1 Department of Cardiovascular Surgery and National Clinical Research Center for Geriatrics, West China Hospital, Sichuan University, 610041 Chengdu, Sichuan, China
2 Integrated Care Management Center, Outpatient Department, West China Hospital, Sichuan University, 610041 Chengdu, Sichuan, China
3 Department of Cardiovascular Surgery, West China Hospital, Sichuan University, 610041 Chengdu, Sichuan, China
4 West China School of Nursing, Sichuan University, 610041 Chengdu, Sichuan, China
5 Outpatient Department, West China Hospital, Sichuan University, 610041 Chengdu, Sichuan, China
†These authors contributed equally.
Abstract
Mitral valve repair is a widely used operation to treat mitral regurgitation. However, the annuloplasty ring used may fail, causing mitral regurgitation to recur several years after the operation. Transcatheter mitral valve-in-ring (MViR) replacement may become a potential therapeutic strategy for those patients with high surgical risks.
This study presents a case report of a patient who underwent transcatheter MViR replacement using a novel Renato valve. A 67-year-old female presented with chest discomfort, and an echocardiogram showed severe mitral regurgitation and symptomatic left ventricular (LV) dysfunction. The patient had a surgical history of mitral repair, and the mitral regurgitation was due to the failed annuloplasty ring. As the patient was a poor candidate for the redo operation, we performed transcatheter MViR replacement using a novel Renato valve at our institution. The post-procedure transesophageal echocardiogram revealed a significant reduction in mitral regurgitation. The patient was discharged uneventfully, and the symptoms were alleviated.
Transcatheter MViR replacement is a safe procedure for patients with a failed annuloplasty ring and LV dysfunction.
Keywords
- mitral regurgitation
- valve-in-ring
- left ventricular dysfunction
- transcatheter valve replacement
- transapical
Mitral valve replacement or repair are both common therapies for treating mitral valve disease; however, mitral valve repair has been chosen by more patients over the past few decades [1]. Meanwhile, the problem of a failed annuloplasty ring gradually occurs years after the initial valve repair, causing patients to suffer again from mitral regurgitation or stenosis, and forcing them to undergo another operation. This presents a dilemma because many patients with mitral regurgitation are older and may not be able to withstand a redo operation. Thus, interventional therapies for these patients have become an urgent need. Recently, advancements in the technique of interventional treatment for valvular heart diseases have led to the emergence of transcatheter mitral valve replacement as a novel therapeutic strategy for patients with high surgical risks. Therefore, this study used a novel balloon-expandable Renato valve to perform transcatheter mitral valve-in-ring (MViR) replacement in an older female patient with mitral regurgitation and severe left ventricular (LV) dysfunction. The valve was implanted successfully, and the patient was discharged uneventfully. To our knowledge, this is the first case study to report a transcatheter MViR replacement in China; hence, this study aimed to discuss the efficacy and safety of transcatheter MViR implantation using the novel Renato valve (Balance Medical Technology Co., Ltd. Beijing, China).
A 67-year-old female presented with chest discomfort and edema in the lower extremities that had persisted for 3 months. The patient had a surgical history of mitral repair 5 years prior. An echocardiogram showed severe mitral regurgitation and LV dysfunction. The preoperative left ventricular ejection fraction (LVEF) was 34%, the LV internal diameter was 60 mm, and the left atrial (LA) internal diameter was 61 mm (Table 1). As the patient was a poor candidate for a redo-operation (the society of thoracic surgeons (STS) score 8.2%), we decided to perform transcatheter MViR replacement.
