1 Department of Anesthesiology, Zhangzhou Affiliated Hospital of Fujian Medical University, 363000 Zhangzhou, Fujian, China
2 Department of Cardiac Surgery, Fujian Medical University Union Hospital, 350001 Fuzhou, Fujian, China
3 Fujian Key Laboratory of Cardio-Thoracic Surgery (Fujian Medical University), Fujian Province University, 350001 Fuzhou, Fujian, China
4 Fujian Provincial Special Reserve Talents Laboratory, Fujian Province University, 350001 Fuzhou, Fujian, China
†These authors contributed equally.
Abstract
Prolonged mechanical ventilation (PMV) is a common and serious complication after heart valve surgery, associated with increased morbidity, mortality, and healthcare resource utilization. Although several predictive models exist, many are limited by population homogeneity or lack of intraoperative variables. This study aimed to develop and validate a practical predictive model for PMV risk stratification to facilitate early intervention and optimize resource allocation.
This was a retrospective study of adult patients who underwent elective heart valve surgery between January 2013 and January 2023. Patients from Center A were randomly assigned to a training cohort (n = 349) or an internal validation cohort (n = 149, with a 7:3 ratio). PMV was defined as mechanical ventilation lasting more than 48 hours postoperatively. Preoperative, intraoperative, and early postoperative variables were analyzed. Univariate and multivariate logistic regression analyses were used to identify independent predictors in the training cohort. A predictive nomogram was subsequently developed. Model performance was evaluated using discrimination (area under the receiver operating characteristic (AUROC) curve), calibration (calibration plots, Hosmer–Lemeshow test), and clinical utility (decision curve analysis (DCA) and clinical impact curve (CIC)).
Data were analyzed from 498 patients (training: n = 349; internal validation: n = 149). The incidence of PMV was 32.7% in the training cohort. Multivariate analysis identified six independent predictors: age (per 1-year increase), body mass index (per 1 kg/m2 increase), chronic obstructive pulmonary disease severity (per 1-grade increase), forced expiratory volume in 1 s (per 1% increase), left ventricular ejection fraction (per 1% increase), and cardiopulmonary bypass time (per 10 minute increase). The nomogram demonstrated strong discrimination, with area under the curve (AUC) values of 0.847 (95% confidence interval (CI), 0.798–0.882) in training and 0.891 (95% CI, 0.858–0.927) in internal validation. Calibration was good across cohorts (Hosmer–Lemeshow p > 0.05). The DCA and CIC indicated that the model provided meaningful clinical benefit compared with treating all or no patients when the predicted probability threshold ranged from 40% to 100%.
PMV was associated with higher in-hospital mortality, increased healthcare resource utilization, and reduced long-term survival. The proposed predictive model may aid in optimizing perioperative management, thereby improving outcomes and reducing costs.
Keywords
- prolonged mechanical ventilation
- heart valve surgery
- predictive model
- risk stratification
- nomogram
Prolonged mechanical ventilation (PMV), defined as postoperative ventilatory support lasting more than 24 hours, remains a significant complication following heart valve surgery [1]. Contemporary studies report PMV incidence rates as high as 22%, with associated mortality exceeding 40% among affected patients [2, 3, 4, 5, 6]. PMV markedly increases the risk of ventilator-associated pneumonia, prolongs intensive care unit (ICU) stays, and escalates healthcare costs, posing considerable clinical and economic burdens [7, 8].
Early identification of patients at high risk for PMV can optimize perioperative management through targeted resource allocation, closer monitoring, and timely preemptive strategies [9]. Although several predictive models have been proposed, their clinical applicability remains limited [10, 11]. Many were derived from relatively homogeneous populations or relied on complex variables with limited feasibility in routine care [12, 13]. Additionally, intraoperative factors—important determinants of postoperative respiratory outcomes—have not been comprehensively integrated into most existing risk models.
To address these limitations, we developed and internally validated a novel risk-prediction model specifically for patients undergoing valve surgery. Using a contemporary, heterogeneous cohort from a tertiary cardiac center, we incorporated both preoperative characteristics and intraoperative variables. The model emphasizes clinically accessible predictors to ensure practical application, while internal validation using separate training and testing cohorts methodological robustness. This tool aims to support individualized risk stratification, improve perioperative decision-making, and guide efficient resource utilization.
