1 Department of Cardiothoracic Surgery, Circle Health Group Ross Hall Hospital, G52 3NQ Glasgow, UK
2 Department of Cardiothoracic Surgery, Golden Jubilee University National Hospital, G81 4DY Glasgow, UK
3 Cardiac Surgery Department, University of Cincinnati College of Medicine, Cincinnati, OH 45267, USA
4 Division of Cardiac Surgery, UT Health Houston Heart & Vascular, Houston, TX 77030, USA
5 Section of Cardiac and Thoracic Surgery, The University of Chicago Medicine, Chicago, IL 60637, USA
Abstract
A minimally invasive surgical technique for aortic valve replacement using a custom surgical retractor specifically designed for transcervical approach to cardiothoracic surgery has previously been described. We hypothesized that the adjunct and integration of robotic technology may improve surgical dexterity and render the transcervical approach advantageous over lateral approaches for robotic aortic valve replacement (AVR). We therefore sought to evaluate its feasibility. This is a preclinical cadaveric feasibility study; no human outcomes are reported. Clinical validation will be addressed in future trials.
A dry lab was firstly set up in the robotic operating room (OR) to explore the concept, with chest phantom incorporating synthetic aortic root mounted atop the surgical table. The minimally invasive surgery was then repeated on five fresh frozen cadavers using an identical setup. The specially designed transcervical retractor system was mounted on the table for exposure. A da Vinci Xi robot was docked from the side and arms allocated for instrumentation or camerascope. Key steps of AVR were performed by console and bedside surgeons working in harmony. Objective procedural metrics were prospectively recorded for each cadaver, including pericardial opening time, aortotomy creation, leaflet excision, prosthesis delivery, inspection and deployment, and aortotomy closure. All times were measured from video timestamps by two independent observers.
Quantitative findings are summarised. A transient loss of visualisation occurred once when adipose tissue obscured the 30° scope; switching to a 0° lens resolved the issue. No difficulty was encountered passing the prosthesis through the uniportal access; however, in two cadavers mild resistance required momentary elevation of the sternum via the retractor ratchet. The cadaver provided a realistic representation of transcervical anatomy, surgical approach for detailing instrumentation and OR setup, similar to live surgery. Key steps of the aortic valve replacement procedure were successfully executed by console surgeon and bedside assistant working in harmony and integrating the use of the robot with the specially designed transcervical retractor system.
Advanced Videoscopic Aortic surgery, Transcervical Approach, Robot assisted (AVATAR) AVR looks feasible. Key steps were easily performed with the robot when used in cooperation with the robot enabling retraction system.
Keywords
- aortic valve replacement
- minimally invasive surgery
- minimally invasive cardiac surgery
- robotic surgery
- robotic heart surgery
- transcervical approach
- suprasternal approach
Recent robotic surgical aortic valve replacement (SAVR) via lateral thoracotomy, while promising, remains limited by restricted exposure, instrument arm collision, and prolonged aortotomy closure times, as highlighted by Badhwar et al. (2021) [1] and others. A midline transcervical route offers a straight, coaxial view of the aortic root, potentially reducing those limitations. Accordingly, we explored whether robotic assistance could exploit this central access to improve ergonomics and reproducibility.
Transcervical approach to cardiothoracic surgery was championed by Cooper in transcervical thymectomy (TCT). This technique represented a step change in outcomes from old full sternotomy approach to thymectomy at the time and continues to be performed with patients typically discharged on the day of surgery with little or no analgesic requirement [2].
Recent comprehensive reviews on the evolution of surgical aortic valve replacement, such as the analysis by Cabrucci et al. (2025) [3], have specifically highlighted transcervical robot-assisted techniques as a promising frontier in minimally invasive AVR.
