1 Liaoning Academy of Traditional Chinese Medicine Science, Liaoning University of Traditional Chinese Medicine, 110847 Shenyang, Liaoning, China
2 College of Traditional Chinese Medicine, Liaoning University of Traditional Chinese Medicine, 110847 Shenyang, Liaoning, China
†These authors contributed equally.
Abstract
Sleep is essential for human physiological functions; however, the prevalence of sleep disorders has increased in recent years. Traditional Chinese Medicine has gained widespread attention owing to the reduced observed side effects and significant efficacy following administration. Thus, this study aimed to provide a new treatment option for sleep disorders using a drug pairing of Radix Ginseng and Semen Ziziphi Spinosae Drug pair (R–S).
A total of 60 mice were randomly divided into six groups. An enzyme-linked immunosorbent assay (ELISA) was used to measure the SS (somatostatin), SP (substance P), np-y (neuropeptide Y), 5-HTR4 (5-hydroxytryptamine (serotonin) receptor 4) and 5-HTR3 (5-hydroxytryptamine (serotonin) receptor 3) levels in the small intestine tissues of the mice. Real-time quantitative PCR (RT-qPCR) was employed to assess the Piezo1 and Piezo2 mRNA expression levels. Hematoxylin and eosin (H&E) staining was used to observe cell morphology, and immunohistochemistry was employed to detect brain-derived neurotrophic factor (BDNF) and glial fibrillary acidic protein (GFAP) expression in the small intestine. Data were analyzed using SPSS 27.0 software. A p-value < 0.05 was considered statistically significant.
The treatment groups presented higher levels of np-y (p < 0.01) and lower levels of SS, SP, np-y, 5-HTR4 and 5-HTR3 (p < 0.01) compared to the model group. Meanwhile, the treatment groups showed a decreasing trend in Piezo1 and Piezo 2 mRNA expression, an elevated positive expression of BDNF, and a reduced positive expression of GFAP proteins (p < 0.05, p < 0.01).
R–S treatment can regulate 5-HTR/Piezo/BDNF/GFAP to improve the sleep of mice with PCPA (para-chlorophenylalanine)-induced insomnia mice, which provides experimental evidence for studying R–S in the treatment of insomnia.
Keywords
- insomnia
- Radix Ginseng
- Semen Ziziphi Spinosae Drug Pair
- Chinese medicine
- small intestine
- 5-HTR/Piezo/BDNF/GFAP
Good sleep is crucial for overall physical and mental health. However, with increasing life stress, the prevalence of sleep disorders, characterized mainly by insomnia, is rising. Short-term insomnia can lead to mood disturbances, while chronic insomnia can significantly increase the risk of severe conditions such as depression, anxiety, chronic pain and cardiovascular diseases [1]. In Traditional Chinese Medicine (TCM), insomnia is categorized under “Bu Mei”, with contributing factors including external pathogenic influences, emotional stress and irregular diet. The understanding of insomnia in traditional Chinese medicine has been well-established since the “Huangdi Neijing” [2]. Surveys indicate that 10–40% of young and middle-aged populations experience varying degrees of insomnia and the prevalence in the elderly is even higher, ranging from 40–70%. Previous studies have found that prolonged sleep deprivation can lead to irritability, anxiety and depression in adolescents and may even indirectly result in self-harm behaviors [3].
The Traditional Chinese Medicine (TCM) adopts a holistic approach and follows the principles of syndrome differentiation and treatment. Through multi-drug formulations, TCM can target multiple pathways to regulate neurotransmitters that affect sleep, demonstrating good clinical efficacy. Ginseng and Semen Ziziphi Spinosae, two commonly used herbs in traditional remedies for insomnia, both possess calming effects and have shown promising results in clinical treatment [4]. Additionally, TCM herbs generally have fewer side effects and lower toxicity compared to Western medications, addressing some of the limitations of Western treatments.