This retrospective cohort study included adult patients (
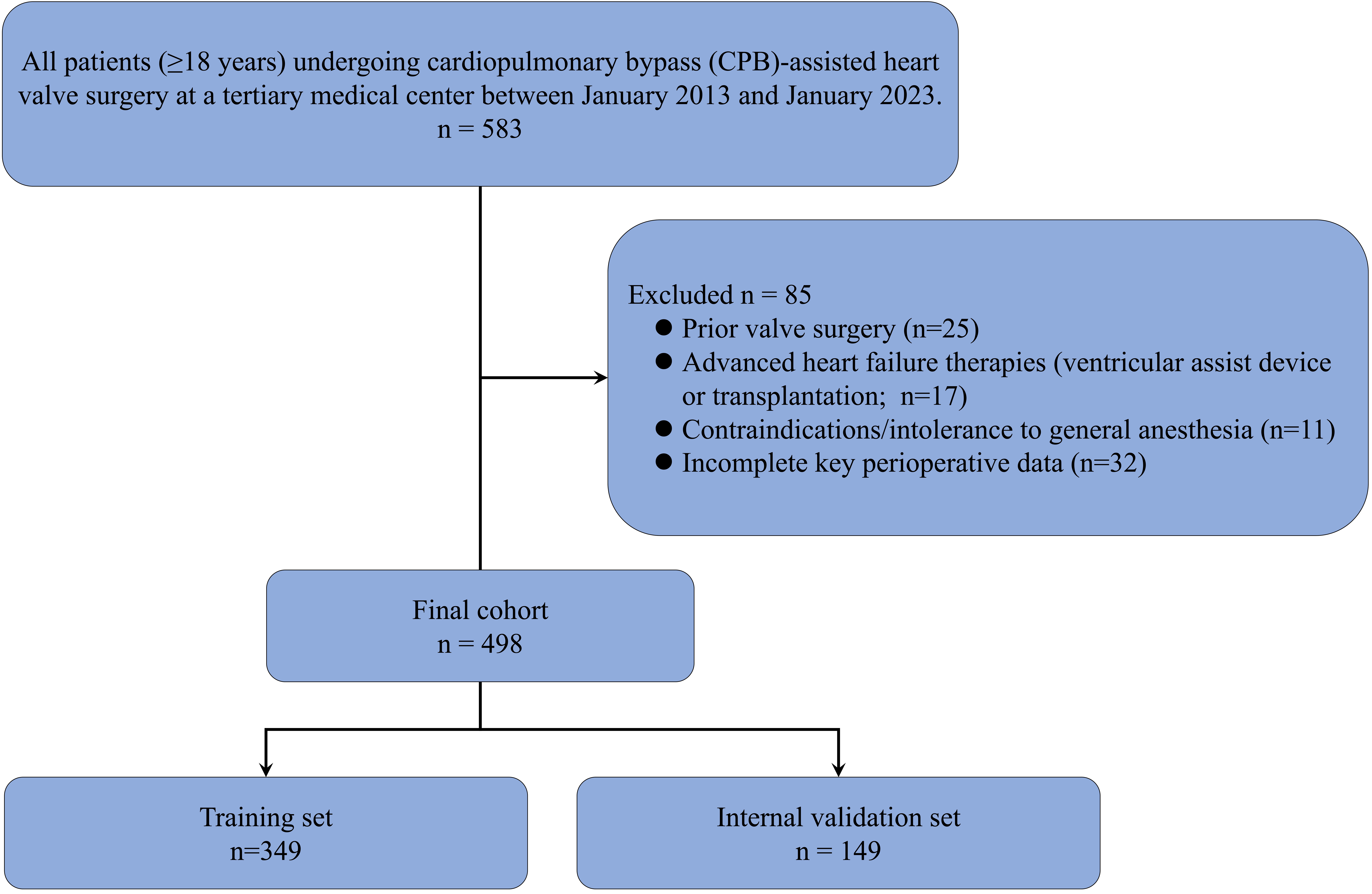 Fig. 1.
Fig. 1. Flowchart of patient enrollment and cohort allocation. A schematic overview of inclusion and exclusion criteria and cohort stratification. A total of 583 patients who underwent CPB-assisted heart valve surgery between January 2013 and January 2023. After applying exclusion criteria (n = 85), 498 patients were included and randomly assigned to the training cohort (n = 349) or internal validation cohort (n = 149).