Based on this experience, it was surmised that SAVR performed by transcervical approach could also deliver a similar step change in outcomes. However, SAVR presents additional challenges over thymectomy and therefore a custom retractor system (CoreVista Robot Enabling Platform™, CardioPrecision Ltd., Glasgow, UK) was developed to allow a wide range of cardiothoracic surgery procedures to be performed by transcervical approach. The equipment incorporates a set of lights that sequentially illuminate different zones of the operative field at specific operative steps, an on-table surgical monitor and a robust framework for attachment of accessories. By way of explanation, a first light setting illuminates structures around the entrance of the incision, a second setting provides generalised illumination further inside the mediastinum whilst also extinguishing the first light to minimise glare, and a third light setting is specifically designed to illuminate the aortic valve through the open aortotomy.
The concept of totally endoscopic SAVR by transcervical approach was first articulated by Sutherland and Sutherland [4] and then performed and reported by Dapunt et al. [5]. However further uptake has been slow. It is believed that this is because cardiac surgeons find transition to totally endoscopic procedures challenging with conventional long-shafted minimally invasive instruments and 2D imaging alone. In addition, cardiac surgeons are generally unfamiliar with the transcervical approach as thymectomy, mediastinoscopy and other similar procedures more commonly performed by general thoracic surgery subspecialists.
