1 Department of Cardiology, Nanjing First Hospital, Nanjing Medical University, 210006 Nanjing, Jiangsu, China
2 Department of Critical Care Medicine, Nanjing First Hospital, Nanjing Medical University, 210006 Nanjing, Jiangsu, China
3 Department of Thoracic and Cardiovascular Surgery, Nanjing First Hospital, Nanjing Medical University, 210006 Nanjing, Jiangsu, China
4 Department of Anesthesiology, Perioperative and Pain Medicine, Nanjing First Hospital, Nanjing Medical University, 210006 Nanjing, Jiangsu, China
5 Department of Echocardiography, Nanjing First Hospital, Nanjing Medical University, 210006 Nanjing, Jiangsu, China
†These authors contributed equally.
Abstract
To evaluate the neuroprotective efficacy of combining unilateral antegrade selective cerebral perfusion with percentage-controlled flow regulation during aortic arch reconstruction surgery for aortic dissection.
A retrospective analysis was conducted using clinical data from 226 consecutive patients who underwent surgery for acute aortic dissection with arch reconstruction at our hospital between January 2020 and January 2021. Based on the cerebral protection strategy used, patients were divided into two groups: the percentage-flow cerebral perfusion group (n = 89) and the control group (n = 137). The severity of neurological impairment was rigorously evaluated using standardized biomarker assessments, including serial measurements of serum S100β protein and neuron-specific enolase (NSE) levels. These biomarkers were systematically analyzed and compared between the two groups at two critical time points: preoperatively (baseline) and postoperatively during follow-up. Multivariate analysis was subsequently performed to identify independent risk factors associated with postoperative neurological dysfunction following surgical repair.
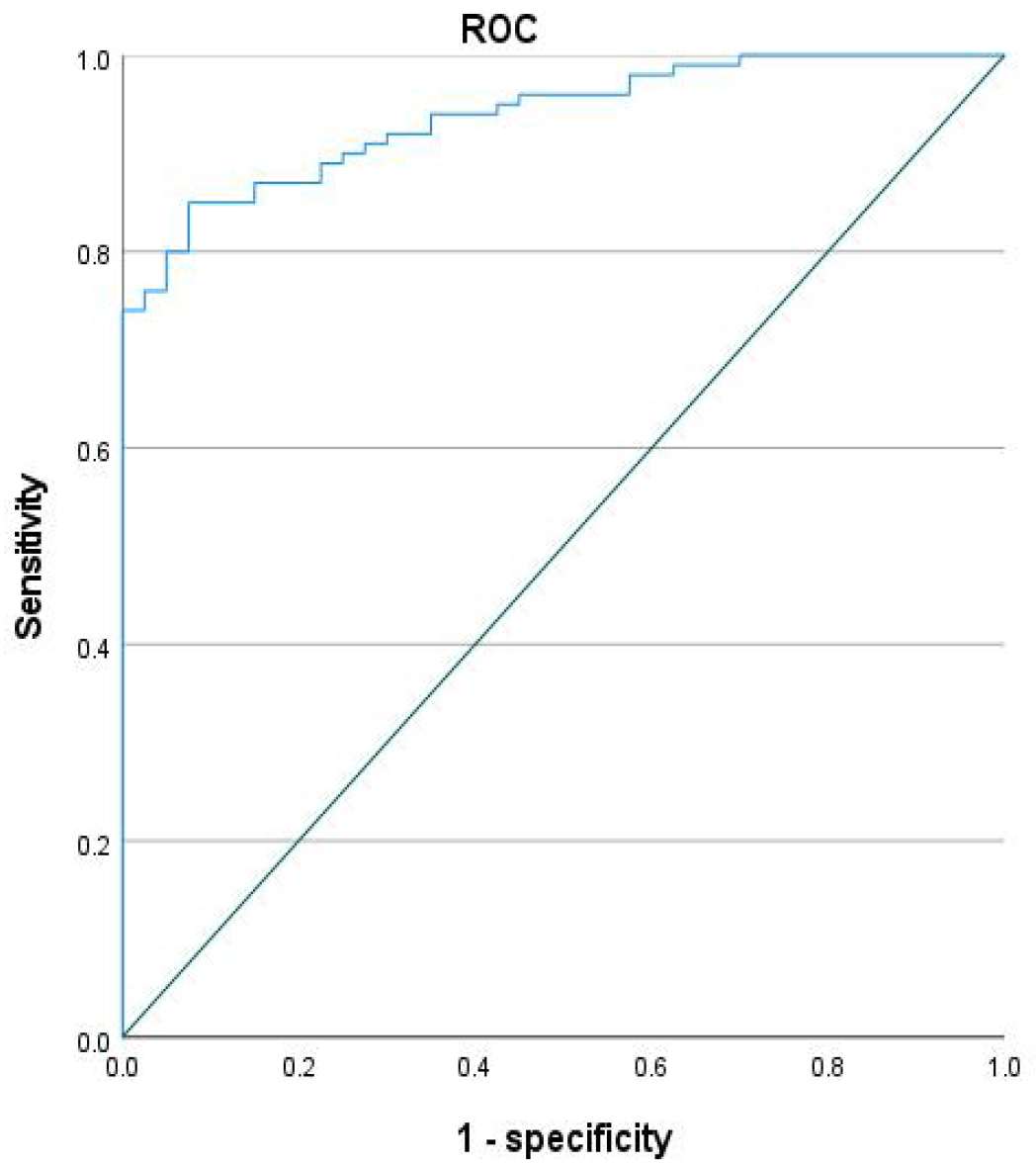
No statistically significant differences were observed in baseline characteristics or intraoperative parameters between the two groups (all p > 0.05). Postoperative mortality was comparable (4.5% vs. 4.4%, p = 0.915). However, the percentage-flow cerebral perfusion group showed a significantly lower incidence of neurological dysfunction—including both temporary and permanent deficits—compared to the conventional control group (8.98% vs. 18.98%, p = 0.031). Additionally, these patients demonstrated significantly shorter times to wakefulness and extubation (both p < 0.05). Serum biomarker analysis further indicated markedly elevated levels of S100β and NSE in the control group relative to the percentage-flow group (both p < 0.05). Univariate and multivariate regression analyses identified age, unilateral cerebral perfusion time, and cardiopulmonary bypass (CPB) time as independent risk factors for postoperative neurological injury. A predictive model incorporating these variables exhibited strong discriminative power (area under the curve, AUC = 0.838) and good stability (p = 0.256).
