1 Department of Cardiovascular Surgery, Istanbul University-Cerrahpasa Institute of Cardiology, 34098 Istanbul, Turkey
2 Department of Cardiovascular Surgery, Istanbul Prof. Dr. Cemil Tascioglu City Hospital, 34384 Istanbul, Turkey
Abstract
The neutrophil–lymphocyte ratio (NLR), platelet–lymphocyte ratio (PLR), and lymphocyte–monocyte ratio (LMR) are recognized markers linked to inflammation and have been studied across various fields. This study aimed to evaluate which systemic inflammation indices are more specific to post-cardiac surgery mortality and morbidity.
A total of 1528 cardiac surgery patients were retrospectively analyzed, including a subset of 1205 patients who underwent coronary artery bypass grafting (CABG). This study assessed the associations between the NLR, PLR, and LMR and postoperative complications. The predictive accuracy of indices was also specifically compared within the CABG subgroup. While the NLR and PLR indicated the occurrence of events, the LMR, with a receiver operating characteristic curve below 0.5, was interpreted as “free of events”. The cut-off values were determined as NLR: 3.10, PLR: 143.9, and LMR: 3.52.
The NLR correlated with higher mortality and complications, whereas the PLR showed no significant relation to neurological complications. The LMR was found to be related to the non-occurrence of events. Patients with higher NLR and PLR values experienced increased mortality and major adverse cardiac and cerebrovascular events, as well as a higher incidence of complications, including postoperative revision, atrial fibrillation (AF), and renal issues. Conversely, higher LMR values corresponded with lower rates of such complications.
The NLR has emerged as a crucial indicator for predicting mortality and complications in cardiac surgery patients, more so than the PLR and LMR. Specifically, the NLR can be used to indicate the risk of mortality and complications in cardiac surgery. This prediction can be supported further using the PLR and LMR.
Keywords
- neutrophil–lymphocyte ratio
- platelet–lymphocyte ratio
- lymphocyte–monocyte ratio
- systemic inflammation indices
- cardiac surgery complications
It is increasingly accepted that systemic inflammation plays a fundamental role in the pathophysiology and clinical course of cardiovascular diseases [1]. Chronic inflammation is reported in the literature as a key factor in the destabilization of atherosclerotic plaques, thrombus formation, and myocardial damage [2]. In addition, numerous studies have studied the relationship between systemic inflammatory processes and valve [3] and aortic diseases [4]. Inflammation indices such as the neutrophil–lymphocyte ratio (NLR), platelet–lymphocyte ratio (PLR), and lymphocyte–monocyte ratio (LMR), which have recently increased in popularity, have been used to predict the outcome of cardiac and many other surgical procedures [5, 6, 7, 8].
However, to our knowledge, no comparative study has been presented in the literature to determine which of these indices is the most powerful tool in predicting mortality and morbidity after cardiac surgery. Therefore, this study is based on a large patient population that has undergone various cardiac surgeries, and aimed to evaluate the relationships between the NLR, PLR, and LMR and 1-year mortality and morbidity after cardiac surgery to determine which of these indices has the highest predictive value.
This study received ethical approval from the Istanbul University-Cerrahpasa Non-Interventional Clinical Research Ethics Committee, chaired by Prof. Dr. Aysem Kaya, on June 7, 2023, under approval number 2023/93.
The study included all cardiac surgery patients, retrospectively screened at our university clinic over the last 10 years. The study included adult patients (aged
Peripheral venous blood samples were collected from all patients within 48 hours before cardiac surgery. Hematological parameters were analyzed using the Beckman Coulter DxH 800 automated hematology analyzer (Beckman Coulter Inc., Brea, CA, USA); meanwhile, biochemical analyses were performed using the Roche Cobas e411 analyzer (Roche Diagnostics, Mannheim, Germany) according to the manufacturer’s standard protocols. All analyses were conducted in the central laboratory in the hospital by trained personnel in accordance with institutional quality control standards. Reference ranges for hematological and biochemical parameters were consistent with current laboratory guidelines.
Atrial fibrillation (AF): The presence of complications was accepted in patients with any postoperative AF episode lasting
Myocardial infarction (MI): During the first 72 hours following coronary artery bypass grafting (CABG), the diagnosis of MI associated with CABG is accepted when creatine kinase and troponin levels exceed five times the 99th percentile of the normal reference range, combined with new pathological Q-waves, the emergence of a new left bundle branch block, or newly documented graft or natural coronary artery occlusion via angiography [10]. In the postoperative period, patients meeting these criteria and requiring revascularization through surgical or percutaneous interventions are considered to have the complication.
