1 Yunnan Provincial Key Laboratory of Entomological Biopharmaceutical R&D, College of Pharmacy, Dali University, 671000 Dali, Yunnan, China
†These authors contributed equally.
Abstract
Periplaneta americana is an important traditional medicine in China. Moreover, Periplaneta americana is effective in treating multiple tumor types. Thus, this study aimed to investigate the combined effects of Periplaneta americana extract CII-3 (an active component from Periplaneta americana) and 5-fluorouracil (5-FU) on liver cancer in mice, focusing on anti-tumor and immunomodulatory properties.
Mice were divided into five groups: normal, model, CII-3, 5-FU and 5-FU+CII-3. Continuous treatment for 14 days included monitoring tumor volume and weight, calculating spleen and thymus indices, and observing tumor tissue morphology. Immunological assays evaluated natural killer (NK) cell cytotoxicity, T/B lymphocyte proliferation, and the proportions of CD3+, CD4+, and CD8+ cells. ELISA was employed to assess serum levels of IgA, IgM, IgG, and interleukin-8 (IL-8). The reverse transcription quantitative polymerase chain reaction (RT-qPCR) and Western blot assay were utilized to analyze the mRNA and protein expressions, respectively, of toll-like receptor 4 (TLR4), nuclear factor-kappa B (NF-κB), tumor necrosis factor receptor-associated factor 6 (TRAF6), myeloid differentiation primary response protein 88 (MyD88) and tumor necrosis factor-α (TNF-α) in tumors.
Compared to the 5-FU group, the 5-FU+CII-3 group showed reduced tumor mass and increased spleen and thymus indices. Tumor tissue in the treatment groups exhibited a loose structure, characterized by nuclear fragmentation and the presence of lipid vacuoles. The 5-FU+CII-3 group exhibited enhanced NK cell cytotoxicity, T/B lymphocyte proliferation, CD3+ proportion, CD4+/CD8+ ratio and serum levels of IgA, IgM, and IgG, alongside a reduction in IL-8 content. The levels of TLR4, NF-κB, MyD88, and TNF-α mRNA and protein expressions were downregulated, while TRAF6 mRNA expression was also notably decreased in the tumor tissue.
The CII-3 and 5-FU combined treatment synergistically exerts anti-tumor and immunomodulatory effects, potentially mediated through the regulation of TLR4, NF-κB, TRAF6, MyD88, and TNF-α.
Keywords
- Periplaneta americana extract
- 5-fluorouracil
- hepatocellular carcinoma
- synergism
- immunoregulation
Hepatocellular Carcinoma (HCC) ranks as the third leading cause of cancer-related mortality, with a 5-year survival rate of
The expression of Toll-Like Receptor 4 (TLR4) has been linked to the progression, invasion, metastasis and adverse prognosis of HCC [6, 7]. The TLR4 is expressed on the surface of tumor cells in many different tissues and is associated with the establishment and maintenance of tumor immune microenvironment. The activation of TLR4 signaling pathway in tumor tissue can cause the increase of immunosuppressive factors, promote cancer cell proliferation and immune escape and lead to the growth and metastasis of cancer cells [8, 9]. The TLR4 can activate downstream signals through myeloid differentiation primary response protein 88 (MyD88), triggering the activation of important proteins such as nuclear factor-kappa B (NF-
Chemotherapy remains one of the most effective treatment options for advanced-stage HCC and 5-Fluorouracil (5-FU) is a commonly used chemotherapy drug in clinical practice [11]. The 5-FU intervenes in the biological behavior of HCC cells through multiple pharmacological mechanisms. Firstly, it is converted into active metabolites that disrupt the synthesis of purines and pyrimidines, causing abnormal DNA and RNA synthesis, thereby hindering the normal proliferation of cancer cells. Secondly, the binding of active metabolites to DNA induces DNA strand damage, triggering apoptosis in cancer cells [12]. Additionally, the 5-FU metabolic product, 5-Fluoro-2′-Deoxyuridine Monophosphate (FdUMP), inhibits thymidylate synthase, affecting DNA synthesis and repair [13]. However, despite the significant inhibitory effects of 5-FU in HCC treatment, its usage is associated with a range of potential toxic side effects. The 5-FU can lead to bone marrow suppression, immune suppression, gastrointestinal toxicity, immune system suppression, skin reactions and neurotoxicity [9, 14]. Moreover, with prolonged treatment courses, issues such as decreased drug sensitivity and drug resistance leading to poor efficacy may arise [15].
Numerous studies have indicated that the combination of traditional Chinese medicine (TCM) and chemotherapy can synergistically enhance therapeutic effects, alleviate chemotherapy side effects, improve the quality of life for patients and prolong survival time [16, 17]. The TCM can suppress the occurrence of HCC by inhibiting the expression of MyD88, TRAF6, NF-
Given this background, this study explored the synergistic effects and immunomodulatory actions of the Periplaneta americana extract CII-3 in combination with 5-fluorouracil in the treatment of HCC. This study aimed to investigate the changes in the TLR4/MyD88/NF-
The current work was performed at the Yunnan Provincial Key Laboratory of Entomological Biopharmaceutical R&D, Dali University, China from October, 2023 to January, 2024.
The CII-3 extract (Batch: 20200814) was generously provided by Associate Professor Zhengchun He from the School of Pharmacy at Dali University. The extraction process involved ethanol extraction and concentration of dried Periplaneta americana bodies to obtain a crude extract. Further purification was achieved through chromatography using large-pore adsorption resin, alcohol elution and vacuum concentration, resulting in a refined substance primarily composed of small peptides with anti-tumor activity [27].
The SPF-grade KM mice (n = 30, both genders, body weight: 18–22 g) were supplied by Hunan Slake Jingda Experimental Animal Co. Ltd. The H22 hepatocellular carcinoma cell line and Yac-1 cell line were generously provided by the Pharmacology Laboratory at Dali University. All cell lines were validated by STR profiling and tested negative for mycoplasma.
The RPMI-1640 medium (ThermoFisher Biochemical Products, Beijing, China, Co., Ltd., No: C11875500BT); Fetal bovine serum (Hangzhou Sijiqing Biotechnology Materials, Hangzhou, China, Co. Ltd., No.: 11012-8611); Lipopolysaccharide (Beijing Solabe Biotechnology, Beijing, China, Co., Ltd., No.: L8880); Concanavalin A (Sigma, St. Louis, MO, USA, Lot: SLBL3798V); FITC Hamster Anti-Mouse CD3e, APC Rat Anti-Mouse CD4, PerCP-Cy5.5 Rat Anti-Mouse CD8a (BD, San Diego, CA, USA, No.: 553061, 553051, 551162); Total RNA extraction kit, cDNA first-strand synthesis kit, SuperReal fluorescent quantitative pre-mixed kit (Tiangen Biotech, Beijing, China, Co. Ltd., No.: DP419, KR116, FP205); Mouse IgA Elisa KIT, Mouse IgM Elisa KIT, Mouse IgG Elisa KIT (Shanghai Enzyme-linked Biotechnology, Shanghai, China, Co. Ltd., No.: ml037606, ml063597, ml037601); TLR4 Monoclonal antibody, MYD88 Monoclonal antibody, TNF-
Instruments comprised an inverted microscope (CKX41SF, Olympus, Windsor, Hachioji-shi, Tokyo Metropolis, Japan), a microplate reader (255939, Bio-Teck, Winooski, VT, USA), a CO2 incubator (5510E, NUAIRE, Plymouth, MN, USA), a flow cytometer (FACSCantoⅡ, BD, Franklin Lakes, NJ, USA), a protein electrophoresis apparatus (1658004, Bio-Rad, Hercules, CA, USA), a gel chemiluminescence imaging system (GelView6000Pro, Bo Lu Teng, Guangzhou, China) and a real-time fluorescence quantitative PCR analysis instrument (2720, Applied Biosystems, Carlsbad, CA, USA).
