1 Department of Guidance and Psychological Counseling, Institute of Educational Sciences, Ataturk University, 25240 Erzurum, Turkey
2 Department of Pharmacology, Faculty of Medicine, Erzincan Binali Yildirim University, 24100 Erzincan, Turkey
3 Department of Physiology, Faculty of Medicine, Erzincan Binali Yildirim University, 24100 Erzincan, Turkey
4 Department of Biochemistry, Faculty of Medicine, Erzincan Binali Yildirim University, 24100 Erzincan, Turkey
Abstract
Doxorubicin (DOX) use can promote neurobehavioral changes and neurodegeneration, which have been attributed to oxidative stress. Thus, this study aimed to examine the effect of Hippophae rhamnoides L., fruit extract (HRe), against possible oxidative brain damage and behavioral disorders in rats caused by DOX.
A total of 24 male Sprague-Dawley rats were utilized in this study and were divided randomly into four groups (n = 6 in each groups): CG, healthy control; HRe, 50 mg/kg HRe; DOX, 5 mg/kg i.p., in a single intraperitoneal dose of DOX; Hre + DOX, 50 mg/kg HRe + 5 mg/kg DOX. HRe was administered orally once a day for two weeks, while DOX was administered intraperitoneally twice a week for two weeks. Subsequently, behavioral tests were performed—the sucrose preference test (SPT) and pole test—to assess depression-like behaviors and motor function, respectively. Then, the level of oxidative stress was biochemically evaluated in the brain tissues of the rats. One-way analysis of variance (ANOVA) was conducted, followed by a post hoc Tukey’s test for the statistical analysis. A p-value < 0.05 was considered statistically significant.
The HRe treatment markedly reduced DOX-induced depression-like behaviors and improved motor dysfunction. The HRe treatment also restored the impaired antioxidant response by inhibiting the DOX-related malondialdehyde increase and reducing the decrease in total glutathione levels, as well as superoxide dismutase and catalase activities.
The present study indicates that HRe treatment has beneficial effects on motor dysfunction as well as depression-like behavior associated with neurodegeneration following DOX-induced brain damage. Possible mechanisms underlying these beneficial effects include lipid peroxidation inhibition and restoration of antioxidant defense mechanisms by HRe.
Keywords
- behavioral impairment
- doxorubicin
- Hippophae rhamnoides L.
- neurodegeneration
- oxidative stress
Doxorubicin (DOX) is a powerful anti-cancer anthracycline antibiotic that is often used to treat several types of cancer, including breast, prostate and lung cancer [1]. As part of its multifaceted mechanism of action, DOX disrupts DNA synthesis by acting on cancer cells during their S phase, as well as prevents cell division by inhibiting topoisomerase 2 [2]. The production of reactive oxygen radicals (ROS) by DOX by interaction with enzymes such as oxidases and reductases in malignant cells is also considered to be an anticancer mechanism [3, 4]. Although DOX is still the cornerstone of many cancer chemotherapies, its clinical use is limited due to multiple systemic side effects and drug resistance phenomena [5, 6]. Patients treated with DOX have been reported to develop severe cardiac adverse effects, including dilated cardiomyopathy [6], congestive heart failure [7], acute ventricular dysfunction [6] and cardiogenic shock [8]. In addition, a growing body of literature in basic and clinical research suggests that DOX exposure also has significant toxic effects on brain tissue [9, 10, 11].
The presence of neurobehavioral disorders such as cognitive impairment, depression and anxiety has been reported in patients treated with DOX, despite its limited ability to cross the blood-brain barrier [12, 13, 14]. Therefore, following DOX chemotherapy, neurobehavioral changes and associated neuropsychological disorders may make it difficult for the individual to perform routine tasks [15]. Moreover, oxidative stress damage in brain tissue has been reported in the pathogenesis of motor function and behavioral disorders in in vivo studies [16, 17]. The DOX has been revealed to cause oxidative damage by increasing malondialdehyde (MDA) levels and decreasing antioxidant levels such as Glutathione (GSH), superoxide dismutase (SOD) and catalase (CAT) in brain tissue [18, 19]. Thus, these conditions may lead to neurochemical changes along with various neurobehavioral disorders, ultimately resulting in neuronal cell death. Despite extensive research, effective treatment strategies to manage DOX-associated neurobehavioral disorders and neurodegeneration remain elusive.