Ginseng’s primary active component, ginsenosides, has been shown to promote sleep according to modern pharmacological studies [5]. Semen Ziziphi Spinosae helps calm the mind, nourish the heart and generate fluids. Its active components include saponins, flavonoids, fatty acids, alkaloids and proteins, which contribute to its neuroprotective, antidepressant, anti-inflammatory, antioxidant, antihypertensive, lipid-lowering, anxiolytic, sleep-improving and sedative properties [6]. Research indicates that brain activity is significantly influenced by gut microbiota, potentially involving neurotransmitters, immune factors and neuropeptides related to the gut-brain axis [7]. Therefore, this study investigated the relationship between R–S by examining the regulation of 5-HTR and various intestinal biomarkers (SS (somatostatin), SP (substance P), np-y (neuropeptide Y), 5-HTR4 (5 hydroxytryptamine (serotonin) receptor 4), 5-HTR3 (5 hydroxytryptamine (serotonin) receptor 3)) in mice, providing clinical and theoretical support for TCM treatment of insomnia.
The experiment began on March 15, 2021 and ended on March 15, 2024 and was completed at the Innovation Engineering Technology Center of Liaoning University of Traditional Chinese Medicine.
The 60 mice, SPF grade, weighing 20
Starting from day 4, all groups except the blank group were intraperitoneally injected with PCPA (PCPA:4- Chlorophenylacetic acid) suspension (1 mL/100 g, 300 mg/kg) at 9:00 AM daily for 7 consecutive days.
After the final PCPA injection, the mice were observed. Except for the blank group, which exhibited blank activity and circadian rhythms, the other groups showed loss of circadian rhythm, increased daytime activity, agitation and fur loss, indicating successful modeling. On the following morning at 9:00 AM, gavage administration began. Dosing for each group was calculated based on an adult weight of 60 kg, with the mouse dose being 1/10th of the adult equivalent. The dosages were determined based on the formula for oral administration [8], the 2020 edition of the Chinese Pharmacopoeia and clinical experience. The daily dose for adults is 9 g of ginseng and 9 g of Semen Ziziphi Spinosae. For the control group, Sanchi Capsules were administered at 0.0075 mg/kg. The herbal groups received ginseng-Semen Ziziphi Spinosae solution at 0.06 g/kg. The blank and model groups were gavaged with distilled water at 1 mL/100 g. Treatment continued for 14 days.
Radix Ginseng (No.20160039, Hebei Mingzhu Pharmaceutical Co., Ltd., Baoding, Hebei, China); Semen Ziziphi Spinosae (No.20170365, Bozhou Sanfeng Traditional Chinese Medicine Decoction Pieces Co., Ltd., Bozhou, Anhui, China); Diazepam (No.14200901, Shanghai Xinyi Pharmaceutical Co., Ltd., Shanghai, China); 5HTR, ss, sp, np-y, HTR4, HTR3 (No.AE90976MU, No.AE92233Mu, No.AE90561Mu, No.AE91047Mu, No.AE90973Mu, No.AE90977Mu, Shanghai Lianshuo Biotechnology Co., Ltd. Shanghai, China); BDNF & GFAP (No.ab108319, No.ab207165, ABCAM, Cambridge, UK); PCPA (No.HY-101456, MedChemExpress LLC, NJ, USA); PrimeScript® RT Reagent Kit with gDNA Eraser (No.RR047A, Takara Bio, Shiga, Japan); SYBR®Premix Ex Taq™ II (Tli Rnase H Plus, Takara Bio, Shiga, Japan); ROX plus (No.RR42LRJ, Takara Bio, Shiga, Japan); Trizol (No.BK5005, Takara Bio, Shiga, Japan).
High-throughput Tissue Grinder (MM400, Shanghai Verder Instruments and Equipment Co., Ltd., Shanghai, China); Low-temperature high-speed centrifuge (MR1822, Jouan SA, Saint-herblain, Pays de la Loire, France); Microplate reader (Multiskan FC, Thermo Fisher Scientific Inc., Waltham, MA, USA); Optical Microscope (DM2000, Leica, Germany); Real-Time Quantitative PCR Instrument (Stratagene Mx3000P, Agilent Technologies, Inc., Palo Alto, CA, USA); Nucleic acid protein analyzer (DU640, Beckman Coulter, Inc., Pasadena, CA, USA); Electronic Balance (BL610, Beijing Sartorius Instrument System Engineering Co., Ltd., Beijing, China); Centrifugal (TDL-5-4, Shanghai Anting Scientific Instrument Factory, Shanghai, China); Electric glass homogenizer (DY89-2j, Ningbo Scientz Biotechnology Co., Ltd. Ningbo, Zhejiang, China); ultra micro UV analysis system (BioSpec-nano, Shimadzu Corporation, Kyoto, Japan).