We sought to overcome these challenges by engaging a robot to improve technical dexterity and applying surgical expertise from a thoracic surgeon experienced in robotic surgery to build familiarity with the transcervical approach in a cadaver lab. To this end, we undertook detailed analysis of all operative steps of the transcervical SAVR procedure previously described in detail by Sutherland et al. [6], followed by a proof of concept on a phantom chest model. Success in the dry lab was finally replicated using a series of five fresh frozen cadavers in simulated operating theatre environment.
Firstly, a dry lab was set up in the robotic operating room (OR) with a chest phantom incorporating synthetic aortic root mounted atop the surgical table. The CoreVista Robot Enabling Platform™ (Fig. 1) was mounted on the table for transcervical aortic exposure and on-screen bedside visualisation for the assistant surgeon. This provided a realistic modelling of the transcervical anatomical approach for initial proof of concept.
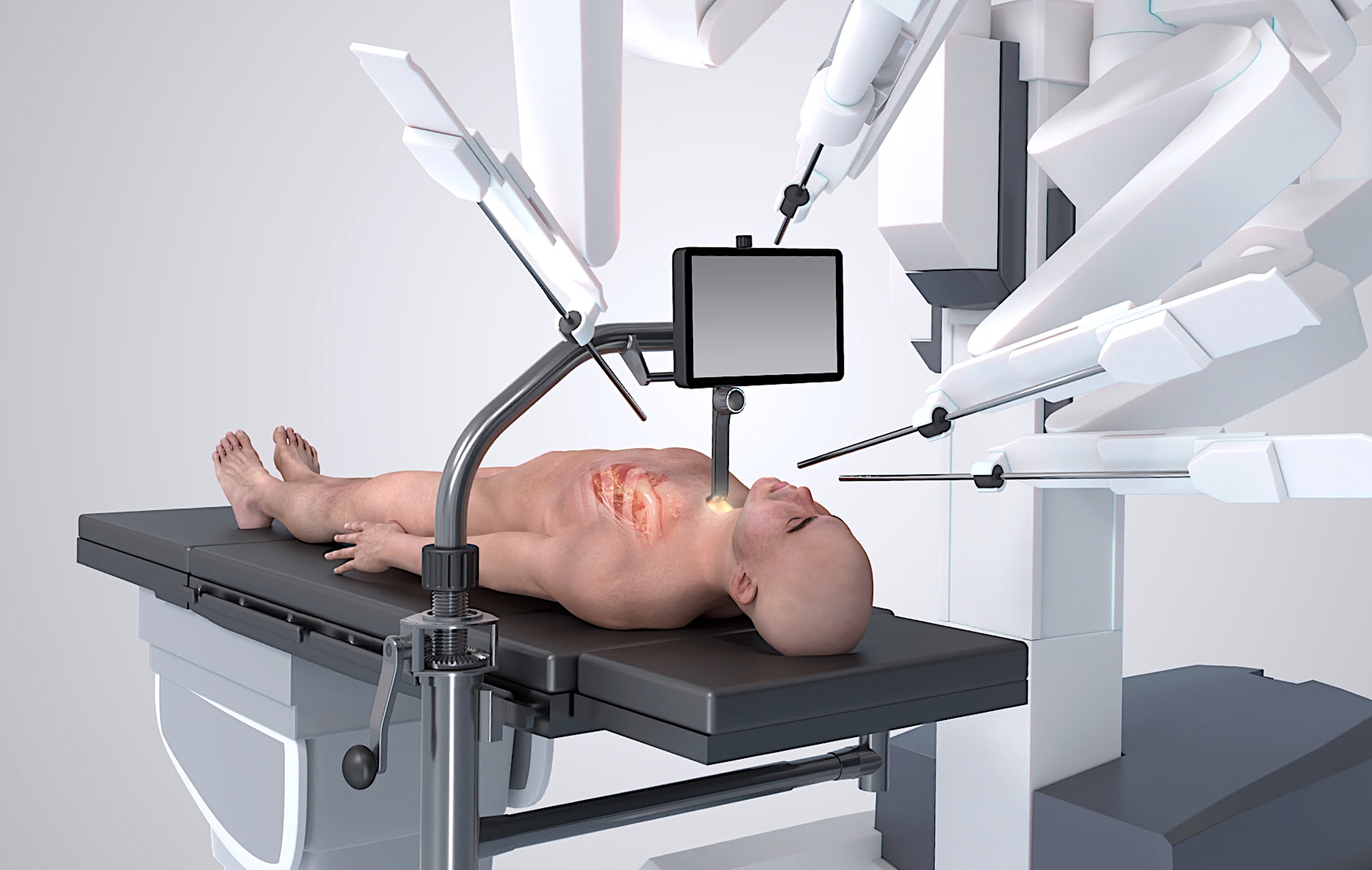 Fig. 1.
Fig. 1. CoreVista Robot Enabling Platform™ mounted on operating table and set up in combination with a generic surgical robot. The retractor provides sternal elevation and ‘uniportal’ access for robot arms, camera and delivery of heart valve prosthesis. Also, illumination for completion of non-robotic surgical tasks. Surgical display shows camera feed from the robot, immediately above the task space, for fast and accurate execution of assisting tasks by bedside surgeon.
The work was then repeated on a cadaveric model in a dedicated robotic training lab (Fig. 2). Fresh frozen cadavers were sourced comprising full torso, head and neck. Cadavers were positioned supine on the robotic OR table with the head section depressed to optimise exposure of the neck. Subject characteristics include male-to-female ratio 1:4, mean age 77 years (range 53 to 90 years), mean height 5’4” (range 5’0” to 5’8”), mean weight 145 lb (range 105 to 206 lb).