The H22 mouse hepatocellular carcinoma cells were resuscitated, adjusted to a density of 1
All the experiments were performed based on the approved animal protocols and guidelines established by the Medicine Ethics Review Committee for Animal Experiments of Dali University under the approval (number: 2023P2260).
Apart from the normal group, tumor-bearing mice were randomly divided into the model group, CII-3 125 mg/kg group, 5-Fluorouracil (5-FU) 45 mg/kg group and 5-FU 45 mg/kg+CII-3 125 mg/kg group. The blank and model groups received intragastric saline and intraperitoneal injection of 5-FU, while the CII-3 group received intragastric administration of 125 mg/kg CII-3 once a day. The combination group received both 125 mg/kg CII-3 orally and intraperitoneal injection of 45 mg/kg 5-FU every 2 days.
Tumor dimensions were measured on days 6, 8, 10, 12 and 14 after administration. Tumor volume was calculated using the formula:
Volume = (Length
Mice were euthanized by cervical dislocation 24 hours after the final administration, in accordance with institutional ethical guidelines. The weights of tumors, spleens, and thymuses were recorded. Tumor inhibition rate, spleen index, and thymus index were subsequently calculated.
Tumors were harvested, washed in physiological saline, fixed in 10% paraformaldehyde, processed through dehydration, paraffin embedding, sectioning, H&E staining and neutral gum sealing and observed under an optical microscope.
Spleens were obtained and cell suspensions were prepared by gently grinding through a cell strainer. The cell concentration was adjusted to 5
Spleen cell suspensions were prepared and the cell concentration was adjusted to 5
Mouse eyeballs were removed, blood was collected to prepare serum and ELISA assays were performed to determine the levels of IgA, IgM, IgG and IL-8 according to the instructions of the respective ELISA assay kits.
Total RNA was extracted from tumors using a total RNA extraction kit. Reverse transcription was performed using a cDNA synthesis kit and quantitative PCR was carried out using the SuperReal fluorescence quantitative kit. The 2-ΔΔCt method was used to calculate the relative expression of mRNA. The qPCR primer sequences were shown in Table 1.
| Genes | Sequence (5′-3′) |
| TLR4 | |
| Forward | ATGGCATGGCTTACACCACC |
| Reverse | GAGGCCAATTTTGTCTCCACA |
| NF-κB | |
| Forward | ATGGCAGACGATGATCCCTAC |
| Reverse | CGGAATCGAAATCCCCTCTGTT |
| TRAF6 | |
| Forward | TCATTATGATCTGGACTGCCCAAC |
| Reverse | TTATGAACAGCCTGGGCCAAC |
| MYD88 | |
| Forward | TACAGGTGGCCAGAGTGGAA |
| Reverse | GCAGTAGCAGATAAAGGCATCGAA |
| TNF- | |
| Forward | CAGGCGGTGCCTATGTCTCA |
| Reverse | GGCTACAGGCTTGTCACTCGAA |
| Forward | GGCTGTATTCCCCTCCATCG |
| Reverse | CCAGTTGGTAACAATGCCATGT |
qPCR, Quantitative Polymerase Chain Reaction; TLR4, Toll-Like Receptor 4; NF-
Tumor tissues were homogenized and total protein was extracted using RIPA lysis buffer. Protein concentration was determined using the BCA protein assay. The SDS-PAGE gel electrophoresis, PVDF membrane transfer, milk blocking, overnight incubation with primary antibodies, secondary antibody incubation, chemiluminescence imaging and grayscale value analysis using ImageJ software 1.54 (NIH, Bethesda, MD, USA ). were performed. The
The SPSS 27.0 (IBM-SPSS Statistics, Chicago, IL, USA) was used for data analysis. Measurement data were expressed as Mean
As shown in Fig. 1a, on the 6th day of administration, there was no significant difference in tumor volume among the groups of mice; with the extension of administration time, tumor volume gradually showed differences. The combined administration group exhibited significantly lower tumor volume than the model group (p
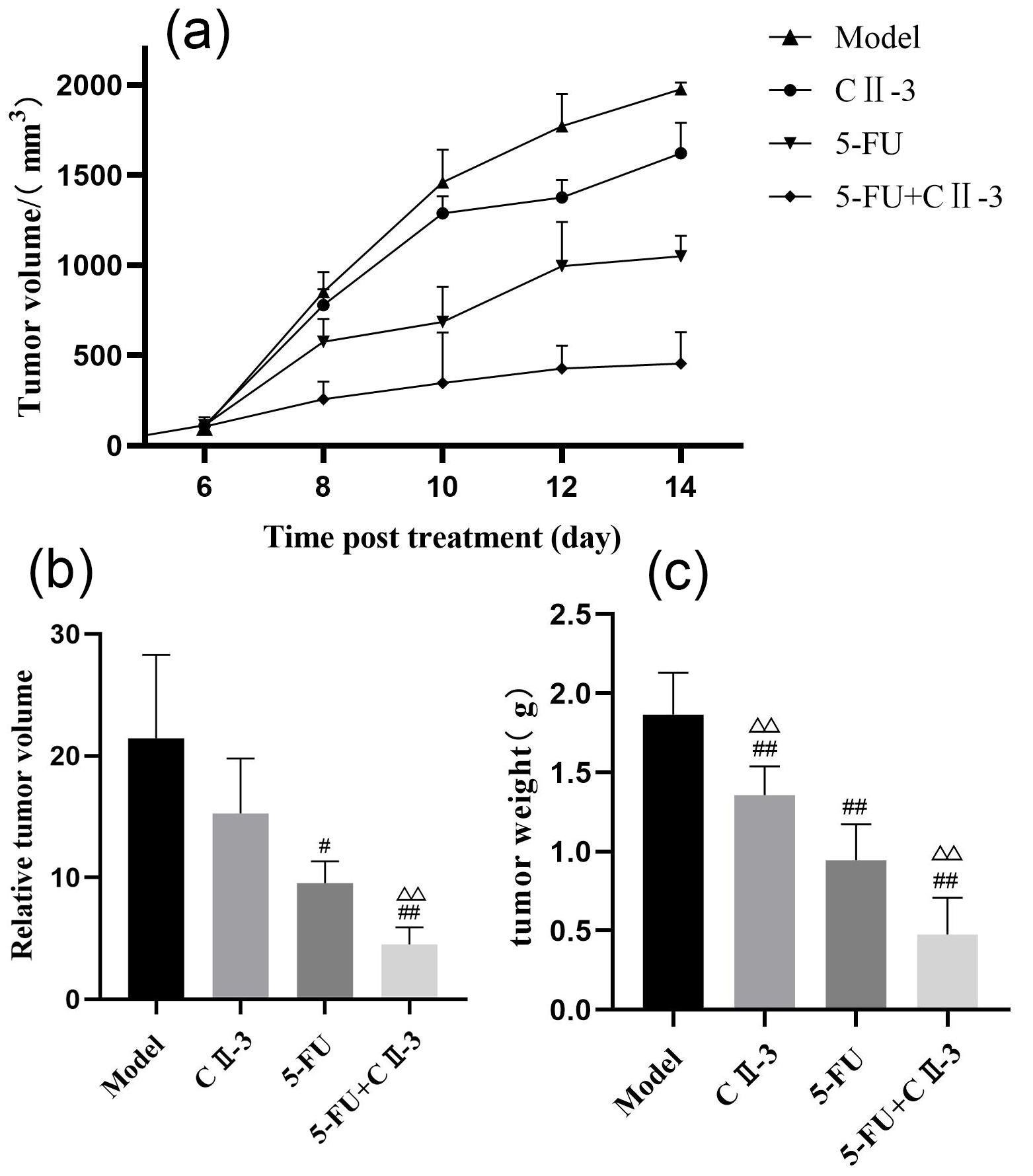 Fig. 1.