| LVEF | Vmax | Area | PGmean | VC | LV | LA | PVL | KCCQ | NYHA | |
| Preoperative | 34 | 1.6 | NA | NA | NA | 60 | 61 | NA | 67.71 | IV |
| Postoperative | 35 | 2.1 | 3.1 | 2 | 4 | 60 | 56 | Mild to moderate | NA | NA |
| 3-month | 37 | 2.2 | 3.3 | 5 | 5 | 60 | 63 | Moderate | NA | NA |
| 6-month | 36 | 2.0 | 4.0 | 4 | 5 | 56 | 59 | Moderate | NA | NA |
| 12-month | 37 | 2.7 | 2.8 | 12 | 3 | 59 | 58 | Moderate | 76.04 | II |
| 18-month | 37 | 2.6 | 3.3 | 10 | 3 | 57 | 51 | Moderate | NA | NA |
| 24-month | 32 | 2.1 | 3.4 | 8 | 3 | 60 | 52 | Moderate | 78.13 | II |
| 30-month | 29 | 1.9 | 3.8 | 8 | 4 | 64 | 57 | Moderate | NA | NA |
| 36-month | 43 | 2.7 | 3.2 | 8 | 3 | 60 | 63 | Mild to moderate | 81.25 | II |
| 42-month | 37 | 2.0 | 5.4 | 5 | 4 | 60 | 58 | Mild to moderate | 83.33 | II |
LVEF, left ventricular ejection fraction; PGmean, mean pressure gradient; VC, vena contracta; LV, left ventricle; LA, left atrium; PVL, perivalvular leak; KCCQ, Kansas City Cardiomyopathy Questionnaire; NYHA, New York Heart Association functional classification; NA, not application.
The ring is a complete, semi-rigid 28 mm ring (Edwards Physio II). In previous studies, it is recommended that 20% of the valve be anchored in the atrial position [2]. We noticed that when using a valve with an outer skirt, part of the ring may be located on the outer skirt, and the other part may be positioned on the metal frame. Considering that the failed annuloplasty ring is D-shaped and curved, this may lead to imbalanced radial forces in different parts of the ring, thereby raising the risk of valve dislocation and paravalvular leak (PVL). Hence, we used a novel Renato valve (Balance Medical), which has a completely exposed metal frame and an inner skirt to avoid left ventricular outflow tract (LVOT) obstruction (Fig. 1).
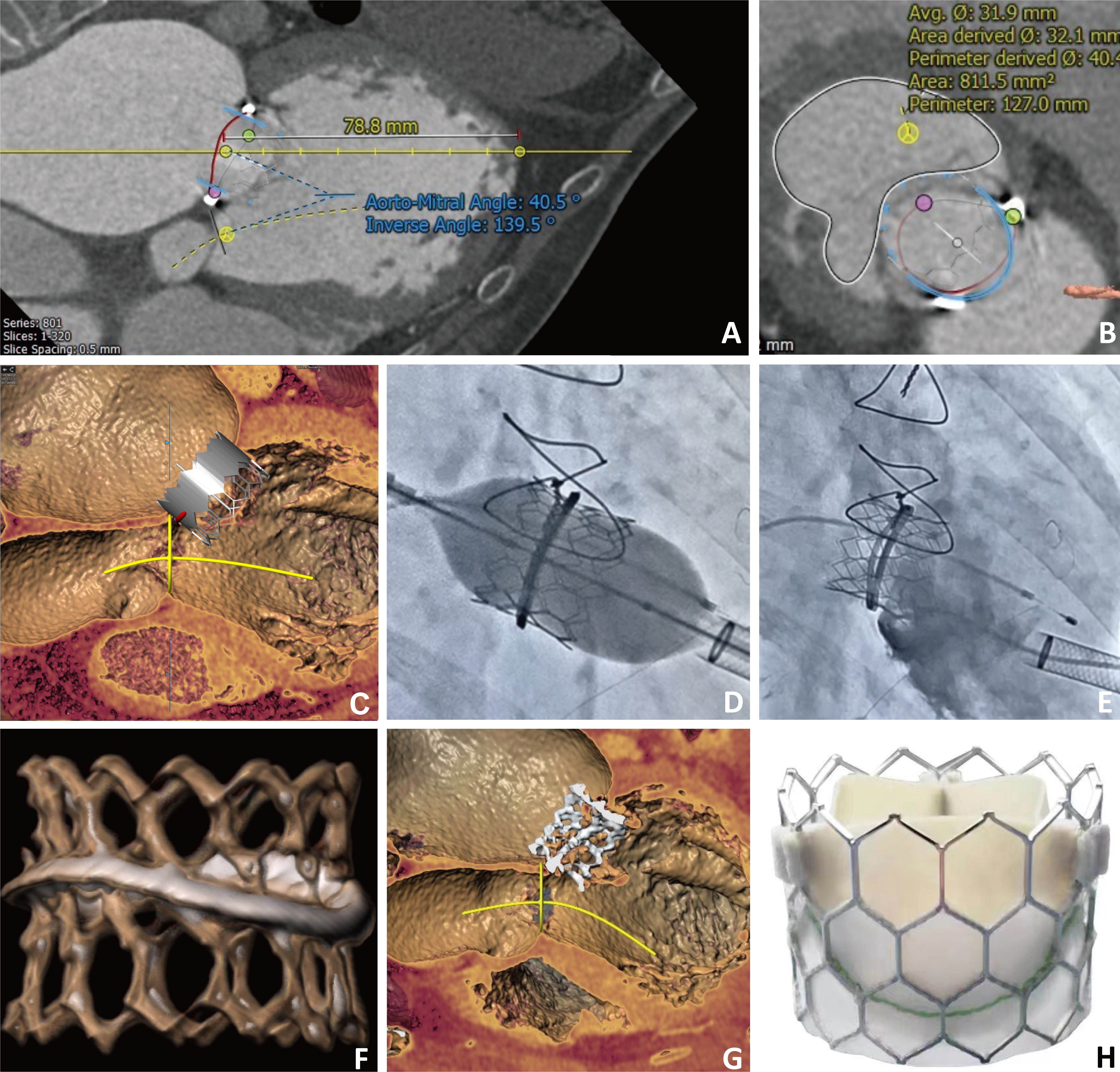 Fig. 1.
Fig. 1. Imaging evaluation of Renato valve implantation in mitral valve-in-ring. (A) Preprocedural computed tomography (CT) scan showed that the aortomitral angle was 40.5°. (B) A CT-simulated 27 mm Renato valve was implanted. The estimated neo-left ventricular outflow tract (LVOT) was 811.5 mm2, and no LVOT was observed. (C) Simulated valve implantation in the left ventricular long-axis view. (D) The fluoroscopy image of the valve expansion. (E) Angiography showed no residual mitral regurgitation. (F) Post-procedural three-dimensional reconstruction CT scan of mitral valve-in-ring (MViR). (G) Post-procedural CT scan showed no LVOT obstruction. (H) Image of the Renato valve.
