1 Department of Surgery, Division of Thoracic and Cardiovascular Surgery, University of Virginia School of Medicine, Charlottesville, VA 22908, USA
‡Deceased.
Abstract
The intra-aortic balloon pump (IABP) is a relatively economical device for providing temporary cardiac support in cases of cardiac dysfunction or of ongoing cardiac ischemia.
This brief review describes the techniques and strategies for using the IABP to help trainees, nurses, intensivists, and other practitioners who may lack familiarity with it and need to assist in managing patients who have had one of these devices inserted in a catheterization lab or operating room.
These devices have proven useful in supporting cardiac patients and can be managed by all who care for these patients.
Keywords
- cardiac dysfunction
- mechanical circulatory support
- economical cardiac support device
Everything should be made as simple as possible, but not simpler.
—Albert Einstein
The intra-aortic balloon pump (IABP) is a relatively simple and economical device that temporarily supports cardiac function in the setting of myocardial ischemia or impaired cardiac function [1]. The development of the IABP is generally attributed to Dr. Adrian Kantrovitz and his surgical team at the Maimonides Hospital in Brooklyn, New York, in the 1960s. The first report of the use of the IABP was published in 1968 [2, 3]. The advantages of the IABP include the relative lack of need for anticoagulation in the short term, as well as the usefulness of these devices in ‘under-resourced’ environments, where ventricular assist devices or extracorporeal membrane oxygenation (ECMO) may not be available or affordable. All cardiac surgeons, as well as cardiologists, should be familiar with using the IABP device. Although cardiologists do not infrequently place these pumps in catheterization laboratories to facilitate a transfer to a center that can provide a higher level of care, this overview will focus primarily on the use of the IABP during and after cardiac surgical operations, with the target audience being team members with limited experience with these devices.
Every cardiac surgical trainee should have a good understanding of the concept of coronary artery perfusion pressure. Obviously, most coronary artery perfusion happens during diastole. Therefore, the coronary perfusion pressure is approximately the gradient between the pressure in the aortic root during diastole and the pressure in the right atrium, where the coronary sinus empties, as defined by the right atrial or central venous pressure. As a general rule, a coronary perfusion pressure of less than 25 mmHg is an unsustainable physiological state in most patients, unless this situation is addressed [4].
In the early days of cardiac surgery, some surgeons placed IABPs prophylactically before performing cardiac surgical operations. This practice was common for a time at some of the early pre-eminent cardiac surgery programs. Recently, some surgeons have used a prophylactic IABP for patients undergoing cardiac surgery who are known to have tight left main coronary artery stenosis or severe aortic stenosis, with the hope of enhancing coronary perfusion pressure during the initial phases of those operations. However, few surgeons currently place IABPs prophylactically [5, 6].
Counterpulsation with an IABP can also be used to stabilize a patient with unstable angina or complications of myocardial infarction, such as a ventricular septal defect (VSD) induced by a myocardial infarction or acute mitral regurgitation caused by a papillary muscle rupture. Under these conditions, an IABP is often placed in a cardiac catheter laboratory by cardiologists as a part of a cardiac catheterization procedure. However, the most common reason that cardiac surgeons will utilize an IABP is to facilitate separation from cardiopulmonary bypass when the heart is ‘struggling’ following a cardiac surgical operation.
Recently, other forms of temporary circulatory support have gained wider use, particularly ECMO [7]. However, ECMO requires that the patient be fully anticoagulated; meanwhile, full anticoagulation can sometimes be delayed transiently if the patient has a significant coagulopathy, as may be the case after a complex or prolonged cardiac operation. Furthermore, the IABP remains a valuable option for temporary circulatory support in ‘under-resourced’ environments where ECMO and other circulatory support devices are less likely to be available. These IABPs are also occasionally used in centers with cardiac catheterization labs, but without cardiac surgical programs, to stabilize patients for transfer to a center where cardiac surgical care is available. Furthermore, despite the intuitive appeal of devices that may seem to provide more complete circulatory support, such as the Impella device, recent reports suggest that the use of the Impella is actually associated with a higher risk of adverse events and mortality compared to the use of an IABP [8, 9, 10, 11].