Fig. 1. Changes in weight and volume of mice tumour tissue under different exposure conditions. (a) Changes in tumor volume over time, (b) Relative tumor volume and (c) Weight of tumor tissue after 14 days . Relative tumor volumes were standardized with the tumor volume at day 6 as the baseline. n = 6. #p
 Fig. 2.
Fig. 2. Tumor tissue of mice after 14 days of different exposure conditions. (a) Model group, (b) CII-3 group, (c) 5-FU group and (d) 5-FU+CII-3 group. Scale bar = 1 cm. n = 6.
As shown in Fig. 3, compared to the normal group, all groups exhibited a significant increase in spleen index (Fig. 3a, p
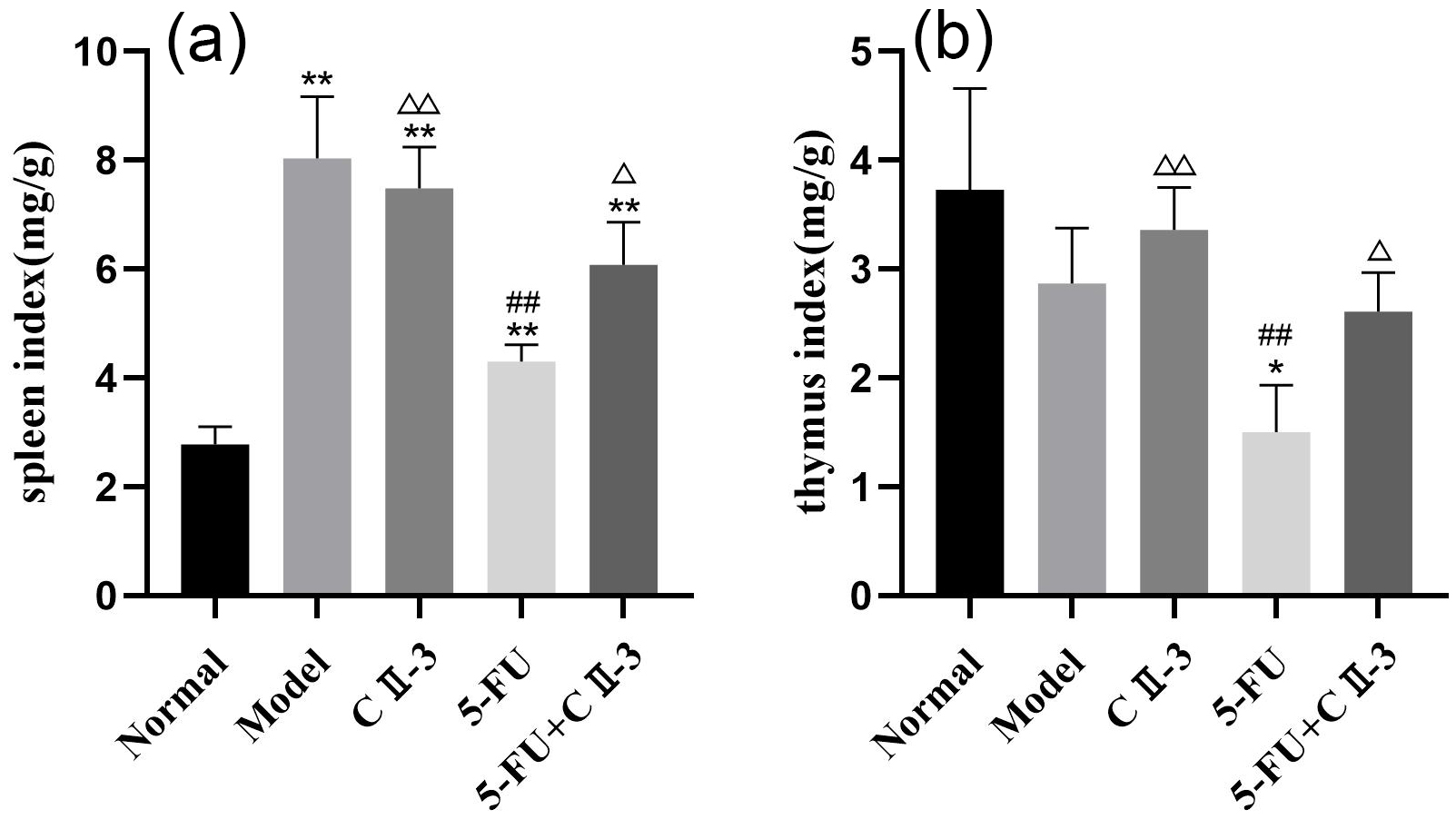 Fig. 3.
Fig. 3. Changes in organ index under different exposure conditions. (a) Spleen index and (b) Thymus index. n = 3. *p
As shown in Fig. 4a, H&E staining results revealed that the tumor tissues in the model group were characterized by abundant blood vessels, well-preserved cell status with diffuse distribution, no necrotic cells and varying degrees of nuclear staining, clear nucleoli and increased mitotic activity. As shown in Fig. 4b, the CII-3 group displayed loosely arranged tumor tissues with reduced blood supply. As shown in Fig. 4c, following 5-FU treatment, tumor tissues showed areas of necrosis and fragmented tumor cells, a significant decrease in cell number, irregular arrangement of tumor cells and nuclear condensation. As shown in Fig. 4d, the combined administration group exhibited extensive tumor tissue necrosis, significant appearance of lipid vacuoles and obvious nuclear fragmentation.