After the treatment period, behavioral tests were conducted on the mice, followed by euthanasia (Intraperitoneal injection of 3% pentobarbital sodium 200 mg/kg). The small intestine tissues were collected. Portions of the small intestine were washed with physiological saline to remove blood, then stored at –80 °C for ELISA and real-time quantitative PCR. The remaining tissues were fixed in 4% paraformaldehyde and stored at room temperature for Hematoxylin and Eosin (H&E) staining and immunohistochemistry.
Small intestine tissues were sectioned coronally, de-waxed and washed with
distilled water. The sections were then stained with H&E. After staining, the tissues were cleared, dehydrated and mounted with a
coverslip. The morphology of the intestinal cells was observed under a light
microscope at 200
The small intestine tissues from mice were homogenized and then centrifuged at 12,000 rpm at 4 °C for 15 min. The supernatant was collected. The ELISA procedure was carried out according to the instructions provided with the assay kits.
Small intestine tissues from mice were homogenized and centrifuged to extract
total RNA, which was then reverse-transcribed into cDNA for amplification. The
PCR conditions were as follows: pre-denaturation at 95 °C for 30
seconds; denaturation at 95 °C for 5 sec, annealing at 60 °C
for 30–60 sec, for 45 cycles; final denaturation at 95 °C for 1 min,
annealing at 55 °C for 10 sec, with a gradual increase of 0.5
°C per cycle over 80 cycles to generate a melting curve.
| Gene | Primer | bp |
|---|---|---|
| Piezo1 | Forward GCCGTCGGGAACCAGAG | 155 |
| Reverse CCGTGGCTAAAGGGAGGTG | ||
| Piezo2 | Forward TCAACCAACCCCTGGATGTG | 158 |
| Reverse AAATTGCATTGCGCCACTGT | ||
| Forward CACTGTCGAGTCGCGTCCA | 88 | |
| Reverse CATCCATGGCGAACTGGTGG |
Small intestine tissues from mice were embedded in paraffin, sectioned, de-waxed
and hydrated. Antigen retrieval was performed using high pressure. The sections
were incubated with hydrogen peroxide for 10 min and then washed with PBS.
Primary antibodies were applied overnight, followed by washing with PBS. The DAB
(3,3′-diaminobenzidine) was used for color development and the sections were
washed with tap water. Hematoxylin was used for counterstaining and sections were
washed with PBS. Dehydration was carried out with ethanol at varying
concentrations, followed by clearing with xylene. After mounting, three random
fields (200
The R–S can improve insomnia behavior in mice, exerting an anti-insomnia effect. The experiment administered R–S via gavage to PCPA mice. The active ingredients enter the intestine, where the gut microbiota, aided by neurotransmitters, pass through the intestinal epithelium. The whole stages and results of this experiment were shown in Fig. 1.