It has been demonstrated that many natural compounds have antioxidant properties that have the potential to diminish and/or ameliorate the toxic effects of chemotherapeutic drugs without reducing their antitumor properties [20]. Among the medicinal plants in the Elaeagnaceae family, Hippophae rhamnoides L. (HR) was tested against DOX-induced neurotoxicity in this study [21]. As a result of the presence of flavonoids, carotenoids, organic acids, vitamins A, C, E and K, trace elements, monosaccharides and amino acids in the fruits of HRe, it has been deemed to be a unique pharmacological agent [22, 23, 24, 25]. Low molecular weight flavonoids are natural compounds with a variety of pharmacological properties, such as anti-inflammatory, antibiotic and antioxidant properties [26]. Additionally, flavonoids have been demonstrated to inhibit or attenuate neurodegeneration by modulating oxidative stress mediators in animal models of neurodegeneration [27]. According to Wang et al. [21], total flavonoids from HR fruit exhibit antioxidant activity in vitro and in vivo, as well as potential neuroprotective properties. Moreover, HR has been shown to alleviate depression-like symptoms in experimental animals [28] and protect brain tissue from oxidative stress by suppressing lipid peroxidation (LPO) and strengthening antioxidant defenses [29]. In light of these findings, HR may prove useful in the treatment of DOX-induced organ tissue toxicity. No information is available in the literature on the protective effect of HR fruit extract (HRe) against doxorubicin-induced brain damage and behavioral disturbances in rats. The study investigated the effects of HRe against potential oxidative brain damage and behavioral disturbances induced by doxorubicin in rats.
The experimental steps of this research were carried out in the laboratories of the Experimental Animals Application and Research Centre, Erzincan Binali Yildirim University, Erzincan, Turkey, in January, 2025.
In this study, 24 male Sprague-Dawley rats (3–4 months old; weighing 230
The DOX was obtained (50 mg/25 mL vial) from Saba Pharmaceuticals (Istanbul,
Turkey), HRe from PhytoLab (Vestenbergsgreuth, Germany) and sucrose
(
The experiment consisted of 4 different groups with 6 male rats in each group. The choice of the rats was random. The groups were designed as: Healthy control (CG), 50 mg/kg HRe-treated (HRe), DOX-injected (DOX) and HRe-treated+DOX-injected (HRe+DOX). The CG group received corn oil by oral gavage once a day for two weeks. The animals in the HRe group received 50 mg/kg HRe [29] by gavage once daily for two weeks. In the DOX group, DOX (5 mg/kg; a total of four injections; cumulative dose, 20 mg/kg) was injected intraperitoneally (i.p.) twice a week for two weeks. The HRe+DOX group received HRe (50 mg/kg, gavage) once daily and DOX (5 mg/kg, i.p.) twice weekly for two weeks. The DOX administration and HRe administration were initiated at the same time. The dose and duration of administration were determined based on a previous study that established DOX-induced neurotoxicity and depression-like behaviors [30]. When determining the HRe dose, the dose showing a neuroprotective effect in acrylamide-induced neurotoxicity was selected [29]. The HRe was dissolved in corn oil and DOX was prepared in 0.9% normal saline. The solutions were prepared freshly before administration to ensure their potency.
At the end of all treatments, on the 15th day, a pole test was performed to assess motor activity and on the 16th–18th days, a sucrose preference test was conducted to elevate antidepressant-like effects. The experimental protocol was presented in Fig. 1.
