1 Department of Emergency Medicine, Hangzhou Third People’s Hospital, 310009 Hangzhou, Zhejiang, China
Abstract
Acute myocardial infarction (AMI) remains a global health challenge. This has driven innovation toward precision medicine, including major advances in several key areas. Precision Reperfusion: Intravascular ultrasound (IVUS), optical coherence tomography (OCT) and fractional flow reserve (FFR) can be used to optimize stent deployment, thereby reducing thrombosis and restenosis. Bioabsorbable stents and drug-coated balloons (DCBs) show promise in minimizing long-term complications. Mechanical Circulatory Support (MCS): Early use of Impella and veno-arterial extracorporeal membrane oxygenation (VA-ECMO) has been shown to improve survival in select AMI-cardiogenic shock patients, although device selection and timing require further validation. Antiplatelet Personalization: Genotyping (e.g., CYP2C19) and testing of platelet function enables tailored dual antiplatelet therapy (DAPT), thus balancing ischemic and bleeding risks. Regenerative Therapies: Extracellular vesicles (EVs) from stem cells or cardiac progenitors have shown cardioprotective effects in preclinical models, addressing limitations of cell-based approaches. Artificial intelligence (AI)-driven platforms can optimize EV delivery and tissue repair. AI-Enhanced Diagnostics: Machine learning models improve Electrocardiogram (ECG) interpretation, risk stratification, and the detection of ST-segment elevation myocardial infarction (STEMI). This review aims to provide a theoretical foundation for practical clinical applications in the treatment of AMI.
Keywords
- acute myocardial infarction
- precision medicine
- individualized antiplatelet therapy
- artificial intelligence
- extracellular vesicles
- mechanical circulatory support
Acute myocardial infarction (AMI) continues to be a primary contributor to illness and death across the globe. The incidence of AMI varies geographically, with a higher prevalence in wealthier nations, largely driven by an older demographic and lifestyle-related risk factors such as high blood pressure, diabetes, and obesity. Conversely, in low- and middle-income countries (LMICs), the increasing incidence of AMI is the result of urbanization and shifts in disease patterns. Globally, AMI is responsible for roughly 15% to 20% of all deaths from cardiovascular disease (CVD). While improvements in healthcare have reduced mortality rates in well-resourced areas, LMICs still experience elevated fatality rates due to insufficient access to timely medical intervention. ST-segment elevation myocardial infarction (STEMI) and non-ST-segment elevation myocardial infarction (NSTEMI) show distinct patterns of distribution, with NSTEMI becoming more prevalent in older populations [1]. Despite advances in the management of AMI, significant clinical challenges persist. The availability of diverse treatment options contrasts sharply with the absence of personalized therapies, leading to suboptimal outcomes. This review seeks to outline developments over the past three years in optimizing reperfusion strategies, innovative antiplatelet medications, cardiac regeneration approaches, and artificial intelligence (AI)-driven decision support. The overarching aim is to improve the precision and effectiveness of AMI treatment on a global scale.
Intravascular imaging modalities, including intravascular ultrasound (IVUS), optical coherence tomography (OCT), and fractional flow reserve (FFR), significantly improve the outcomes of percutaneous coronary intervention (PCI) compared to angiography alone, particularly in patients with left main artery disease or complex multi-vessel lesions. These techniques promote optimal stent deployment, minimize dissection of the coronary artery, and reduce the incidence of suboptimal stent apposition. However, direct comparisons of OCT versus IVUS and FFR in terms of efficacy remain inconclusive. Previous studies have highlighted the anatomical advantages of IVUS. For example, the Adaptive Design for Drug Evaluation and Study (ADAPT-DES) study (n = 8583) found that IVUS-guided drug-eluting stent (DES) implantation was associated with reduced rates of stent thrombosis [2]. Additionally, IVUS-guided PCI significantly lowered the rate of target vessel failure at one year relative to angiography-guided PCI [3].
OCT provides high-resolution intracoronary imaging, enabling detailed visualization of atherosclerotic plaques, stent deployment, and procedural complications. Key applications of OCT include identifying vulnerable plaques (e.g., thin-cap fibroatheromas), optimizing PCI, and elucidating mechanisms of acute coronary syndrome (ACS), such as plaque erosion or calcified nodules. Although OCT is superior to angiography for lesion assessment and stent placement compared to IVUS, its utility is still under debate due to concerns about cost, technical expertise, and contrast agent-induced nephrotoxicity. Emerging trends involve the integration of OCT with AI for automated plaque analysis and the exploration of hybrid imaging systems. Despite its limitations, OCT plays a critical role in precision cardiology, especially for complex lesions and for optimizing long-term PCI outcomes [4].
A randomized trial enrolled patients undergoing PCI (n = 2008) and assigned them to either OCT-guided or IVUS-guided groups. The primary endpoint was a composite of cardiac death, target vessel myocardial infarction, or ischemia-driven revascularization after one year. Analysis demonstrated that OCT-guided PCI met the non-inferiority criterion compared to IVUS, with comparable rates of bleeding and contrast agent-induced nephropathy. The OCT group experienced fewer procedural complications and no imaging-related adverse events. OCT-guided PCI was non-inferior to IVUS in reducing ischemic events at one year, while exhibiting equivalent safety, although procedural efficiency favored OCT. Analysis of long-term outcomes and cost-effectiveness requires further investigation [5].
FFR and IVUS represent two pivotal strategies for guiding PCI, and are applicable to patients with moderate coronary artery stenosis (angiographic stenosis of 40%–70%). In the FLAVOUR trial, Asian patients (n = 1682) with moderate coronary artery stenosis were randomly assigned to FFR-guided or IVUS-guided PCI. FFR-guided PCI demonstrated non-inferiority compared to IVUS-guided PCI, achieving comparable clinical outcomes while reducing interventional procedures and bleeding risk. However, its applicability in higher-risk lesions requires further validation [6]. Although IVUS-guided PCI improves outcomes over angiography alone, the incremental value of angiography-based physiological assessment during IVUS-guided procedures remains uncertain. Post hoc analysis of the FLAVOUR trial revealed that angiography-derived physiological characteristics could identify functionally irrelevant lesions, thereby optimizing IVUS-guided PCI by avoiding unnecessary stent implantation in low-risk patients. Therefore, combining anatomical and physiological assessments may enhance the accuracy of lesion selection [7].
