmstadt, Germany) and absorbance at 540 nm was measured via HBS-1101 microplate reader (Nanjing Detie Experimental Equipment Co., Ltd., Nanjing, China).
A total of 30 adult male New Zealand white rabbits (4–6 kg) were anesthetized for the procedure using 3% sodium pentobarbital (Catalog No. P3761, Sigma-Aldrich, St. Louis, MO, USA) at a dose of 0.05 g/kg, and the animals were purchased from Shanghai Jiagen Biotechnology Co. Ltd., China. A constriction ring (custom-made, Suzhou Weirui Medical Device Technology Co., Ltd., Suzhou, China) was placed at the distal end of the left coronary artery under assisted breathing conditions using a small animal ventilator (Catalog No. SAR-830, CWE Inc., Ardmore, PA, USA). Post-operative disinfection was performed with 75% ethanol (Catalog No. E7023, Sigma-Aldrich, St. Louis, MO, USA) and anti-inflammatory treatment were administered with ceftriaxone sodium (Catalog No. C5793, Sigma-Aldrich, St. Louis, MO, USA) to establish a CAD animal model [14]. Two-dimensional ultrasound was employed using a small animal ultrasound system (Catalog No. Vevo 2100, FUJIFILM Visual Sonics Inc., Toronto, ON, Canada) to visualize the vascular orientation, lumen endoscopy, vessel wall thickness and plaque formation.
The thorax was directly sutured for the Sham group using surgical sutures (Catalog No. 11-0, Ethicon Inc., Somerville, NJ, USA), while animals subjected to modeling and atherosclerotic plaque formation were divided into the Model group and the Stent group (treatment with Meto-PLGA/PTMC DES), with 10 rabbits in each group. Pre-operatively, the Stent group received aspirin (Catalog No. A2093, Sigma-Aldrich, St. Louis, MO, USA) at 25 mg/day and clopidogrel (Catalog No. C3487, Sigma-Aldrich, St. Louis, MO, USA) at 12.5 mg/day. Three days post-operation, angiography was performed under X-ray fluoroscopy using the 1725AX small animal X-ray imaging system (Shanghai Yuyan Scientific Instrument Co. Ltd., Shanghai, China), with a microcatheter (Catalog No. MPC-1.2F, Cook Medical Inc., Bloomington, IN, USA) introduced and the DES positioned at the site of arterial stenosis. Routine intramuscular injections of 4.5 million U penicillin (Catalog No. P3032, Sigma-Aldrich, St. Louis, MO, USA) were administered for 3 days. After recovery, animals were housed in cages (Catalog No. RCC-01, Suzhou Fengshi Laboratory Animal Equipment Co., Ltd., Suzhou, China) and continued a high-fat diet (Catalog No. D12492, Research Diets Inc., New Brunswick, NJ, USA) for 30 days. The 1725AX small animal X-ray imaging system was sourced from Shanghai Yuyan Scientific Instrument Co. Ltd., China.
Post-treatment, 3 mL of venous blood was collected from the ear margin using a 5 mL disposable syringe (Catalog No. 100500, Shanghai Kohama Medical Devices Co., Ltd., Shanghai, China) with a 22G needle (Catalog No. 302220, BD Medical, Franklin Lakes, NJ, USA), anticoagulated with heparin sodium (Catalog No. H3149, Sigma-Aldrich, St. Louis, MO, USA) at a final concentration of 10 U/mL and centrifuged at 1200 rpm for 10 min using a centrifuge (Catalog No. 5810R, Eppendorf AG, Hamburg, Germany).The supernatant was utilized to measure serum Interleukin-8 (IL-8) levels via radioimmunoassay using an IL-8 radioimmunoassay kit (Catalog No. RK00012, Shanghai Enzyme Linked Bio, Shanghai, China), while serum Vascular Cell Adhesion Molecule-1 (VCAM-1), Intercellular Adhesion Molecule-1 (ICAM-1) and Tumor Necrosis Factor-Alpha (TNF-
Twelve hours after treatment, the animals were anesthetized with 0.05 g/kg of 3% sodium pentobarbital (Sigma-Aldrich, USA). The right common carotid artery and left femoral artery were isolated. A polyethylene (PE-20) catheter was inserted through a right common carotid artery into the left ventricle, with the other end connected to a physiological recorder (Shanghai Renyi Biotechnology Co. China). This setup measured Left Ventricular Systolic Pressure (LVSP), LV end-diastolic pressure (LVEDP) and the maximum rates of pressure increase and decrease (
Twenty-four hours after cardiac function assessment, the animals were euthanized under 3% sodium pentobarbital (Catalog No. P3761, Sigma-Aldrich, St. Louis, MO, USA) anesthesia. Coronary artery tissues were fixed in 4% paraformaldehyde (Catalog No. P6148, Sigma-Aldrich, St. Louis, MO, USA) for 24 hrs. Tissues were rinsed twice with PBS (pH 7.2, Catalog No. P1020, Solarbio Science & Technology Co., Ltd., Beijing, China) , dehydrated through a graded ethanol series (50%: Catalog No. E7023-500ML, Sigma-Aldrich, St. Louis, MO, USA; 70%: Catalog No. 10009218, Sinopharm Chemical Reagent Co., Ltd., Shanghai, China; 80%: Custom-prepared, Sinopharm Chemical Reagent Co., Ltd., Shanghai, China; 90%: Custom-prepared, Sinopharm Chemical Reagent Co., Ltd., Shanghai, China; 100%: Catalog No. 10009218, Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) and cleared in xylene (Catalog No. X1500, Solarbio Science & Technology Co., Ltd., Beijing, China). They were embedded in paraffin (Catalog No. P1461, Solarbio Science & Technology Co., Ltd., Beijing, China) using a tissue embedding machine (Catalog No. EG1150H, Leica Biosystems, Wetzlar, Germany) and sectioned into 4 µm thick slices with a microtome (Catalog No. RM2235, Leica Biosystems, Wetzlar, Germany).