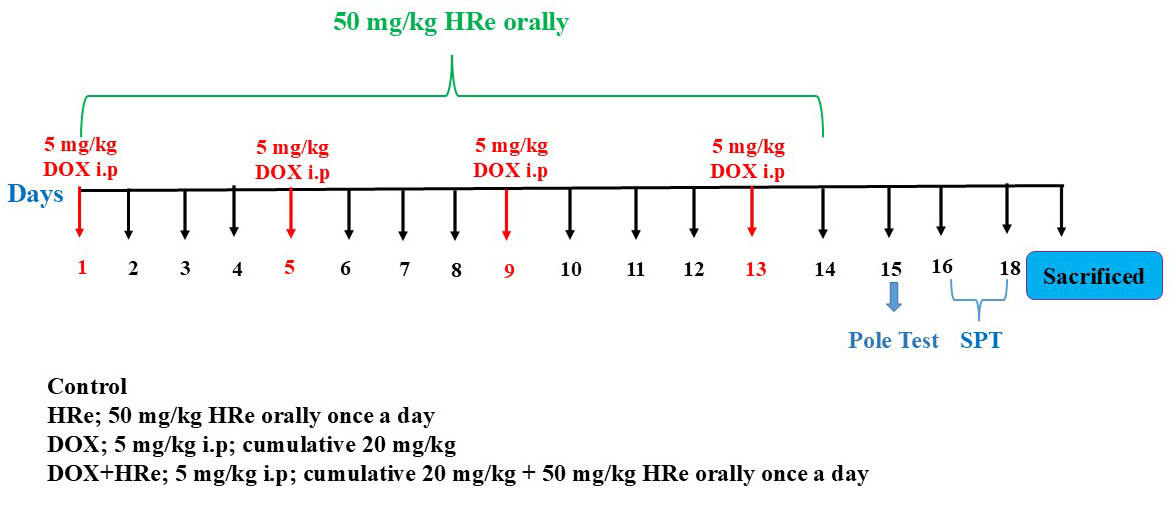 Fig. 1.
Fig. 1.
Experimental procedure of the present study. Hre, Hippophae rhamnoides L., fruit extract; DOX, Doxorubicin; SPT, sucrose preference test.
To reduce stress in rats, no test was conducted consecutively and rats were taken to the experimental environment 30 min before the tests to acclimatize, thereby minimizing the effects of anxiety and fear on rats.
The SPT was used to measure the anhedonia effect, a key symptom of major depression in rats. During the adaptation process, the rats were exposed to two bottles having identical characteristics on each side, containing 1% sucrose liquid, for 48 hrs. After 14 hrs of water withdrawal, the animals received two pre-weighed bottles, one containing drinking water (tap water) and one containing 1% sucrose fluid. The bottles were switched left-right in case the animals preferred a particular direction for drinking. The bottles of drinking water and 1% sucrose solution were weighed and placed in cages. The bottles were weighed again after one hour to determine the amount of liquid consumed.
To calculate the percentage preference for sucrose, the following formula was used [31]:
To assess the impairment of exploratory behavior and motor signs in animals, the pole test method of Mohammad et al. [32] was used with minor modifications. A metal pole measuring 63 cm in length and 10 mm in diameter was wrapped with a bandage in order to facilitate a better hold by the animal. The rats were positioned at the top of the rod with their heads facing upward. As they remained in this position, the time (t-total) until they completely turned back and moved toward the ground was recorded.
At the end of the experimental protocol, rats were sacrificed with sodium thiopental (50 mg/kg, i.p.). Brains were carefully excised from the skull, cleaned with cold saline and snap-frozen in liquid nitrogen. Homogenates were centrifuged (3000 rpm, 15 min) and the separated clear filtrate was stored at –80 °C for biochemical analysis. The supernatants were used to assess levels of oxidative stress markers including MDA tGSH SOD and CAT.