In summary, IVUS improves stent placement, optimizes stent deployment and apposition, and detects vulnerable plaques through plaque burden assessment. OCT offers superior resolution imaging and near-micron-level details of plaques, restenosis, and thrombi, thus helping to determine the etiology of ACS and optimizing PCI in ACS. However, caution is advised when using OCT in patients with retinal disease, significant renal impairment, or contrast agent allergy. IVUS is recommended for complex lesions, whereas OCT demonstrates potential in personalized treatment approaches. FFR combined with OCT is mainly used for functional assessment, to guide non-surgical vascular interventions, and to reduce excessive medical treatment.
The potential for future applications and clinical significance of bioabsorbable stents and drug-coated balloons (DCBs) in interventional therapy remains a focal point in cardiovascular research. PCI continues to be the preferred approach for restoring patency in occluded arteries and reestablishing blood flow. However, restenosis remains a critical factor limiting the success of PCI. First-generation drug-eluting stents (DES) significantly reduced restenosis rates, but were associated with late stent thrombosis due to persistent polymers. Second-generation DES improved biocompatibility through thinner struts and biodegradable polymers (e.g., everolimus/zotarolimus), further decreasing the incidence of restenosis. Bioabsorbable stents demonstrated promising short-term outcomes, but failed to achieve consistent long-term efficacy due to the development of neoatherosclerosis. Polymer-free DES addressed inflammation concerns while retaining metallic platforms [8]. The use of DES remains central to managing ACS and PCI. Significant advancements have been made in DES design, including thin-strut stents with compact biocompatible or bioresorbable polymer layers. Ultra-thin strut DES further improves upon this concept, demonstrating superior performance compared to traditional DES in some studies, although challenges such as recoil in complex lesions like chronic total occlusion (CTO) persist. A meta-analysis comparing titanium nitride oxide-coated stents (TiNOS) with DES in 2743 ACS patients across three randomized trials revealed comparable outcomes with respect to Major Adverse Cardiovascular Events (MACE) and target lesion revascularization (TLR). Additionally, TiNOS significantly reduced cardiac death, myocardial infarction (MI), and stent thrombosis rates compared to DES [9].
Intravascular imaging techniques (IVUS, OCT) and FFR are significantly superior to traditional angiography, enabling optimal PCI stent placement and improving the prognosis of patients with complex lesions. IVUS reduces the risk of thrombosis, but has lower resolution. OCT has extremely high resolution, clearly identifying plaques and thrombi, but is costly and not suitable for patients with renal failure. FFR can avoid over-treatment of non-functional significant stenosis, but does not provide anatomical information. Applicable scenarios are that IVUS is suitable for complex lesions, OCT for precise identification of plaques and optimization of stents, and FFR for evaluating moderate stenosis. Combination of the three can achieve synergistic assessment of anatomy and function, but is limited by the increased operational complexity and cost. Future research directions include hybrid imaging that integrates OCT/IVUS, integration of AI for automated analysis, conducting long-term efficacy and cost-benefit studies, and exploring combinations with new technologies such as bioresorbable stents. Therefore, integration rather than selection may be the way forward through combined applications (IVUS/OCT + FFR), technological integration (hybrid imaging systems), and AI-assisted automated analysis. This should allow progress towards safer, more precise, and more efficient interventional treatment for coronary heart disease (Table 1).
| Parameter | Intravascular ultrasound (IVUS) | Optical coherence tomography (OCT) | Fractional flow reserve (FFR) |
| Principle | Ultrasound | Near-infrared light | Pressure-derived index of coronary blood flow |
| Resolution | Intermediate (100–200 µm) | High (10–20 µm) | N/A (Functional assessment only) |
| Penetration depth | Deep (4–8 mm) | Superficial (1–2 mm) | N/A (Functional assessment only) |
| Key strengths | Robust clinical evidence; vessel remodeling; plaque burden assessment | Unparalleled resolution; plaque morphology; stent strut apposition, thrombus visualization | Objective ischemia assessment; avoids unnecessary stenting in non-flow-limiting lesions |
| Primary limitation | Lower resolution; limited detail on superficial microstructure | Limited penetration; requires contrast flush (CI in renal impairment); higher cost | No anatomical guidance; does not inform stent sizing or optimization |
| Ideal clinical scenario | Left main disease; bifurcation lesions; long lesions; stent optimization | Identification of vulnerable plaques (e.g., TCFA); mechanistic insight into ACS; assessment of stent complications | Guidance for revascularization of angiographically intermediate stenosis (40–70%) |
| Impact on outcomes | Reduces stent thrombosis, target vessel revascularization, and MACE | Non-inferior to IVUS for reducing ischemic events; fewer procedural complications; long-term data pending | Reduces unnecessary interventions, achieving comparable clinical outcomes to anatomy-guided strategies |
Note: TCFA, thin-cap fibroatheroma; MACE, Major Adverse Cardiovascular Events; IVUS, intravascular ultrasound; ACS, acute coronary syndrome; CI, Contraindicated; N/A, Not Applicable.
To address limitations of permanent metallic stents, such as late thrombosis and restricted vascular movement, bioresorbable stents (BRS) have emerged as a transformative option. However, rigorous validation is required in complex lesions and diverse populations. For diabetic patients with ACS and multi-vessel disease, Lhermusier T et al. [10] highlighted that BRS and minimally invasive coronary artery bypass grafting may improve treatment outcomes. While BRS performs well in small vessels and AMI, issues remain with stent thickness, fracture, and inconsistent long-term efficacy. A meta-analysis involving 10,510 patients found that use of Absorb Bioresorbable Vascular Scaffold (ABSORB BVS) in PCI was associated with higher risks of definite/probable stent thrombosis and MI compared to DES, with no significant difference in mortality [11]. Long-term randomized trials are essential to clarify the safety profile of BVS versus DES, especially in high-risk populations.
The BIOSOLVE-IV trial evaluated the Magmaris magnesium alloy sirolimus-eluting
scaffold in 1075 patients with de novo coronary artery disease. At 12
months, the target lesion failure (TLF) rate was 4.3%, with a low
definite/probable stent thrombosis rate of 0.5%. Strict adherence to
implantation protocols minimized complications. Notably, 4 of the 5 reported
thrombotic events occurred after premature discontinuation of dual antiplatelet
therapy (DAPT). Compared to ABSORB BVS, Magmaris exhibited fewer thrombotic
features, likely due to rapid magnesium degradation and reduced inflammation.