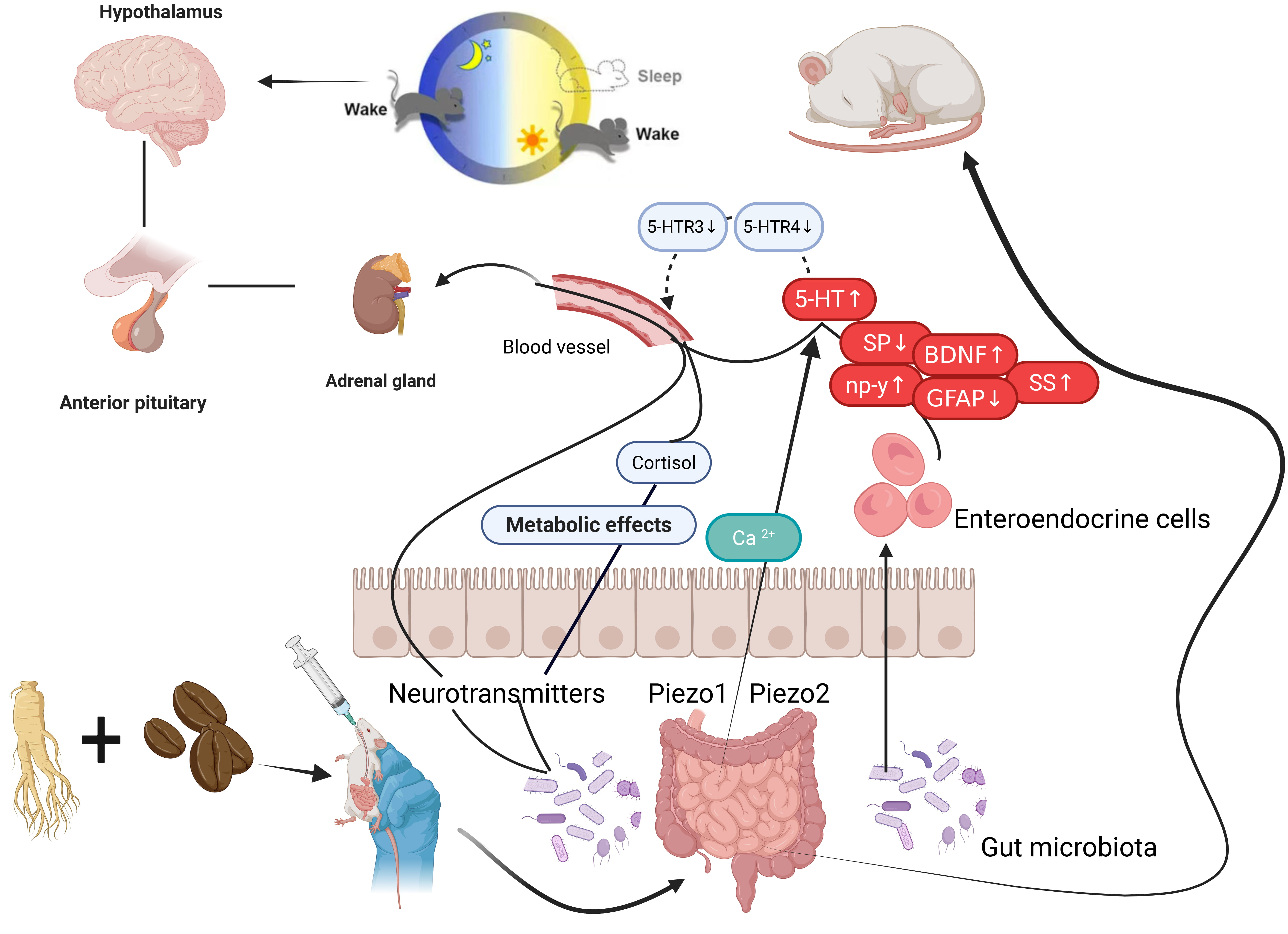 Fig. 1.
Fig. 1.
Mechanisms of R–S in PCPA-induced insomnia mice by regulating 5-HTR/Piezo/BDNF/GFAP. HTR, 5-Hydroxytryptamine receptor; Piezo, Piezoelectric ion channel; BDNF, Brain-derived neurotrophic factor; GFAP, Glial fibrillary acidic protein; SS, somatostatin; SP, substance P; np-y, neuropeptide Y.
Data were analyzed using SPSS 27.0 software (IBM Corp., Chicago, IL, USA).
Measurement data were described as Mean
In the blank group, the intestinal cells displayed a regular morphology, with round and clear nuclei and orderly arrangement (Fig. 2a). In contrast, the model group mice showed intestinal cells of varying sizes, with some cells elongated, nuclei shriveled, irregular shapes and sparse arrangement (Fig. 2b). Compared to the model group, the treated groups exhibited an increased number of intestinal neurons, more closely arranged cells and generally blank morphology. Pathological damage in the intestinal tissues of the mice was improved to varying extents (Fig. 2c–f). The results are shown in Fig. 2.