Staining was conducted according to the instructions of the Hematoxylin-Eosin staining kit (Catalog No. G1120, Shanghai Enzyme Linked Bio, Shanghai, China).The coronary artery tissue morphology was visualized via a CX23 Microscope (Olympus, Japan) equipped with a digital camera (Catalog No. DP27, Olympus, Tokyo, Japan) and image analysis software (Image-Pro Plus 6.0, Media Cybernetics, Rockville, MD, USA) was employed to evaluate intimal thickness, lumen area and stenosis severity.
All data were presented as Mean
Fig. 1a shows the Fourier Transform Infrared Spectroscopy (FTIR) spectrum of Meto-PLGA/PTMC DES. The spectrum indicated a notable presence of PLLA in Meto-PLGA/PTMC DES, with a prominent C = O peak at 1758/cm and a notable -CH2 peak at 1431/cm. Fig. 1b depicts that the glass transition temperature (Tg) of Meto-PLGA/PTMC DES is 56.9 °C. Fig. 1c presents the tensile strength is 1831.5 MPa. Fig. 1d illustrates the storage modulus of Meto-PLGA/PTMC DES as a function of temperature, showing a decreasing trend with increasing temperature, with a sharp decline in storage modulus observed above 40 °C. Fig. 1e displays the weight loss versus time curves for Meto-PLGA/PTMC DES during degradation in water and proteinase K. Over time, the weight loss of Meto-PLGA/PTMC DES increased gradually (Fig. 1e), with a higher weight loss rate in proteinase K compared to water (Fig. 1f).
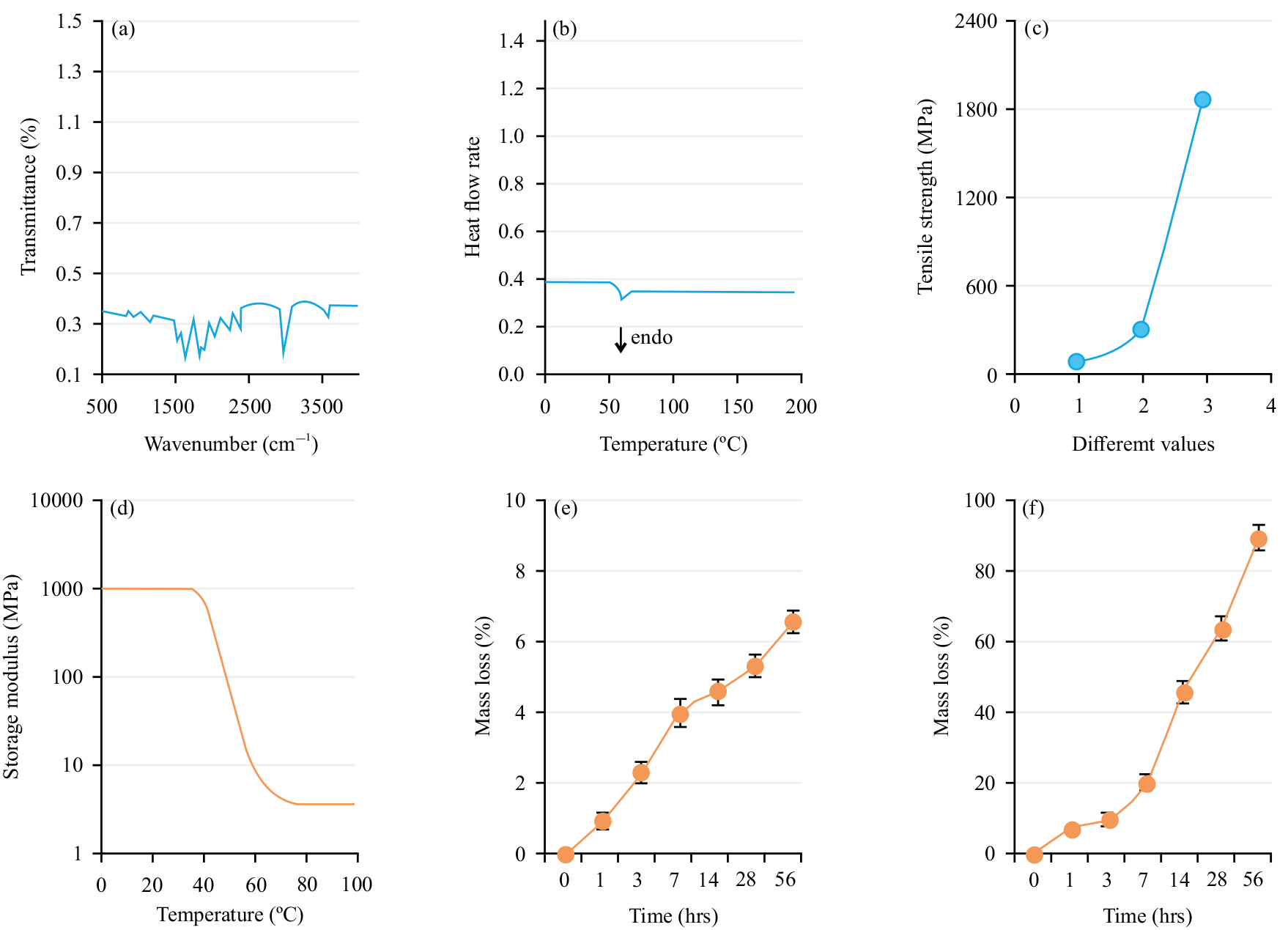 Fig. 1.