Optimized cerebral perfusion flow significantly shortens the time to awakening and extubation in patients undergoing acute aortic dissection repair, while reducing neurological injury, as supported by decreased serum levels of the biomarkers S100β and NSE. These results indicate a considerable neuroprotective benefit. Moreover, multivariate analysis confirmed that age, unilateral cerebral perfusion duration, and cardiopulmonary bypass (CPB) time are independent risk factors for postoperative neurological impairment. A predictive model integrating these factors exhibited strong clinical applicability.
Keywords
- acute aortic dissection
- flow percentage
- unilateral antegrade selective cerebral protection
- arch reconstruction
- model prediction
Acute aortic dissection (AAD) is among the most life-threatening emergencies in cardiovascular medicine, with the highest mortality rate of all cardiac surgical conditions [1]. Without immediate surgical intervention, mortality can exceed 90% [2]. Sun’s procedure—which entails replacement of the ascending aorta, total arch reconstruction, and distal stent-graft implantation—has become the preferred surgical approach for AAD owing to its favorable clinical outcomes [3, 4]. Nevertheless, because the procedure requires reconstruction of the cerebral vascular supply, postoperative neurological complications such as anxiety, stroke, and delirium continue to pose significant challenges [5]. Therefore, optimizing cerebral protection strategies has become a crucial research priority for improving perioperative organ preservation in aortic dissection repair. Current clinical practice utilizes various cerebral protection strategies, such as deep hypothermic circulatory arrest, retrograde cerebral perfusion via the superior vena cava, unilateral antegrade selective cerebral perfusion (uSACP), and bilateral antegrade selective cerebral perfusion (bSACP) [6]. Although our previous studies have confirmed the neuroprotective benefits of uSACP combined with moderate hypothermia in acute aortic dissection surgery, no standardized perfusion protocol has been widely established. Notably, significant inter-institutional variation remains in flow management strategies—with rates typically ranging from 5 to 15 mL/kg—and the assumed linear relationship between cerebral blood flow and patient body weight becomes unreliable beyond certain physiological thresholds. These observations highlight the importance of implementing goal-directed perfusion management to refine cerebral protection strategies [7, 8].
In aortic dissection surgery, cerebral oxygen saturation serves as an indicator of cerebral metabolic status. However, its clinical utility for guiding cerebral perfusion is limited by significant measurement variability, which hinders accurate quantification of optimal perfusion flow. Similarly, pressure-based flow selection strategies are constrained by the inherent inaccuracies of current pressure monitoring technologies, compromising their reliability in ensuring adequate neurological perfusion. In contrast, percentage-based flow management enables personalized perfusion strategies that may offer superior neuroprotection. Building upon this theoretical framework, the present study investigates percentage-flow guided unilateral antegrade selective cerebral perfusion (uSACP) in acute aortic dissection surgery, with the objective of establishing optimized parameters for neurological protection and functional outcomes.
A retrospective analysis was conducted on the clinical data of 226 patients who underwent acute aortic dissection and concomitant arch reconstruction surgery in our cardiovascular surgery department from January 2020 to January 2021. Based on the cerebral protection strategies used, the patients were divided into a flow percentage cerebral perfusion group and a traditional group. Inclusion criteria: ① diagnosis of acute aortic dissection confirmed by arterial Coronary Computed Tomography Angiography(CTA); ② absence of involvement of the innominate artery, coronary arteries, valvular heart disease, or left common carotid artery; ③ age over 20 years; and ④ performance of arch reconstruction during surgery. Exclusion criteria: ① mortality within 24 hours post-surgery; ② preexisting hepatic or renal dysfunction; ③ preoperative neurological impairment; and ④ history of neurological disorders. All patients were informed and consented to this study, which was approved by Nanjing First Hospital’s ethics committee (Ethics Approval No. KY20220805).