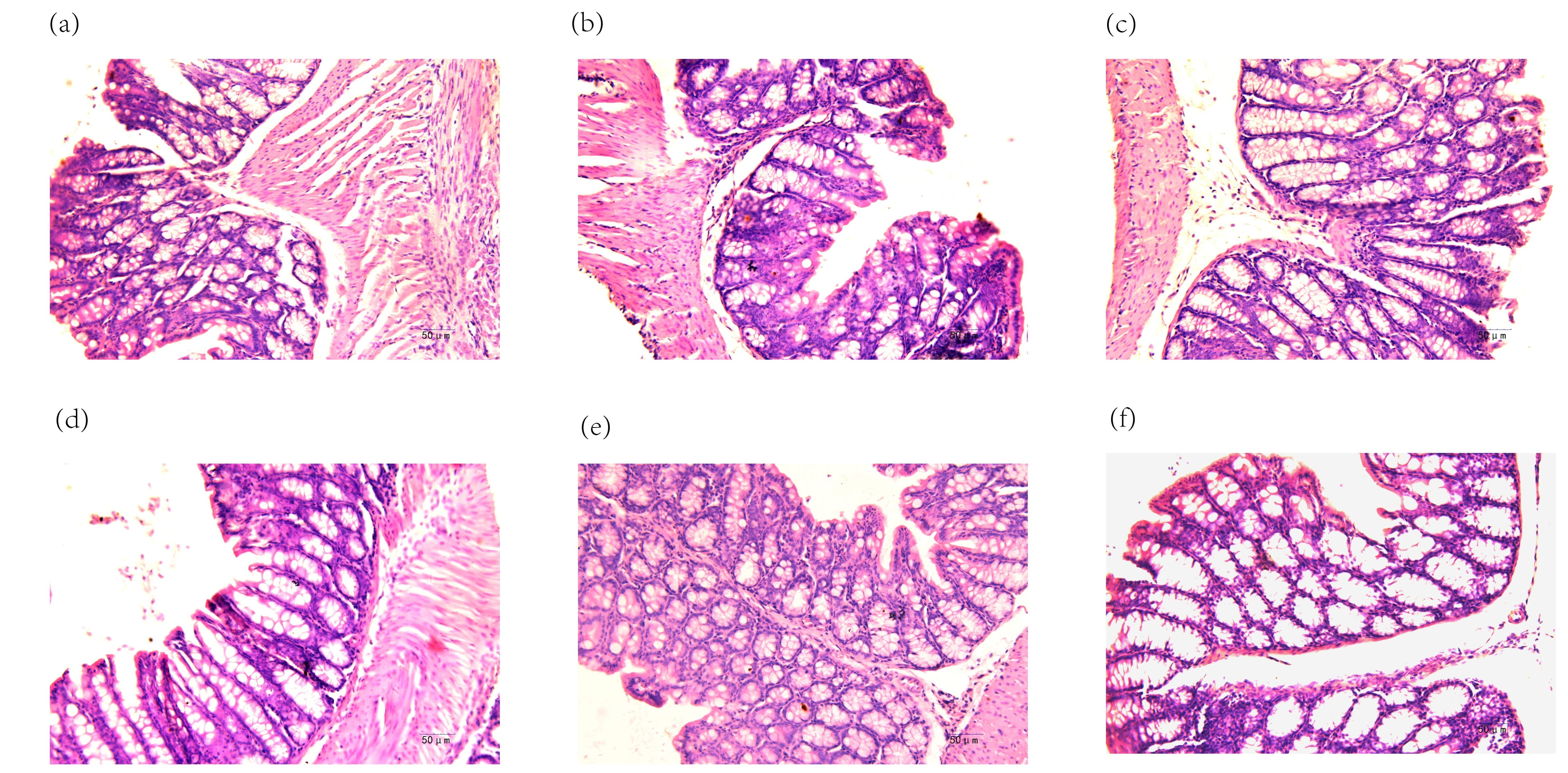 Fig. 2.
Fig. 2.