Mechanical limitations were observed in cases of overlapping stents, highlighting
the need for careful deployment strategies. Although short-term efficacy was
confirmed, longer-term follow-up is necessary to assess neoatherosclerosis and
late lumen patency. Randomized trials comparing Magmaris with durable DES are
warranted to define its role in complex lesions [12]. BIOSOLVE-IV prospectively
enrolled 2066 patients at 106 centers. The results from this trial showed a TLF
rate of 6.8% at 24 months, with higher rates in NSTEMI patients.
Definite/probable stent thrombosis occurred in 0.8% of cases, with half of these
linked to premature DAPT cessation. Only one late thrombosis (
DCBs are emerging as an alternative for small vessel disease, avoiding permanent implantation and reducing DAPT duration. They exhibit a lower thrombotic burden compared to DES and enable shorter DAPT regimens. In the REC-CAGEFREE II trial, 1948 ACS patients treated with DCBs alone demonstrated non-inferiority of a step-down DAPT regimen (1 month of aspirin plus ticagrelor, followed by 5 months of ticagrelor monotherapy) compared to the standard 12-month DAPT regimen. No statistically significant differences in adverse clinical events were observed between the groups, while the step-down group experienced significantly reduced Bleeding Academic Research Consortium (BARC) 3/5 bleeding. This confirms that DCBs can shorten DAPT duration while balancing ischemic and bleeding risks, offering a safer option for patients with high risk of bleeding [14]. A meta-analysis of 485 patients comparing DCBs with stents in AMI found similar MACE rates but lower MI incidence and less late lumen loss with DCBs compared to bare-metal stents (BMS). Angiographic follow-up revealed smaller postoperative minimum lumen diameters with DCBs [15]. Overall, DCBs demonstrate promise as an alternative therapy for small vessel disease, reducing DAPT duration and potentially minimizing restenosis or thrombotic events. The “leave-nothing-behind” strategy using DCBs appears feasible in AMI, particularly for patients transitioning from BMS. However, the efficacy of DCBs in diabetic patients remains controversial, with challenges such as delayed endothelialization, malalignment, and calcified lesions requiring further investigation. Hybrid strategies focusing on nano therapies combining DES/DCBs and computational modeling may optimize treatment outcomes. Future innovations will focus on technology, smart stents, and regenerative therapies to enhance safety and enable personalized medicine.
MCS plays a critical role in improving hemodynamic stability and enhancing survival rates in patients with cardiogenic shock (CS) secondary to acute myocardial infarction (AMI-CS). However, the optimal selection of devices and appropriate patient stratification remain areas of ongoing debate. In recent years, the application of Impella devices and the timing of extracorporeal membrane oxygenation (ECMO) have emerged as key topics of discussion. Evidence from the IABP-SHOCK II trial indicates that intra-aortic balloon pump (IABP) does not provide a survival benefit in AMI-CS patients [16], although it may still be beneficial for chronic heart failure-related CS. Similarly, VA-ECMO did not show a significant impact on 30-day mortality in AMI-CS patients, as demonstrated in the Extracorporeal Life Support in Shock (ECLS-SHOCK) trial [17], even though it was associated with higher risks of bleeding and limb ischemia. There is increasing utilization of percutaneous microaxial flow pumps, such as Impella, in the management of CS, high-risk PCI, and right ventricular failure. While Impella improves hemodynamics in CS patients, it carries a higher risk of bleeding and hemolysis compared to IABP. Randomized controlled trials, including ISAR-SHOCK and IMPRESS, have not demonstrated a survival advantage of Impella over IABP. The ECMELLA concept, which combines Impella with VA-ECMO, shows promise in refractory shock but complicates vascular access. For high-risk PCI, animal studies suggest that Impella-assisted left ventricular unloading reduces infarct size. However, the PROTECT II trial failed to demonstrate superiority over IABP. Data regarding the efficacy of right ventricular Impella in pulmonary embolism or myocardial infarction remain limited [18]. Routine use of Impella in high-risk PCI is not supported, and careful patient selection is essential until large-scale randomized controlled trials (RCTs) provide definitive evidence. The PROTECT IV and DTU-STEMI trials aim to further clarify the role of Impella. Key challenges include optimizing the timing and technique to minimize complications, and obtaining robust evidence through large RCTs. Notably, a study found that Impella-supported high-risk PCI in elderly patients achieved comparable short-term efficacy, emphasizing the need to balance procedural feasibility with patient frailty, comorbidities, and difficult decision-making [19]. For elderly candidates undergoing high-risk PCI with Impella, individualized risk-benefit assessments are necessary that consider frailty, multimorbidity, and treatment preferences to optimize outcomes. Impella is a percutaneously implanted micro-ventricular assist device that reduces 6-month mortality in ST-elevation myocardial infarction-related cardiogenic shock (STEMI-CS) patients when left ventricular blood flow is compromised [20]. Ongoing trials, such as PROTECT IV and DTU-STEMI, aim to further clarify the role of Impella in clinical practice.
The ECMO-CS trial compared immediate VA-ECMO initiation with early conservative treatment (with delayed ECMO if hemodynamics deteriorated) in 117 patients with severe/refractory CS. No significant differences were observed in outcomes between the two groups, including all-cause mortality and serious adverse events. Notably, 39% of patients in the conservative treatment group eventually required ECMO due to worsening shock. These findings emphasize the importance of tailoring the deployment of ECMO to balance risks and benefits, reserving it for refractory cases [21].
Despite the significant progress in revascularization strategies for ACS, the
mortality rate among patients with AMI-CS remains unacceptably high, ranging from
40% to 50% within 30 days. Selecting the optimal MCS is crucial, as
approximately half of these patients may recover without device assistance. A
recent study on MCS in AMI-CS highlights that Impella CP can reduce 180-day
mortality, particularly in younger patients (
In summary, BRS offers transient vessel support with reduced long-term risks, but faces challenges due to thrombogenicity and mechanical performance, especially in complex lesions. They are suited to simple de novo lesions with strict adherence to implantation protocol. DCBs provide a stent-free alternative and are ideal for small vessels and high-bleeding-risk patients due to the shortening of DAPT. However, their efficacy in diabetes and calcification remains uncertain. MCS devices stabilize critical hemodynamics in CS or high-risk PCI, but lack a survival benefit and increase the risk of bleeding. Selection should be phenotype-driven. Future research should prioritize long-term outcomes, RCTs comparing BRS/DES, hybrid MCS strategies, and personalized approaches based on patient profiles and vessel characteristics.