All heart valve procedures were performed under CPB by the center’s surgical team, typically composed of 3 to 4 members, including chief physicians, attending surgeons, and residents. CPB was established via femoral arterial and venous cannulation. Valve interventions (aortic, mitral, tricuspid, or multiple) involved either replacement or repair, with mitral and tricuspid repairs performed whenever anatomically feasible. Concomitant atrial fibrillation ablation and prosthesis selection (mechanical vs. bioprosthetic) were guided by preoperative and intraoperative findings, as well as patient-specific factors such as age, comorbidities, and preferences. Intraoperative efforts focused on minimizing CPB duration and ensuring meticulous hemostasis. Transesophageal echocardiography was routinely employed to assess valve function and confirm procedural success. Postoperatively, patients were transferred to the ICU for continuous hemodynamic monitoring, fluid management, and inotropic support as needed. Multimodal analgesia—combining opioids, non-opioid agents, and regional techniques such as epidural or paravertebral blocks—was prioritized to promote early mobilization and facilitate timely extubation. Standardized ICU protocols guided the management of prolonged ventilation, including daily weaning assessments and prevention of complications (e.g., ventilator-associated pneumonia). Clinical guidelines informed the detection and management of postoperative complications, including bleeding, infection, and arrhythmias. Patients were closely monitored for respiratory distress, hemodynamic instability, and other factors potentially contributing to prolonged ventilation.
PMV was defined as invasive mechanical ventilation lasting more than 48 h after surgery. This included patients who remained continuously intubated for
The study cohort comprised 498 patients from the Zhangzhou Affiliated Hospital of Fujian Medical University. Patients were randomly assigned to a training cohort (70%) and an internal validation cohort (30%). Comparative analyses between patients requiring PMV and those not requiring PMV (non-PMV) were performed using Fisher’s exact test or the
This study included 498 adult patients (
| Variable | Non-PMV (n = 235) | PMV (n = 114) | p value | ||||
| N, mean or median | %, | N, mean or median | %, | ||||
| Preoperative Characteristics | |||||||