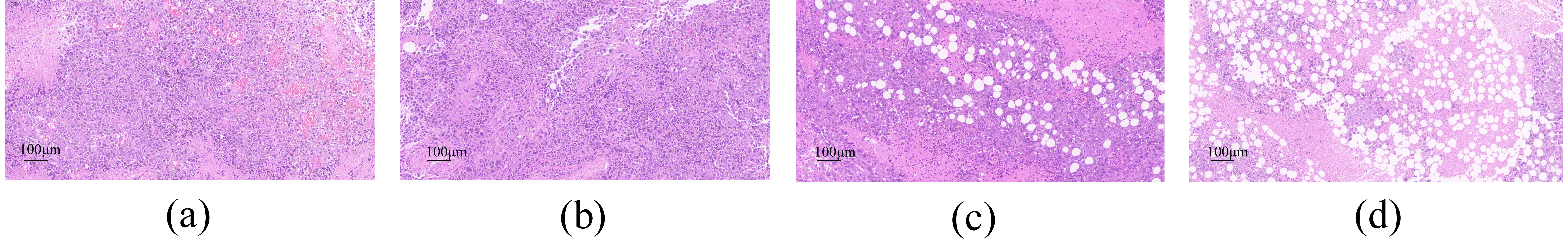 Fig. 4.
Fig. 4. Changes in histopathological morphology of tumor mass in H22 bearing mice under different exposure conditions (H&E,
As shown in Fig. 5, compared to the normal group, both NK cell cytotoxicity (Fig. 5a) and T-cell proliferation capacity (Fig. 5b) significantly decreased in the model group (p
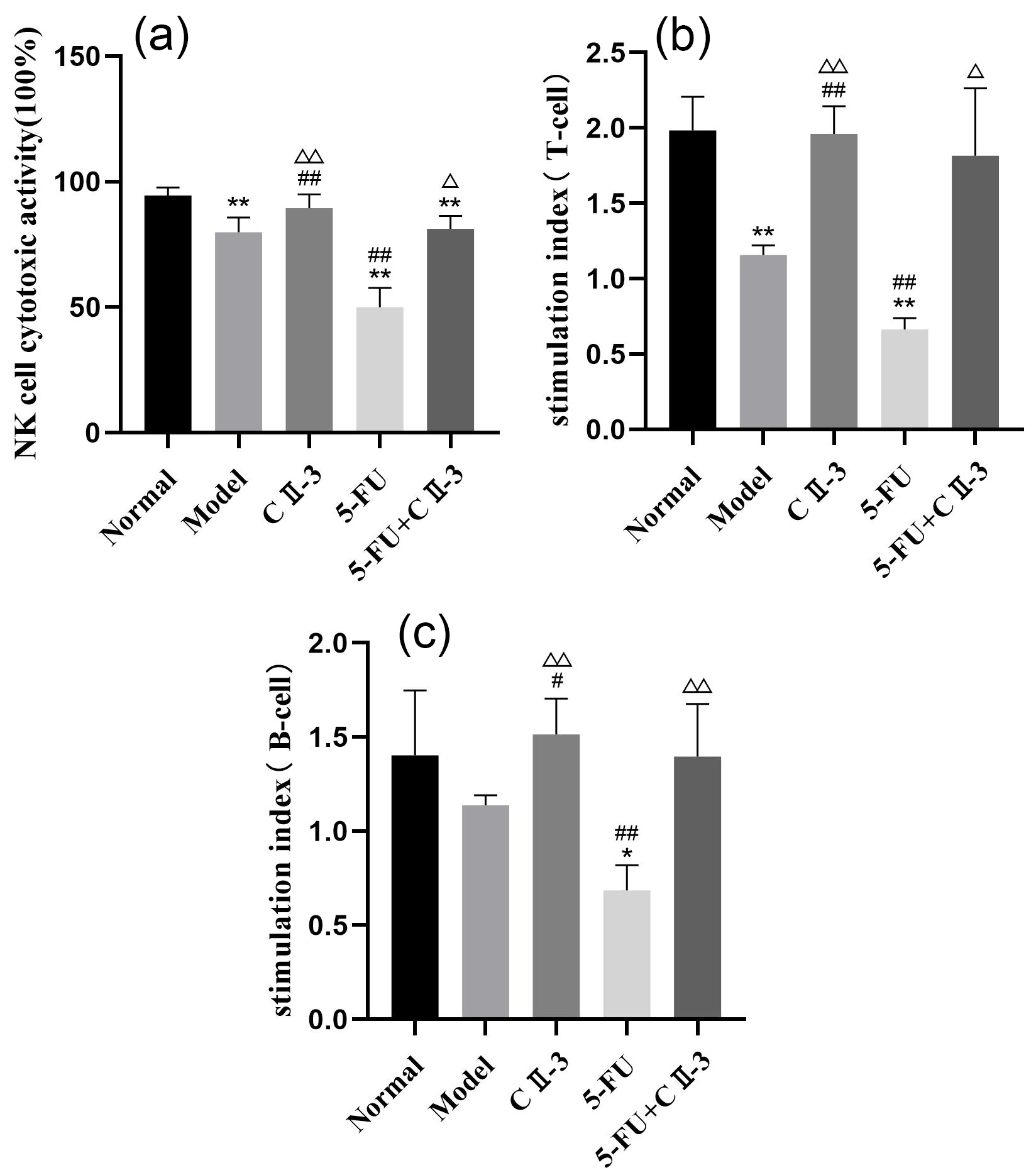 Fig. 5.