Antiplatelet therapy for AMI continues to face significant challenges in balancing the reduction of thrombotic events with the control of bleeding risks. While traditional DAPT effectively reduces ischemic risk, its “one-size-fits-all” approach has notable limitations. In recent years, individualized treatment strategies based on platelet function testing (PFT), genotyping, and dynamic risk stratification have emerged as promising approaches to optimize clinical decision-making.
Current guidelines recommend adjusting the duration of DAPT dynamically according to ischemic and bleeding risks. Although DAPT reduces ischemic events following stent implantation, it also increases bleeding risks, particularly in patients with ACS or high bleeding risk (HBR). A trial randomly assigned 6002 patients to prasugrel monotherapy following PCI using everolimus-eluting stents. Patient outcomes after 1 month of prasugrel monotherapy were compared to those after 1 month of DAPT. The results showed that prasugrel monotherapy did not significantly reduce bleeding risk compared to DAPT, despite demonstrating non-inferiority in ischemic outcomes. However, it was associated with an increased risk of stent thrombosis and revascularization, especially among ACS patients. Consequently, DAPT remains the standard therapy for early ischemic protection [23]. The PRECISE-DAPT score integrates 7 indicators, including hemoglobin levels, renal function, and bleeding history. It is used to classify patients into non-high, high, and extremely high bleeding risk categories, providing a standardized basis for de-escalation therapy [24]. For instance, HBR patients receiving 1 month of prasugrel monotherapy exhibited significantly lower major bleeding risks (particularly in the ACS subgroup) compared to prolonged DAPT, with non-inferior ischemic endpoints [25]. Nevertheless, the applicability of this score to East Asian populations requires further validation.
The timing, time window, and efficacy of antiplatelet therapy in AMI patients
remain controversial topics. Specifically, the optimal timing of pre-treatment
with the P2Y12 inhibitor in STEMI patients is still being debated. A
registry study involving 1624 patients undergoing direct PCI demonstrated that
pre-PCI administration, as compared to intra-procedural administration, reduced
the 30-day MACE risk, particularly when administered
Precision interventions guided by PFT and genotyping have shown improved outcomes. CYP2C19 gene polymorphisms significantly influence clopidogrel metabolism, with carriers of loss-of-function (LOF) alleles exhibiting a 30% higher ischemic risk [27]. A prospective study confirmed that a genotyping-guided de-escalation strategy (switching LOF carriers to ticagrelor or prasugrel) reduced ischemic events by 22% and bleeding risks by 53% at 12 months [28]. Despite its cost-effectiveness, the application of CYP2C19-guided strategies is limited by poor access to genetic testing, especially in developing countries, and insufficient standardization of result interpretation. AI-assisted decision-making systems may accelerate the analysis of genotype-phenotype associations, although the generalizability of these algorithms requires further verification. Additionally, rapid genotyping platforms, such as point-of-care testing, can expedite precise decision-making but must address challenges such as the potential omission of rare variants and delays in clinical decision-making (median genotyping time: 48 h).
Current antiplatelet strategies for AMI are designed to balance the reduction of thrombotic risk with the potential for bleeding complications. A standardized DAPT regimen is not universally recommended due to interpatient variability. Dynamic risk stratification tools, such as the PRECISE-DAPT score, may help to determine optimal treatment duration and guide de-escalation strategies, particularly in HBR patients. Precision medicine approaches, including the use of CYP2C19 genotyping to inform the selection of P2Y12 inhibitors, can improve clinical outcomes among poor metabolizers of clopidogrel. Personalized therapeutic strategies improve the overall risk-benefit profile, and genotyping has demonstrated cost-effectiveness in this context. However, challenges remain regarding the accessibility and turnaround time of genotyping technologies. Future directions include the broad implementation of rapid point-of-care genetic testing, as well as the integration of AI-based clinical decision support systems to facilitate real-time, individualized treatment (Table 2).
| Strategy | Rationale and advantages | Limitations and risks | Level of evidence |
| Standard 12-month DAPT | Established efficacy in reducing early stent thrombosis and ischemic events. Simplicity of a uniform approach. | Increased risk of major bleeding, especially in HBR or complex patients. “One-size-fits-all” paradigm. | High (A) Based on numerous RCTs and meta-analyses. |
| Short-term DAPT (1–3 month) → De-escalation | Significantly reduces bleeding risk in appropriately selected patients (e.g., HBR), particularly with PRECISE-DAPT score guidance, without sacrificing ischemic protection. | Risk of under-treatment in patients with high ischemic burden. Requires accurate risk stratification tools. | High (A) Supported by RCTs |
| PRECISE-DAPT Score Guidance | Standardized, validated tool integrating clinical factors (e.g., age, CrCl, bleeding history) to objectively quantify bleeding risk and guide DAPT duration. | Limited validation in East Asian populations. Primarily assesses bleeding, not ischemic risk. | Moderate (B) Based on post-hoc analyses and prospective registry data. |
| Genotype-Guided De-escalation | Personalized approach for clopidogrel hypo-responders. Reduces both ischemic (22%) and bleeding (53%) events by switching *CYP2C19* LOF carriers to potent P2Y12 inhibitors. | Low adoption rate (access, cost, turnaround time). Risk of missing rare variants. Lack of standardized implementation protocols. | Moderate (B) Supported by prospective trials (e.g., POPular Genetics, TAILOR-PCI). |
Note: Dynamic risk stratification tools, such as the DAPT Score; (A) High: Multiple RCTs/Meta-analyses; (B) Moderate: Limited RCTs/Registry data. DAPT, dual antiplatelet therapy; HBR, high bleeding risk; LOF, loss-of-function.