| Age, years | 55.1 | 5.9 | 68.1 | 4.3 | |||
| Male, n (%) | 114 | 48.5% | 69 | 60.5% | 0.040 | ||
| BMI, kg/m2 | 24.9 | 1.9 | 28.9 | 1.6 | |||
| Current smoker, n (%) | 58 | 24.7% | 43 | 37.7% | 0.016 | ||
| COPD severity, n (%) | |||||||
| None | 192 | 81.7% | 70 | 61.4% | |||
| Mild | 28 | 11.9% | 17 | 14.9% | |||
| Moderate | 12 | 5.1% | 20 | 17.5% | |||
| Severe | 3 | 1.3% | 7 | 6.1% | |||
| FEV1% predicted | 74.7 | 8.5 | 56.0 | 9.0 | |||
| NYHA class, n (%) | |||||||
| I/II | 148 | 63.0% | 39 | 34.2% | |||
| III/IV | 87 | 37.0% | 75 | 65.8% | |||
| LVEF, % | 54.4 | 5.8 | 39.3 | 6.1 | |||
| Serum albumin, g/L | 36.7 | 3.0 | 37.3 | 3.0 | 0.101 | ||
| eGFR, mL/min/1.73 m2 | 70.3 | 17.1 | 68.0 | 17.1 | 0.245 | ||
| Hemoglobin, g/dL | 11.9 | 1.2 | 12.0 | 1.1 | 0.900 | ||
| Pulmonary hypertension, n (%) | 77 | 32.8% | 58 | 50.9% | 0.002 | ||
| Diabetes, n (%) | 49 | 20.9% | 23 | 20.2% | 0.884 | ||
| Hypertension, n (%) | 100 | 42.6% | 42 | 36.8% | 0.353 | ||
| CAD, n (%) | 41 | 17.4% | 20 | 17.5% | 0.982 | ||
| Previous MI, n (%) | 20 | 8.5% | 14 | 12.3% | 0.336 | ||
| Stroke, n (%) | 26 | 11.1% | 14 | 12.3% | 0.724 | ||
| Liver dysfunction, n (%) | 11 | 4.7% | 10 | 8.8% | 0.152 | ||
| PVD, n (%) | 34 | 14.5% | 14 | 12.3% | 0.623 | ||
| Cancer, n (%) | 12 | 5.1% | 9 | 7.9% | 0.340 | ||
| Atrial fibrillation, n (%) | 73 | 31.1% | 60 | 52.6% | |||
| Endocarditis, n (%) | 12 | 5.1% | 12 | 10.5% | 0.072 | ||
| Intraoperative Characteristics | |||||||
| Surgery type, n (%) | |||||||
| MVR | 142 | 60.4% | 50 | 43.9% | |||
| MVP | 36 | 15.3% | 9 | 7.9% | |||
| MVR + TVP | 36 | 15.3% | 34.00 | 29.8% | |||
| AVR + MVR | 9 | 3.8% | 8.00 | 7.0% | |||
| AVR + MVR + TVP | 3 | 1.3% | 8.00 | 7.0% | |||
| MVP + TVP | 9 | 3.8% | 5.00 | 4.4% | |||
| Operative time, min | 201.6 | 29.7 | 237.9 | 37.0 | |||
| CPB time, min | 119.1 | 17.7 | 154.1 | 20.2 | |||
| Aortic cross-clamp time, min | 89.0 | 17.2 | 90.9 | 17.4 | 0.333 | ||
| Blood transfusion, units | 3 | 1–5 | 4 | 2–5 | 0.109 | ||
| Crystalloid infusion, mL | 2745.9 | 435.0 | 2798.5 | 428.5 | 0.288 | ||
| Lowest SpO2, % | 93.8 | 2.3 | 93.73 | 2.4 | 0.880 | ||
| Mean PEEP, cmH2O | 3.4 | 0.9 | 3.5 | 0.8 | 0.291 | ||
| Pleural adhesion, n (%) | 27 | 11.5% | 21 | 18.4% | 0.097 | ||
| AF ablation, n (%) | 52 | 22.1% | 30 | 26.3% | 0.420 | ||
| Postoperative Characteristics | |||||||
| PaO2/FiO2 ratio | 270.7 | 29.4 | 165.7 | 29.4 | |||