Major adverse cardiovascular and cerebrovascular events (MACCEs): A composite outcome including MI, stroke, cardiovascular death, and the need for urgent revascularization.
Prolonged intubation: Patients who required mechanical ventilation for more than 24 hours after surgery were considered to have prolonged intubation.
Acute kidney injury (AKI): The presence of complications was acknowledged if a diagnosis of AKI was made within 7 days postoperatively according to the Acute Kidney Injury Network (AKIN) criteria [11]; this includes an increase in creatinine to 1.5–2 times the preoperative level, a rise of 0.3 mg/dL within 48 hours, or a decrease in urine output to less than 0.5 mL/kg/hour.
Transient ischemic attack (TIA): The presence of complications was acknowledged in patients who experienced a transient neurological deficit that resolved within 24 hours without sequelae and did not show findings on magnetic resonance imaging (MRI) [12].
Cerebrovascular event (CVE): The presence of complications was acknowledged in patients who experienced a neurological deficit accompanied by findings on magnetic resonance or computed tomography imaging [12].
Statistical Package for the Social Sciences (SPSS) version 21 software (IBM Corp., Armonk, NY, USA) was used for statistical analyses. Descriptive statistical methods (median, frequency, percentage, 25th, and 75th percentile) were employed to evaluate the study data. The suitability of quantitative data for normal distribution was tested using the Kolmogorov–Smirnov and Shapiro–Wilk tests, as appropriate, and further examined graphically. For comparisons between two groups of quantitative variables, the Mann–Whitney U test was used because the data did not exhibit a normal distribution. Categorical data were compared using the Chi-square test and Fisher’s exact test. Receiver operating characteristic (ROC) analysis was applied to determine estimation accuracy. To control for the risk of Type I error due to multiple comparisons across inflammatory indices, Bonferroni correction was used where applicable. The Bonferroni-corrected significance level was set at p
The NLR was found to be statistically significant (p
The demographic and clinical characteristics of the 1528 patients included in the study are presented in Table 1. When comparing the NLR, PLR, and LMR based on demographic and clinical characteristics, comorbidities, and medication usage, it was observed that patients with a NLR
| n (%) | Mean | Median (Q1–Q3) | ||
| Gender | ||||
| Male | 1152 (75.4) | |||
| Female | 376 (24.6) | |||
| Age | 61.1 | 61 (55–68) | ||
| Height | 167.5 | 168 (161–174) | ||
| Weight | 79.7 | 79 (70–87) | ||
| BMI | 28.4 | 27.8 (25.1–30.8) | ||
| BMI | 475 (31.1) | |||
| Procedure | ||||
| Isolated CABG | 1205 (79) | |||
| Combined CABG | 1319 (86.3) | |||
| Aortic valve | 172 (11.3) | |||
| Mitral valve | 178 (11.6) | |||
| Tricuspid valve | 61 (4) | |||
| Ascending aorta | 61 (4) | |||
| Hypertension | 1279 (83.7) | |||
| Diabetes mellitus | 975 (63.8) | |||
| Hyperlipidemia | 1077 (70.5) | |||
| COPD | 361 (23.6) | |||
| CVE | 70 (4.6) | |||
| Peripheral artery disease | 122 (8) | |||
| MI | 629 (41.2) | |||
| Renal failure | 200 (13.1) | |||
| EF | 54.2 | 60 (48–60) | ||
| EF | 137 (9) | |||
| Medication | ||||
| Antihypertensive | 991 (64.9) | |||
| Beta blockers | 635 (41.6) | |||
| Calcium channel blocker | 274 (17.9) | |||
| Antiaggregant | 900 (41.6) | |||
CABG, coronary artery bypass grafting; CVE, cerebrovascular event; MI, myocardial infarction; BMI, body mass index; EF, ejection fraction; COPD, chronic obstructive pulmonary disease.