If there is aortic regurgitation (AR; aortic insufficiency (AI)), which is more than trivial, the balloon pump may create more of a hindrance than a solution, as the IABP can worsen the AI and overload the left ventricle (LV). Dissections (acute or chronic) or aneurysms involving the descending thoracic aorta may not allow the safe placement or effective function of an IABP. Furthermore, diffuse vascular disease, such as calcification in the thoracic aorta or occlusive disease in the infrarenal aorta and the iliac or femoral arteries, is also a relative contraindication for the use of an IABP. Finally, using an IABP in very small adults or children is generally difficult, since their vessels are often too small to accommodate the typical IABP.
In a preoperative note, the entire surgical team should be alerted by email to the possibility that an IABP may be needed postoperatively [12]. The operating room team should ensure that the electrocardiogram (EKG) leads, which are optimal for use with an IABP, are attached to the patient before prepping, as these balloon leads are often different from the standard monitoring leads. The insertion of a femoral arterial line should be considered if the eventual need for an IABP is even a remote possibility, both for accurate blood pressure monitoring and for ease of access should an IABP be needed. All IABP supplies (and the machine) should be in the operating room before the planned operation begins. Not only will this preparation make the eventual use of an IABP at the conclusion of an operation more efficient, but some surgeons will also assert that there may be an additional benefit simply by having the device in the room. The size of the patient must also be considered, so a smaller balloon (30 cc) is available for patients who are small.
Inserting an IABP into a femoral artery will, of course, be more expeditious if there is already a femoral arterial line in place. Therefore, placing a femoral arterial line should be considered at the beginning of a cardiac surgical operation if there is a suspicion that an IABP might be required. Furthermore, a femoral arterial line will almost always provide more reliable pressure measurements than a radial arterial line. If an IABP must be placed while the patient is still undergoing cardiopulmonary bypass (CPB) (when there are no palpable arterial pulses) and if a femoral arterial line has not already been placed, the process of gaining access to the femoral artery should begin by marking the relevant anatomy on the patient with a felt-tipped pen. The spine of the anterior superior iliac crest, the pubic tubercle, and the midline should be marked. Then, the location of the inguinal ligament should be marked, which lies between the iliac spine and the pubic tubercle. The femoral artery will lie approximately halfway between the iliac crest and the midline. A more detailed description of this anatomy can be found in a prior publication [13].
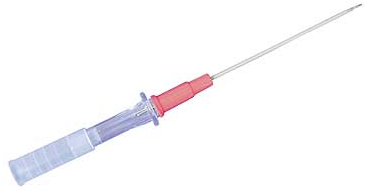
Once the relevant anatomy has been marked, the Seldinger technique should be employed to access the femoral artery, using a long 18-gauge Angiocath, which is similar to the one displayed below (Fig. 1):
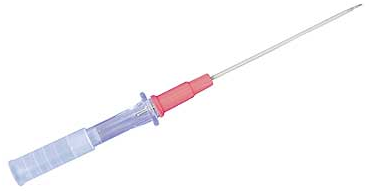 Fig. 1.
Fig. 1.
A long 18-gauge Angiocath.
The plug should be removed from the back of this catheter, which will permit the free flow of blood out of it, once the artery is entered. It is best to enter the skin below the intended site of entry into the common femoral artery, with the trajectory of the needle being about 45 degrees. It is also optimal to avoid compressing the skin over the expected site of entry as the angiocath needle is inserted, as doing so may distort the tissues between the skin and the artery. The catheter should be inserted slowly while watching for a flash of blood from the angiocath. The angiocath should be pushed in a bit further, perhaps even going through the back wall of the artery. The needle is then removed, and the catheter is withdrawn very slowly, watching for pulsatile arterial blood. Once pulsatile arterial blood is observed coming out of the catheter, the catheter should be carefully stabilized, and an assistant should insert a short guide wire through it, while ensuring that this wire advances easily. The angiocath should then be slid a bit further over the wire, which must be held steady during this process, ensuring that the angiocath advances smoothly. The catheter should cease being inserted if any resistance is encountered to the movement of either the wire or the angiocath. Once the decision has been made to proceed with the insertion of an IABP, the long, relatively stiff wire that comes with the IABP can then be passed through the angiocath, ensuring that the wire advances easily. If a transesophageal echo probe is in place, it can be used to confirm the proper positioning of the wire tip near or slightly within the aortic arch [14].
Next, the track from skin to artery should be enlarged slightly so that the IABP can be inserted without a sheath, if possible. This is because inserting the IABP without a sheath significantly reduces the possibility of obstructing flow in the femoral artery, which can lead to leg ischemia. The next step is to nick the skin with a number 11 scalpel blade to facilitate the passing of vascular dilators, ensuring that the skin is not so tightly adherent that it impedes the subsequent passage of a series of these dilators, which are similar to those depicted here (Fig. 2):
 Fig. 2.