Fig. 5. Changes in immune cells in mice spleen under different exposure conditions. (a) NK cell cytotoxicity, (b) T lymphocyte proliferation capacity and (c) B lymphocyte proliferation capacity. n = 6. *p
Fig. 6 shows the results of flow cytometry experiments. As shown in Fig. 7, compared to the normal group, the CD3+ (Fig. 7a) and CD4+ (Fig. 7b) cell proportions in both the model group and the 5-FU group significantly decreased (p
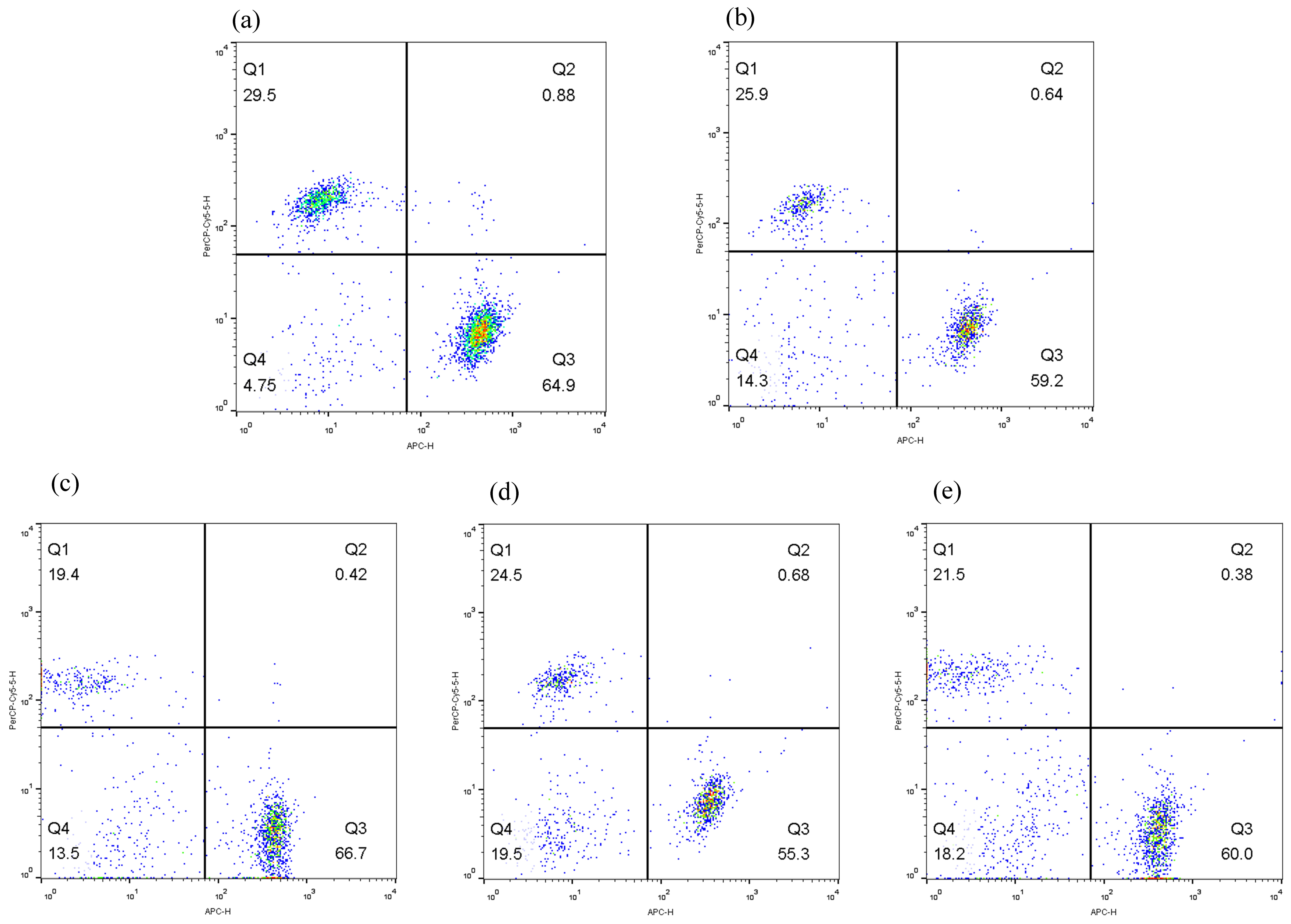 Fig. 6.
Fig. 6. Flow cytogram of CD3+, CD4+ and CD8+ cells. (a) Normal, (b) Model, (c) CII-3, (d) 5-FU and (e) 5-FU+CII-3. n = 6.
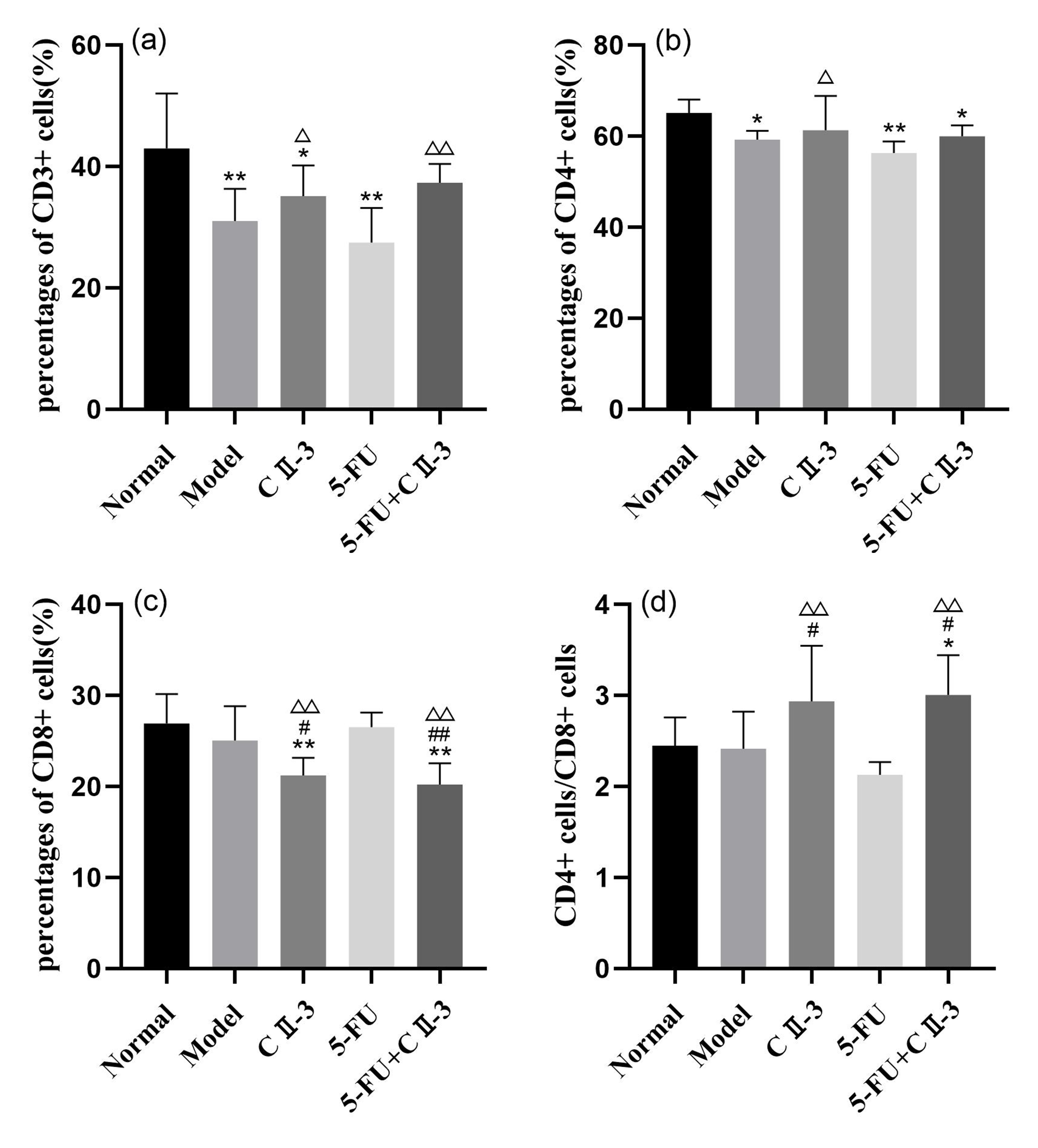 Fig. 7.