Pathological findings in mouse small intestine (H&E staining,
Compared to the blank group, the model group mice had reduced levels of ss, sp,
HTR4 and HTR3 (p
| Group | ss | sp | np-y | HTR4 | HTR3 |
| Blank group | 5.32 |
16.62 |
65.59 |
36.72 |
45.65 |
| Model Group | 9.23 |
29.11 |
47.76 |
65.74 |
87.99 |
| Positive Control Group | 6.00 |
20.60 |
63.24 |
44.30 |
56.28 |
| Low-Dose Group | 7.82 |
24.58 |
54.57 |
55.41 |
69.34 |
| Middle-Dose Group | 6.87 |
22.28 |
55.77 |
51.36 |
65.20 |
| High-Dose Group | 6.45 |
20.86 |
58.07 |
45.58 |
60.37 |
Compared to the normal group, ##p
Compared to the Normal Group, the Model Group showed increased expression of
Piezo1 and Piezo2 mRNA in the small intestine (p
| Group | Piezo1 | Piezo2 |
| Blank group | 1.00 |
1.00 |
| Model Group | 2.31 |
2.18 |
| Positive Control Group | 1.43 |
1.40 |
| Low-Dose Group | 1.68 |
1.69 |
| Middle-Dose Group | 1.79 |
1.63 |
| High-Dose Group | 1.54 |
1.43 |
##p
Compared to the Normal Group (Fig. 3a), the Model Group exhibited increased
positive expression of BDNF and GFAP proteins in the small intestine (p
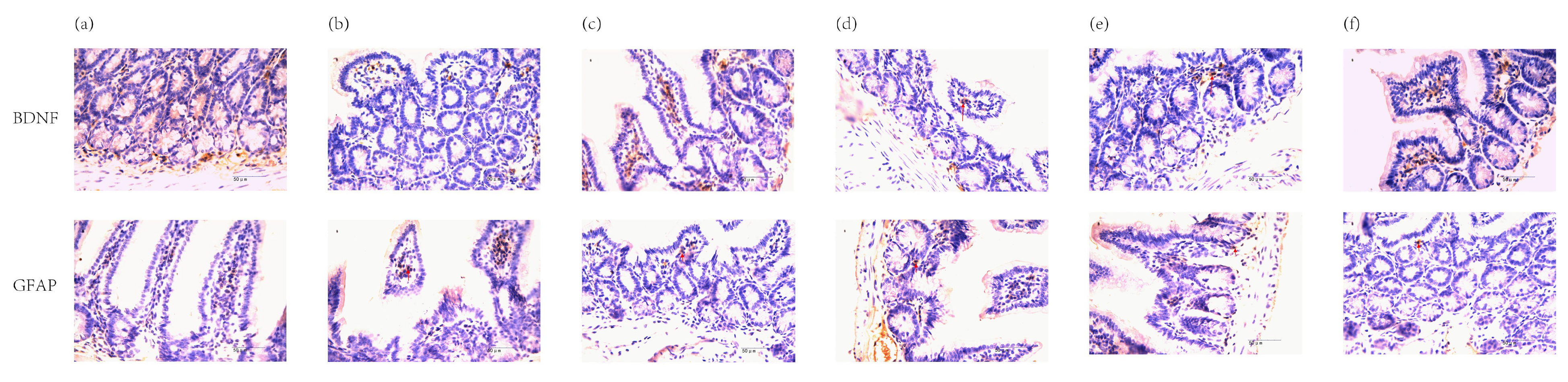 Fig. 3.
Fig. 3.
Positive expression of BDNF and GFAP proteins in mouse small
intestine (immunohistochemistry, junction of gland and basal layer,
| Group | BDNF | GFAP |
| Blank group | 0.360 |
0.174 |
| Model Group | 0.260 |
0.303 |
| Positive Control Group | 0.346 |
0.210 |
| Low-Dose Group | 0.277 |
0.254 |
| Middle-Dose Group | 0.309 |
0.248 |
| High-Dose Group | 0.345 |
0.248 |
##p
Insomnia, categorized as “Bu mei” in Traditional Chinese Medicine (TCM), is attributed to various factors such as external pathogenic influences or internal injuries that lead to dysfunction in the heart, brain, liver, gallbladder, spleen, stomach and kidneys. This results in inadequate nourishment of the mind and unrest in the five viscera, causing disturbances in the spirit and leading to insomnia [7]. The pathogenesis of insomnia has traditionally been summarized as a disruption in the balance of yin and yang and abnormal qi flow. Any factor affecting the circulation of qi and blood can lead to insomnia. When qi flows smoothly and the interaction between yin and yang is balanced, sleep is regular and restorative. Conversely, disruptions in qi flow and imbalances between yin and yang lead to restlessness and difficulty sleeping [10].