The management of AMI centers primarily on repairing damaged myocardium and
inhibiting adverse remodeling processes. Conventional cell-based therapies
encounter challenges such as immunogenicity, arrhythmia risks, and difficulties
in large-scale production. In contrast, extracellular vesicles (EVs), also known
as exosomes, have emerged as an exciting prospect in regenerative medicine due to
their immunomodulatory, pro-angiogenic, and non-toxic properties. EVs can
precisely regulate inflammatory responses and cellular fate within the infarcted
microenvironment by delivering functional nucleic acids (e.g., miRNA) and
proteins. Mesenchymal stem cell-derived EVs (MSCATV-EVs), pre-treated with
atorvastatin, carry high levels of miR-139-3p, which drives macrophage
polarization from the pro-inflammatory M1 type to the reparative M2 type by
inhibiting the Stat1 signaling pathway [29]. In rat models, intramyocardial
injection of MSCATV-EVs reduces the infarct area by 28%, increases left
ventricular ejection fraction (LVEF) by 15%, and significantly decreases the
expression of pro-inflammatory cytokines such as IL-1
Small EVs derived from human cardiac progenitor cells (CPC-sEVs) and produced
under Good Manufacturing Practice (GMP) standards represent a breakthrough in
clinical-grade, “off-the-shelf” therapies. CPC-sEVs are compatible with
coronary artery (CA) delivery during PCI procedures. In pig AMI models, IC
infusion of CPC-sEVs (2
EVs derived from mesenchymal/stromal cells or cardiac progenitor cells exhibit immunomodulatory, pro-angiogenic, and cardioprotective effects, offering advantages such as stability, scalability, and reduced immunogenicity compared to conventional cell therapies. Preclinical studies in large animal models (pigs, primates) demonstrate that EVs delivered via IC, intramyocardial (IM), or through engineered stents reduce infarct size, improve left ventricular function, and mitigate adverse remodeling. The key mechanisms include regulation of macrophage polarization, inhibition of fibrosis, and promotion of angiogenesis. While IC delivery aligns with PCI workflows and provides sustained benefits of up to 3 months, IM injection carries risks of procedural damage [31].
Despite the promising preclinical outcomes with EVs, significant translational challenges remain. A primary hurdle is the scalable manufacturing of clinical-grade EVs under strict GMP conditions. This includes standardizing the isolation methods (e.g., ultrafiltration, size-exclusion chromatography), defining critical quality attributes (e.g., particle concentration, surface markers, miRNA cargo), and ensuring batch-to-batch consistency [32]. Furthermore, optimal dosing regimens (single vs. multiple doses), delivery routes (intracoronary vs. systemic), and long-term biodistribution and safety profiles in humans are yet to be fully established [33].
Several early-phase clinical trials are directly addressing these challenges. The EV-AMI trial is evaluating the safety and efficacy of allogeneic MSC-derived exosomes administered intravenously in patients with AMI. Similarly, the SECRET-HF trial is investigating the impact of cardiosphere-derived cell (CDC)-exosomes in patients with non-ischemic cardiomyopathy. These studies will provide crucial initial data on safety, tolerability, and preliminary efficacy in humans.
A recent study developed a composite hydrogel-EV system, DHPM(4APPC)-EVs, which
addresses the limitation of short retention times. This system was constructed
using CXCR2-overexpressing exosomes and responds to acidic pH/H2O2/MMP9
signals in the infarct microenvironment, enabling the following sequential
release: pH-triggered gelation captures exosomes at the infarct site, followed by
H2O2-induced stabilization and MMP9-mediated sustained release. In rat
AMI models, DHPM(4APPC)-EVs reduce infarct size, enhance LVEF, suppress
pro-inflammatory cytokines (IL-1
EVs purified from pooled human platelet concentrates (SCPL-EVs) exhibit potent cardioprotective effects in MI/reperfusion (I/R) injury models. In a rodent model, SCPL-EVs reduced cardiomyocyte apoptosis, enhanced angiogenesis, and improved cardiac function. Crucially, platelet concentrates are clinical-grade materials with established safety profiles for transfusion, thus streamlining the regulatory process. Preliminary human trials of platelet-derived EVs for wound healing found no safety concerns, supporting their translational potential. The scalable and cost-effective production of SCPL-EVs, coupled with their stability and low immunogenicity, makes them promising candidates for rapid advancement into clinical trials for AMI [35].
EVs derived from MSC/CPC sources mitigate myocardial injury via targeted
delivery of miRNAs/proteins, thereby promoting macrophage M2 polarization,
angiogenesis, and antifibrotic effects. Intracoronary infusion optimizes delivery
[36], while smart hydrogels enhance retention (
The integration of AI into precision medicine has significantly transformed the diagnosis and risk assessment of CVDs. In 2021, Chang et al. [37] introduced a multi-label AI system designed to detect arrhythmias and STEMI. By leveraging a long short-term memory (LSTM) model, this system simultaneously diagnosed 12 types of arrhythmias and STEMI using 12-lead ECGs. It demonstrated very high accuracy (AUC of 0.987), outperforming both cardiologists and commercial algorithms. This highlights its potential for real-time triage of patients with chest pain [37].
Such systems can be integrated into emergency department workflows to provide immediate decision support, helping clinicians prioritize high-risk patients for urgent catheterization based on AI-interpreted ECG findings. This practice is increasingly being reflected in recent chest pain guidelines that emphasize the use of advanced diagnostic technologies.
Expanding on this work, Han et al. [38] in 2021 tackled the challenge of asynchrony in smartwatch ECG signals by developing a residual neural network enhanced with a self-attention mechanism. Their model demonstrated diagnostic accuracy comparable to traditional 12-lead ECGs (AUC ranging from 0.845 to 0.880) across various configurations, enabling outpatient settings to conduct real-time risk stratification. This technology allows continuous ambulatory monitoring of individuals with suspected arrhythmias, aligning with the 2023 ACC/AHA Guidelines on the Management of Patients with Chronic Coronary Disease, which endorse the use of wearable devices for enhancing patient surveillance and early intervention [39].
In 2023, De Michieli et al. [40] validated the diagnostic efficacy of an artificial intelligence Electrocardiogram (AI-ECG) for identifying left ventricular systolic dysfunction (LVSD) in a study of 1977 emergency department patients. This tool identified high-risk individuals with a MACE rate of 48% within two years, showcasing its complementary prognostic value alongside high-sensitivity troponin T (hs-cTnT) biomarkers and highlighting the role of AI in addressing diagnostic gaps in acute care scenarios. The above findings support the recommendations of the 2021 European Society of Cardiology (ESC) Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure, which encourage the use of multidimensional risk stratification tools to identify high-risk patients who may benefit from early advanced imaging or intervention [41].
Large-scale observational studies further confirm the scalability of AI. For instance, the 2022 EURObservational program by the ESC analyzed data from 3620 NSTEMI patients across 59 countries, revealing variations in guideline adherence and patient outcomes [42]. Although not directly AI-driven, such registry studies emphasize the need for AI tools in standardizing care for high-risk populations and minimizing variability. AI-driven clinical decision support systems (CDSS) can now be embedded within electronic health records (EHRs) to alert physicians when guideline-recommended treatments such as DAPT or statin use are overlooked, thereby improving adherence and reducing practice variation.