All surgical procedures were performed by a dedicated vascular surgery team at our center following standardized protocols. Upon arrival in the operating room, patients received routine intravenous access along with simultaneous arterial pressure monitoring via cannulation of the left radial and dorsalis pedis arteries. Cardiopulmonary bypass (CPB) was established using a Sorin S5 heart-lung machine (Sorin Group Deutschland GmbH, Germany), an adult membrane oxygenator (Sorin Group, Saluggia, Italy), myocardial protection perfusion tubing (Weigao, Shandong, China), and extracorporeal circulation tubing (Tianjin Plastic Research Institute, Tianjin, China). The circuit was primed with a solution consisting of 1000 mL succinylated gelatin injection (Nanjing Chia Tai Tianqing Pharmaceutical Co., Ltd., Nanjing, China), 500 mL Ringer’s acetate (Shijiazhuang Fourth Pharmaceutical Co., Ltd., Shijiazhuang, China), 150g of 20% human albumin (Wuhan Zhongyuan Ruide Biological Products Co., Ltd., HuBei, China), 500 mg methylprednisolone (Pfizer Inc, Liaoning, China), and 10 mg torasemide (Yangtze River Pharmaceutical Group, Jiangsu, China). Surgical cannulation was performed via the right axillary artery, with two-stage venous drainage through the right atrium. Myocardial protection was achieved using Del Nido crystalloid cardioplegia administered at a 4:1 crystalloid-to-blood ratio. The dosage was 20 mL/kg for patients weighing less than 50 kg and 1000 mL for those weighing 50 kg or more, supplemented by 500 mL of root perfusion. Arch reconstruction was initiated when the nasopharyngeal temperature reached 25 °C and bladder temperature approximated 28 °C.
After the repair, rewarming was commenced at a rate not exceeding 0.5 °C/min, with a water–nasopharyngeal temperature gradient maintained below 6 °C. This process began only after achieving mixed venous oxygen saturation (SvO2) above 70%, completing the left common carotid artery anastomosis, and repaying the oxygen debt. During rewarming, 500 mg of methylprednisolone was administered, and 250 mL of 20% mannitol was infused when the patient’s temperature reached 32 °C.
Subsequent vascular reconstructions included proximal anastomosis of the ascending aorta, followed by repair of the left subclavian and innominate arteries. Cardiopulmonary bypass was discontinued after the core temperature exceeded 35 °C and adequate cardiac function was confirmed by transesophageal echocardiography. Throughout the procedure, mean arterial pressure was maintained between 60–80 mmHg. Blood gas management followed alpha-stat principles, switching to pH-stat during deep hypothermic circulatory arrest, and then returning to alpha-stat after rewarming, with PaCO2 maintained between 35–45 mmHg.
In this study cohort, all patients received intraoperative oxygen delivery (DO2)
management according to the following protocol: moderate hypothermia combined
with unilateral selective cerebral perfusion was utilized. Throughout the cooling
and rewarming phases, DO2 was maintained above 280 mL/(m2
For the percentage-flow cerebral perfusion group, the initial unilateral perfusion flow was set at 15% of the pre-deep hypothermic circulatory arrest (DHCA) average flow rate. The pre-DHCA average flow was calculated by multiplying each recorded flow value during the cardiopulmonary bypass (CPB) period prior to DHCA by its corresponding time interval, summing these products, and dividing by the total duration. This 15% threshold was selected based on the physiological assumption that cerebral blood flow comprises approximately 15–20% of total cardiac output.
In the conventional group, perfusion was managed according to body surface
area–based flow targets (1.8–2.8 L/m2
The incidence of neurological injury was compared between the groups and
classified as either temporary or permanent dysfunction. Temporary neurological
dysfunction (TND) included delayed awakening, anxiety, agitation, and delirium.
Delirium was assessed at least twice daily using the Confusion Assessment Method
for the ICU (CAM-ICU). Anxiety and irritability were evaluated with the Hospital
Anxiety and Depression Scale (HADS) and the Richmond Agitation-Sedation Scale
(RASS), respectively. Permanent neurological dysfunction (PND) encompassed
stroke, coma, and epilepsy. The diagnosis of epilepsy adhered to the
International League Against Epilepsy (ILAE) criteria and was confirmed in all
cases by routine electroencephalography (EEG), with independent review by two
neurologists. The incidence rate of neurological dysfunction was calculated as:
(number of cases with neurological dysfunction / total number of cases)
Serum levels of neurological injury biomarkers—S100
Statistical analyses were conducted using SPSS version 22.0 (IBM Corp., Armonk,
NY, USA). Continuous variables are expressed as mean
The two groups exhibited similar baseline characteristics, with no statistically
significant differences in preoperative demographics, intraoperative
parameters—such as perfusion time, aortic cross-clamp time, and cerebral
perfusion duration—or the incidence of perioperative complications (all
p
| Variables | Flow percentage cerebral perfusion group (n = 89) | Control group (n = 137) | p value | |
| Gender Male/Female (n) | 67/22 | 93/44 | 1.093 | 0.296 |
| Age (years) | 59.8 |
61.3 |
1.811 | 0.071 |
| BMI (kg/m2) | 26.8 |
27.3 |
1.973 | 0.051 |
| Hypertension (n) | 78 | 121 | 0.141 | 0.707 |
| Diabetes (n) | 6 | 11 | 0.010 | 0.920 |
| Hyperlipidemia (n) | 26 | 51 | 1.206 | 0.272 |
| Left ventricular ejection fraction (%) | 56.7 |
57.0 |
0.432 | 0.666 |
| Death (n) | 4 | 6 | 0.011 | 0.915 |
| Length of stay in ICU (d) | 1.2 |
1.3 |
1.356 | 0.176 |
| Acute renal failure (n) | 7 | 13 | 0.032 | 0.857 |
| Number of CRRT cases beside the bed (n) | 2 | 4 | 0.014 | 0.908 |
| Number of re-thoracotomies (n) | 1 | 2 | 0.144 | 0.705 |
| Mechanical assisted cardiac function (IABP/ECMO) (n) | 3 | 5 | 0.066 | 0.797 |
BMI, Body Mass Index; Mechanical support includes intra-aortic balloon pumping (IABP) and extracorporeal membrane oxygenation (ECMO).