The predictive values of the NLR, PLR, and LMR were evaluated in relation to in-hospital mortality, 1-month, 6-month, and 12-month mortality, as well as MACCEs and complications. Upon examination of the ROC curves, the NLR and PLR were found to be the most successful in predicting 12-month mortality associated with the complications (Fig. 1). Since the LMR curve fell below 0.5, the ROC curve was interpreted as representing an absence of events. Moreover, the analysis of the ROC curve revealed that the LMR was most effective in predicting the non-occurrence of renal complications (Fig. 2). The ROC analysis results for the NLR, PLR, and LMR revealed no significant overall differences in prognostic performance between males and females, as well as between younger (
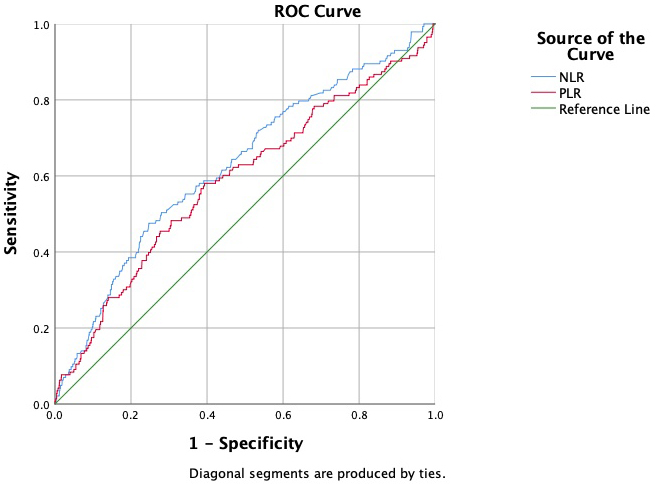 Fig. 1.
Fig. 1. NLR and PLR ROC curves for 12-month mortality. NLR AUC: 0.628; 95% CI: 0.578–0.677; PLR AUC: 0.587; 95% CI: 0.535–0.640. NLR, neutrophil–lymphocyte ratio; PLR, platelet–lymphocyte ratio; ROC, receiver operating characteristic; CI, confidence interval; AUC, area under the curve.
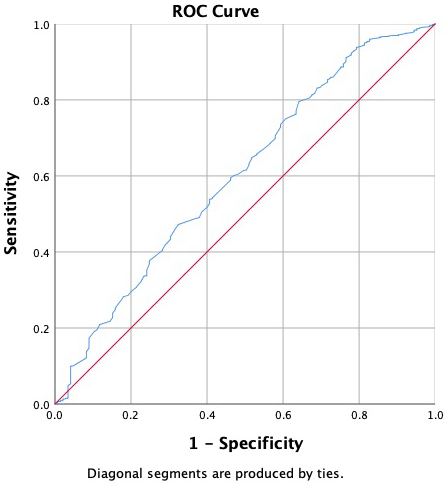 Fig. 2.
Fig. 2. LMR ROC curve for the non-occurrence of renal complications. The red line corresponds to the reference line, and the blue line represents the LMR. LMR AUC: 0.603; 95% CI: 0.553–0.654. LMR, lymphocyte–monocyte ratio.
The comparisons for the NLR, PLR, and LMR in relation to mortality and postoperative complications revealed that patients with a NLR
Furthermore, an elevated NLR (
| Postoperative complication | NLR (n) | PLR (n) | LMR | |||||||||
| p-value | BAS | p-value | BAS | p-value | BAS | |||||||
| Revision | 48 (4.5) | 32 (7) | 0.0471 | NS | 47 (4.4) | 33 (7.4) | 0.0161 | NS | 53 (5.5) | 27 (4.8) | 0.6061 | NS |
| AF | 261 (24.4) | 147 (32) | 0.0021 | S | 264 (24.4) | 144 (32.1) | 0.0021 | S | 284 (29.2) | 124 (22.3) | 0.0031 | NS |
| MI | 79 (7.4) | 43 (9.3) | 0.1971 | NS | 85 (7.9) | 37 (8.3) | 0.7991 | NS | 81 (8.3) | 41 (7.4) | 0.4961 | NS |
| Pneumonia | 150 (14) | 116 (25.2) | S | 165 (15.3) | 101 (22.5) | 0.0011 | S | 182 (18.7) | 84 (15.1) | 0.0691 | NS | |
| Prolonged intubation | 115 (10.8) | 94 (20.4) | S | 132 (12.2) | 77 (17.2) | 0.0101 | NS | 143 (14.7) | 66 (11.8) | 0.1151 | NS | |
| AKI | 75 (5) | 69 (15) | S | 77 (7.1) | 67 (15) | S | 109 (11.2) | 35 (6.3) | 0.0011 | S | ||
| Dialysis (RRT) | 20 (1.9) | 21 (4.6) | 0.0031 | NS | 20 (1.9) | 21 (4.7) | 0.0021 | S | 34 (3.5) | 7 (1.3) | 0.0091 | NS |
| TIA | 47 (4.4) | 43 (9.3) | S | 57 (5.3) | 33 (7.4) | 0.1141 | NS | 67 (6.9) | 23 (4.1) | 0.0271 | NS | |
| CVE | 42 (3.9) | 37 (8) | 0.0011 | S | 49 (4.5) | 30 (6.7) | 0.0831 | NS | 60 (6.2) | 19 (3.4) | 0.0191 | NS |
| Superficial wound infection | 89 (8.3) | 42 (9.1) | 0.6101 | NS | 91 (8.4) | 40 (8.9) | 0.7491 | NS | 79 (8.1) | 52 (9.3) | 0.4201 | NS |