The preprocedural computed tomography (CT) scan showed that the aortomitral angle was 40.5°, which implies a low LVOT obstruction risk. Following CT simulation of a 27 mm Renato valve implantation, no risk of LVOT obstruction was demonstrated. The preoperative evaluation revealed that the height of the interatrial septum puncture was less than 3.5 cm, indicating that this approach would pose challenges for the operation. Consequently, the apical approach was selected due to its ease of delivery and superior coaxiality. The procedure was conducted through transapical access, and the delivery catheter was introduced into the left atrium with approximately one-third of the valve in the atrial position. A post-procedural transesophageal echocardiogram showed no residual mitral regurgitation, mild to moderate paravalvular leak, and no LVOT obstruction. The echocardiogram indicated the presence of a perivalvular regurgitation jet (vena contracta = 3 mm) originating from the 10 to 11 o’clock direction. We hypothesize that the cause of the PVL may be attributed to a misalignment in the height of the mitral valvuloplasty ring, resulting in an imbalance of support between the implanted Renato valve and the mitral valvuloplasty ring, leading to local voids. This finding was further corroborated by CT (Fig. 1). Intervention may be necessary if the PVL worsens, which can include PVL closure or reimplantation of the Renato valve. The expandable valve stent design of the Renato valve, aligned with the concept of whole-life cycle management, helps prevent the occurrence of prosthesis-patient mismatch (PPM), as shown in Fig. 2 and Supplementary Video 1. The follow-up examination, conducted three months later, indicated an improvement in the cardiac function of the patient compared to previous assessments. However, a moderate PVL was present, and the forward blood flow for the mitral valve was not accelerated. Subsequent echocardiograms performed at regular intervals did not reveal a further increase in the degree of perivalvular leakage. By the 4-year follow-up, the PVL had progressed to a mild to moderate degree. The cardiac function of the patient showed continued improvement following the operation, with the New York Heart Association classification decreasing from preoperative class IV to class II and the Kansas City Cardiomyopathy Questionnaire (KCCQ) score increasing from 67.71 to 83.33 (Table 1). Notably, the patient did not experience bleeding, stroke, or readmission for heart failure during the follow-up period. Given the absence of significant heart failure symptoms, no further intervention was pursued for the PVL.