Fig. 2.
Vascular dilators.
These dilators typically come in sets of four or five, with each dilator being slightly larger than the previous one. The optimal vascular dilators are those that have no more than a minimal ‘shoulder’, which is an advantage of these dilators. The goal should be to dilate the track to the size of the balloon by comparing each dilator used to the balloon, ensuring that the track is neither under- nor over-dilated. The balloon should be laid out on the patient before the insertion is attempted to determine how far the balloon should be inserted. The tip of the balloon should end up at about the level of the sternal–manubrium junction (also called ‘The Angle of Louis’).
Ensuring that the balloon has been deflated as much as possible before insertion is essential to facilitate the insertion of the device. A large syringe should be used to deflate the balloon. Additionally, air must be flushed from the central lumen of the balloon with heparinized saline at this point. When handling the fully deflated balloon, the balloon should not be allowed to unfurl, even slightly, as this will make the insertion more difficult. At this point, the balloon can start to be inserted over the guide wire, with care taken not to create a kink. The trick to avoiding kinking the wire is to have someone hold it very securely ‘in space’ so that the wire is not pushed in as the balloon is inserted. The wire needs to be a ‘rail’ over which the balloon is inserted. As the balloon is slowly inserted, one can intermittently move the guide wire in and out slightly to ensure that it has not been kinked. However, this technique is secondary to the strategy of holding the wire securely, as described above. If the wire kinks, even slightly, reinsertion of the original catheter into the artery should be attempted, and the kinked wire should be exchanged for a fresh one. However, sometimes this is not feasible, which is disappointing. Nonetheless, if a catheter cannot be reinserted over the wire, performing a small cutdown can still ‘save the day’, since the wire can be used to guide the insertion down onto the femoral artery. Please strive to avoid abandoning the access to the artery once access has been obtained.
After fully inserting the IABP, but before securing it in place with skin sutures, an assistant should turn the balloon inflation device on to ensure the balloon fills. If the balloon does not fill, it may be in too far, perhaps with its tip having entered the left subclavian artery. The position of the device should also be corroborated using the transesophageal echocardiogram (TEE), if possible. The tip of the balloon should ideally be positioned about two centimeters distal to the orifice of the left subclavian artery. When IABP is satisfactorily positioned, it should be secured in several places to the leg with skin sutures. Remember that the IABP may need to be slightly repositioned later, when a chest radiograph (CXR) is taken (usually when the patient has been moved to the intensive care unit (ICU)), so the stabilizing sutures should be placed in a manner that permits them to be easily cut and replaced, if necessary. It is important to remember that the IABP should be ‘paused’ so that it is not inflated when repositioning the device in the aorta.
Occasionally, sites other than the common femoral artery are used to place an IABP. These alternative sites are used for two primary reasons: when significant iliac or femoral vascular disease is present, or when there is hope of permitting the patient to be more mobile after IABP insertion, which may allow them to use a bedside commode or, in some cases, to ambulate to some degree. One alternative insertion site that can be considered is the common iliac artery. The approach used to access this site is the same incision as commonly used for a kidney transplant. When this approach is used, a vascular graft is sutured to the common iliac artery and is then progressed out through the lower abdominal wall or through a groin cutdown incision. This approach has been described in greater detail previously [15, 16].
An IABP can also be inserted into a subclavian artery, which is sometimes conducted percutaneously in a cardiac catheterization laboratory; however, the insertion can also be performed using a cutdown on the subclavian or axillary artery. This insertion site is sometimes chosen to allow the patient to ambulate as the patient either recovers or moves toward a more definitive outcome, such as the placement of a left ventricular assist device or a heart transplant [17].
An IABP can also be inserted through the ascending aorta with the use of a graft that is sutured to the aorta, usually during an operation conducted through a median sternotomy. This approach is occasionally used when femoral or iliac artery access is not feasible for various reasons, such as aortoiliac occlusive disease. If this approach is used, the IABP catheter can be led out of the right second interspace. This strategy will often allow a sternotomy to be closed. The disadvantage of this strategy is that the IABP will traverse the aortic arch, which can increase the risk of cerebral embolization. For this reason, this approach is not frequently used.