The MDA, GSH and SOD in the tissue samples were determined with Enzyme-Linked Immunosorbent Assay (ELISA) kits for rats (MDA catalog No. 10009055; GSH catalog No. 703002; SOD catalog No. 706002; Cayman Chemical Company) (MI, USA). The CAT determination was performed according to the method proposed by Góth [33].
All statistical analyses were performed in the statistical program “SPSS for
Windows, 22.0” (IBM Corp., Armonk, NY, USA). Data were presented as Mean
The DOX administration resulted in a decrease in sucrose preference as compared
to the CG (p = 0.003). The DOX group administered with HRe demonstrated
a reduction in sucrose preference (p
| Experimental groups | ||||||
| Parameter | CG (Mean |
HRe (Mean |
DOX (Mean |
HRe+DOX (Mean |
F (3.20) | p-values |
| Pole test | 9.67 |
8.67 |
19.83 |
14.00 |
20.22 | |
| Sucrose preference test | 83.83 |
82.67 |
56.67 |
76.33 |
7.32 | 0.002 |
*p
Time to movement (bradykinesia severity) was evaluated in the Pole test used for
the determination of motor activity. It was found that rats in the DOX-treated
group had a longer time to land on the ground according to the CG group
(p
The Pole test data of HRe and HRe+DOX groups and
the data of CG group were close to each other (p
Based on Table 2, DOX administration increased MDA levels in rats’ brain tissue
(p
| Experimental groups | ||||||
| Parameter | CG (Mean |
HRe (Mean |
DOX (Mean |
HRe+DOX (Mean |
F (3.20) | p-values |
| MDA | 3.42 |
2.95 |
4.98 |
3.52 |
35.95 | |
| tGSH | 5.41 |
5.90 |
3.30 |
5.13 |
163.73 | |
| SOD | 7.30 |
7.92 |
4.25 |
7.11 |
532.21 | |
| CAT | 5.75 |
6.29 |
3.27 |
5.72 |
344.91 | |
*p
The tGSH levels in the DOX group decreased compared to the CG (Table 2;
p
According to the comparison of SOD enzyme activity between the groups, DOX
significantly decreased SOD activity in the brain tissue of rats compared to the
CG group (Table 2; p
As seen in, Table 2, CAT enzyme activity in the DOX group showed a significant
decrease compared to the CG group (p
The present study investigated the behavioral and biochemical effects of HRe on DOX-induced neurotoxicity in rats. Based on our experimental results, HRe significantly reduced DOX-induced changes in oxidants, enzymatic antioxidants and nonenzymatic antioxidants. Further, it alleviated DOX-induced motor dysfunction and depression-like behaviors. The long-term use of DOX, an important chemotherapeutic drug [3, 34], has been associated with decreased cognitive function, depression and anxiety-like neuropsychological disorders [9, 10, 11, 12, 13, 14]. In experimental models, it has been reported that animals subjected to behavioral tests exhibit impaired or altered responses depending on the degree of neurodegeneration caused by DOX [16, 17, 31]. Data from preclinical [19, 35] as well as clinical studies [13] indicate that DOX administration may be associated with anxiety and depression. The SPT is a commonly used method for detecting anhedonia behavior, which is one of the symptoms of depression in rodents [31]. As a result of DOX administration, depression-like behaviors were observed in conjunction with decreased sucrose consumption scores in SPT in our study. There was, however, a significant increase in sucrose consumption scores in the HRe+DOX group. Currently, there is no research on HRe use and its effect on depression-like behaviors associated with DOX. Nevertheless, a study conducted reported that HRe markedly improved anxiety index values by improving the elevated plus maze test and forced swim test scores, which were in favor of our study [36]. Despite studies emphasizing that motor dysfunction accompanies the anxious behaviors caused by DOX in experimental animals [17, 19, 35, 37, 38], interestingly, there are also findings reporting that DOX does not affect motor activity [35, 38]. In the current report, DOX administration significantly prolonged the time for rats to turn back and land on the ground compared to the control and HRe groups in the pole test, which assessed motor activity. It is believed that these results are indicative of bradykinesia, which is a condition in which voluntary movements are slowed and is an indicator of neurological damage. There are no studies in the literature that examine the effect of DOX on motor activity using the pole test. Nevertheless, cisplatin, an anticancer drug, was found to significantly prolong the landing and movement times of experimental animals compared to the control group in the pole test [39]. In accordance with findings from the literature [17, 37], DOX induction of depression-like effects in rats was accompanied by a decrease in motor activity. Furthermore, depending on the protocol applied in the current report, the dose and duration of DOX treatment may cause a significant change in motor functions. Furthermore, HRe, which was investigated in the context of DOX-induced movement disorders, reversed the slowing of movement caused by DOX. The research demonstrated that hypokinetic behaviors observed in experimental animals with depression model [36] and treated with haloperidol [40] were improved when HR extract was administered.