| DSWI | 21 (2) | 12 (2.6) | 0.4281 | NS | 20 (1.9) | 13 (2.9) | 0.1991 | NS | 19 (2) | 14 (2.5) | 0.4711 | NS |
| In-hospital mortality | 55 (5.1) | 43 (9.3) | 0.0021 | S | 57 (5.3) | 41 (9.2) | 0.0051 | S | 72 (7.4) | 26 (4.7) | 0.0351 | NS |
| 1-month mortality | 47 (4.4) | 43 (9.3) | S | 48 (4.4) | 42 (9.4) | S | 66 (6.8) | 24 (4.3) | 0.0471 | NS | ||
| 6-month mortality | 67 (6.3) | 68 (14.8) | S | 74 (6.9) | 61 (13.6) | S | 101 (10.4) | 34 (6.1) | 0.0041 | NS | ||
| 12-month mortality | 71 (6.6) | 72 (15.7) | S | 78 (7.2) | 65 (14.5) | S | 107 (11) | 36 (6.5) | 0.0031 | NS | ||
| MACCEs | 143 (13.4) | 106 (23) | S | 156 (14.4) | 93 (20.8) | 0.0021 | S | 176 (18.1) | 73 (13.1) | 0.0111 | NS | |
| Intensive care length of stay (days) | 2 (2–3) | 2 (2–5) | S | 2 (2–3) | 2 (2–4) | 0.0032 | S | 2 (2–4) | 2 (2–3) | 0.0752 | NS | |
| Hospital length of stay | 6 (6–8) | 6 (5–9) | 0.3622 | NS | 6 (5–8) | 6 (5–9) | 0.1372 | NS | 6 (5–8) | 6 (6–8) | 0.9782 | NS |
1 Chi square test; 2 Mann–Whitney U test; BAS, Bonferroni-adjusted significance; S, statistically significant after Bonferroni correction (adjusted
When examining 1205 patients who underwent isolated CABG, comparisons between the NLR, PLR, and LMR and mortality and morbidity variables and postoperative complications revealed that, unlike in the entire patient cohort, the RRT requirement became statistically non-significant. Similarly, the relationship between the PLR and both AF and prolonged intubation no longer exhibited significance (Table 3).
| Postoperative complication | NLR (n) | PLR (n) | LMR (n) | |||||||||
| p-value | BAS | p-value | BAS | p-value | BAS | |||||||
| Revision | 28 (3.2) | 20 (5.9) | 0.0361 | NS | 29 (3.3) | 19 (6) | 0.0331 | NS | 31 (4.1) | 17 (3.8) | 0.8431 | NS |
| AF | 146 (16.9) | 75 (22) | 0.0391 | NS | 160 (18) | 61 (19.2) | 0.6291 | NS | 155 (20.3) | 66 (14.9) | 0.0191 | NS |
| MI | 64 (7.4) | 37 (10.9) | 0.0521 | NS | 70 (7.9) | 31 (9.8) | 0.2961 | NS | 66 (8.7) | 35 (7.9) | 0.6461 | NS |
| Pneumonia | 102 (11.8) | 72 (21.1) | S | 111 (12.5) | 63 (19.9) | 0.0011 | S | 114 (15) | 60 (13.5) | 0.5001 | NS | |
| Prolonged intubation | 72 (8.3) | 61 (17.9) | S | 89 (10) | 44 (13.9) | 0.0601 | NS | 92 (12.1) | 41 (9.3) | 0.1321 | NS | |
| AKI | 47 (5.4) | 38 (11.1) | S | 49 (5.5) | 36 (11.4) | S | 65 (8.5) | 20 (4.5) | 0.0091 | NS | ||
| Dialysis (RRT) | 12 (1.4) | 10 (2.9) | 0.0711 | NS | 11 (1.2) | 11 (3.5) | 0.0111 | NS | 20 (2.6) | 2 (0.5) | 0.0071 | NS |
| TIA | 33 (3.8) | 25 (7.3) | 0.0101 | NS | 42 (4.7) | 16 (5) | 0.8211 | NS | 42 (5.5) | 16 (3.6) | 0.1371 | NS |
| CVE | 25 (2.9) | 23 (6.7) | 0.0021 | S | 33 (3.7) | 15 (4.7) | 0.4271 | NS | 37 (4.9) | 11 (2.5) | 0.0421 | NS |
| Superficial wound infection | 70 (8.1) | 28 (8.2) | 0.9501 | NS | 71 (8) | 27 (8.5) | 0.7701 | NS | 60 (7.9) | 38 (8.6) | 0.6661 | NS |