No statistically significant differences (all p
| Variables | Flow percentage cerebral perfusion group (n = 89) | Control group (n = 137) | t value | p value |
| Cerebral perfusion minimum temperature | ||||
| Minimum Nasopharyngeal temperature (°C) | 24.6 |
24.8 |
1.846 | 0.066 |
| Minimum Bladder temperature (°C) | 27.6 |
27.7 |
1.469 | 0.143 |
| Cerebral perfusion flow (L/min) | 0.62 |
0.56 |
1.661 | 0.098 |
| Unilateral brain perfusion time (min) | 22.5 |
22.9 |
0.589 | 0.556 |
| Minimum intraoperative Hb (g/L) | 88.1 |
86.9 |
0.677 | 0.499 |
| Minimum intraoperative DO2 (L/m2·min) | 289.8 |
287.3 |
1.571 | 0.071 |
| CPB time | 156.7 |
158.4 |
0.525 | 0.600 |
| Aortic cross-clamp time | 89.6 |
88.4 |
0.747 | 0.456 |
Hb, Hemoglobin; DO2, Oxygen transport index; CPB, cardiopulmonary bypass.
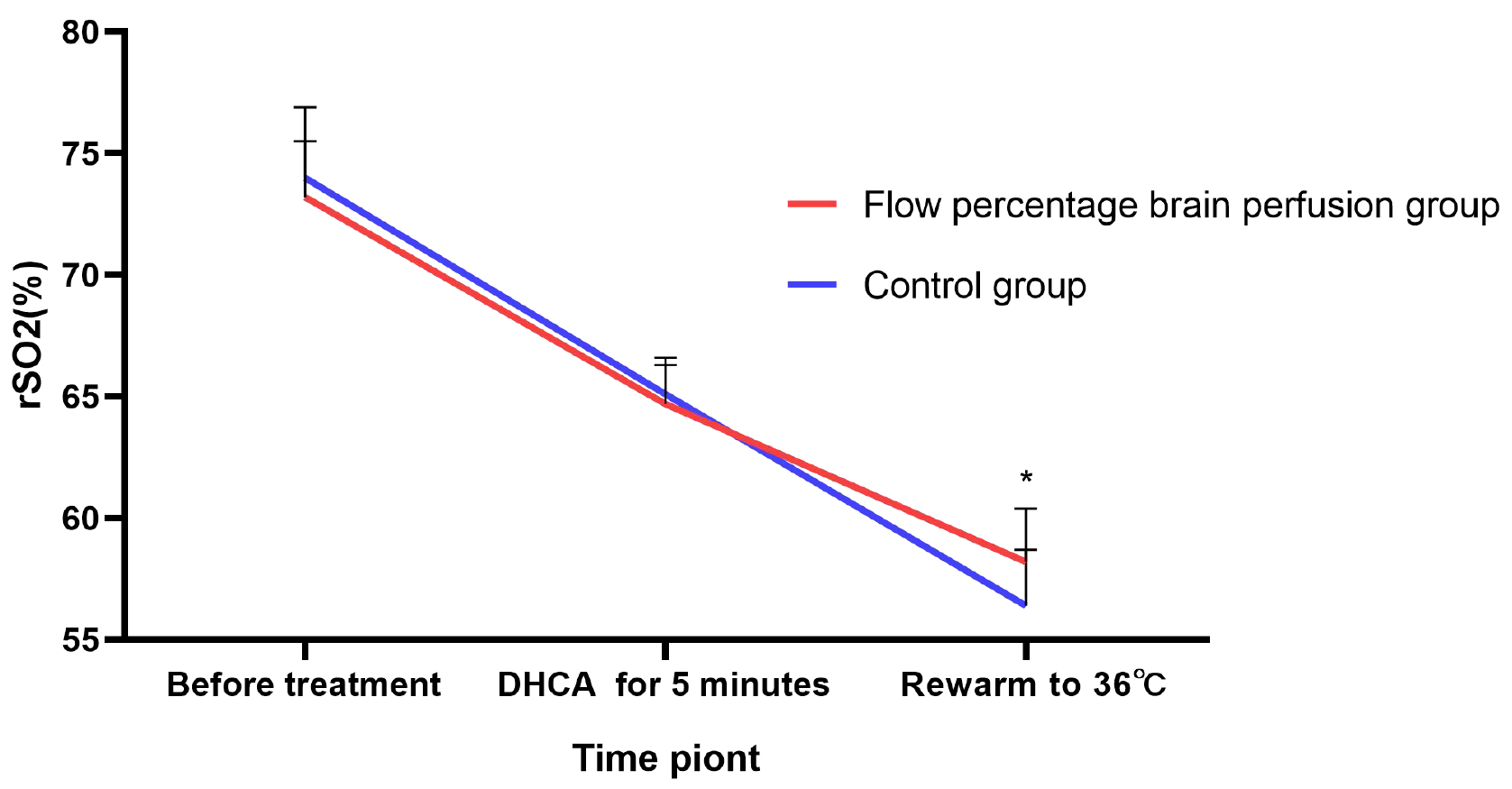
The results indicated no statistically significant differences in regional
cerebral oxygen saturation (rSO2) between the two groups either preoperatively or
at 5 minutes after the initiation of deep hypothermic circulatory arrest (all