| Peak airway pressure, cmH2O | 18.8 | 2.7 | 19.1 | 2.8 | 0.467 | ||
| Plateau airway pressure, cmH2O | 15.1 | 2.3 | 17.1 | 4.8 | 0.078 | ||
| VIS score | 12 | 8–15 | 28 | 21–35 | |||
| Temperature, °C | 36.5 | 0.7 | 36.4 | 0.9 | 0.312 | ||
| RASS score | –2 | –3 to –1 | –2 | –3 to –1 | 0.156 | ||
| Pain score (0–10) | 4 | 3–5 | 3 | 2–5 | 0.546 | ||
| Chest tube drainage, mL | 494 | 396–616 | 502 | 395–603 | 0.819 | ||
| Respiratory inflammation, n (%) | 0.196 | ||||||
| None | 138 | 58.7% | 60 | 52.6% | |||
| Mild | 66 | 28.1% | 32 | 28.1% | |||
| Moderate | 22 | 9.4% | 16 | 14.0% | |||
| Severe | 9 | 3.8% | 6 | 5.3% | |||
| Pulmonary edema, n (%) | 31 | 13.2% | 18 | 15.8% | 0.515 | ||
| Atelectasis, n (%) | 53 | 22.6% | 35 | 30.7% | 0.115 | ||
| Pneumothorax, n (%) | 12 | 5.1% | 4 | 3.5% | 0.595 | ||
| IABP, n (%) | 0 | 0.0% | 15 | 13.2% | |||
| ECMO, n (%) | 0 | 0.0% | 9 | 7.9% | |||
| Tracheostomy, n (%) | 4 | 1.7% | 30 | 26.3% | |||
| Reintubation, n (%) | 7 | 3.0% | 45 | 39.5% | |||
| 30-day mortality, n (%) | 9 | 3.8% | 32 | 28.1% | |||
| Overall mortality, n (%) | 4 | 1.7% | 45 | 39.5% | |||
PMV, prolonged mechanical ventilation (
| Variable | Non-PMV (n = 101) | PMV (n = 48) | p value | ||||
| N, mean or median | %, | N, mean or median | %, | ||||
| Preoperative characteristics | |||||||
| Age, years | 54.8 | 5.9 | 67.3 | 4.2 | |||
| Male, n (%) | 60 | 59.4% | 35 | 72.9% | |||
| BMI, kg/m2 | 24.8 | 1.7 | 29.3 | 1.6 | |||
| Current smoker, n (%) | 18 | 17.8% | 16 | 33.3% | 0.039 | ||
| COPD severity, n (%) | |||||||
| None | 82 | 81.2% | 29 | 60.4% | |||
| Mild | 13 | 12.9% | 6 | 12.5% | |||
| Moderate | 5 | 5.0% | 10 | 20.8% | |||
| Severe | 1 | 1.0% | 3 | 6.3% | |||
| FEV1% predicted | 74.6 | 8.5 | 66.9 | 14.9 | |||
| NYHA class, n (%) | 0.002 | ||||||
| I/II | 64 | 63.4% | 17 | 35.4% | |||
| III/IV | 37 | 36.6% | 31 | 64.6% | |||
| LVEF, % | 54.1 | 5.9 | 49.2 | 9.5 | |||
| Serum albumin, g/L | 36.9 | 2.9 | 37.0 | 3.0 | 0.730 | ||
| eGFR, mL/min/1.73 m2 | 70.2 | 16.5 | 69.3 | 17.4 | 0.643 | ||
| Hemoglobin, g/dL | 13.4 | 1.8 | 11.8 | 2.5 | |||
| Pulmonary hypertension, n (%) | 25 | 24.8% | 24 | 50.0% | 0.003 | ||
| Diabetes, n (%) | 19 | 18.8% | 12 | 25.0% | 0.395 | ||
| Hypertension, n (%) | 42 | 41.6% | 30 | 62.5% | 0.017 | ||
| CAD, n (%) | 19 | 18.8% | 9 | 18.8% | 0.862 | ||
| Previous MI, n (%) | 7 | 6.9% | 7 | 14.6% | 0.145 | ||