| DSWI | 15 (1.7) | 7 (2.1) | 0.7111 | NS | 15 (1.7) | 7 (2.2) | 0.5541 | NS | 11 (1.4) | 11 (2.5) | 0.1941 | NS |
| In-hospital mortality | 30 (3.5) | 26 (7.6) | 0.0021 | S | 33 (3.7) | 23 (7.3) | 0.0101 | NS | 42 (5.5) | 14 (3.2) | 0.0621 | NS |
| 1-month mortality | 26 (3) | 26 (7.6) | S | 28 (3.2) | 24 (7.6) | 0.0011 | S | 40 (5.2) | 12 (2.7) | 0.0361 | NS | |
| 6-month mortality | 37 (4.3) | 43 (12.6) | S | 45 (5.1) | 35 (11) | S | 61 (8) | 19 (4.3) | 0.0121 | NS | ||
| 12-month mortality | 41 (4.7) | 47 (13.8) | S | 49 (5.5) | 39 (12.3) | S | 67 (8.8) | 21 (4.7) | 0.0091 | NS | ||
| MACCEs | 101 (11.7) | 75 (22) | S | 114 (12.8) | 62 (19.6) | 0.0041 | NS | 125 (16.4) | 51 (11.5) | 0.0201 | NS | |
| Intensive care length of stay (days) | 2 (2–3) | 2 (2–4) | 0.0252 | NS | 2 (2–3) | 2 (2–3) | 0.0692 | NS | 2 (2–3) | 2 (2–3) | 0.2752 | NS |
| Hospital length of stay | 6 (5–7) | 6 (5–8) | 0.6192 | NS | 6 (5–7) | 6 (5–8) | 0.5052 | NS | 6 (5–7) | 6 (5–7) | 0.5172 | NS |
1 Chi square test; 2 Mann–Whitney U test; BAS, Bonferroni-adjusted significance; S, statistically significant after Bonferroni correction (adjusted
Cardiac surgery is a major surgical procedure and, as expected, carries risks of postoperative complications and mortality. Therefore, accurately assessing risk factors, predicting postoperative outcomes, and minimizing risks is critically important for success. Traditional biomarkers such as high-sensitivity C-reactive protein (hs-CRP), erythrocyte sedimentation rate, and fibrinogen levels have been extensively studied for their predictive value in both coronary artery disease and the postoperative course of cardiac surgery. Indeed, in the most recent meta-analysis involving 7068 cases, hs-CRP was associated with coronary heart disease, although this association appeared weaker than that of fibrinogen [13]. Meanwhile, another meta-analysis indicated a significant but weak-to-moderate correlation between preoperative and postoperative fibrinogen levels and postoperative blood loss in cardiac surgery [14]. In contrast, erythrocyte sedimentation rate generally does not affect postoperative morbidity or mortality unless elevated to
We designed our study to examine on-pump cardiac surgery; however, prior studies have explored whether these inflammatory indices have predictive value in off-pump CABG. Indeed, the NLR and MLR are considered significant predictors of all-cause long-term mortality following surgical revascularization performed with the off-pump technique, particularly when combined with demographic factors (ages above 62 years) and echocardiographic parameters [16]. Additionally, studies have demonstrated that the NLR and PLR are important hematological parameters for predicting poor outcomes after Off Pump CABG [17].