Fig. 1. Physicochemical properties of Metoprolol-loaded poly (lactic-co-glycolic acid)-poly (trimethylene carbonate)-poly (glycolic acid) drug-eluting stent (Meto-PLGA/PTMC DES). (a) Fourier Transform Infrared Spectroscopy (FTIR) spectrum; (b) Differential Scanning Calorimetry (DSC) curve; (c) Static mechanical properties (the x-axis labels 1, 2, and 3 represent the mass ratios of PLLA:PTMC:PGA as 1:1:1, 1:1:2 and 1:1:3, respectively); (d) Dynamic mechanical properties; (e) Weight loss versus time curve in water and (f) Weight loss versus time curve in proteinase K.
Fig. 2a shows the results of cell proliferation assays for rabbit cardiac fibroblasts cultured in a normal medium and in extracts from Meto-PLGA/PTMC DES. Over time, proliferation activity increased markedly in both conditions, with higher proliferation in the normal medium. The effect of Meto-PLGA/PTMC DES on cell proliferation was minimal, indicating good cytocompatibility and absence of cytotoxicity. The hemolysis rate of Meto-PLGA/PTMC DES was 3.09%, which was below the 5% threshold, indicating that the composite materials meet the hemolysis requirements for medical biomaterials. Fig. 2b presents the dynamic clotting time curve for Meto-PLGA/PTMC DES, which shows a similar gradual decrease as observed in the normal control, indicating comparable clotting behavior.
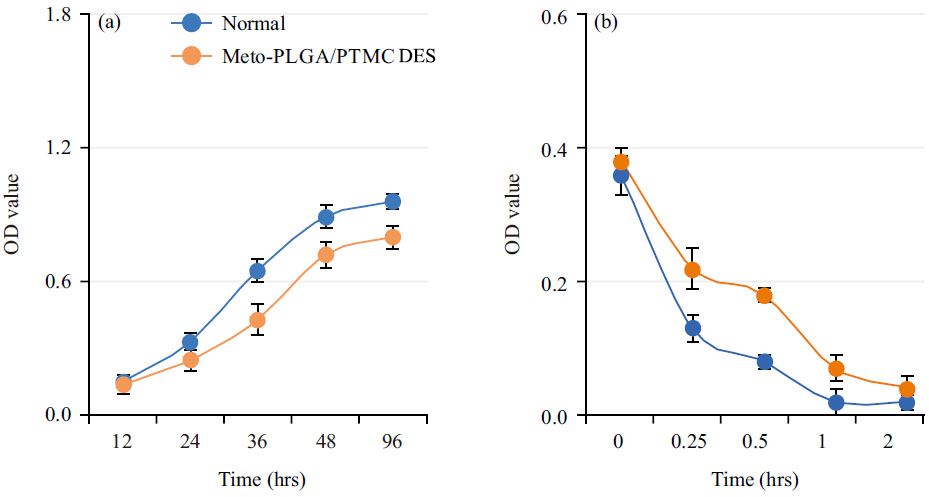 Fig. 2.