p
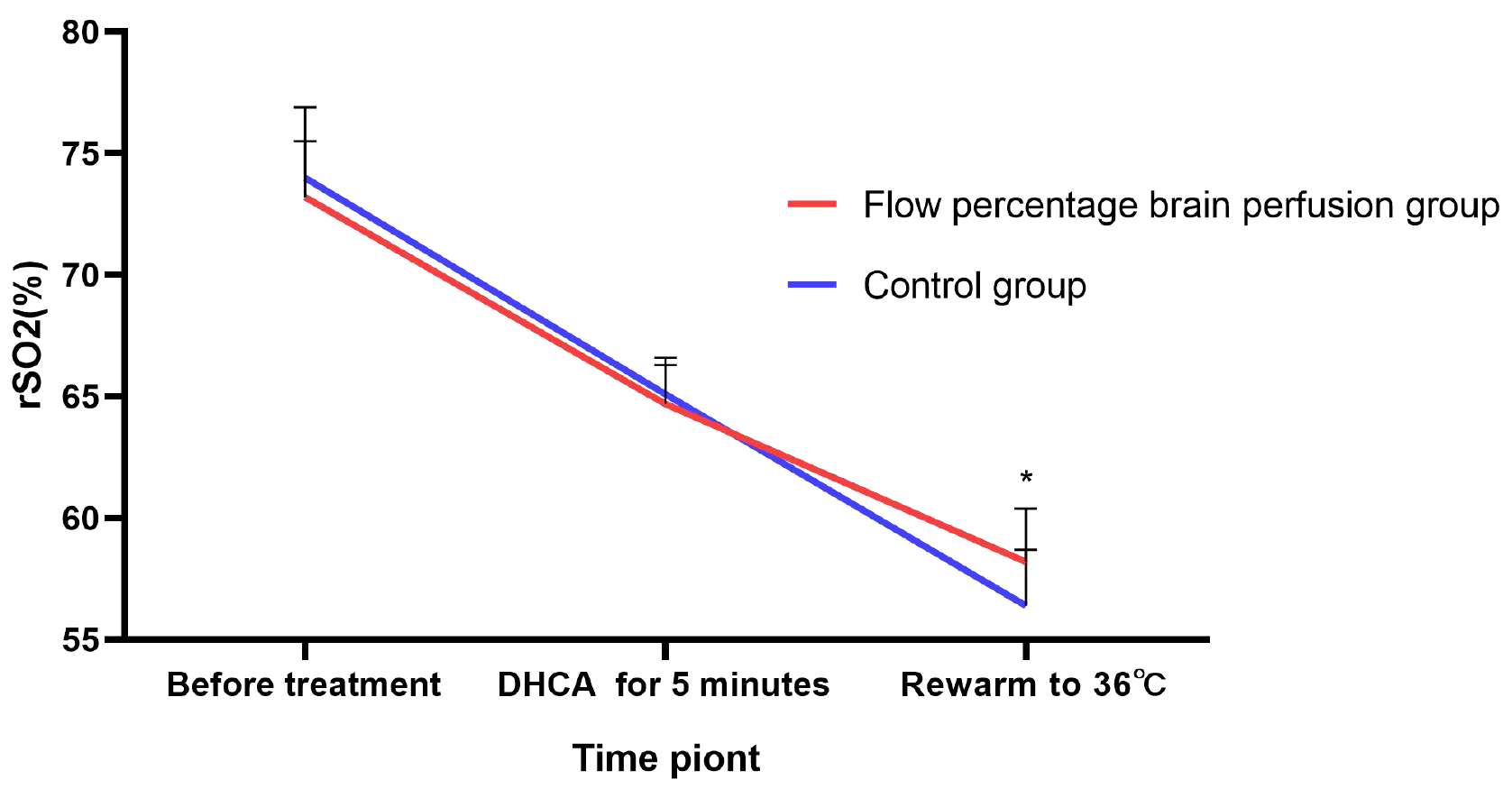 Fig. 1.
Fig. 1.
Comparison of regional cerebral oxygen saturation (rSO2)
levels at different time points during the surgery between the two groups of
patients. *: p
The percentage-flow cerebral perfusion group showed superior clinical outcomes
relative to the conventional management group, with statistically significant
reductions in the incidence of both temporary and permanent neurological
dysfunction, as well as shorter median wake-up times and expedited extubation
durations (all p
| Group | Awake time (h) | Extubation time (h) | TND components | PND components | Total | |||||
| Awakening Delay | Anxiety | Irritable | Delirium | Stroke | Coma | Epilepsy | ||||
| Flow percentage cerebral perfusion group (n = 89) | 8.77 |
12.6 |
2 | 2 | 2 | 2 | 0 | 1 | 1 | 10 |
| Control group (n = 137) | 9.89 |
14.9 |
4 | 8 | 7 | 7 | 1 | 1 | 2 | 30 |
| 4.510 | 5.780 | 4.210 | 4.210 | |||||||
| p value | 0.040 | 0.040 | ||||||||
TND, Temporary nervous dysfunction; PND, Permanent nervous dysfunction.