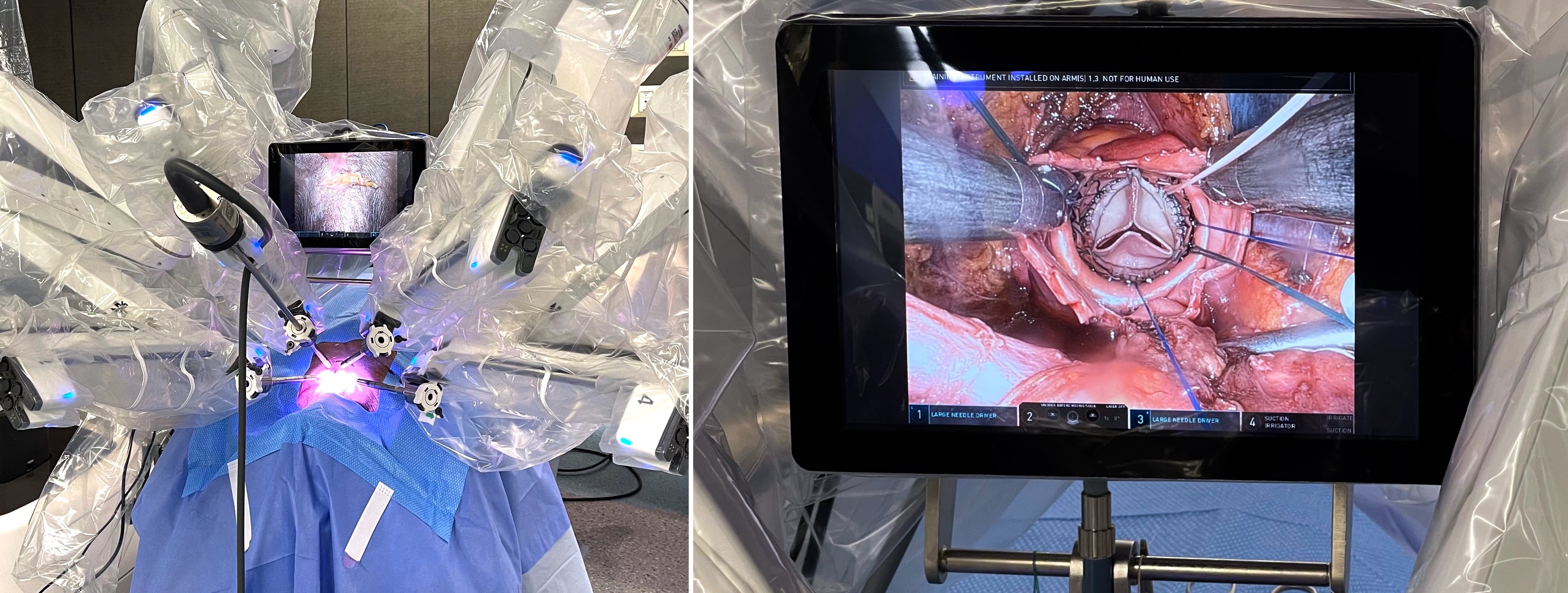 Fig. 2.
Fig. 2. Surgical set up for Advanced Videoscopic Aortic surgery, Transcervical Approach, Robot assisted (AVATAR) procedure with robotic arms docked and ready to start the procedure (left panel); successful implantation of sutureless Perceval (Corcym) heart valve prosthesis AVATAR AVR (right panel). AVR, aortic valve replacement.
One surgeon assumed the role of console surgeon (CS) and another surgeon assumed the role of bedside surgeon (BS). The bedside surgeon’s position is the same previously described for non-robotic transcervical SAVR [5, 6]. The CoreVista monitor has a direct cable connection to the robotic cart and displays identical views available to the console surgeon, but in 2D. This is mounted on the same framework where the retractor is attached and a sterile disposable monitor drape allows placement conveniently facing the BS at sit-down eye level; crystal clear image view is ensured via a peel away window in the sterile monitor drape.
The robot was air docked from the left side with the boom roughly positioned over the incision. The camerascope was allocated to arm 2, and arms 1 and 3 were utilised for standard robotic instrumentation tools such as Cadière forceps, Maryland forceps, needle holder and/or scissors. Instrumentation in arm 4, which was docked for the entirety of the dry lab work, found particular utility as a soft tissue retractor in specific operative steps.
Skin incision was made as for mediastinoscopy by the bedside surgeon. Execution of the procedure then switched to the console surgeon. Dissection into the chest and opening of the pericardium were performed as previously described [4, 5, 6] but using robotic instrumentation in place of conventional MICS instruments. The small incision in the neck ‘uniportal’ access for all robot arms, camera and delivery of heart valve prosthesis (Fig. 3). There are no separate robotic ports.