Fig. 2. Cell compatibility of Metoprolol-loaded poly (lactic-co-glycolic acid)-poly (trimethylene carbonate)-poly (glycolic acid) drug-eluting stent (Meto-PLGA/PTMC DES) complex. (a) Effect of Meto-PLGA/PTMC DES on the growth of rabbit cardiac fibroblasts and (b) Dynamic clotting time curve of Meto-PLGA/PTMC DES.
Fig. 3 shows the in vitro drug release curve of Meto-PLGA/PTMC DES. Over time, the cumulative drug release rate of the stent gradually increased (Fig. 3a), while the total amount of drug released gradually decreased (Fig. 3b), indicating that the stent enables slow and sustained drug release with a stable release profile.
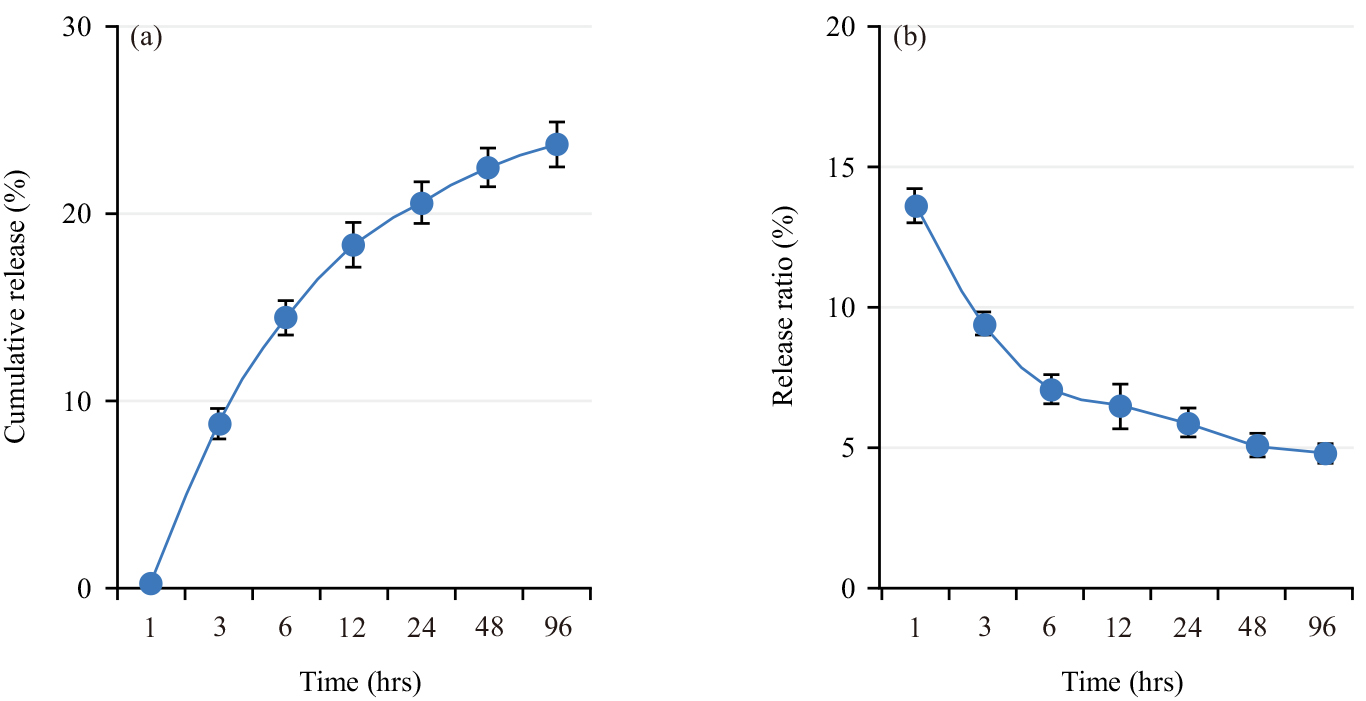 Fig. 3.
Fig. 3. In vitro drug release characteristics of Metoprolol-loaded poly (lactic-co-glycolic acid)-poly (trimethylene carbonate)-poly (glycolic acid) drug-eluting stent (Meto-PLGA/PTMC DES). (a) Cumulative drug release rate and (b) Amount of drug released.