| Stroke, n (%) | 10 | 9.9% | 3 | 6.4% | 0.550 | ||
| Liver dysfunction, n (%) | 3 | 3.0% | 4 | 8.3% | 0.213 | ||
| PVD, n (%) | 18 | 17.8% | 8 | 16.7% | 0.284 | ||
| Cancer, n (%) | 3 | 3.0% | 0 | 0% | 0.551 | ||
| Atrial fibrillation, n (%) | 35 | 34.7% | 19 | 39.6% | 0.557 | ||
| Endocarditis, n (%) | 7 | 6.9% | 4 | 8.3% | 0.755 | ||
| Intraoperative Characteristics | |||||||
| Surgery type, n (%) | 0.008 | ||||||
| MVR | 62 | 61.4% | 20 | 41.7% | |||
| MVP | 15 | 14.9% | 4 | 8.3% | |||
| MVR + TVP | 15 | 14.9% | 15 | 31.3% | |||
| AVR + MVR | 3 | 3.0% | 4 | 8.3% | |||
| AVR + MVR + TVP | 1 | 1.0% | 4 | 8.3% | |||
| MVP + TVP | 5 | 5.0% | 1 | 2.1% | |||
| Operative time, min | 197.5 | 27.8 | 241.6 | 33.5 | |||
| CPB time, min | 118.7 | 16.9 | 146.6 | 18.9 | |||
| Aortic cross-clamp time, min | 89.9 | 16.4 | 87.4 | 15.2 | 0.372 | ||
| Blood transfusion, units | 3 | 1–5 | 3 | 1–6 | 0.676 | ||
| Crystalloid infusion, mL | 2808.4 | 430.4 | 2713.6 | 439.4 | 0.214 | ||
| Lowest SpO2, % | 94.4 | 2.3 | 93.8 | 2.3 | 0.123 | ||
| Mean PEEP, cmH2O | 3.5 | 0.9 | 3.6 | 0.9 | 0.218 | ||
| Pleural adhesion, n (%) | 12 | 11.9% | 13 | 27.1% | 0.018 | ||
| AF ablation, n (%) | 25 | 24.8% | 13 | 27.1% | 0.841 | ||
| Postoperative characteristics | |||||||
| PaO2/FiO2 ratio | 273.9 | 27.8 | 169.3 | 28.4 | |||
| Peak airway pressure, cmH2O | 16.8 | 2.8 | 16.8 | 2.7 | 0.995 | ||
| Plateau airway pressure, cmH2O | 13.9 | 2.4 | 15.1 | 2.2 | 0.556 | ||
| VIS score | 12 | 9–17 | 27 | 23–35 | |||
| Temperature, °C | 36.3 | 0.5 | 36.4 | 0.3 | 0.118 | ||
| RASS score | –2 | –2 to –1 | –2 | –2 to –1 | 0.370 | ||
| Pain score (0–10) | 3 | 2–5 | 3 | 2–4 | 0.705 | ||
| Chest tube drainage, mL | 486 | 403–576 | 516 | 391–600 | 0.504 | ||
| Respiratory inflammation, n (%) | 0.002 | ||||||
| None | 65 | 64.4% | 21 | 43.8% | |||
| Mild | 26 | 25.7% | 15 | 31.3% | |||
| Moderate | 9 | 8.9% | 9 | 18.8% | |||
| Severe | 1 | 1.0% | 3 | 6.3% | |||
| Pulmonary edema, n (%) | 18 | 17.8% | 3 | 6.3% | 0.077 | ||
| Atelectasis, n (%) | 19 | 18.8% | 9 | 18.8% | 0.993 | ||
| Pneumothorax, n (%) | 4 | 4.0% | 1 | 2.0% | 0.552 | ||
| IABP, n (%) | 0 | 0.0% | 7 | 14.6% | |||
| ECMO, n (%) | 0 | 0.0% | 2 | 4.2% | |||
| Tracheostomy, n (%) | 1 | 1.0% | 18 | 37.5% | |||
| Reintubation, n (%) | 6 | 5.9% | 26 | 54.2% | |||
| 30-day mortality, n (%) | 1 | 1.0% | 12 | 25.0% | |||