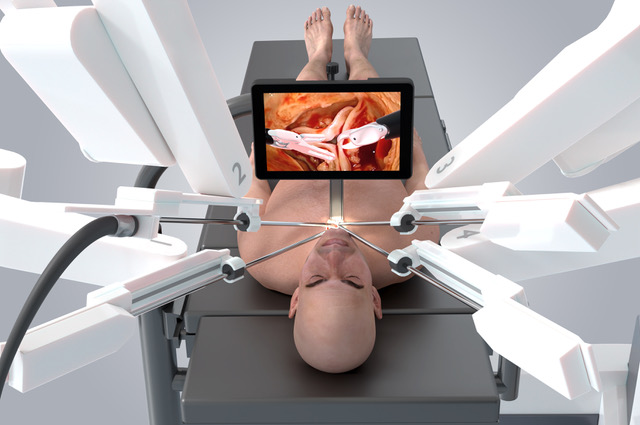 Fig. 3.
Fig. 3. AVATAR procedure showing the location of the small neck incision. All robotic instruments are directed through the incision creating what is effectively a uniportal access.
A 30° camerascope facing upwards was used to begin the procedure, with bipolar forceps in the two main operative arms to dissect whilst maintaining a dry field. Monopolar spatula in dominant hand was found to be equally suitable, subject to individual surgeon preference. The camerascope was positioned at 30° away from the vertical from the start. As the procedure progressed into the chest the camerascope was progressively brought towards the horizontal plane and switched to a 0 degrees scope when approaching the aorta.
The pericardium was opened over the main pulmonary artery with robotic spatula and the incision in pericardium widened laterally (Video 1). The aorta was next dissected free from its pericardial attachments and a 6 mm Nylon tape passed around for control. A Chitwood transthoracic clamp was placed through the first right intercostal space by the bedside surgeon standing to the patient’s side and directed across the aorta with visual endoscopic/direct view coordination; opening and inspection of the right pleura from the surgical field seemed helpful in some instances. The tape was then removed and a cardioplegia cannula was inserted into the aorta below the clamp by the bedside surgeon with careful direction from the console. It was also possible to directly cannulate coronary ostia through the open aorta.
Procedural video. This video summarizes the steps of the Transcervical Robot Assisted Aortic Valve Replacement procedure using a sutureless aortic prosthesis. The video captures the phases described in the text including the approach to the chest cavity, the pericardium opening, the preparation for cardioplegic arrest, the aortotomy, the removal of the valve leaflets, the implant of the sutureless device and the final aortotomy closure. Video associated with this article can be found, in the online version, at https://doi.org/10.31083/HSF47572.
After cardioplegia delivery, the catheter was removed and robotic scissors used to extend the aortotomy in a longitudinal direction towards the right coronary sinus and in retrograde direction towards the clamp. Stay sutures were placed on each side of the aortotomy for exposure. Additional stay sutures were optionally placed at each of the commissures. The aortic valve was excised using Cadière forceps and robotic scissors. The aortic annulus was sized using standard sutureless valve sizers without modification (Video 1).
Two needle holders were used to place guiding sutures into the nadir of each sinus for the sutureless valve and brought out through the neck. An appropriately sized valve was crimped, guiding sutures passed through the eyelets on the prosthesis and the valve passed into the native aortic annulus by the bedside surgeon. The valve was followed with the camerascope and deployed inside the aortic root under direction of the console surgeon. A heart valve was successfully implanted in all five cadavers.
A careful inspection and adjustment of the valve was performed by the console surgeon, aided by the suction irrigation tool. The aorta was then closed with a running 4/0 Prolene sutures (Video 1). After aortic closure it was possible to place a pacing wire onto the surface of the right ventricle before de-airing and release of the aortic cross clamp. The neck incision was then closed in a standard fashion.
Surgical aortic valve replacement (SAVR) is characterised by a well-defined series of operative steps. The dry lab model demonstrated that key operative steps encountered during routine SAVR could be performed with the robot through a uniportal transcervical incision with the aid of a custom designed retractor system. This was confirmed in several cadaver models with different body shapes.