The changes in the expression levels of IL-8 (Fig. 4a), TNF-
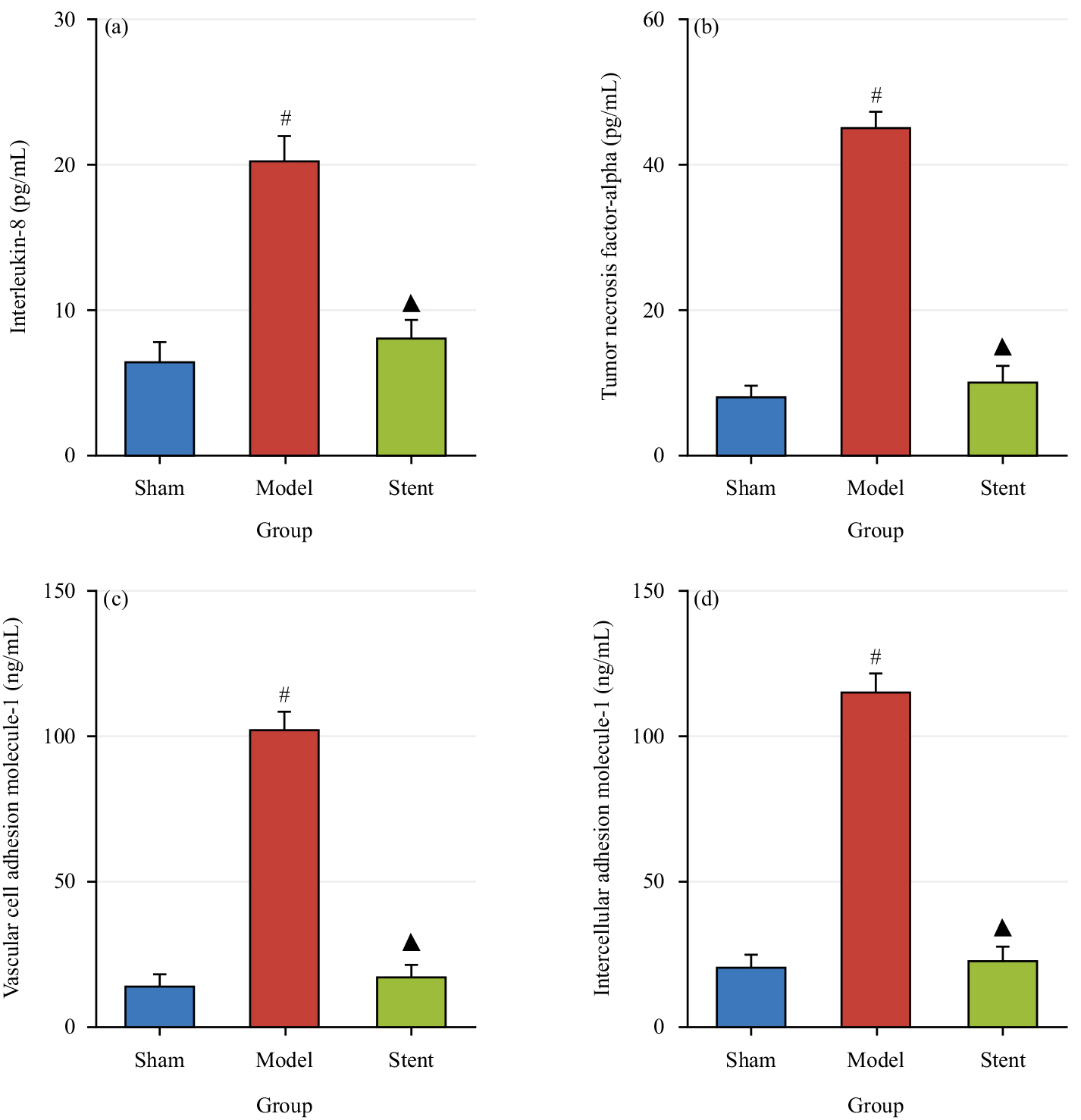 Fig. 4.
Fig. 4. Contrast of serum levels of related factors across groups. (a) Expression levels of Interleukin-8 (IL-8); (b) Expression levels of Tumor Necrosis Factor-Alpha (TNF-
Fig. 5 shows the differences in cardiac function parameters LVSP (Fig. 5a), LVEDP (Fig. 5b), +dp/dtmax (Fig. 5c), –dp/dtmax (Fig. 5d), BPs (Fig. 5e) and BPd (Fig. 5f) across groups. In Model group, LVSP, +dp/dtmax, –dp/dtmax, BPs and BPd were drastically inferior to Sham group, while LVEDP was markedly higher (p
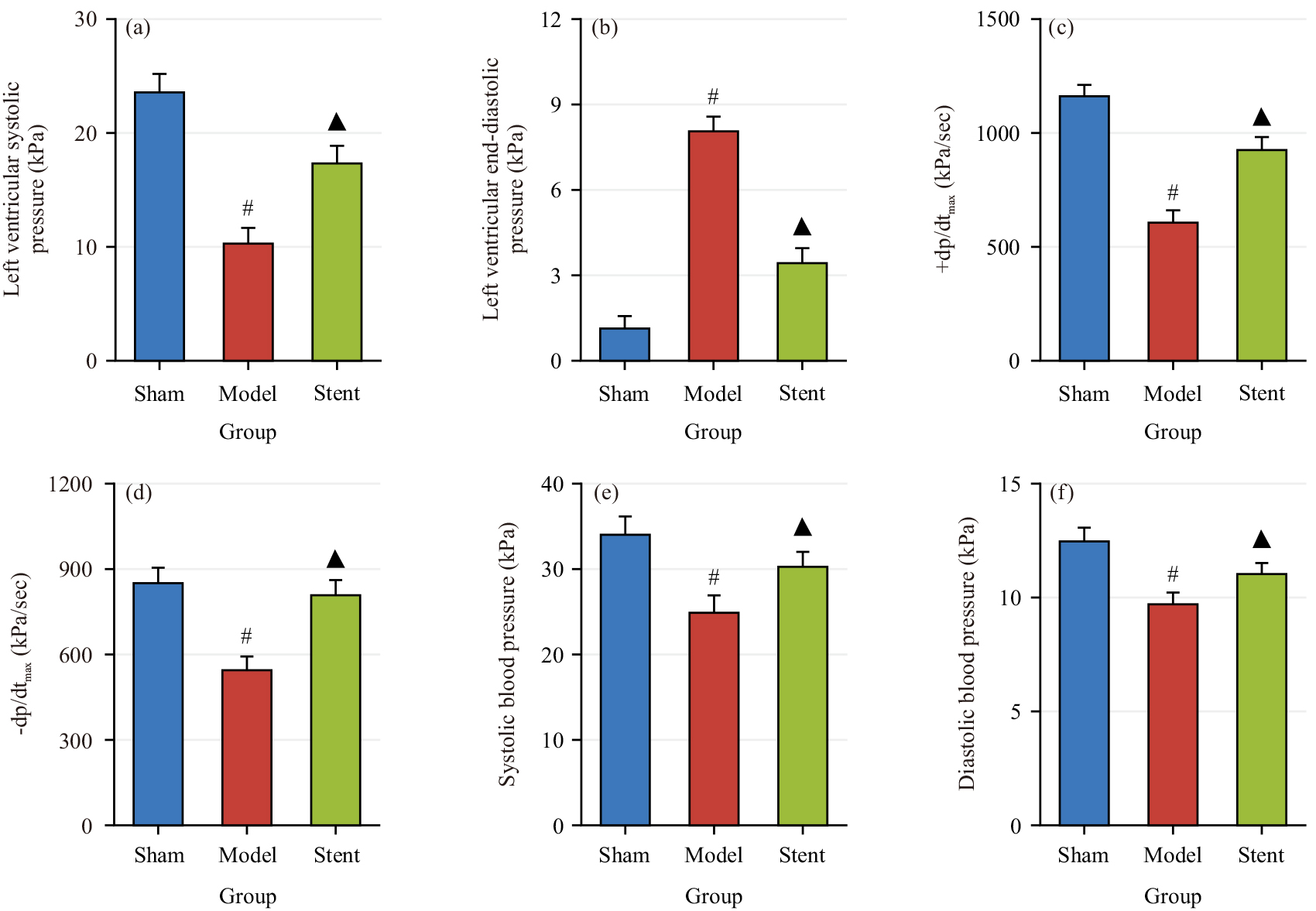 Fig. 5.
Fig. 5. Comparison of cardiac function parameters across groups. (a) Left Ventricular Systolic Pressure (LVSP); (b) LV end-diastolic pressure (LVEDP); (c) +dp/dtmax; (d) –dp/dtmax; (e) systolic blood pressure (BPs) and (f) diastolic BP (BPd). #p
Fig. 6 shows the comparison of the intimal thickness (Fig. 6a), lumen area (Fig. 6b), intimal area (Fig. 6c) and degree of stenosis (Fig. 6d) across groups. The model group exhibited notably greater intimal thickness and area and considerably smaller lumen area relative to the Sham group (p
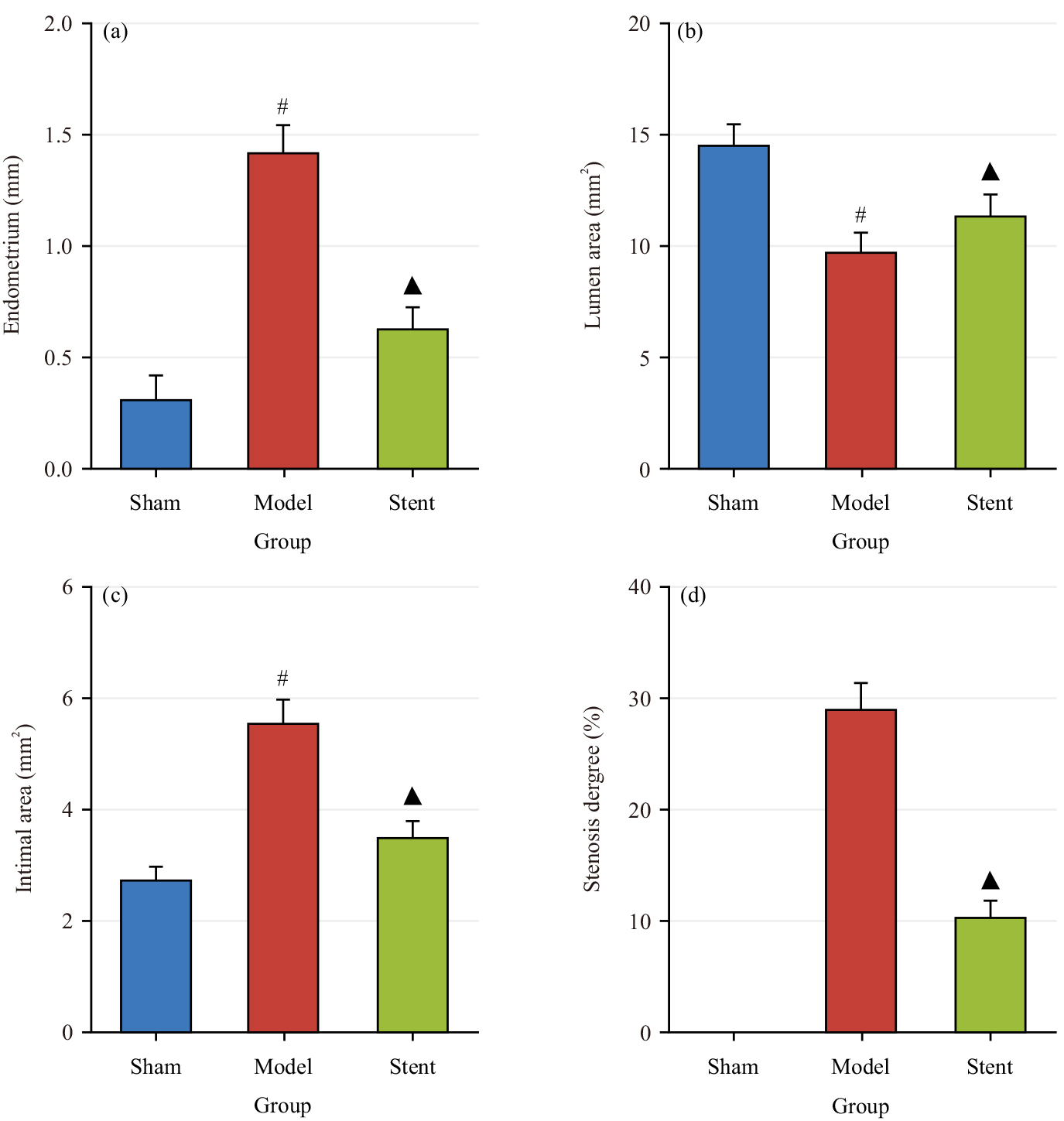 Fig. 6.
Fig. 6. Comparison of coronary artery wall and intimal across groups. (a) Intimal thickness; (b) Lumen area; (c) Intimal area and (d) Degree of stenosis. #p