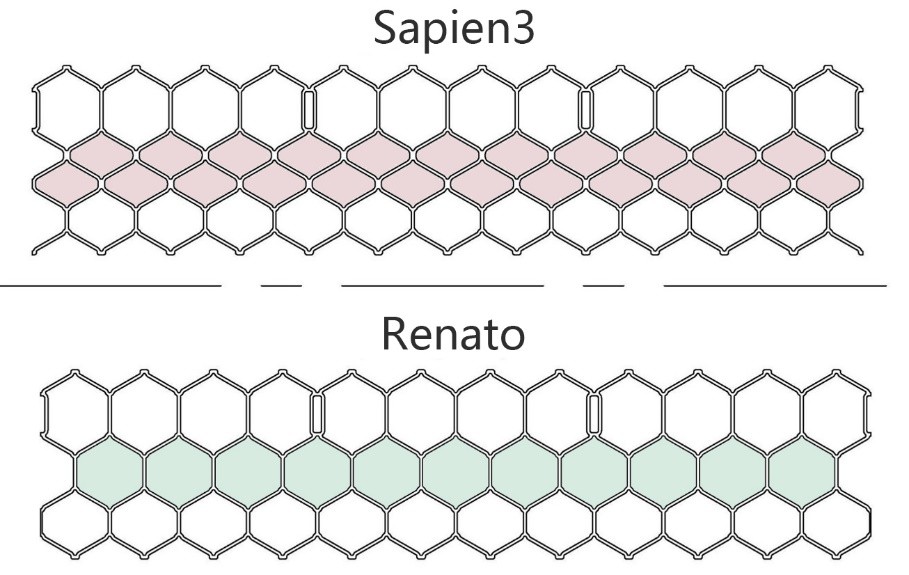 Fig. 2.
Fig. 2. Differences in the stent structural design of the Sapine3 valve and Renato valve. The Renato valve stent exhibits improved expandability and can reduce the insufficient device expansion in the reimplanted transcatheter heart valve (THV).
Over the past two decades, various mitral valvuloplasty rings have been developed and implanted. However, until recently, all cases of failed mitral valve prostheses were managed through redo surgery. Subsequently, the emergence and advancement of transcatheter procedures have provided an effective alternative, particularly for high-risk surgical patients. Similar to the transcatheter mitral valve-in-valve (MViV) concept, the treatment for repair failure involves implanting the transcatheter heart valve (THV) device within the ring, a procedure known as MViR [3]. Kamioka et al. [4] conducted a retrospective study comparing MViV with redo mitral valve surgery. The mitral valve pressure gradient was higher in the MViV group (7.2
In recent studies, researchers focused on the possibility of transcatheter MViV, MViR, and valve-in-mitral annular calcification in patients with high surgical risks. However, patients undergoing transcatheter MViR presented a far from satisfying mortality rate. In three large cohort studies, the 1-year all-cause mortality rate for the MViR group ranged from 20% to 30% [5, 6, 7], with the 2-year all-cause mortality as high as 50% in one study [8]. This poor prognosis can result from the following challenges, including anatomic differences in the shape of the mitral valves compared to transcatheter bioprostheses, variations in the annuloplasty rings previously sutured, the length of the anterior mitral leaflet, and post-procedural antithrombosis therapy. These challenges may lead to residual mitral regurgitation, LVOT obstruction, bioprosthetic valve thrombosis, and increased mortality [9].
The patient in this study was a high-risk candidate for the redo operation and had severe LV dysfunction. Considering the cardiac function, the impact of previous surgery, and the will of the patient, transcatheter MViR was chosen over surgical mitral replacement. The success of valve deployment showed that transcatheter MViR is a safe procedure for patients with severe LV dysfunction. However, the previously chosen ring has a significant impact on the prognosis of transcatheter MViR.