Access to the aorta was achievable using the robotic instruments. The stability and ability to control the camerascope directly by the operating surgeon at the console was also hugely advantageous. Access to the aorta was achievable using the robotic instruments Table 1.
| Robot arm | Instrument | Function |
| Arm 2 | 30°/0° Endoscope | Visualization |
| Arm 1 | Cadière or Maryland Forceps | Tissue manipulation |
| Arm 3 | Needle Holder/Scissors | Suturing & cutting |
| Arm 4 | Tip-Up Fenestrated Grasper | Dynamic retraction |
Robot arm assignments and instrument allocation are detailed in Table 1 to facilitate reproducibility.
We adopted a longitudinal aortotomy. This provided a clear exposure of the aortic valve and may provide a better exposure and prove quicker to close than conventional transverse or hockey stick aortotomy incisions. This is in stark contrast with to reports of aortotomy closure from a lateral approach which can be challenging and time consuming [1].
With the aorta open, it was possible to cannulate coronary ostia with a flexible cardioplegia cannula. This was especially true for the right coronary artery, which can be small and difficult to visualise in other minimally invasive approaches to aortic valve. Flexible cannulae were preferred to rigid cardioplegia cannulae which may be difficult to manipulate using the robot within the confines of the aortic root.
A particular advantage of the robot is that right and left hands can be used interchangeably without loss of control. For example, two needle holders were used to place the guiding sutures. The left hand was used to place the suture at the non-coronary sinus and the right hand for the left and right coronary sinuses. This may be substantially easier to perform than doing the same manoeuvres using conventional long shafted minimally invasive cardiac surgical (MICS) instruments. 2/0 sutures with 17.4 mm needles (Ethibond Excel, Ethicon) were used in preference to the larger 23 mm needles often favoured during conventional surgery. We found that the smaller needle can be manipulated within the confines of the aortic root when using the robotic instrumentation. Sutures were passed into the surgical field by the bedside assistant and placed by the console surgeon.
It should come as no surprise that it was observed to be a ‘straight shot’ into the aortic annulus which greatly facilitates sizing and later delivery of the valve prosthesis. It is pertinent that sizers must not be inserted off axis; otherwise incorrect sizing can result.
Excision of aortic valve leaflets is executed by the console surgeon, grasping individual leaflets with Cadière forceps and skirting around the edge of calcium with monopolar scissors using low electrocautery when necessary as we have previously reported in robotic AVR using traditional upper left chest ports. The magnified view afforded by the robot and fine, controlled movement of robotic instruments facilitates enucleation of calcium deposits.
Quantitative findings are summarised in Table 2. There were no instrument collision events. A transient loss of visualisation occurred once when adipose tissue obscured the 30° scope; switching to a 0° lens resolved the issue. No difficulty was encountered passing the prosthesis through the uniportal access; however, in two cadavers mild resistance required momentary elevation of the sternum via the retractor ratchet.
| Procedural step | Mean time | Range |
| Pericardial opening | 3.1 | 1.7–5.0 min |
| Aortotomy | 2.2 | 1.0–3.0 min |
| Leaflet excision | 2.5 | 1.7–3.7 min |
| Prosthesis delivery, deployment and inspection | 4.6 | 2.2–6.0 min |
| Aortotomy closure | 21.3 | 15.7–28.3 min |
Below summarises objective procedural metrics which were prospectively recorded for each cadaver, including pericardial opening time, aortotomy creation, leaflet excision, prosthesis delivery, inspection & deployment, and aortotomy closure. All times were measured from video timestamps by two independent observers.
Advanced Videoscopic Aortic surgery, using Transcervical Approach and Robot assistance (AVATAR) aortic valve replacement (AVR) looks feasible and reproducible. Key steps of valve implantation are easily performed with the robotic platform working in cooperation with the transcervical retractor system. A team of two surgeons (or one surgeon and an experienced bedside assistant) working in harmony is required as in all robotic cardiac surgical procedures.
In relation to setup of the operating room, trans-oral robotic surgery (TORS) is now routinely performed [7] with a very similar setup wherein a retractor is positioned in the mouth and the tonsillar area is approached with the robotic instruments from the head area, with triangulation occurring outside of the body. A key difference between our approach and TORS is that the orientation of the arms in TORS tends to be close to the vertical whereas in transcervical SAVR the arms are oriented closer to the horizontal plane.