Awasthi et al. [43] reported in 2023 a series of deep learning models
that utilized 7.1 million ECGs to detect coronary artery calcification (CAC
Bock et al. [44] showcased advancements in multimodal integration by combining clinical variables and exercise stress test ECGs to predict functional coronary artery disease (fCAD) [43]. Herman et al. [45] validated an AI-based, 12-lead ECG model in an international cohort for detecting acute coronary occlusion myocardial infarction (OMI). This model outperformed STEMI criteria (sensitivity 80.6% vs. 32.5%) and matched expert interpretations (accuracy 90.9%), demonstrating its utility in prioritizing urgent revascularization for NSTEMI patients [45]. This approach supports the 2023 ESC Guidelines for the Management of Acute Coronary Syndromes, which stress the importance of early detection of OMI regardless of ST-segment elevation, particularly in patients with ongoing symptoms and ambiguous ECG changes.
Approximately 24% to 35% of patients with non-ST-segment elevation acute coronary syndrome (NSTE-ACS) experience complete coronary artery OMI requiring immediate reperfusion. Current reliance on ST-segment elevation (STE) for diagnosing OMI often results in delayed treatment and higher mortality rates. Researchers recently developed a machine learning (ML) model using data from three U.S. cohorts comprising 7313 patients. This ML model detected subtle ECG patterns that were overlooked by clinicians, such as ST-segment depression and T-wave inversion. When combined with clinical judgment, the OMI score reclassified one-third of chest pain patients, reducing uncertain evaluations. This framework improved OMI detection, thereby facilitating earlier reperfusion and optimizing resource allocation, although further prospective trials are necessary to validate clinical outcomes [46]. Incorporating such ML-based scores into existing ACS algorithms could help emergency physicians identify candidates for urgent angiography more accurately, thereby implementing the Class IIa recommendation in current guidelines regarding early invasive strategy in high-risk NSTE-ACS patients. Zepeda-Echavarria et al. [47] developed a portable four-electrode mini-ECG device capable of detecting AMI caused by different culprit vessels (left anterior descending artery, right coronary artery, and left circumflex artery) with a sensitivity of 65% and specificity of 92%. This innovation enables rapid and targeted ischemia assessment in emergency settings, reducing the intervention time [47]. Their integrated model, employing Shapley Additive Explanations (SHAP) analysis to pinpoint key predictors like age and coronary heart disease history, outperformed cardiologists (AUC 0.71 vs. 0.64), highlighting the importance of integrating AI into clinical decision-making. Such portable devices are especially valuable in resource-limited settings or pre-hospital environments, allowing paramedics to perform ECG acquisition and AI-supported analysis en route to the hospital. This shortens the door-to-balloon time, a key performance indicator in STEMI care systems.
The role of AI in clinical workflows is expected to expand significantly by 2025. The ROMIAE study applied an AI ECG algorithm to 8493 emergency patients, achieving diagnostic accuracy comparable to the HEART score (AUROC 0.878 vs. 0.877) while combining high-sensitivity troponin data to enhance risk stratification, giving a net reclassification improvement of 19.6% [48]. This suggests that AI can improve clinical decision-making by prioritizing high-risk patients and minimizing unnecessary catheterizations. The 2022 AHA/ACC Key Data Elements and Definitions for Chest Pain and Acute Myocardial Infarction also advocates the incorporation of AI-derived metrics into existing risk scores to improve triage accuracy and reduce low-yield cardiac testing [49].
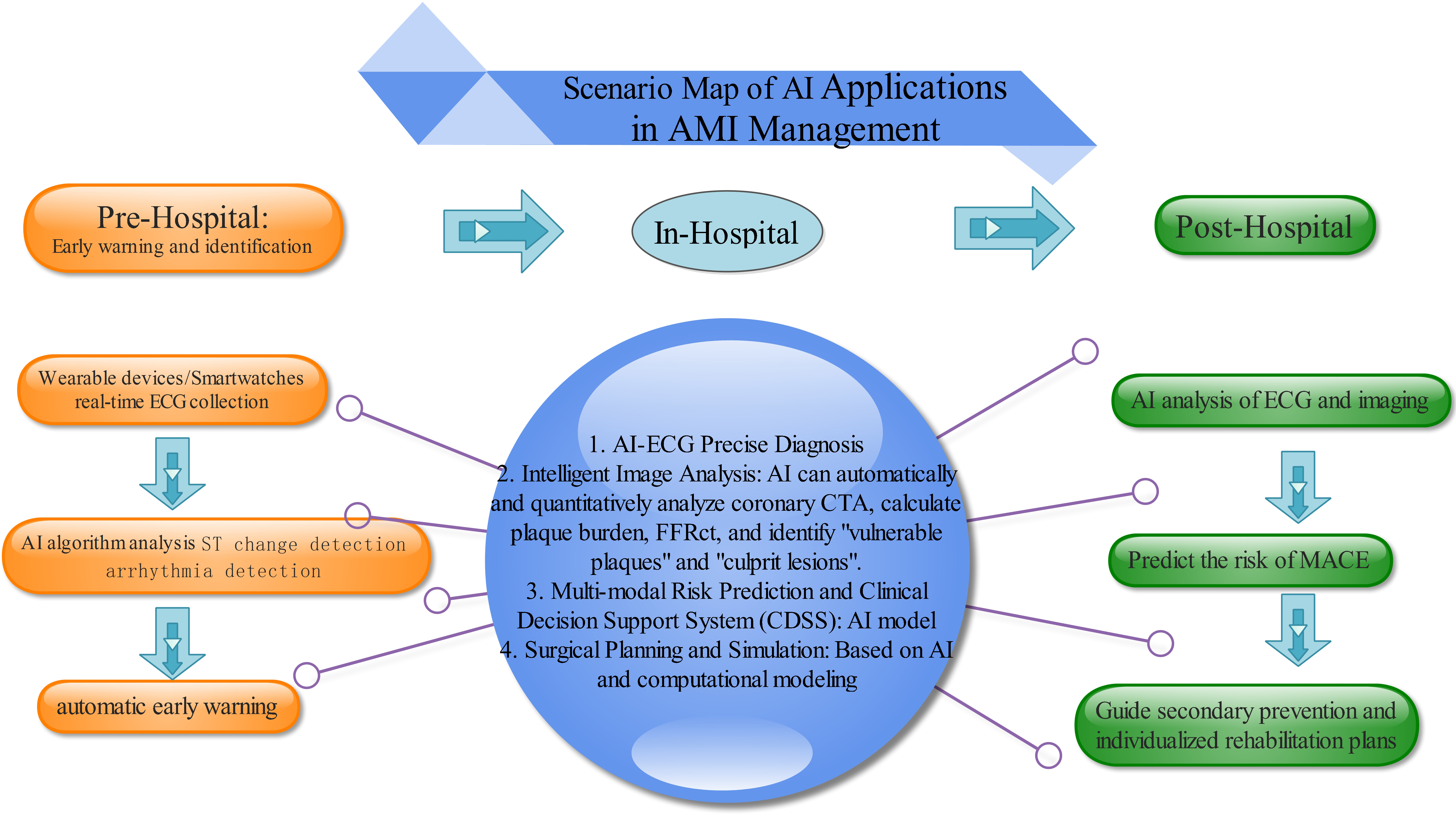
Collectively, these studies highlight the transformative impact of AI on precision medicine—from enhancing risk prediction through multimodal data integration, to enabling immediate diagnosis via wearable/mobile devices. Despite challenges related to demographic biases and ensuring universal applicability, the trend toward AI-driven personalized cardiovascular care continues to gain momentum (Fig. 1).