A rigid-ring complete cannot be rounded, which may result in incomplete expansion, deformation, and THV leakage. Interestingly, the rigid-ring incomplete is similar to the rigid-ring complete; however, in some cases, the open end may allow for a greater degree of roundness, resulting in more favorable outcomes. A semi-rigid ring can be completely rounded or nearly round, making this ring ideal for MViR [10]. In this study, we also performed MViR using a semi-rigid ring complete (Edwards Physio II, Edwards Lifesciences Corporation, One Edwards Way, Irvine, California, American) and achieved favorable hemodynamic outcomes. More importantly, the patient exhibited significant improvement in cardiac function over the 4-year follow-up, without developing LVOT obstruction, valvular thrombosis, or adverse cardiovascular events. A recent echocardiogram indicated mild to moderate PVL, but the patient showed no symptoms of heart failure, meaning no further intervention was required. However, attention should be given to the PVL cause following MViR. Currently, all available THVs are designed for the aortic valve. Anatomically, the aortic valve annulus is more circular in shape compared to the mitral valve annulus. The mismatch between the shape of the valvuloplasty ring and the THV increases the likelihood of a gap forming, which can impair hemodynamics, particularly in rigid and non-circular rings. This mismatch can lead to residual mitral regurgitation and insufficient device expansion [11]. Additionally, THVs in the mitral valvuloplasty ring are exposed to higher pressures from the left ventricle during systole, which may reduce anchoring stability and the effectiveness of dilatation [12], potentially leading to complications such as thrombosis or early degeneration. The Renato valve utilized in this study offers the potential advantage of mitigating the aforementioned risks. Firstly, the Renato valve features a fully exposed metal frame and an inner skirt, which provide more effective anchorage of the mitral valvuloplasty ring and help prevent LVOT obstruction. Secondly, the Renato valve incorporates an expandable stent design, which reduces the risk of incomplete expansion. This characteristic is particularly beneficial if the patient requires another THV implantation in the future. Such features align with the concept of whole-life cycle management.
Our study has several important limitations that should be considered. First, this study did not include a control group, such as patients undergoing alternative treatments for mitral valvuloplasty ring failure (e.g., repeat surgical replacement or repair). Additionally, we did not collect data on cases of surgical valve or mitral valvuloplasty ring failure that were managed solely with medical treatment, excluding MViR. Furthermore, while the current follow-up duration was 4 years, longer-term follow-up (e.g., 5–10 years) and data from a larger cohort are needed to improve the assessment of the outcomes of the Renato valve in MViR.
Our experience suggests that transcatheter MViR may be a possible therapeutic strategy for patients with failed annuloplasty rings combined with severe LV dysfunction.
Data is accessible through our system at our institution and is not publicly available.
TQC and BYL contributed to the study conception and design, as well as the writing and critical revision of the manuscript. SYH, YQW, LLL and JS were responsible for the acquisition, analysis, and interpretation of data and image collection. They also participated in drafting and critically reviewing the manuscript. DJ and YQG contributed to data interpretation, manuscript revision, and provided administrative support. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work. All authors read and approved the final manuscript.
Typically, case reports require ethical approval; however, the Biomedical Ethics Committee of West China Hospital of Sichuan University has waived this requirement for our case report. Based on the patient’s enrollment in the West China-VHD Registry (Registration No. 20232422), which has obtained ethical approval, and the patient’s consent for the use of medical records for research publication, this study qualifies for ethical exemption. The corresponding exemption approval number is 20232422. Consent for publication was obtained directly from the patient. The patient has agreed to the publication of her photo and medical records in the journal. She has read the manuscript or understood a general description of its contents and has reviewed all photographs, illustrations, videos, or audio files (if applicable) related to her for publication. The patient is aware that her name will not be published, and that articles published in the case report may be republished in other media and freely redistributed for any lawful purpose, including academic exchange, translation, and commercial use.
Not applicable.
This manuscript was supported by National Clinical Research Center for Geriatrics, West China Hospital, Sichuan University (No. Z2024YY001), and 1.3.5 project for disciplines of excellence from West China Hospital of Sichuan University (No. ZYGD22010).
The authors declare no conflict of interest.
Supplementary material associated with this article can be found, in the online version, at https://doi.org/10.31083/HSF47014.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.