The transcervical retractor system functions as a robot enabling technology by providing essential access to the aorta and the aortic valve through the neck [4, 5, 6]. The on-table monitor replicates the image seen by console surgeon. Location of the monitor screen within the surgical field, immediately above the task space and at eye-level, is known to improve hand-eye coordination and facilitate speed and accuracy of complex motor tasks by the bedside surgeon [8] significantly aiding seamless coordination between console and table side surgeons.
The retractor provides precisely adjustable sternal elevation, particularly precious in this set up, not only in for the deeper robotic dissection manoeuvres, but also for valve prosthesis sizing and delivery. Its multiple light settings also maintain illumination when the robot undocked, allowing for completion of non-robotic surgical tasks. Alternative systems such as the Rultract® retractor have been tested in simulation but failed to deliver equivalent working space or bedside visualization and, therefore, are not recommended for clinical use. In fact, the CoreVista retractor remains for its characteristic able to create the uniportal corridor, elevate the sternum, and provide the necessary calibrated lighting and exposure also to enable the critical role of the bedside surgeon.
The console surgeon benefits from the enhanced visualisation in 3D and operative dexterity afforded by the robotic platform, as has been reported across a number of cardiac surgical procedures [9].
In future clinical cases it is envisaged that the lead surgeon would establish and manage cardiopulmonary bypass via peripheral cannulation, applying the cross clamp, delivering myocardial protection, managing sutures and executing valve implantation. To this end the on-table monitor facilitated accurate choreography between the actions of bedside surgeon and console surgeon.
In our cadaver experience the neck dissection, entry into the pericardium and exposure of the aorta could be done by a robotic thoracic surgeon at the console if necessary. For the next steps a robotic cardiac surgeon would be required to open the aorta, excise the valve leaflets and place sutures in the aortic annulus, confirm the position of the valve, and finally close the aorta and conduct separation from cardiopulmonary bypass. In many robotic cardiac surgery programs a trained physician assistant conducts the bedside activities in traditional robotic mitral and aortic valve procedures [10].
A significant challenge reported with some previous attempts at traditional robotic SAVR has been closure of the aortotomy using the robot, with suggestion that this can take up to 40 min even in expert hands using a lateral chest approach [1]. The transcervical approach adopts a longitudinal aortotomy for improved access and we found this much quicker and easier to execute, also thanks to the more direct anatomical presentation of the aortic valve. This however would need to be validated in clinical cases.
We expected conflict between the robotic arms. However, in practice we did not encounter instrument conflicts as most of the required movement is at the instrument tips, in common with other successful uniportal robotic procedures [11].
The cadaveric data demonstrate technical feasibility but cannot predict clinical safety. Potential risks unique to the transcervical route include injury to cervical neuro-vascular structures and limited working distance in patients with short necks—factors that will require careful evaluation in forthcoming first-in-human trials.
Compared with lateral mini-thoracotomy robotic AVR, our midline approach avoided complex suture line angulation and reduced aortotomy closure time (15–28 min versus the 25–40 min reported by Badhwar et al. [1]). Conversely, the transcervical route offers no direct access for central cannulation at the moment, and therefore mandates reliable peripheral cardiopulmonary bypass (CPB) strategies.
A realistic expectation is that approximately 10–20 clinical cases will be required for a primary operator already experienced in robotic cardiac surgery to achieve procedural fluency. The value of dedicated cadaveric rehearsal cannot be overstated in shortening this curve and ensuring safe translation to the operating room. We recommend a structured three-step pathway: first, achieve confidence with transcervical neck access and mediastinal exposure; second, perform valve implantation with a sutureless prosthesis under robotic guidance; third, achieve familiarity with vertical aortotomy closure under the robot. Partnering with a local thoracic surgeon familiar with cervical anatomy can further accelerate proficiency.