As noted earlier, assessing the vascular status of the patient before all cardiac surgical operations is essential, with particular attention being paid to the femoral arteries and potential atherosclerotic disease in the aorta and iliac arteries. The procedure for accessing one of the femoral arteries to insert an IABP percutaneously should include:
The aim is to enter the common femoral artery while avoiding entry into either the iliac or superficial femoral arteries.
An attempt to insert the balloon without a sheath (which ‘takes up space’ inside the artery entered), although this approach is not always feasible.
Frequently checking the position of the balloon tip after its insertion, using daily chest X-rays, as the position of the balloon can change, potentially interfering with the perfusion of the left subclavian or the visceral arteries. This issue is of particular concern after the patient has been moved, as when studies in another part of the hospital are necessary.
Performing frequent exams of the abdomen and legs, checking for palpable pulses, Doppler signals in the leg vessels, skin temperature of the leg, and abdominal signs or symptoms of bowel ischemia.
Heparinizing systemically within a few days after a cardiac surgical operation, or sooner, especially if the patient has not had an operation that required cardiopulmonary bypass, with the usual accompanying coagulopathy.
Considering local heparinization via a side arm on the IABP insertion sheath, especially if a sheath with a side arm was used, and especially if the initiation of systemic heparinization has been delayed for any reason.
If an insertion sheath is in a femoral artery and perfusion of the leg is questionable, pulling the sheath back slightly should be considered to position it just outside the artery, which may allow increased blood flow around the balloon shaft.
Conversely, a limited cutdown could instead be performed by placing a purse string for hemostasis around the entry site of the balloon into the artery, controlling the purse string with a Rummel tourniquet, and then pulling the sheath back so that it is outside the artery, which will usually improve perfusion of the leg [13].
Optimally positioning the balloon in the aorta, once the balloon has been inserted through a femoral artery, can be more challenging than one might expect. The first estimation for positioning involves measuring the balloon and its catheter on the body surface of the patient before their introduction into the artery, as noted earlier. A transesophageal echo probe can be used to fine-tune the position of the tip of the IABP device, once the balloon is near the estimated depth of insertion. The position of the balloon needs to be corroborated with a chest X-ray once the patient has been transported to the ICU. The currently available balloons have radiopaque markers at their tips; however, these balloons can sometimes be frustratingly difficult to visualize on a CXR. One trick to enhance the ability to observe the tip is to request an over-penetrated chest film specifically for the purpose of checking the IABP position. Furthermore, a new CXR should be taken every time the patient is moved, especially if the patient has been transferred away from the ICU, as this may be necessary to obtain various studies. As anyone who transports patients knows, everything protruding from a patient is at risk for getting caught on door knobs! Additionally, being aware of whether the balloon is inserted too far (i.e., with the tip in the left subclavian artery orifice) is essential; the pressure alarm on the device will sound. If the alarm sounds, the problem can usually be resolved by pulling the balloon back slightly. Postoperative transesophageal echocardiography can also be useful if there is any doubt about the position of the balloon tip.
The balloon inflation tubing from the IABP machine must be connected to the balloon after the device has been inserted and properly positioned. The arterial line sidearm of the device must be aspirated to evacuate any remaining air and to fill the central lumen of the device with blood. Considerable care should be taken when flushing this lumen after insertion, since even a tiny air bubble coming out of the tip of the balloon up near the aortic arch could make its way into the cerebral circulation. Once the lumen has been aspirated, it should be flushed gently with heparinized saline. However, this line should never be used for arterial blood gases, as aspirating blood from it requires subsequent flushing, which, as noted, should be minimized due to the risk of air embolization.
The inflation volume of the balloon can be adjusted, as may be necessary in a small patient. While some clinicians may also lower the inflation volume as part of the eventual process of weaning off the support provided by the balloon, most try to avoid that strategy because of the concern that it might increase the possibility of a clot forming on a partially inflated balloon.
Once the balloon is properly positioned, it must be initially synchronized with the EKG. This timing should be performed with the balloon in the 1:2 mode. The balloon can be set to inflate at the peak of the T wave on the EKG, since systole will certainly be complete at that point. Then, the balloon can be set to deflate just before the beginning of the QRS, since systole cannot occur until depolarization has started. If the intrinsic heart rate is relatively high, the balloon may track the heart rate and rhythm better if the timing is switched from 1:1 to 1:2.