The COVID-19 pandemic, which has persisted for 3 years, has severely impacted sleep quality. Data indicates that the insomnia detection rate among the general adult population reached 29.2% during and after the pandemic. This rate is even higher among family members of infected or suspected cases, averaging 2.5 to 3 times greater than the general population [11]. In outpatient settings, patients recovering from COVID-19 have reported increased instances of insomnia, with a noticeable decline in quality of life due to both physical weakness and sleep disturbances [12]. Although Benzodiazepines such as clonazepam and alprazolam remain common treatments for insomnia [13], they are associated with side effects including drowsiness, ataxia, strong potential for addiction and severe withdrawal symptoms.
Consequently, natural products with high safety and tolerability profiles are being explored as alternatives for insomnia treatment. Traditional Chinese medicine offers advantages in treating insomnia with herbal remedies [14]. Ginseng and Semen Ziziphi Spinosae, used together, are widely employed in TCM for this purpose. Historical texts such as the Pu Ji Fang describe a formula for treating insomnia with sweating: combining equal parts of Semen Ziziphi Spinosae, Ginseng and Poria and taking it with rice wine. Semen Ziziphi Spinosae calms the mind and reduces sweating, while Ginseng replenishes qi and generates fluids, showing effective results in treating insomnia due to physical weakness. Modern pharmacological studies reveal that Semen Ziziphi Spinosae extract can decrease levels of norepinephrine (NE) and dopamine (DA) in the mouse brain [15], thereby improving sleep. Additionally, ginsenosides, the active components of Ginseng, can increase levels of serotonin (5-HT) and DA in the rat hippocampus and their metabolic products [16], thus contributing to insomnia treatment.
Modern medical research has revealed the existence of a bidirectional neuroendocrine network between the central nervous system and the gastrointestinal tract, known as the “brain-gut axis”. As a sleep disorder, insomnia profoundly affects the composition and abundance of gut microbiota. Brain-gut peptides such as 5-hydroxytryptamine (5-HT), somatostatin (SS), substance P (SP) and neuropeptide Y (NPY) play significant roles in regulating the sleep-wake cycle and are widely used as key indicators in sleep research. Among these, SS and SP are important inhibitory neurotransmitters that not only influence the secretion of digestive fluids and gastrointestinal motility but also impair sleep [17, 18]. Conversely, NPY promotes sleep. Therefore, using brain-gut peptides as indicators in Traditional Chinese Medicine (TCM) research on insomnia can provide reliable and objective results and help uncover the potential mechanisms of TCM in treating insomnia.
Studies have shown that the monoamine neurotransmitter 5-HT is closely related to sleep and also affects gastrointestinal motility and secretory reflexes [19]. 5-HT, which is predominantly distributed in the pineal gland and hypothalamus [20], plays a crucial role in the sleep-wake mechanism and is recognized as a “sleep-promoting factor” [21]. In 1967, Jouvet [22] proposed the 5-HT insomnia theory, suggesting that 5-HT regulates various gastrointestinal functions through different 5-HT receptor (5-HTR) families. Specifically, 5-HTR3 and 5-HTR4 play key roles in gastrointestinal secretion, motility, immune function and the regulation of the sleep-wake cycle [23, 24, 25]. The experimental results show that insomnia is associated with the upregulation of 5-HTR3 and 5-HTR4 in the small intestine of mice. After treatment with R–S in PCPA-induced insomnia mice, the levels of 5-HTR3 and 5-HTR4 in the small intestine decreased to varying degrees.