Although the current series employed sutureless devices to simplify deployment, the workspace permits the use of conventional sutured valves. The latter is undeniably more crowded because of multiple sutures, but with meticulous suture management, such as color-coded sutures and suture-gate systems, we consider it feasible. Alternatively, a semi-continuous technique might be possible.
For a first-in-human program we would exclude patients with prior neck or upper-mediastinal surgery, marked obesity, or a short neck that limits extension. All candidates should undergo a contrast-enhanced computed tomography (CT) angiogram of the chest and neck with 3D reconstruction to delineate the relationship of the ascending aorta to the sternum, brachiocephalic artery take-off, and related anatomy.
A single mediastinal drain can be routed through the cervical incision. Should the right pleural space be opened—for example, during Chitwood clamp placement—an additional 19-Fr Blake drain can be placed via the anterior axillary line through the same intercostal space.
We believe that the main benefit of the transcervical approach for the surgeon is its centralised, single port access and more direct access to the aorta and aortic valve which should be well suited to the use of robotic instrumentation.
One of the foreseeable benefits from patients’ perspective is that incisions in this area of the body typically heal quickly and without pain, which might theoretically facilitate faster recovery and discharge from hospital [12]. However, while promising, these results should be interpreted strictly as preclinical; patient-centered benefits remain hypothetical pending clinical study.
The use of anatomical and cadaveric modelling is an obvious limitation to this exploratory study.
Future work may include large-animal validation, human feasibility, and development of a procedure-specific simulation curriculum.
We have identified a novel approach to SAVR. Initial feasibility testing has been successfully carried out in a dry lab followed by multiple cadaveric samples. We have called this procedure AVATAR AVR to reflect the integration of robotic and non-robotic advanced videoscopic technology, aimed at improving the delivery of complex cardiac surgery procedures via the innovative and minimally invasive transcervical route.
All data points generated or analyzed during this study are included in this published article.
FS conceived the idea for the study, provided insight from the world’s first clinical cases of transcervical aortic valve replacement performed without the use of robotic instrumentation, participated in execution of the study and arranged logistical support. RB provided unique insight on robotic instrumentation from a thoracic surgical perspective, advanced preliminary dry lab work on how to navigate the transcervical anatomy using robotic instrumentation prior to executing the study on cadavers, participated in execution of the study and in drafting the first draft of the manuscript. CS provided insight from experimentation on cadavers and early clinical experience of transcervical aortic valve replacement and transcervical transcatheter aortic valve interventions, assisted in drafting the first draft of the manuscript and revisions. HB executed the very first surgery from the console, arranged participation of his clinical team and provided insight from his extensive clinical practice of robotic cardiac surgery and the world’s first clinical robotic aortic valve replacement. DR provided insight from his extensive experience of robotic cardiac surgery, provided logistical arrangement for his own clinical team to attend and executed some of the surgeries from the console. All authors contributed to execution of the surgery and writing of the manuscript, to critical revisions of the manuscript and important intellectual content. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
The study was carried out in accordance with the University of Illinois Chicago Surgical Innovation Training Laboratory (SITL) protocol for cadaver and recognizable human body parts use for education and research purposes.
The authors are grateful to the director and staff of the Surgical Innovation Training Laboratory (SITL) of the University of Illinois Chicago for providing access to their facility to execute this study and for their enthusiastic and professional support throughout this study.
This research received no external funding.
The authors declare no conflict of interest. Cristiano Spadaccio is serving as one of the Editorial Board members of this journal. We declare that Cristiano Spadaccio had no involvement in the peer review of this article and has no access to information regarding it’s peer review. Full responsibility for the editorial process for this article was delegated to Ho Young Hwang and Giuseppe Santarpino. Fraser Sutherland is a consultant for Cardioprecision Ltd. However, the company had no role in the handling or conduct of the study. The authors had full access to all data in the study and take full responsibility for the integrity of the data and the accuracy of the data analysis.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.