Fig. 7. Changes in the T-cell population in mice spleen under different exposure conditions. (a) CD3+ cells, (b) CD4+ cells, (c) CD8+ cells and (d) CD4+ cells/CD8+ cells. n = 6. *p
As shown in Fig. 8a–c, compared to the model group, the levels of IgA, IgM and IgG in the serum of the 5-FU group mice all decreased (p
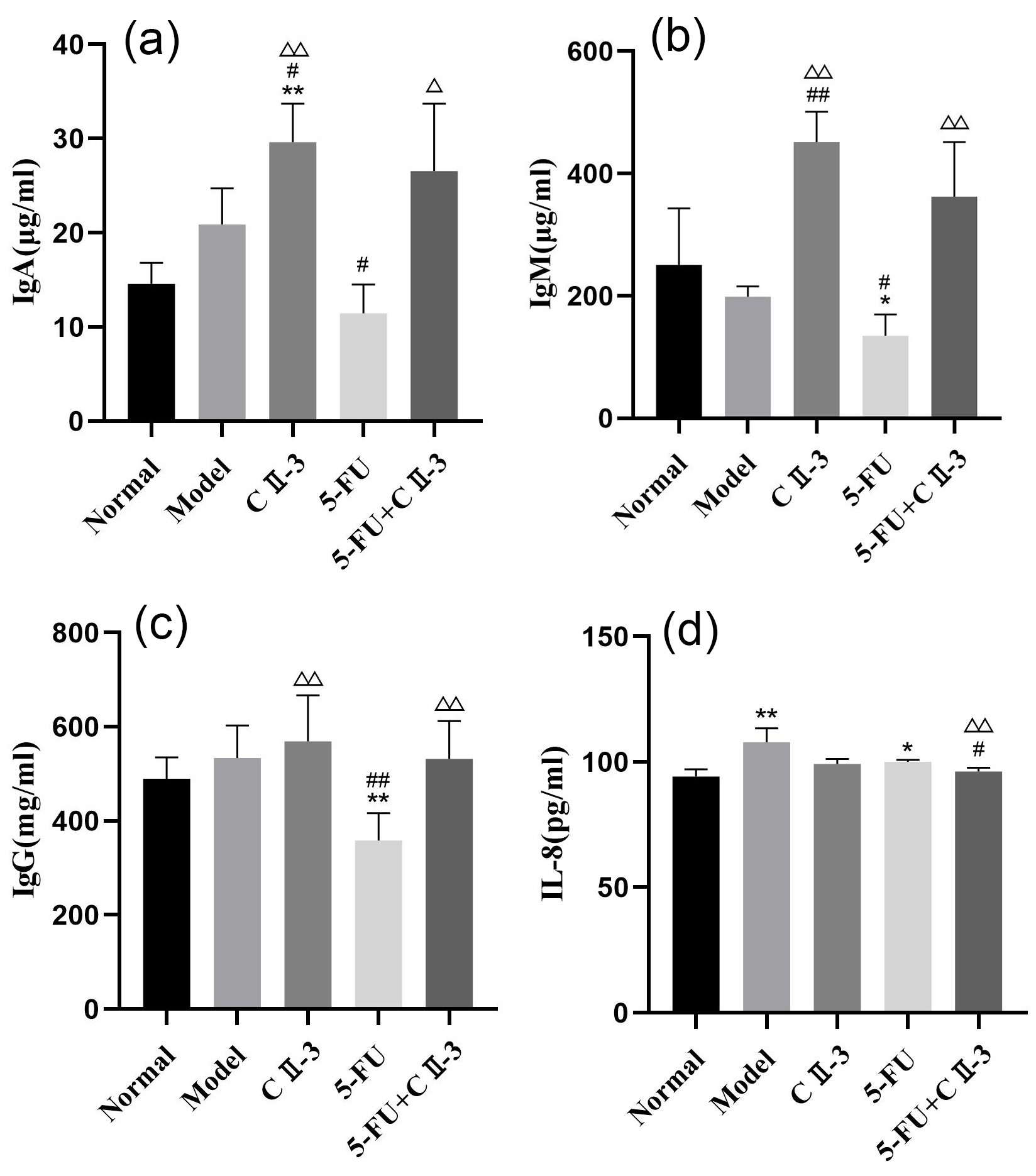 Fig. 8.
Fig. 8. Changes in the B-cell population and cytokine of mice serum under different exposure conditions. (a) IgA, (b) IgM, (c) IgG and (d) IL-8. n = 6. *p
As shown in Fig. 9, compared to the model group, the expression of TLR4 (Fig. 9a), MYD88 (Fig. 9d) and TNF-
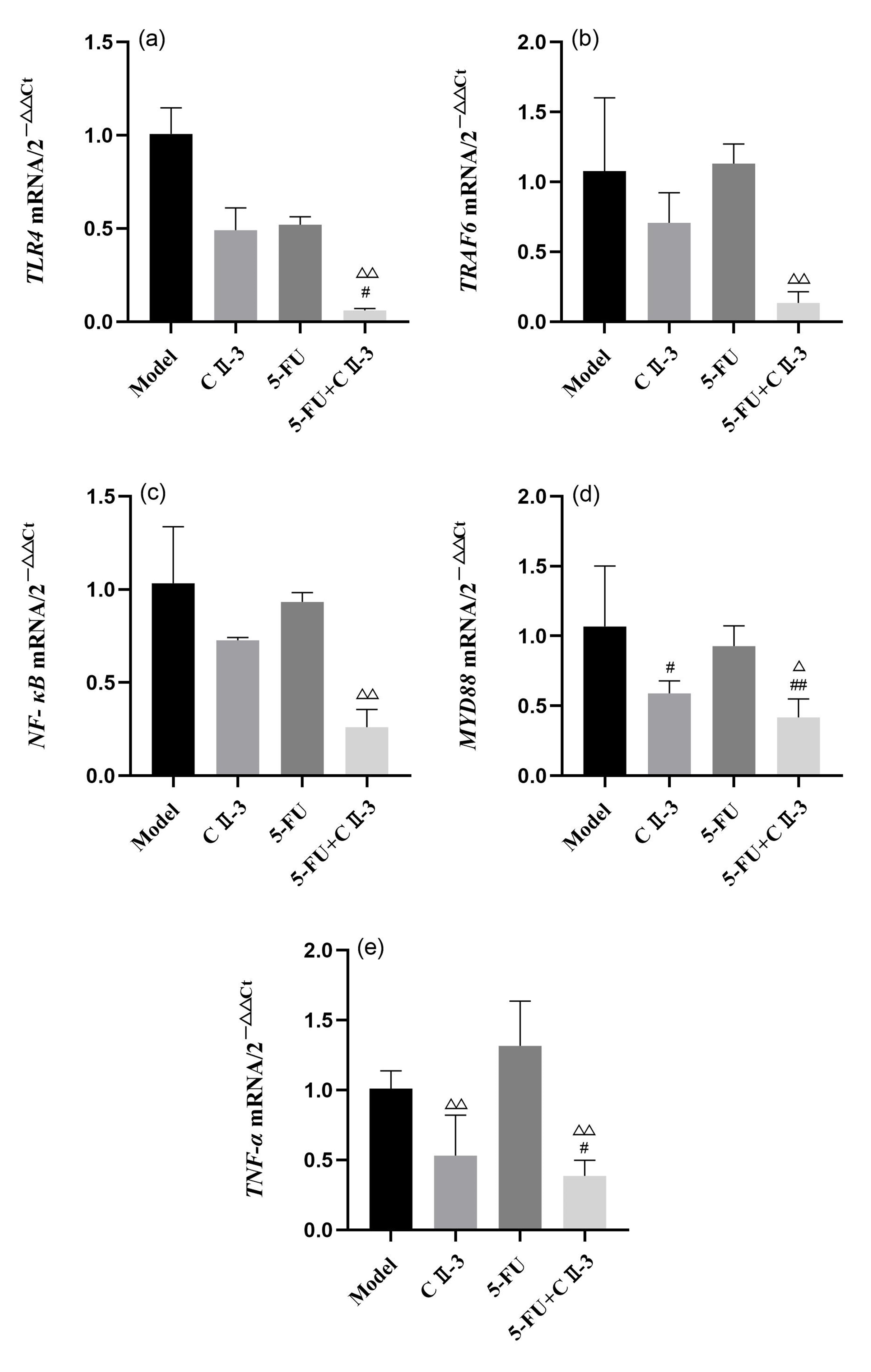 Fig. 9.