| Overall mortality, n (%) | 4 | 4.0% | 20 | 41.7% | |||
Univariate analysis identified 13 factors significantly associated with PMV (all p
| Variable | Univariate | Multivariate | |||
| OR (95% CI) | p value | OR (95% CI) | p value | ||
| Preoperative characteristics | |||||
| Age (per 1-year increase) | 1.90 (1.60–2.26) | 2.18 (1.32–3.61) | 0.002 | ||
| Male (vs female) | 1.63 (1.03–2.56) | 0.036 | |||
| BMI (per 1 kg/m2 increase) | 4.54 (3.11–6.63) | 2.05 (1.22–3.46) | 0.007 | ||
| Current smoker (vs non-smoker) | 1.85 (1.14–2.99) | 0.012 | |||
| COPD severity (per 1-grade increase) | 1.57 (1.23–2.01) | 2.22 (1.28–3.84) | 0.004 | ||
| FEV1 (per 1% increase) | 1.76 (1.10–1.81) | 3.05 (1.78–5.22) | |||
| NYHA III/IV (vs I/II) | 3.27 (2.08–5.23) | ||||
| LVEF (per 1% increase) | 1.56 (1.48–1.66) | 2.41 (1.40–4.15) | 0.001 | ||
| Serum albumin (per 1 g/L increase) | 1.07 (0.99–1.45) | 0.101 | |||
| eGFR (per 1 mL/min/1.73 m2 increase) | 0.99 (0.98–1.00) | 0.112 | |||
| Hemoglobin (per 1 g/dL increase) | 1.01 (0.83–1.23) | 0.9 | |||
| Pulmonary hypertension (yes vs no) | 2.23 (1.35–3.36) | ||||
| Diabetes (yes vs no) | 1.04 (0.60–1.82) | 0.884 | |||
| Hypertension (yes vs no) | 1.27 (0.80–2.01) | 0.309 | |||
| CAD (yes vs no) | 1.01 (0.56–1.81) | 0.982 | |||
| Previous MI (yes vs no) | 1.51 (0.73–3.10) | 0.268 | |||
| Stroke (yes vs no) | 1.13 (0.56–2.25) | 0.738 | |||
| Liver dysfunction (yes vs no) | 1.96 (0.81–4.76) | 0.138 | |||
| PVD (yes vs no) | 1.21 (0.62–2.35) | 0.578 | |||
| Cancer (yes vs no) | 0.63 (0.26–1.54) | 0.308 | |||
| Atrial fibrillation (yes vs no) | 2.47 (1.56–3.91) | ||||
| Endocarditis (yes vs no) | 2.19 (0.95–5.03) | 0.066 | |||
| Surgery type | 2.42 (1.47–4.00) | 0.001 | |||
| Operation time (per 10-min increase) | 1.04 (1.03–1.04) | ||||
| CPB time (per 10-min increase) | 1.10 (1.08–1.13) | 3.12 (1.85–5.26) | |||
| Aortic cross-clamp time (per 10-min increase) | 1.01 (0.99–1.02) | 0.332 | |||
| Blood transfusion (per 1-unit increase) | 1.09 (0.98–1.20) | 0.109 | |||
| Crystalloid infusion (per 500-mL increase) | 1.08 (0.98–1.19) | 0.287 | |||
| Lowest SpO2 (per 1% increase) | 0.99 (0.90–1.09) | 0.879 | |||
| Mean PEEP (per 1 cmH2O increase) | 1.15 (0.89–1.50) | 0.29 | |||
| Pleural adhesion (yes vs no) | 1.74 (0.94–3.24) | 0.08 | |||
| AF ablation (yes vs no) | 1.52 (0.2–2.51) | 0.101 | |||
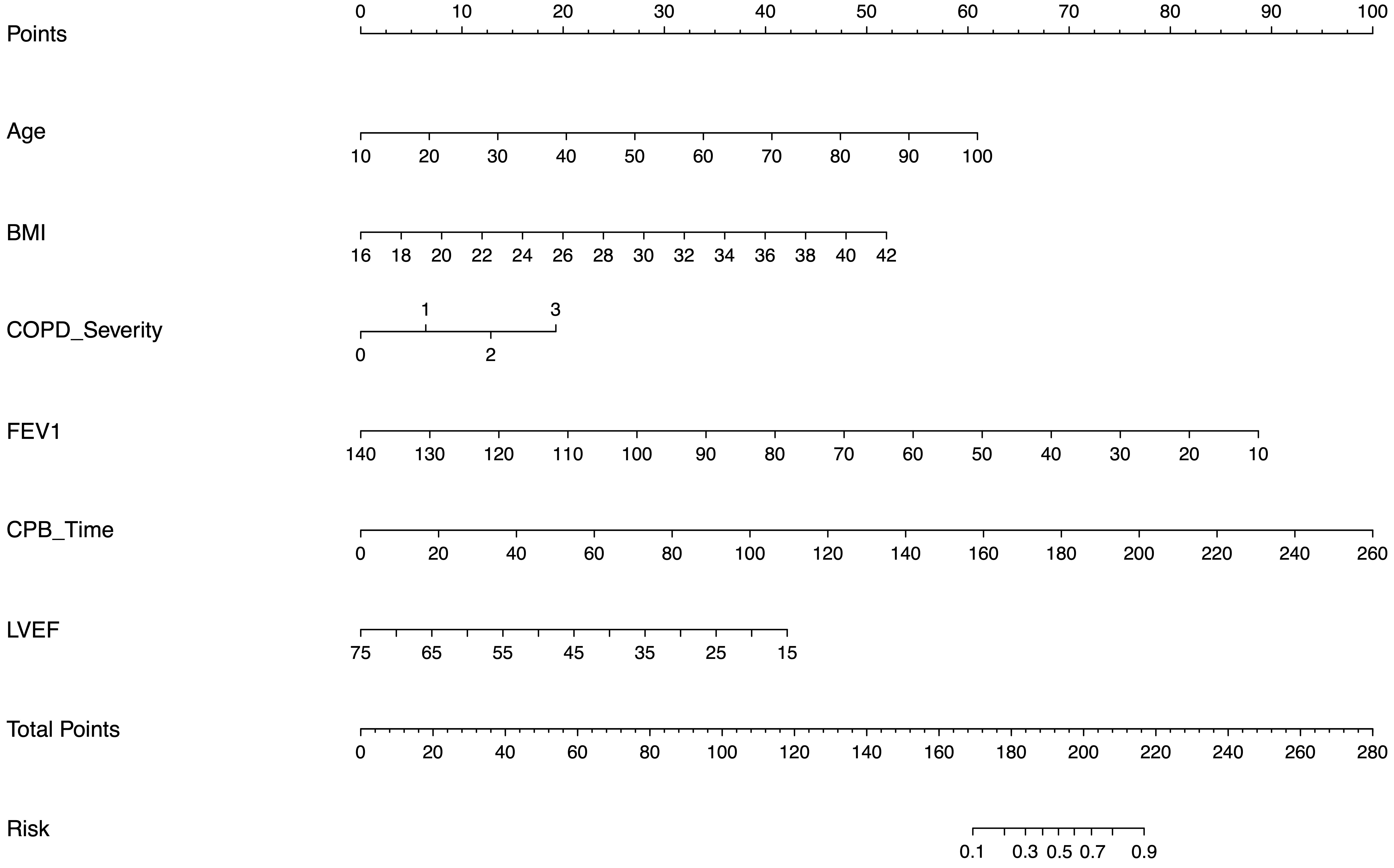 Fig. 2.