In our study, the relationships between the NLR, PLR, and LMR indices and mortality and morbidity within 12 months after adult cardiac surgery were evaluated, and predictive values for each were calculated for routine clinical practice. However, instead of calculating a predictive value for each complication, the mortality and complication risks were assessed in groups to enhance suitability for daily practice and facilitate comparisons, aiming to determine a single predictive value. These values were calculated as 3.10 for the NLR, 143.9 for the PLR, and 3.5 for the LMR.
In the meta-analysis conducted by Perry et al. [18], 12 studies were combined to assess the predictive value of the NLR, with the predictive values ranging between 2.6 and 3.4 [5]. For the PLR, studies examining predictive values encountered values between 119.3 and 142.6 [19, 20, 21]. The predictive value was distinctly set at 86.2 only in the study by Navani et al. [22], which evaluated the relationship between the PLR and AF, which differs from that in other existing literature. The researchers reported that this unique predictive value was chosen to question the independent relationship between the PLR and AF, due to differences in the statistical model used in the analysis of the study [22]. However, neither Şaşkın et al. [20] nor Gungor et al. [21] disclosed the statistical model that was employed to determine the predictive values presented in their studies; meanwhile, neither study provided the PLR distribution data for their cohorts. When examining the literature for the predictive value of the LMR, we encountered values consistent with our study, ranging between 2 and 4.8 [6, 23, 24, 25, 26].
Notably, a significant relationship between a high NLR and monthly mortality was found in three separate studies conducted by Wang et al. (2707 patients) [27], Haran et al. (1694 patients) [28], and Silberman et al. (3207 patients) [29]. In contrast, to our knowledge, no study linking preoperative PLR value with short-term mortality after cardiac surgery has been found in the literature. Only in the study by Tzikos et al. [19], involving 189 patients, were postoperative PLR values found to be related to three-month mortality. In the study led by Zhou et al. [6], which included 1701 patients who were followed for four years, low LMR values were noted to be related to in-hospital, one-month, and three-month mortality. When comparing the ability of the indices to predict short-term mortality, the NLR was the best test for predicting both in-hospital and one-month mortality.
When investigating the relationship between the NLR and both short-term and long-term mortality after cardiac surgery, a meta-analysis conducted by Perry et al. [18], which included eight studies with a total of 9528 patients, found that high NLR values were associated with increased mortality. In a study conducted with 916 CABG patients, Şaşkın et al. [20] found that high PLR values were associated with mortality. Additionally, a study investigating the relationship between the LMR and four-year mortality indicated that a low LMR was associated with higher mortality [6]. In our research, the NLR and PLR were found to be associated with six-month and one-year mortality; meanwhile, the LMR was significantly related to the absence of mortality over similar periods. Among 1205 patients who underwent isolated CABG, those with a NLR
In the study conducted by Gurbuz et al. [30], which followed 751 CABG patients for eight years, it was reported that the NLR could predict MACCEs independently of the risk status of the patients. In a study led by Abanoz and Engin [31], involving 297 patients, it was noted that the NLR could predict MACCEs, but the PLR could not. No studies in the literature have been found that evaluate the relationship between the LMR and MACCEs following cardiac surgery. In our study, a high NLR and PLR were found to be associated with one-year MACCEs, while a high LMR was related to the absence of MACCEs over one year. When the isolated CABG was examined, results consistent with the total population were observed. When comparing the indices, the NLR was found to be the most suitable index for predicting MACCEs.
When examining studies that investigate the relationship between cardiac complications and systemic inflammatory indices, the most frequently noted association is between the NLR, PLR, and AF [21, 22, 28, 32, 33, 34, 35]. While most studies associate a high NLR with AF, studies conducted by Haran et al. [28] and Durukan et al. [35] found no significant relationship between the NLR and AF. Meanwhile, a disagreement exists in the literature regarding the relationship between the PLR and AF. Some studies argue that a significant relationship exists between a high PLR and AF [21, 33], while others report no significant relationship [22, 32]. In this study, higher incidences of AF were observed in patients above the NLR and PLR predictive values. Thus, we believe it is necessary to evaluate the number of patients included in the study, the type of surgery they underwent, and their preoperative characteristics. When examining the comorbidities and medications of patients according to the NLR and PLR predictive values, our study found a statistically significant difference only in patients with a diagnosis of renal failure and those using antihypertensive medications for the PLR. In contrast, no statistically significant difference was found for the presence of other comorbidities and medication usage. Upon reviewing the literature, researchers identified causality based on pathophysiological foundations between the indices and the frequency of AF; however, only statistical interpretations have been observed.