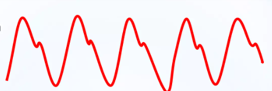
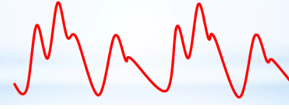
Fine-tuning the timing of the balloon is subsequently based on the arterial trace displayed on the balloon console, which is generated from the central channel of the catheter and, therefore, represents a central aortic pressure trace. This trace is more accurate for this process than the displayed trace of a peripheral arterial line. The balloon should inflate as the aortic valve closes, which is signified by the dicrotic notch on the arterial line pressure trace, as displayed here (Figs. 3,4):
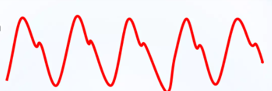 Fig. 3.
Fig. 3.
A typical arterial waveform, without IABP augmentation. Note the dicrotic notch that signifies the closure of the aortic valve after the systolic pressure peaks.
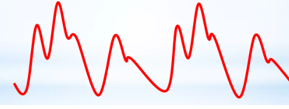 Fig. 4.
Fig. 4.
The typical waveforms in the augmentation created by an IABP, in an ‘every other beat’ pattern, illustrate the balloon inflation after the aortic valve closes, signified by the dicrotic notch.
Ensuring that the balloon deflates before the next systolic ejection of the heart is essential to prevent impairing that ejection and to minimize the resistance against which the heart must work. This second beat after balloon inflation should have a lower systolic pressure than the one before it, and this beat is called an assisted beat because this beat occurs in the setting of the reduced pressure in the aorta that follows balloon deflation. Furthermore, it is worth noting that the enhanced diastolic pressure created by the balloon inflation will increase the perfusion pressure of the coronary arteries.
Occasionally, a previously placed IABP will need to be exchanged, usually because the balloon is not functioning properly or has ruptured. This process is considerably easier if the original device has been placed through a vascular graft attached to the aorta or an iliac artery, allowing for an expeditious exchange [15, 16].
When weaning from balloon support, gradually change the pump settings from 1:1 assistance to 1:2 and then to 1:4. Notably, leaving the balloon on each of these settings for a while before changing the settings again is advised to ensure that the patient can tolerate the weaning process. The best scenario is to have an oximetric pulmonary artery catheter in place, which continuously records cardiac output. With this type of monitoring, one can be assured that the heart can manage despite the diminishing support from the device, as the patient is weaned from the support provided by the balloon.
Moreover, the more time that has elapsed since the initial operation and the more normalized the capacity of the patient for coagulation has likely become, the more thought should be applied to anticoagulating the patient with a heparin infusion, since a clot is more likely to form when the balloon inflates less often, owing to the longer periods of deflation, which cause wrinkles in the deflated balloon, and may allow clot to form. The concept of coronary perfusion pressure should be continually considered when making decisions about the optimal time to proceed with weaning from balloon support, remembering that coronary perfusion occurs almost entirely during diastole.
When the time comes to remove the balloon, plan, gather the necessary equipment, and assemble a team to assist with the process. Pressure will likely need to be maintained at the arterial insertion site for approximately 30 minutes after the device is removed. It is optimal if no one person has to hold pressure for an extended period. Obviously, the most crucial holding time is just after the balloon is removed. The most experienced person available should be in charge of maintaining pressure during that period, while a less experienced person can handle the last shift on site. Sandbags should not have the primary role in assuring hemostasis immediately after removing a balloon, because the pressure needs to be precisely applied to the site of balloon entry into the artery. The concept that the balloon will have entered the femoral artery at some point proximal to where it entered the skin should be explained to everyone who helps with the removal of an IABP. This concept should be obvious to all, but it is quite common to find someone holding the skin exit site rather than the appropriate spot, at least several centimeters proximal to that site, if they have not received proper instruction. A good way to remind everyone on the team of this concept is to mark the spot where pressure should be applied.
An alternative plan, that may be appropriate at times (such as when a patient has a low platelet count or needs to remain fully anticoagulated), will be to perform a limited cutdown from the skin entry site, anterior to the path of the catheter, which will lead down to the entry site of the balloon catheter into the femoral artery. When the surface of the artery is visible and the entry site on the artery is identified, a small purse string can be placed around that site with a 5-0 Prolene suture. Performing a more extensive dissection of the femoral artery cutdown site should be necessary only rarely, if a purse string has been placed and left untied for the purpose of expediting the eventual removal of the IABP. Setting a figure-of-eight stitch is often a good idea to reinforce the purse-string stitch that was initially placed around the catheter [13].