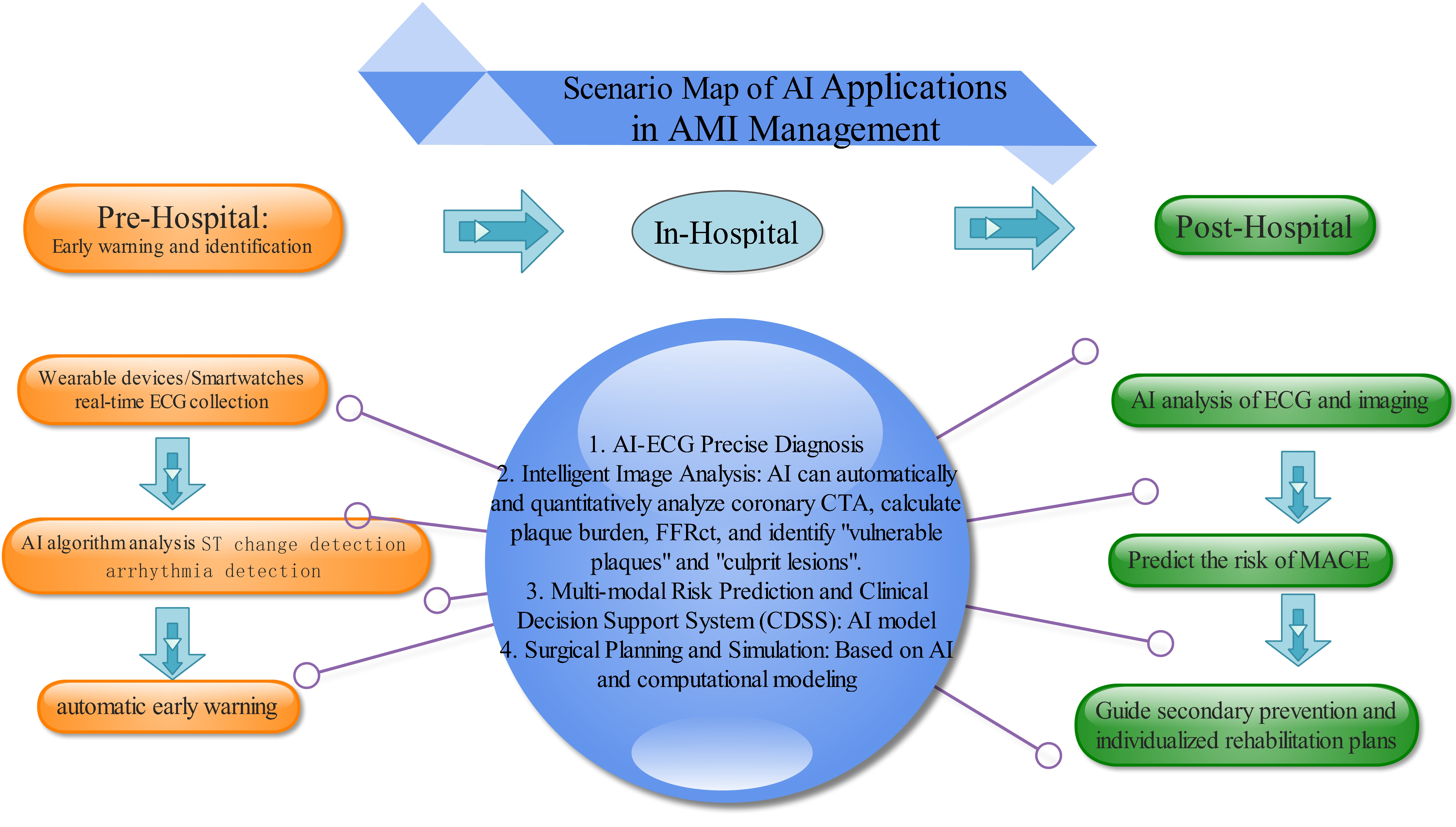 Fig. 1.
Fig. 1.
Scenario map of AI applications in AMI management. AI, artificial intelligence; AMI, acute myocardial infarction; FFR, fractional flow reserve; ECG, Electrocardiogram; ST, ST Segment; AI-ECG, Artificial intelligence-Electrocardiogram; CTA, Computed Tomography Angiography. This figure was created using Edraw Max (www.edrawsoft.com).
Recent advancements in AI have begun to revolutionize precision medicine, particularly in cardiovascular care, offering tools that enhance clinical decision-making and personalize patient management. For example, Samant et al. [50] introduced the Artificial Intelligence-Simulations-Extended Reality (AISER) paradigm, which integrates AI, computational simulations, and extended reality. AISER shows promise in transforming preprocedural planning, virtual trials, and medical education in interventional cardiology. These authors emphasized how AI-driven analytics, in combination with patient-specific simulations and augmented reality (AR), can optimize procedures such as stent deployment and structural heart interventions, providing intuitive visual guidance that supports surgical execution.
Another study employed integrative ML to identify diagnostic genes (AQP9 and SOCS3) from leukocyte transcriptomic data in AMI, which were further validated through correlations with immune cell infiltration [51]. This approach highlights the growing role of AI in biomarker discovery and individualized risk assessment, offering a pathway toward more targeted diagnostic strategies.