Another remarkable point is that when the same complications were examined for isolated CABG, the only difference observed was in the relationship between the PLR and AF, which was not significant. This suggests that the presence of valve surgery in the patient population could alter the outcomes.
The literature does not contain any studies that have investigated the relationship between the LMR and cardiac complications after cardiac surgery. However, while the incidence of AF appeared to be lower in patients with an LMR
When considering cardiac complications in general, the LMR was found to be the most suitable index in terms of predictive power. Meanwhile, the presence of complications is predicted by the NLR, although this index presents a weaker predictive power than other markers.
In a study conducted by Aydınlı et al. [36] involving 1500 patients who underwent heart surgery, a high NLR was found in the group of patients with cardiac complications; these same patients had a statistically significant likelihood of also accompanying pneumonia, pneumothorax, pulmonary embolism, and pleural effusion. Thus, an indirect relationship between a high NLR and pulmonary complications was established. In a study by Giakoumidakis et al. [37] with 145 cardiac surgery patients, a significant relationship was found between high NLR levels and the likelihood of pneumonia and prolonged intubation. Similarly, a meta-analysis reviewing five studies with a total of 3108 patients [7], a study by Wang et al. [27] with 2707 cardiac surgery patients, and a study by Haran et al. [28] all demonstrated a significant relationship between a high NLR and prolonged intubation. Additionally, prolonged intubation and pneumonia data were examined in a study conducted by Lin et al. [38], with 536 patients with acute type A aortic dissection; however, no statistically significant relationship was found with the LMR.
Our study examined rates of pneumonia and prolonged intubation, both under the category of pulmonary complications. Similar to findings in the literature, the NLR predicted complications such as pneumonia and prolonged intubation in patients. The PLR also predicted complications such as pneumonia and prolonged intubation. Consistent with the literature, no significant difference was found for the LMR in relation to pulmonary complications.
When we consider patients undergoing isolated CABG, it is noted that when patients with valve, ascending aorta, or combined surgery are excluded, the relationship between the PLR and prolonged intubation ceases to be significant. The reason for this is that the PLR index is not an independent predictor of prolonged intubation. Meanwhile, when considering pulmonary complications, the NLR has been found to be the most suitable index for clinical use in terms of predictive power.
In the study by Parlar and Şaşkın [39], high preoperative and first-day postoperative NLR and PLR values were found to be more successful in predicting AKI, with the PLR demonstrating better predictive power when compared to other indices. In the study by Aydınlı et al. [36], a similar indirect relationship was established between NLR values and renal complications, similar to the relationship observed with pulmonary complications, while high NLR values were found to be more successful in predicting AKI and the need for RRT. Indeed, the preoperative and postoperative NLR values were investigated in a study by Weedle et al. [34] involving 906 cardiac surgery patients. While no significant relationship was found with the preoperative NLR, high NLR values on the postoperative second day were good predictors of AKI. Meanwhile, a study by Wang et al. [27] with 2707 patients found a statistically significant relationship between a high NLR and the RRT requirement. A study by Chen et al. [23] involving 159 patients with acute type A aortic dissection demonstrated that patients with a high LMR had a lower risk of AKI. A further study by Lin et al. [38] with 536 patients with acute type A aortic dissection found a significant inverse correlation between a high LMR and the likelihood of renal failure.
Our study investigated AKI and the RRT requirement under the category of renal complications. The NLR and PLR predicted postoperative AKI and the RRT requirement in patients. The rates of AKI and the RRT requirement were significantly lower after Bonferroni correction in patients with a LMR
When considering patients undergoing isolated CABG, the RRT requirement ceased to be statistically significant. Meanwhile, upon examining patients requiring RRT, it was observed that the need for RRT in patients undergoing isolated CABG was not significantly different from that in patients who underwent valve or aortic surgery. However, when examining the preoperative characteristics of patients according to the NLR and PLR predictive values, it was found that a high NLR and PLR were significantly associated with preoperative renal insufficiency. It was even noted that half of these patients already had complaints of renal insufficiency. The average age of these patients was calculated to be 73, which was significantly higher than the average of the general population in the study. Although results consistent with the literature were obtained, we believe further commentary is necessary. Moreover, patients should be evaluated separately based on the diagnosis of renal insufficiency; parameters such as preoperative and postoperative creatinine and blood urea nitrogen should be analyzed statistically. When considering renal complications in general, the NLR was found to be the most suitable index for clinical use in terms of predictive power, which aligns with our study.