In insomnia mice, the increase in 5-HTR3 and 5-HTR4 levels may be related to increased brain-derived neurotrophic factor (BDNF) levels. BDNF is a growth factor for 5-HTR ergic neurons and can promote their growth and 5-HTR release and it is also involved in regulating the sleep-wake balance [26]. BDNF levels are positively correlated with sleep quality. The glial fibrillary acidic protein (GFAP) is a cell skeleton protein mainly expressed in astrocytes in regions such as the hippocampus and raphe nuclei, playing roles in the development of the nervous system, neuronal injury, repair and plasticity. During sleep deprivation, astrocytes in the raphe nuclei, particularly the dorsal raphe nucleus, react with enlarged cell bodies and extended processes and the number of GFAP-positive cells increases, serving as an indicator of sleep quality [27]. The results indicate that after treatment with R–S, insomnia mice exhibited significantly improved sleep quality, with increased BDNF levels and decreased GFAP levels.
Piezo1 and Piezo2, important mechanosensitive ion channels, are expressed in the digestive system and are primarily permeable to calcium ions (Ca2+), mediating signal transduction [28]. Activation of Piezo1 and Piezo2 generates mechanosensitive inward non-selective cation currents, which in turn promote the release of 5-HT from the small intestine and improve sleep. Piezo channels play a crucial role in the molecular mechanisms of mechanosensitive 5-HT release. Acute sleep deprivation activates the Piezo1/Ca2+/calpain pathway in the basal forebrain, diminishing the memory-protective effect of increased BDNF expression [29, 30].
The experiment found that R–S significantly reduced the levels of NPY in the small intestine of insomnia mice, while significantly increasing the levels of SS, SP, 5-HTR4 and 5-HTR3. Additionally, the formula increased the expression levels of Piezo1 and Piezo2, effectively alleviating insomnia behavior induced by PCPA. These results suggest that the anti-insomnia effects may be related to the regulation of brain-gut axis mechanisms. However, the current study has limitations and further research is needed to explore the detailed mechanisms by which R–S improves insomnia through modulation of the brain-gut axis.
The R–S can improve insomnia behavior in mice, exerting an anti-insomnia effect. This treatment increases the level of np-y and decreases the levels of SS, SP, 5-HTR4 and 5-HTR3 in the small intestine. It also shows a decreasing trend in Piezo1 and Piezo 2 mRNA expression and reduces the positive expression of BDNF and GFAP proteins. The R–S could regulate 5-HTR/Piezo/BDNF/GFAP to improve the sleep of PCPA-induced insomnia mice, which provides experimental evidence for studying R–S in the treatment of insomnia, it also opens up a new direction for the treatment of insomnia with medicinal and edible homologous Traditional Chinese Medicine, combining daily health care and clinical treatment, reducing patients’ adverse reactions, saving medical costs and generating greater social and economic benefits.
Based on the effective treatment of insomnia with the combination of Ginseng and Ziziphi Spinosae Semen in traditional Chinese medicine, as well as the low side effects due to their medicinal and edible properties, this study explored the mechanism of the Ginseng-Ziziphi Spinosae Semen pair in treating insomnia. The experimental results suggest that the anti-insomnia effect of the Ginseng-Ziziphi Spinosae Semen combination may be related to the regulation of the gut-brain axis, which would be the reference for progressive experimental research and clinical application.
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
BZ designed the research, conducted experiments and contributed to manuscript writing. JC conducted experiments, collected data and wrote the manuscript. MZ, FL conducted experiment and performed data analysis. KL, TQ designed the research and contributed to manuscript writing. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
The animal research was approved by the Experimental Animal Ethics Committee of Liaoning University of Traditional Chinese Medicine No.21000042012024. It was followed by the 3R principles. All animals were handled with human care during the experiment and all treatments were strictly conducted under the supervision of the Experimental Animal Ethics Committee at Liaoning University of Traditional Chinese Medicine.
Not applicable.
This study was supported by grants from the guiding project of the Liaoning Provincial Department of Science and Technology Doctoral Scientific Research Startup Fund Project (No. 2024-BS-135) and the Basic scientific research project of colleges and universities of the Liaoning Provincial Department of Education (LJ232410162009).
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.