Koo et al. [52] developed an AI-enabled, quantitative coronary plaque analysis model (AI-QCPHA) that integrates FFR, plaque burden, and myocardial blood flow. This model outperformed conventional CAD-RADS/HRP scoring in identifying culprit lesions in 351 patients with acute coronary syndrome, demonstrating its potential as a decision-support tool in catheterization laboratories. Similarly, Yang et al. [53] created an explainable XGBoost model to predict in-hospital mortality among Chinese STEMI patients, enhancing interpretability and clinical adoption.
Further expanding the clinical applicability of AI, Lu et al. [54] conducted a multi-center echocardiographic substudy within the PARAGON-HF trial, linking left ventricular mass, filling pressures, and right ventricular dysfunction to renal decline in heart failure with preserved ejection fraction (HFpEF) patients. ML identified LV mass index and E/e’ ratio as independent predictors of renal events, highlighting the value of AI in elucidating complex cardiorenal interactions. In parallel, Aghezzaf et al. [55] deployed a model using transthoracic echocardiography (TTE) parameters—including Left Ventricular Outflow Tract Velocity Time Integral (LVOT VTI), Tricuspid Annular Plane Systolic Excursion (TAPSE), and Systolic Pulmonary Artery Pressures (PAP)—to predict in-hospital MACE in intensive cardiac care unit (ICCU) patients. This model outperformed traditional Thrombolysis In Myocardial Infarction (TIMI) and Global Registry of Acute Coronary Events (GRACE) scores.
Collectively, these studies underscore the expanding role of AI in cardiology—from enhancing risk prediction and biomarker discovery to providing real-time diagnostic support. By improving accuracy and operationalizing data-rich workflows, AI helps to bridge the gap between data and clinical practice. However, challenges remain regarding the generalizability of models across diverse populations, as well as the seamless integration of these tools into routine care. Future efforts should focus on developing standardized reporting guidelines and validating AI systems within real-world clinical pathways to ensure equitable and effective adoption.
AI-enhanced ECG analysis has shown potential in improving the identification of
CVDs, especially in emergency department settings, thereby enabling more rapid
and accurate treatment decisions. However, despite the substantial promise of AI
in cardiology, several limitations and challenges remain. The effective
implementation of AI-based ECG interpretation is constrained by issues such as
systemic bias. Dataset imbalances may introduce biases related to age, gender,
and race. Insufficient representation of demographic diversity can adversely
affect model performance. For example, age-related biases in training datasets
might fail to account for ECG changes associated with aging. Physiological
differences between sexes can also influence ECG patterns, meaning that datasets
skewed toward one gender may compromise accuracy for the other gender.
Furthermore, given that genetic variations can impact ECG readings, it is
essential to ensure racial diversity within datasets. A recent study assessed an
AI-enhanced ECG algorithm for detecting cardiac amyloidosis post-development
[56]. Subtle variations were noted in its performance. The key findings were that
overall AUC decreased from 0.91 (original version) to 0.84 (current validation).
Although robustness was demonstrated across various demographics, including age,
sex, and amyloid subtypes (AL/ATTR), notable discrepancies were identified among
Hispanic patients (AUC 0.66), as well as specific ECG features such as left
bundle branch block (AUC 0.76) and hypertrophy (AUC 0.75). Patterns associated
with low voltage and infarcts maintained high accuracy (
The ideal length of DAPT for patients at high risk of bleeding continues to be a
subject of discussion. Current guidelines (2025) recommend ticagrelor or
prasugrel over clopidogrel for 12 months in ACS patients without high bleeding
risk. Shortened DAPT (1–3 months) followed by ticagrelor monotherapy is favored
in ACS patients with high bleeding risk. In STEMI, prasugrel/ticagrelor is
preferred over clopidogrel during PCI. For patients requiring long-term oral
anticoagulation, aspirin is discontinued 1–4 weeks post-PCI, with continued
P2Y12 inhibition (preferably clopidogrel). Upstream ticagrelor/clopidogrel
may be considered in NSTE-ACS planned for invasive evaluation
While temporary Mechanical Circulatory Support (tMCS) adoption in AMI-CS is
rising, its optimal timing and device-specific efficacy are still being debated.
A large observational study (n = 294,839) using U.S. hospital data (2016–2020)
found that early tMCS initiation (
Next-generation biomarkers such as high-sensitivity troponin, in conjunction with multi-protein panels guided by AI-driven risk scores, are expected to facilitate very early diagnosis of MI and hence significantly reduce the time from hospital admission to balloon angioplasty. Additionally, ML-based risk stratification leveraging AI models that integrate ECG findings, biomarkers, and imaging data will likely improve the accuracy of predicting microvascular obstruction and help to guide the selection of adjuvant therapies. In terms of advanced reperfusion strategies, personalized DAPT regimens informed by pharmacogenomic testing will minimize bleeding risks while preserving ischemic protection. The development of novel bioabsorbable stents with anti-inflammatory properties is anticipated to improve long-term vascular healing following MI. Regarding post-infarction cardiac regeneration, exosome-based therapies, such as the mesenchymal stem cell-derived exosomes currently entering phase II clinical trials, aim to promote myocardial repair and significantly reduce infarct size. Furthermore, the development of wearable devices for continuous biomarker monitoring represents a promising approach for biochemical management and secondary prevention. Several ongoing trials (ANCHOR, ULYSS) should clarify the optimal timing for prophylactic implantation of a cardiac resynchronization defibrillator (MCS). Finally, AI-driven diagnostics and hybrid device strategies (e.g., ECMELLA) may optimize risk-benefit ratios.
Precision medicine is core to the current rapid evolution in AMI treatment, reshaping paradigms from reactive reperfusion to proactive, patient-centric care. The convergence of intravascular imaging, AI-driven diagnostics, and regenerative therapies offers unprecedented opportunities to personalize interventions, reduce disparities, and improve outcomes. However, realizing this potential requires concerted effort to address methodological gaps, validate technologies across diverse populations, and establish unified global standards. By embracing interdisciplinary collaboration and technological innovation, the cardiology community can bridge the divide between benchside discoveries and bedside applications, ultimately transforming AMI management into a paradigm of predictive, preventive, and personalized medicine.
TG contributed to the design of this work and drafted the manuscript. YZ and JH contributed to the conception of the review topic, literature interpretation, and critical revision for important intellectual content. All authors read and approved the final manuscript. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Not applicable.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.