Fig. 9. Changes in expression of, (a) TLR4, (b) TRAF6, (c) NF-
Fig. 10 presented the grayscale images of TLR4, NF-
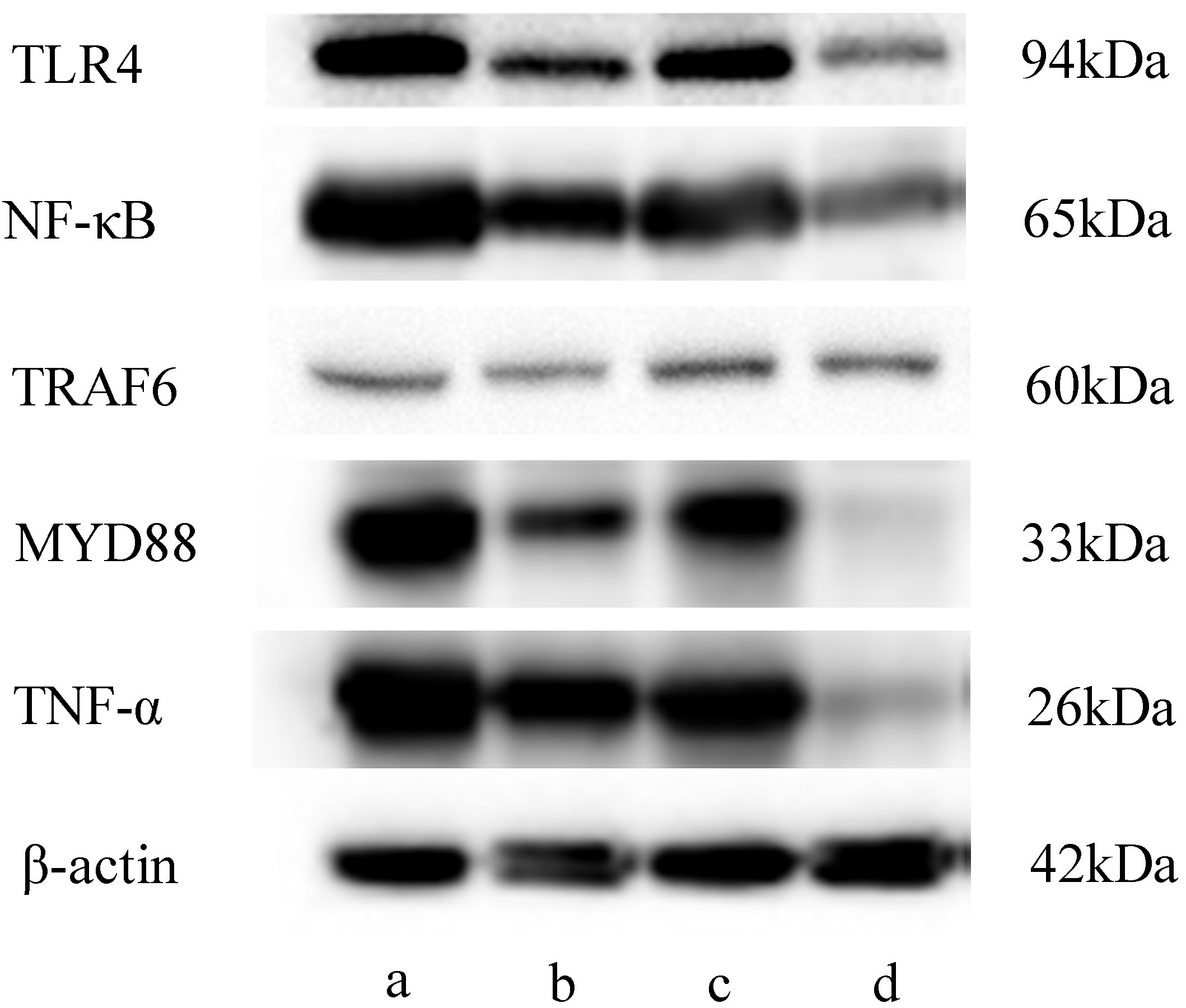 Fig. 10.
Fig. 10. Gray-scale image obtained using western blot. (a) Model group, (b) CII-3 group, (c) 5-FU group and (d) 5-FU+CII-3 group. n = 3.
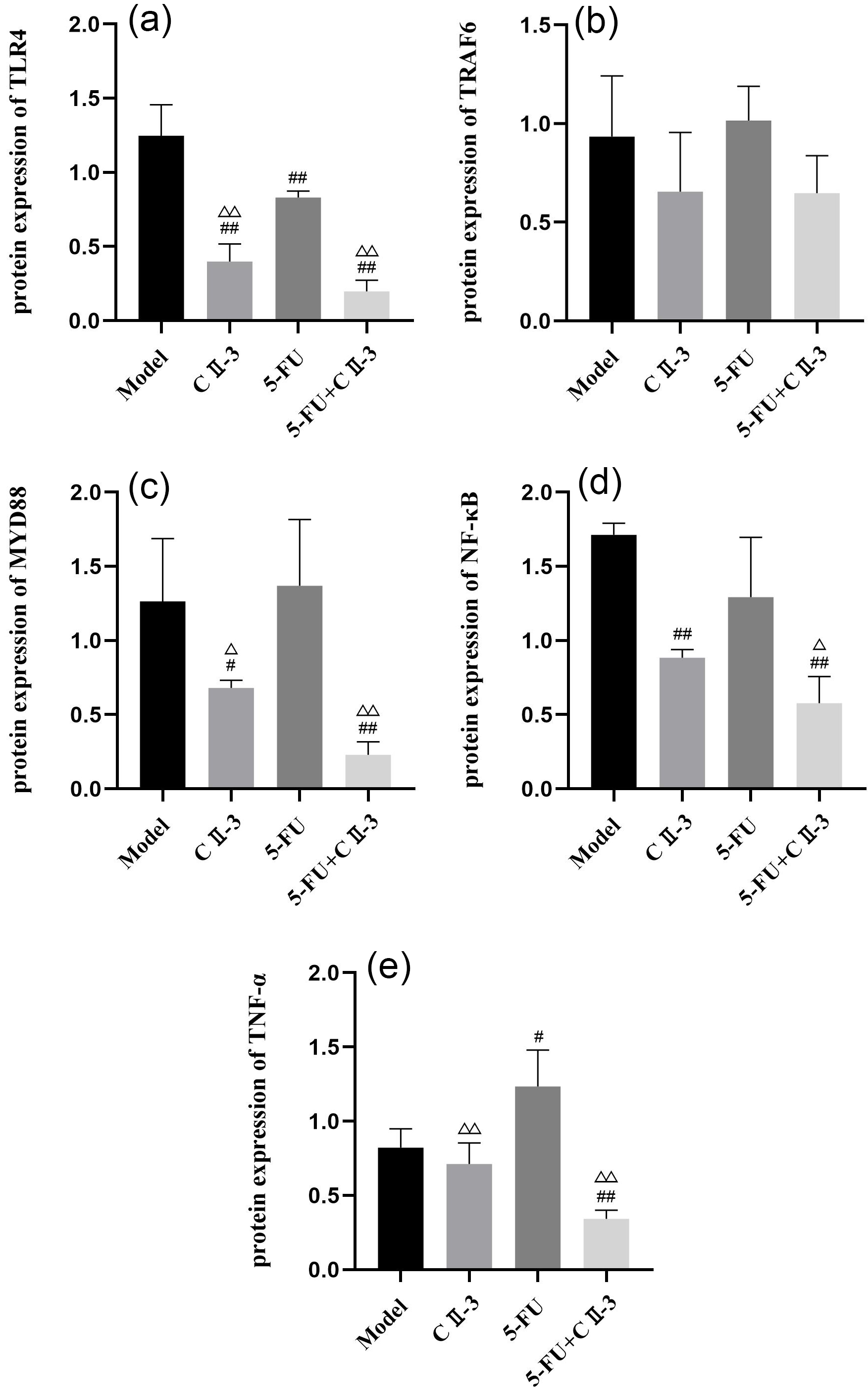 Fig. 11.