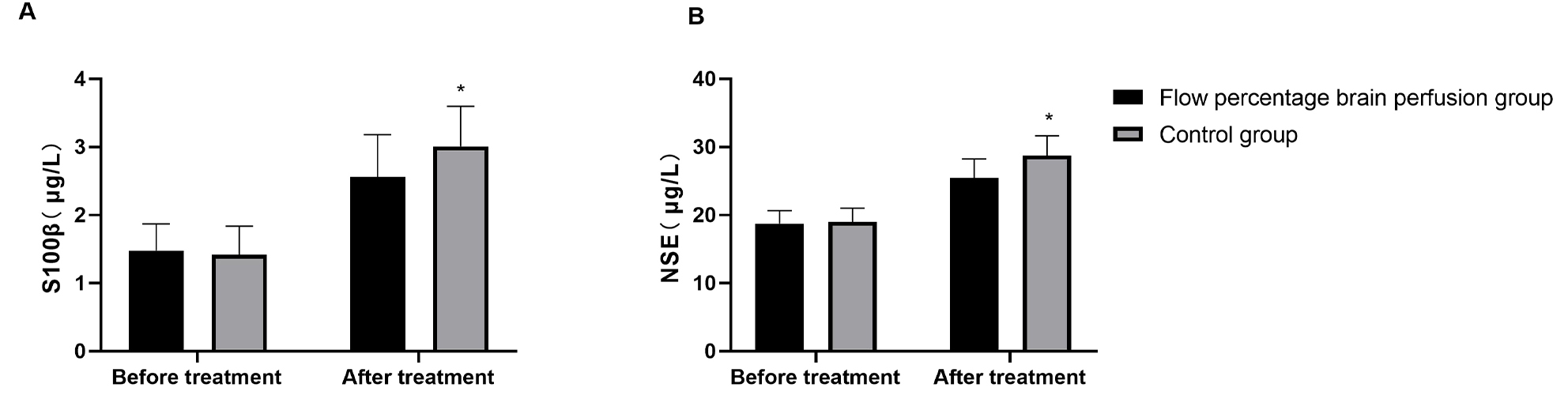
Preoperative serum levels of S100
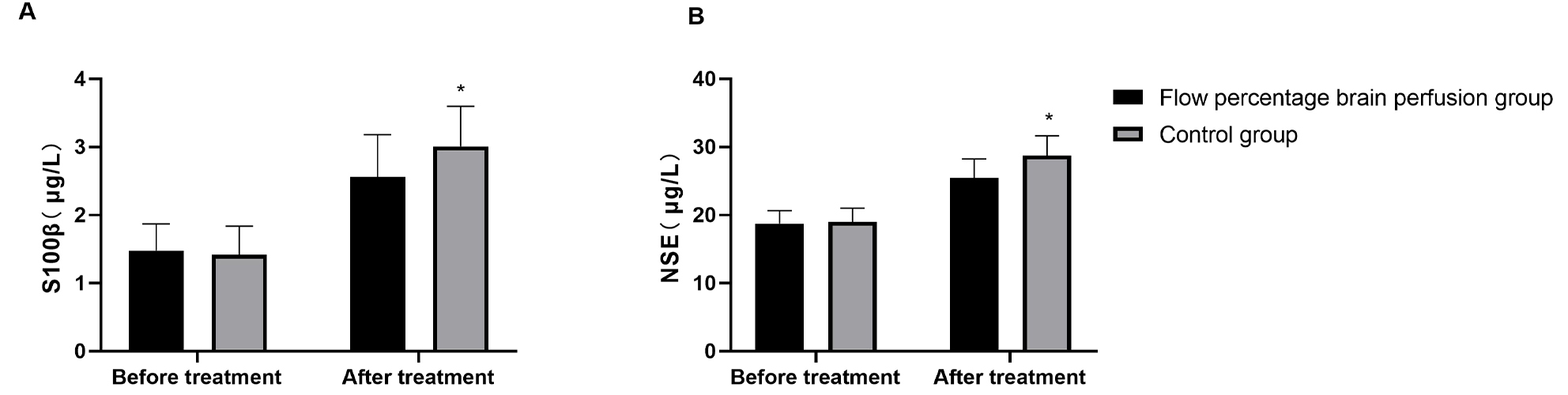 Fig. 2.
Fig. 2.
Markers of neurological injury in both groups of patients. (A) The expression levels of serum S100β in the two groups of patients.
(B) The expression levels of serum NSE in the two groups of patients. *:
p
Our analysis identified several significant predictors of postoperative
neurological dysfunction after acute aortic dissection surgery. Univariate
analysis indicated that patients who developed neurological complications were
significantly older, had a higher prevalence of diabetes, and exhibited longer
cardiopulmonary bypass (CPB) times, aortic cross-clamp durations, and unilateral
antegrade cerebral perfusion periods compared to those without neurological
impairment (all p
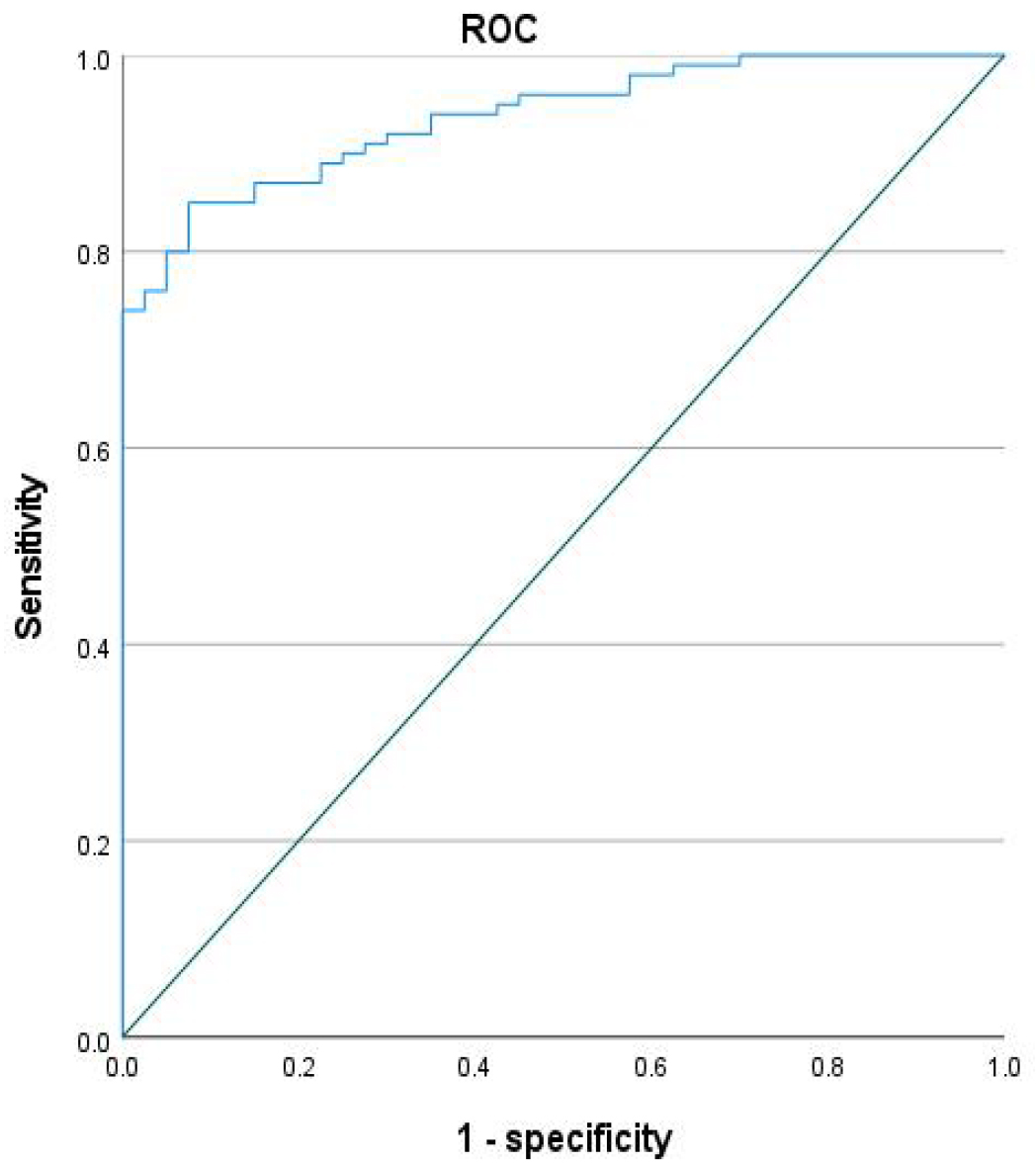 Fig. 3.
Fig. 3.
Area under the curve (AUC) curve of the model for neurological function impairment in acute aortic surgery.
| Variable | Neurological dysfunction(+) | Neurological dysfunction(–) | p value | |
| (n = 40) | (n = 186) | |||
| Gender Male/Female (n) | 29/11 | 131/55 | 1.093 | 0.296 |
| Age (years) | 69.8 |
70.3 |
0.503 | 0.615 |
| BMI (kg/m2) | 27.1 |
27.8 |
1.973 | 0.051 |
| Hypertension (n) | 31 | 168 | 0.141 | 0.707 |
| Diabetes (n) | 6 | 11 | 3.907 | 0.048 |
| Hyperlipidemia (n) | 17 | 60 | 1.206 | 0.272 |
| Left ventricular ejection fraction (%) | 56.8 |
57.3 |
0.432 | 0.666 |
| Cerebral perfusion flow (L/min) | 0.54 |
0.63 |
2.504 | 0.013 |
| Flow percentage method | 10 | 77 | 4.210 | 0.040 |
| Weight Estimation Algorithm | 30 | 109 | ||
| Unilateral cerebral perfusion time (min) | 25.5 |
22.0 |
3.977 | |
| Minimum intraoperative Hb (g/L) | 85.1 |
86.9 |
1.340 | 0.182 |
| Minimum intraoperative DO2 (L/m2·min) | 290.8 |
289.3 |
1.366 | 0.133 |
| CPB Time | 166.7 |
156.4 |
4.5892 |
| Variable | p | OR | 95% CI |
| Per 1-year increase for age | 0.013 | 2.685 | 1.104~4.053 |