This work explores the potential adoption value of Meto-PLGA/PTMC DES in treating coronary heart disease. Experimental results demonstrated that Meto-PLGA/PTMC DES exhibited great advantages in terms of mechanical properties, biocompatibility, drug release characteristics, as well as anti-inflammatory and cardiovascular protective effects.
The PLLA-based material offers excellent biocompatibility and suitable mechanical properties, making it well-suited for cardiovascular stent development. Cardiovascular stents require materials with specific mechanical properties, such as stiffness, strength, elongation, recoil, compliance and degradability [15]. Previous studies confirmed that PLLA-based stent materials can withstand compression forces of 1.3 bars, similar to the strength of metal stents [16]. Kuriakose et al. [17] demonstrated that BPLPL-PLGA-based nanoparticles possess excellent physical and biological properties, making them viable as functional nanocarriers for the treatment and diagnosis of cardiovascular diseases. Kim et al. [18] reported that a polymer mixture of PLLA and phospholipid polymers is used for biodegradable cardiovascular stents, while Kim et al. [19] also highlighted the use of PLLA material as a temporary scaffold for vascular walls. In this study, the glass Tg, tensile strength, elongation at break and Young’s modulus of Meto-PLGA/PTMC DES demonstrated its sufficient mechanical strength and flexibility to withstand the complex stresses in the coronary blood flow dynamics. Particularly, the material’s excellent cellular and blood compatibility suggested that the stent adapted well to the in vivo environment, reducing foreign body reactions and stent-related complications. Young’s modulus is a key physical property for evaluating a material’s resistance to deformation [20]. The results indicated that Meto-PLGA/PTMC DES exhibited good tensile strength and deformation resistance. Viscoelasticity, a distinctive feature of polymer materials, shows strong time and temperature dependency [21]. The synthesized Meto-PLGA/PTMC DES had a Tg above 50 °C, maintaining a frozen molecular state within the normal body temperature range, thereby sustaining high mechanical strength. This study further confirmed the mechanical performance and biocompatibility of Meto-PLGA/PTMC DES, highlighting its mechanical support advantages and its ability to maintain excellent cellular and blood compatibility even after drug loading. These findings align with existing literature and support the material’s potential applications in the cardiovascular field.