Before removing a balloon, enough drapes should be gathered to prevent the bloody balloon from soiling everything nearby when removed. Moreover, it is worth finding a large trash bag in which the balloon will fit, along with all the sheets and drapes that are likely to become bloody when the balloon is removed. Finding personal protective gear for all who will be nearby is also recommended, including eye protection, since the removal of the balloon can sometimes be “colorful”.
When the balloon is ready to be removed, do not forget to cut off the pump and aspirate all the gas from the balloon, so that it does not enlarge the entry site as it is pulled out. Furthermore, do not forget to cut the stabilizing skin sutures. When pulling the balloon, it is often recommended to avoid pressing on the mark designating the entry site into the femoral artery until the balloon is completely out, so that the femoral artery is not compressed against the balloon as it is being removed, with the thought being that, if there is any clot on the balloon, perhaps that clot will be ejected out of the arterial puncture site, rather than moving down the femoral artery. A similar, but possibly more intuitively appealing idea, is to hold pressure, transiently, distal to the arteriotomy, with the hope being that if debris or clot is being wiped off the balloon as it comes out of the femoral artery, that clot may move into the deep femoral artery (where it would be silent) rather than down the superficial femoral artery where it might lodge in the more distal leg vessels and cause lower extremity ischemia. It is important to remember that, since there may have been some degree of ischemia while the balloon is in place, an eye must be kept on the calf muscles after removing the balloon, to ensure that the potentially increased perfusion following that period of ischemia does not result in a compartment syndrome in the lower leg, which can happen in this setting and may need to be addressed with fasciotomies in the lower leg.
Finally, if the balloon has been placed through a conduit (such as a graft sewn onto a femoral or iliac artery or even the ascending aorta), that conduit can be merely oversewn at its most distal end. It will fill and clot, which is unlikely to cause problems later [15]. If the balloon has been placed through a conduit sewn onto the ascending aorta, one should attempt to remove the device as soon as possible, as there will be an ongoing risk of embolization from the movement of the balloon during pumping cycles while it is in the aortic arch.
No successful therapy can be employed without risk.
—O. H. ‘Bud’ Frazier, MD
The helium in the balloon can embolize into the bloodstream if the balloon ruptures, which can occur if it inflates against a calcified plaque in the aorta. Leaked helium bubbles can embolize and obstruct small vessels downstream, leading to severe consequences. Thus, this possibility must be considered if the IABP device console provides a helium loss alert or if blood is observed in the tubing. The appropriate response to a loss of helium in the system or the appearance of blood in the pump tubing is to stop the pump until the situation can be addressed, typically by replacing the device with a new one.
Everyone should also be aware that these IABP balloons can cause aortic dissections, which is a rare but known complication of their use. Moreover, a patient who has had a balloon in place and who is complaining of abdominal pain, even well after its removal, may have some degree of mesenteric ischemia. The worst-case scenarios include situations where a major visceral vessel is obstructed by the balloon, if the balloon is positioned too low in the aorta, or when a clot related to the balloon embolizes into a visceral vessel, especially the superior mesenteric artery. Thus, a small embolus can make its way into the more distal visceral vessels and cause mesenteric ischemia that is less cataclysmic (and, therefore, harder to diagnose) than would be the case in a more typical presentation of mesenteric ischemia. Specifically, small areas of infarction can occur, or the induced ischemia can cause a partial-thickness injury in the bowel, with the mucosa being more susceptible to ischemia than the outer wall of the bowel. These scenarios can be quite difficult to rectify; however, these scenarios must be suspected in patients being supported with an IABP who develop abdominal pain, ileus, or a systemic inflammatory state after the removal of an IABP or even while it is still in use.
The IABP is a mechanical marvel that can be quite useful at times in the practice of cardiology and cardiac surgery. These devices can also play a crucial role in under-resourced medical environments, where more advanced forms of circulatory support may not be readily available. Therefore, cardiologists, cardiac anesthesiologists, cardiac intensivists, and cardiac surgeons need to be familiar with using these devices.
Not applicable.
CT designed the research study and made editorial contributions to the manuscript. The author has participated sufficiently in the work, read and approved the final manuscript and agreed to be accountable for all aspects of the work.
Not applicable.
Not applicable.
This research received no external funding.
The author declares no conflict of interest. Curt Tribble is serving as one of the Editorial Board members of this journal. We declare that Curt Tribble had no involvement in the peer review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to Giuseppe Santarpino.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.