Fig. 11. Changes in expression of, (a) TLR4, (b) TRAF6, (c) MYD88, (d) NF-
In the classification of liver cancer based on clinical symptoms in traditional Chinese medicine, it is categorized into patterns such as qi stagnation and blood stasis, liver depression and spleen deficiency, liver-gallbladder damp-heat, liver-kidney yin deficiency, Zheng deficiency blood stasis, spleen-kidney yang deficiency and damp-heat toxin accumulation [28]. The Periplaneta americana, as documented in the “Shennong Bencao Jing”, is described as having effects such as “controlling blood stasis, solidifying lumps, addressing cold and heat, dispersing accumulations, relieving throat obstruction and treating internal cold and infertility” [29]. Acting on the liver meridian, the Periplaneta americana is believed to possess functions corresponding to breaking down blood stasis, promoting blood circulation and dispelling stagnation, aligning with the patterns seen in traditional Chinese medicine for liver cancer [26, 30]. Modern medical research indicates significant potential of the Periplaneta americana in the treatment of various cancers, particularly in studies related to liver and lung cancers [23].
The CII-3 may exert a comprehensive inhibitory effect on liver cancer through multiple pathways, including immunomodulation and anti-inflammatory effects. Firstly, inflammation in liver cancer has been identified as a crucial factor, playing diverse roles in cancer initiation and progression [31]. Inflammatory processes involve the release of pro-inflammatory cytokines such as TNF-
Secondly, under the context of 5-FU treatment for liver cancer, the impact on the host immune system becomes a critical consideration. Current experimental results indicated that the combination of 5-FU and CII-3 has a pronounced regulatory effect on the murine immune system, particularly on immune cells and factors. Elevated levels of IgA, IgM and IgG in the 5-FU combined with CII-3 group suggest a potential role of CII-3 in modulating humoral immunity, enhancing the body’s defense against infections. Moreover, significant changes were observed in the quantity and activity of immune cells after 5-FU treatment. The NK cells, crucial for nonspecific immune activity, play a vital role in overall immune function [34]. The activation of immune cells is also part of the inflammation in liver cancer [35]. Current results demonstrate that CII-3 can enhance the cytotoxic activity of NK cells, potentially compensating for the inhibitory effect of 5-FU on NK cell activity. This discovery provides a new mechanistic explanation for the immunomodulatory effects of 5-FU combined with CII-3 treatment. The T lymphocytes, especially CD3+, CD4+ and CD8+ cells, may decrease after 5-FU treatment, leading to an imbalance in T lymphocyte subgroups. This aligns with previous research results, indicating the direct impact of chemotherapy drugs on T lymphocytes.
Research suggested that the activation of the TLR4 signaling pathway in liver cancer cells can increase the production of immune inhibitory factors, promoting tumor cell proliferation and immune escape, leading to the growth and metastasis of liver cancer cells [36, 37]. The MyD88-dependent signaling pathway downstream of TLR4 can activate NF-
This study revealed that 5-FU combined with CII-3 can modulate immune factors and immune cells, improving the immune function of tumor-bearing mice and alleviating the immune suppression state. The 5-FU combined with CII-3 can effectively inhibit the growth of liver cancer and the mechanism may involve the inhibition of the TLR4/MyD88/NF-
In summary, this research outcomes unveil the multifaceted actions of CII-3 in the treatment of liver cancer, encompassing immunomodulation, anti-inflammatory effects and tumor inhibition. This provides a theoretical foundation for the clinical application of CII-3, but further investigations are warranted to comprehensively understand its mechanisms of action and its potential prospects in clinical therapy.
The combination of Periplaneta americana extract CII-3 with 5-Fluorouracil (5-FU) demonstrates synergistic anti-tumor and immunomodulatory effects in H22 liver cancer-bearing mice. The CII-3, a complex mixture comprising 25 identified compounds, enhances the inhibitory efficacy on tumor growth, as evidenced by reduced tumor mass and volume. Additionally, CII-3 exerts anti-inflammatory effects by significantly lowering IL-8 levels. These findings provide insights into the potential of CII-3 as an adjunctive therapeutic agent for liver cancer, complementing the effects of conventional chemotherapy. However, caution should be exercised in generalizing these results to human subjects due to species-specific variations. Further research is necessary to unravel the intricate molecular mechanisms underlying CII-3’s actions and to explore its clinical applications.
Hepatocellular Carcinoma (HCC) presents a significant threat to human health and life. While chemotherapy stands as a cornerstone treatment for advanced-stage HCC, its efficacy is often hindered by adverse reactions such as immunosuppression. This study aims to identify therapeutic approaches that can enhance chemotherapy effectiveness while mitigating adverse effects. Our research highlights the synergistic anti-tumor and immunomodulatory effects of combined CII-3 and 5-FU treatment, potentially mediated through the regulation of the TLR4/NF-
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
RG and GW designed the research study. RY, GW, MG, and XL performed the experiments and curated the data. MG and XL provided guidance on experimental procedures. YW, RG and RY conducted the formal analysis and data visualization. RG and GW developed the methodology. MG acquired the funding. RG, RY, and YW drafted the original manuscript. MG, XL, and YW searched references. MG and XL reviewed and edited the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
All the experiments were performed based on the approved animal protocols and guidelines established by the Medicine Ethics Review Committee for Animal Experiments of Dali University under the approval (number: 2023P2260).
Not applicable.
This work was supported by the Special Basic Cooperative Research Programs of Yunnan Provincial Undergraduate Universities’ Association (202101BA070001-119), the Special Basic Cooperative Research Programs of Yunnan Provincial Undergraduate Universities’ Association (202101BA070001-121), the Open project of Yunnan Provincial Key Laboratory of Insect Biomedicine Research and Development (AT2024002) and the Team Project of Yunnan Revitalization Talent Support Program (No.202305AS350001).
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.