In both studies conducted by Haran et al. [28] and Aydınlı et al. [36], a statistically significant relationship was established between a high NLR and the likelihood of new neurological events. In another study by Şaşkin et al. [40], which included 777 CABG patients, preoperative PLR, postoperative PLR, and NLR were associated with delirium; meanwhile, no significant statistical relationship was found between preoperative NLR and delirium. In contrast to the literature, a study by Zhao et al. [24] with 75 individuals identified a direct correlation between a high LMR change and the likelihood of postoperative cognitive changes. The researchers explained this by stating that the perioperative dynamic change in the LMR was calculated, and that patients with higher changes exhibited more complications; therefore, patients with a high LMR, who are more likely to show dynamic changes, need to be carefully monitored [24]. In contrast, Lin et al. [38] did not find a statistically significant relationship between the LMR and CVE. To our knowledge, no study exists in the literature that has investigated the relationship between the PLR and neurological complications after cardiac surgery.
Our study found that the NLR could predict both TIA and CVE in patients. However, no significant relationship was found between the PLR and neurological complications. Considering that an increase in platelet count could enhance the synthesis of adhesion molecules in the vascular endothelium and independently increase the likelihood of thrombotic events [41], the lack of an association between the PLR and neurological complications initially appears unexpected. Nonetheless, we believe that this result can be explained by the fact that all patients underwent surgery under CPB, which is known to cause significant changes in platelet count and function. Additionally, postoperative PLR values were not included in the study. Conversely, the LMR showed a trend toward predicting the one year without TIA and CVE, but this association did not reach statistical significance after Bonferroni correction.When considering neurological complications in general, the NLR was found to be the most suitable index for clinical use in terms of predictive power.
The strengths of our study include a relatively large patient cohort compared to similar studies in the literature, consistency in surgical technique and postoperative care due to the same medical team conducting all procedures, and the longitudinal assessment of complications over 12 months. However, several limitations must be acknowledged. First, the patient cohort was predominantly composed of CABG patients (78.9%), with limited inclusion of valve and aortic surgery cases. This imbalance may confound the generalizability of our findings, particularly for procedures associated with different complication profiles. Second, although we aimed to evaluate a broad range of postoperative complications, the primary endpoint combined heterogeneous events (e.g., mortality, pneumonia, renal failure), which may have masked marker-specific predictive capacities. Additionally, the interpretation of the poor predictive performance of the LMR (ROC
Currently researched systemic inflammatory indices have been evaluated in terms of adult cardiac surgery outcomes and compared against each other. The NLR is superior to other indices in predicting short-term and long-term mortality, as well as MACCEs, pulmonary, renal, and neurological complications. Alternatively, the LMR is superior in predicting the absence of cardiac complications along with survival.
In conclusion, the NLR can serve as an early warning for major complications for the surgical and intensive care team, potentially providing an opportunity to prepare for possible scenarios. The PLR and LMR can be used to support this prediction.
NLR, Neutrophil/Lymphocyte Ratio; PLR, Platelet/Lymphocyte Ratio; LMR, Lymphocyte/Monocyte Ratio; ROC, Receiver Operating Characteristic; AF, Atrial Fibrillation; MI, Myocardial Infarction; CABG, Coronary Artery Bypass Grafting; TIA, Transient Ischemic Attack; CVE, Cerebrovascular Event; AKI, Acute Kidney Injury; RRT, Renal Replacement Therapy; MACCE, Major Adverse Cardiac and Cerebrovascular Events; SPSS, Statistical Package for the Social Sciences; AKIN, Acute Kidney Injury Network.
Data from the study can be provided by the corresponding author upon request.
AOK participated in all aspects of the study, including its conception, design, data collection, analysis, and manuscript writing. AMM and MAY contributed to the study design and provided critical insights throughout the research process. SDO participated in data collection. All authors actively participated in the surgical procedures and contributed to the critical revision of the manuscript for important intellectual content. AOK drafted the manuscript. All authors read and approved the final version of the manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
The study was conducted in accordance with the guidelines of the Declaration of Helsinki and approved by the Istanbul University-Cerrahpasa Non-Interventional Clinical Research Ethics Committee, chaired by Prof. Dr. Aysem Kaya, on June 7, 2023, under approval number 2023/93. Informed consent to participate was obtained from all the participants in the study.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest.
Supplementary material associated with this article can be found, in the online version, at https://doi.org/10.31083/HSF46933.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.