| Per 10-minute increase for uSACP time | 0.001 | 4.765 | 2.474~8.718 |
| Per 10-minute increase CPB time | 0.027 | 3.087 | 1.604~7.393 |
| Cerebral perfusion flow method | 0.039 | 2.014 | 1.336~3.550 |
uSACP, unilateral antegrade selective cerebral perfusion.
Preserving neurological function is a critical priority in aortic arch reconstruction for acute dissection repair [9]. This complex surgical procedure differs fundamentally from routine cardiac operations due to its requirements for prolonged cardiopulmonary bypass, systemic hypothermia, deep hypothermic circulatory arrest of the lower body, and intricate arch reconstruction—all of which substantially disrupt cerebral metabolic homeostasis [10]. Contemporary cerebral protection strategies during arch reconstruction have yielded satisfactory clinical outcomes. Advances in understanding cerebral injury mechanisms and continuous refinement of surgical techniques have led to a consensus favoring progressively higher circulatory arrest temperatures, marking a transition from deep to moderate hypothermia. Substantial evidence indicates that unilateral selective cerebral perfusion combined with moderate hypothermia (24–28 °C) not only improves overall surgical efficacy but also reduces the incidence of stroke [11]. Our data show that cardiopulmonary bypass (CPB) times in both cohorts were only slightly longer than those observed in complex valve procedures, with an overall perioperative mortality rate of 4.6%—consistent with contemporary published outcomes [12]. These results affirm that technical advancements and optimized thermal management can effectively shorten CPB duration without compromising procedural safety. Nevertheless, current evidence remains largely based on single-center experiences, and the lack of standardized cerebral perfusion protocols continues to raise concerns regarding both safety and therapeutic efficacy.