It is claimed that neurobehavioral changes such as anxiety and depression caused by DOX may be related to increased oxidative damage in brain tissue [17, 19, 31, 37]. Metabolic activation of DOX occurs in the cell via nicotinamide adenosine diphosphate (NADPH) cytochrome p450 enzyme and DOX semiquinone is ultimately converted into ROS [9]. The elevation in ROS concentration leads to rise in LPO-related damage [41, 42]. The richness of neuronal membranes in polyunsaturated fatty acids prone to LPO increases the sensitivity of the brain to DOX-induced toxicity [41]. The MDA is a highly reactive and toxic aldehyde formed as a result of LPO and is one of the most important indicators of LPO [43, 44]. Measurement of MDA levels together with antioxidant capacity is recognized as one of the most important parameters determining DOX-induced oxidative brain damage [38, 45]. The MDA levels were measured in this study to determine the amount of LPO caused by oxidative damage. The results of in vitro [46] and in vivo [38] studies of DOX-induced neurotoxicity in the literature reveal that MDA levels are significantly increased. The MDA levels in the brain tissue of the DOX group were higher than those of the CG and HRe groups in the present study, consistent with previous studies. Furthermore, HRe, which was tested for its effect on DOX-induced brain damage, was found to significantly reduce the DOX-induced increase in MDA levels in brain tissue. There is no information in the literature regarding the possible effects of HRe against DOX-induced oxidative damage in the brain. Turan et al. [29] reported, however, that HRe treatment suppressed MDA levels in rats that had been exposed to acrylamide-induced brain damage and showed neuroprotective properties.
Oxidative stress has become one of the most accused mechanisms in the pathogenesis of neurotoxicity caused by various chemotherapeutic drugs in recent years [47]. Therefore, in current study, nonenzymatic and enzymatic antioxidant parameters such as tGSH, SOD and CAT were measured in the brain tissues of rats to evaluate the oxidative damage caused by DOX. The GSH is an important antioxidant normally produced in living organisms. Due to the thiol group in its structure, it protects cells against the damaging effects of oxidation products and is involved in the maintenance of redox homeostasis in neurons [48]. In the literature, there is evidence that GSH levels decreased in the brain tissue of rats in DOX-induced toxicity and this may be related to neurobehavioral disorders [16, 37, 38]. The results obtained in this study also support the previous evidence. In addition, it may be considered that the increase in MDA level may reflect the decrease in brain GSH levels. The GSH is known to play an important role in the detoxification of aldehydes, including MDA [49]. In the present study, the increase in MDA level coincided with the decrease in brain tGSH level after DOX administration. The HRe administration, however, significantly inhibited the DOX-induced decrease in tGSH levels in rat brain tissue. Our findings suggest that in the DOX group treated with HRe, oxidant-antioxidant balance was maintained with the superiority of antioxidants. As reported by Purushothaman et al. [50], the oil derived from the H. rhamnoides seed provided significant protection against hypoxia-induced oxidative damage by suppressing GSH levels significantly, which decreased simultaneously with an increase in ROS and MDA levels in experimental animals exposed to hypoxia-induced cerebral vascular damage. Additionally, it has been reported that HRe protects brain tissue from the toxic effects of ROS products by activating antioxidant enzymes [29, 51].