Beta-blockers are highly effective in controlling ventricular arrhythmias associated with sympathetic nerve excitation, which can be triggered by acute myocardial ischemia, electrolyte disturbances and both psychological and physical stress, thus markedly preventing sudden cardiac death. In various acute coronary syndrome treatment guidelines, beta-blockers are prominently recommended both for acute management and long-term maintenance therapy, establishing them as one of the most used drugs in cardiovascular medicine [22, 23]. Dransfield et al. [24] indicated that Meto is effective in preventing acute exacerbations of COPD. Dybro et al. [25] demonstrated that Meto reduces LV outflow tract obstruction at rest and during exertion and alleviates symptoms in patients. Heck et al. [26] reported that the beta-blocker Meto reduces myocardial troponin levels. Giannakopoulos and Noble [27] noted that early intravenous administration of beta-blockers is safe in hemodynamically stable patients and can prevent malignant arrhythmias. Meto can alleviate heart failure and atrial fibrillation [28, 29]. As a widely applied beta-blocker, the sustained-release characteristics of Meto have been thoroughly validated in this study. Research on a CAD animal model demonstrated that Meto-PLGA/PTMC DES effectively inhibits the release of serum inflammatory factors. This not only confirms the anti-inflammatory effects of Meto but also highlights its potential in alleviating vascular inflammation and mitigating the progression of atherosclerosis in CAD. Mandal et al. [30] reported a myocardial infarction patient who, after receiving Meto and stent therapy, had stable vital signs. Beta-adrenergic blockers like Meto remain the preferred treatment for heart failure, CAD, atrial fibrillation and hypertension-related heart failure, angina or previous myocardial infarction [31]. Studies noted that patients undergoing percutaneous coronary interventions with balloon angioplasty and bare-metal stent implantation experience reduced IL-8 levels [32]. The stent materials used clinically must possess good biocompatibility characteristics, which primarily include cellular and hemocompatibility. Cellular biocompatibility involves evaluating the material’s effects on cell adhesion, growth, differentiation and apoptosis. Blood compatibility requires assessing the material’s anticoagulant properties to minimize thrombosis formation [33]. Thadani [34] demonstrated that stents coated with Meto have a positive impact on chronic stable angina. In vitro dynamic clotting time assays are used to assess the material’s effect on coagulation function, primarily by measuring the activation of intrinsic coagulation factors. A dynamic clotting time curve that gradually declines over an extended period indicates superior anticoagulant performance of the material. Stent implantation not only provides vascular support but also facilitates controlled drug release, enhancing the therapeutic effect of the stent by regulating the drug delivery [35]. The DES offer notable advantages, including significantly reduced restenosis rates, resistance to damage during expansion, independence from raw material supply and timing constraints and low implantation-related side effects [36].
The current study demonstrated that Meto-PLGA/PTMC DES markedly inhibited the release of serum inflammatory factors IL-8, TNF-
The Meto-PLGA/PTMC DES demonstrated excellent cell and blood compatibility. When implanted at the site of CAD lesions in animal models, the sustained release of Meto effectively inhibited the release of serum inflammatory factors, suppressed neointimal hyperplasia and reduced the degree of vascular narrowing. This indicates that Meto plays a crucial anti-inflammatory and vascular protective role in the treatment of CAD. This work primarily focused on the short-term sustained-release effects of Meto and has not yet fully assessed the potential long-term impacts on CAD patients. Future research should address the long-term effects of Meto-eluting stents, including their impact on restenosis, thrombosis and patient quality of life.
The Meto-PLGA/PTMC drug-eluting stent developed in this study offers an efficient and safe drug release solution, ensuring stable drug release while demonstrating exceptional mechanical strength and favorable blood compatibility. These characteristics are critical for enhancing the performance of medical implants. Furthermore, the drug-eluting stent has shown significant effects in reducing inflammation, improving cardiac function and alleviating coronary artery stenosis, presenting a promising new approach to the treatment of cardiovascular diseases. This research has the potential to advance the drug-eluting stent in coronary heart disease, thus providing safer and more effective therapeutic options for patients, with profound clinical and scientific significance.
All relevant data are within the paper and the relevant data be obtained from the first author or corresponding author upon reasonable request.
RL designed the research study. RL, AM and TA performed the research. RL, AM and TA provided help and advice on the ELISA experiments. RL, AM and TA analyzed the data. RL, AM and TA wrote, reviewed and revised the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
The experimental protocol was approved by the Animal Ethical Committee of Kashi Prefecture Second People’s Hospital (Xinjiang, China) (No. 2023-012). All the experimental protocols involved in the current investigation followed the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and the ARRIVE (Animal Research: Reporting of in vivo Experiments) guidelines.
Not applicable.
This work was supported by The Third Phase of The Tianshan Talent Program in Xinjiang Uygur Autonomous Region (No. 2021046).
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.