In this study, we implemented goal-directed perfusion management to optimize tissue oxygenation during surgical intervention. Current evidence indicates that this perfusion strategy enhances neuroprotection in aortic dissection repair [13]. Nevertheless, significant controversy remains regarding optimal cerebral perfusion protocols. Conventional flow rate determination typically relies on patient weight and perfusion pressure parameters, supplemented by intraoperative cerebral oxygen saturation monitoring. This approach has several limitations: (1) cerebral oximetry readings exhibit considerable variability due to anesthetic depth, hypothermic conditions, and individual physiological differences; (2) the inherent latency of saturation monitoring impedes real-time flow precision; and (3) these limitations collectively increase the risk of both cerebral hyperperfusion and hypoperfusion, potentially exacerbating neurological injury.Physiological studies suggest that cerebral blood flow normally accounts for 15–20% of total cardiac output [14]. When normalized to brain tissue mass—approximately 2% of total body weight—percentage-based flow calculation may better reflect actual cerebral perfusion demands. Therefore, our study aimed to evaluate the neuroprotective efficacy of precision-adjusted percentage-flow cerebral perfusion, with the ultimate goal of establishing an optimized brain protection protocol for aortic arch surgery [15].
Guided by existing evidence on goal-directed perfusion in cardiac surgery, we
maintained an intraoperative oxygen delivery (DO2) target of 280
mL/m2
This study further evaluated the expression levels of brain injury biomarkers
under different perfusion strategies. The results demonstrated that postoperative
serum levels of S100
Building upon previous studies examining risk factors for neurological impairment after acute aortic dissection surgery, we analyzed data from the present patient cohort. The analysis identified age, unilateral cerebral perfusion time, and cardiopulmonary bypass (CPB) duration as significant contributors to postoperative neurological injury. Advanced age is associated with a progressive decline in physiological reserve, particularly affecting the cardiovascular and nervous systems. In elderly patients, preexisting cerebral arteriosclerosis may reduce baseline cerebral blood flow, increasing susceptibility to ischemic injury during surgery. Furthermore, the aging brain exhibits reduced tolerance to ischemia, rendering it more vulnerable to irreversible neuronal damage under conditions of inadequate perfusion. Unilateral cerebral perfusion—while useful for reducing overall cerebral perfusion pressure in selected scenarios—carries an inherent risk of hypoperfusion in the non-perfused hemisphere. Prolonged unilateral perfusion may exacerbate oxygen and nutrient deficiency in contralateral brain regions, potentially leading to neurological complications. Additionally, extended CPB time represents a major risk factor for neurological injury. Prolonged bypass can induce systemic hypothermia, acid-base imbalance, and coagulation disorders, all of which may adversely affect nervous system integrity. These findings align with previously published reports [22, 23, 24].
This study has several limitations. As a single-center, retrospective, nested case-control investigation, it is inherently susceptible to certain biases. Moreover, in patients with higher baseline hemoglobin levels, conventional perfusion management may already provide adequate oxygen delivery, potentially diminishing the demonstrated superiority of percentage-flow cerebral perfusion. Variability in blood product transfusion protocols across centers may also affect the comparative outcomes between perfusion strategies. Other potential confounding factors include differences in surgical team experience, anesthesia management, and postoperative care, all of which could influence neurological and operational outcomes. Additionally, the use of near-infrared spectroscopy (NIRS) for perfusion guidance has inherent limitations, such as inter-individual variability and susceptibility to interference from extracranial tissues, which may affect the accuracy of cerebral oxygenation assessment. Extending the follow-up period to validate the long-term neurological outcomes and further corroborate the conclusions of this study remains an important objective for future research.
In summary, the percentage-flow cerebral perfusion strategy in acute aortic dissection surgery enhances intraoperative neuroprotection, reduces the incidence of neurological injury, and shortens both mechanical ventilation duration and time to postoperative awakening. This approach can be recommended for clinical application. Furthermore, this study identified age, unilateral cerebral perfusion time, and cardiopulmonary bypass duration as significant influencing factors for postoperative neurological injury. The predictive model incorporating these variables demonstrated favourable efficacy, supporting its potential utility in risk stratification and perioperative management.
The implementation of percentage-flow-based cerebral perfusion management in acute aortic dissection surgery significantly enhances intraoperative cerebral protection. This strategy not only shortens the duration of postoperative mechanical ventilation and facilitates faster recovery from anesthesia but also effectively reduces the incidence of neurological complications. Furthermore, our results indicate that neurological injury is associated with patient age and the duration of cerebral perfusion during cardiopulmonary bypass. The developed predictive model demonstrates strong clinical utility, providing valuable guidance for optimizing cerebral perfusion strategies in this high-risk surgical population.
The data that support the findings of this study are available from the corresponding author, Wan, upon reasonable request.
YHY: Conceptualization, Methodology, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. JCS: Data curation, Software, Validation, Visualization, Writing – review & editing. LW: Conception, Design, Resources, Supervision, Project administration, Funding acquisition. ZHW: Acquiring the patient data, Interpreting the imaging results. LLW: Investigation, Resources, Data curation, Writing – original draft. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by Nanjing First Hospital’s ethics committee (Ethics Approval No. KY20220805). All patients/participants or their families/legal guardians gave their written informed consent before they participated in the study.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.