In addition, SOD and CAT were the enzymatic antioxidants that decreased in the brain tissues of rats as a result of DOX administration. The SOD, which is involved in the first-line antioxidant defense system against ROS, provides catalytic conversion of superoxide radical (O2¯) or singlet oxygen radical (1O2) to H2O2 and molecular oxygen (O2) [52, 53]. The H2O2, which is highly toxic for body tissues and cells, is broken down into water and O2 by the CAT enzyme, which is abundant in peroxisomes and the damage caused by free radicals is reduced [52, 54]. Therefore, in this study, SOD and CAT enzyme activities were evaluated together to investigate the destructive effect of ROS. Various studies have shown that DOX impairs the cellular antioxidant defense system by decreasing antioxidant enzymes such as SOD and CAT and ultimately causes neuronal damage [16, 18, 19]. In this study, it was found that DOX decreased SOD and CAT enzyme activities in the brain tissues of rats and thus impaired the antioxidant system. However, the data of this study show that HRe treatment together with DOX administration strengthens the antioxidant defense mechanism by inhibiting the decrease in SOD and CAT activities. Literature findings show that HRe has strong antioxidant activity due to bioactive molecules such as flavonoids and carotenoids in its content and it is suggested that these molecules may be responsible for reducing oxidative stress [55]. In the literature, there is no study on the use of HRe and its effect on DOX-associated oxidative brain damage. However, it has been reported in studies that extracts obtained from different parts of HR, such as fruits, leaves and seeds, protect against oxidative damage by strengthening the antioxidant defense system of both the brain and other tissues [21, 23, 29, 56].
Accordingly, the administration of HRe significantly improved depression-like effects caused by DOX, as well as impaired motor function in this study. The beneficial effects of the HRe may be attributed to the prevention of damage caused by increased oxidant and decreased antioxidant levels in brain tissue, as well as the neurobehavioral improvements observed as a result. By elucidating the effects of HRe on the central nervous system induced by DOX, supporting these preclinical data on the efficacy and benefit of HRe in patients receiving DOX chemotherapy with symptoms such as depression and anxiety with further studies, its use not only for DOX-treated individuals but also as adjuvant therapy in patients receiving different chemotherapeutic treatments may open a new solution proposal for reducing and/or preventing neurocognitive symptoms and neurotoxicity such as mood changes caused by anticancer agents.
This study discovered that Hippophae rhamnoides effectively suppresses DOX-induced behavioural changes and neurodegeneration, possibly by regulating antioxidant enzyme activity and suppressing lipid peroxidation. These findings can be beneficial for developing adjunct therapies to mitigate the neurotoxic effects of doxorubicin without compromising its anticancer efficacy. Given the increasing concerns regarding DOX-associated neurobehavioural disorders, identifying a natural neuroprotective agent is of significant clinical importance. This study will help researchers uncover the critical areas of DOX-induced neurotoxicity and its underlying mechanisms that many researchers were not able to explore. Thus, a new theory on the potential neuroprotective role of Hippophae rhamnoides in chemotherapy-induced neurotoxicity may be arrived at.
Further materials related to this study are available from the corresponding author upon reasonable request.
TBB, BC, SB, and HS designed the research study. BC, DA, SB and ATC performed the research. ATC and DA provided help and advice on the ELISA experiments. BC and SB analyzed the data. TBB, DA, ATC, and HS drafted the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
All experiments were carried out following the European Parliament and Council Directive 2010/63/EU and in compliance with the ARRIVE guidelines, and conducted following the decision of Erzincan Binali Yıldırım University’s Animal Experiments Local Ethics Committee, dated 31-10-2024 and numbered 10/45.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.