Fig. 2. Multivariable nomogram for predicting postoperative mechanical ventilation risk. The visual scoring tool incorporates six independent predictors: age (per 1-year increase), body mass index (BMI; per 1 kg/m2 increase), chronic obstructive pulmonary disease (COPD) severity (per 1-grade increase), predicted forced expiratory volume in 1 second (FEV1%; per 1% increase), left ventricular ejection fraction (LVEF%; per 1% decrease), and cardiopulmonary bypass (CPB) time (per 10-minute increase). The total score points correspond to the patient-specific probability of requiring mechanical ventilation for
The predictive model for PMV demonstrated strong calibration performance. In the derivation cohort, the mean absolute error between predicted and observed probabilities was 0.011 (Fig. 3). The model also showed robust discriminatory ability, with an AUC of 0.847 (95% CI: 0.798–0.882) during initial testing (Fig. 4). Internal validation confirmed the model’s stability. The mean absolute error was 0.019 (Fig. 3), and the AUC remained high at 0.891 (95% CI: 0.858–0.927) (Fig. 4).
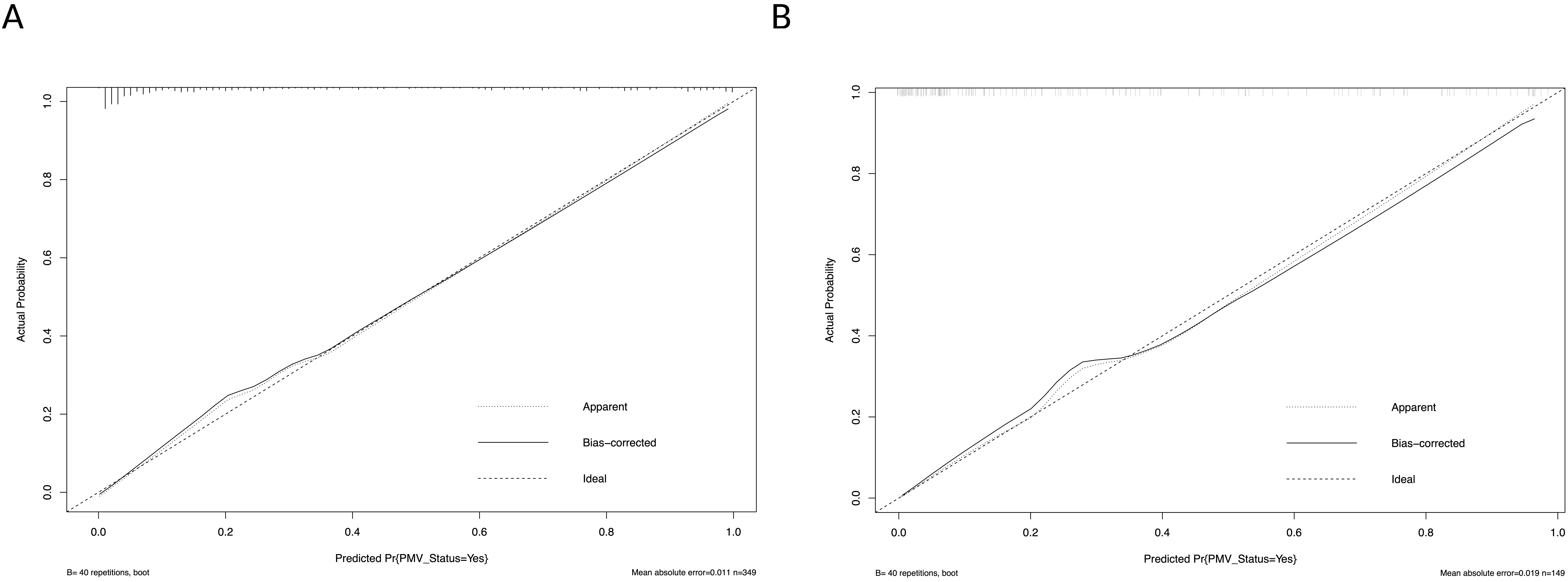 Fig. 3.
Fig. 3. Calibration curves of the prolonged mechanical ventilation (PMV) prediction model. (A) Calibration in the derivation cohort (training set, n = 349) showed strong agreement between predicted probabilities and observed PMV rates, with a mean absolute error (MAE) of 0.011. (B) Internal validation cohort (n = 149) demonstrated comparable calibration performance (MAE = 0.019). The dashed diagonal line represents ideal prediction accuracy. Both curves indicate minimal systematic bias, supporting the nomogram’s reliability for clinical risk stratification. PMV was defined as mechanical ventilation lasting
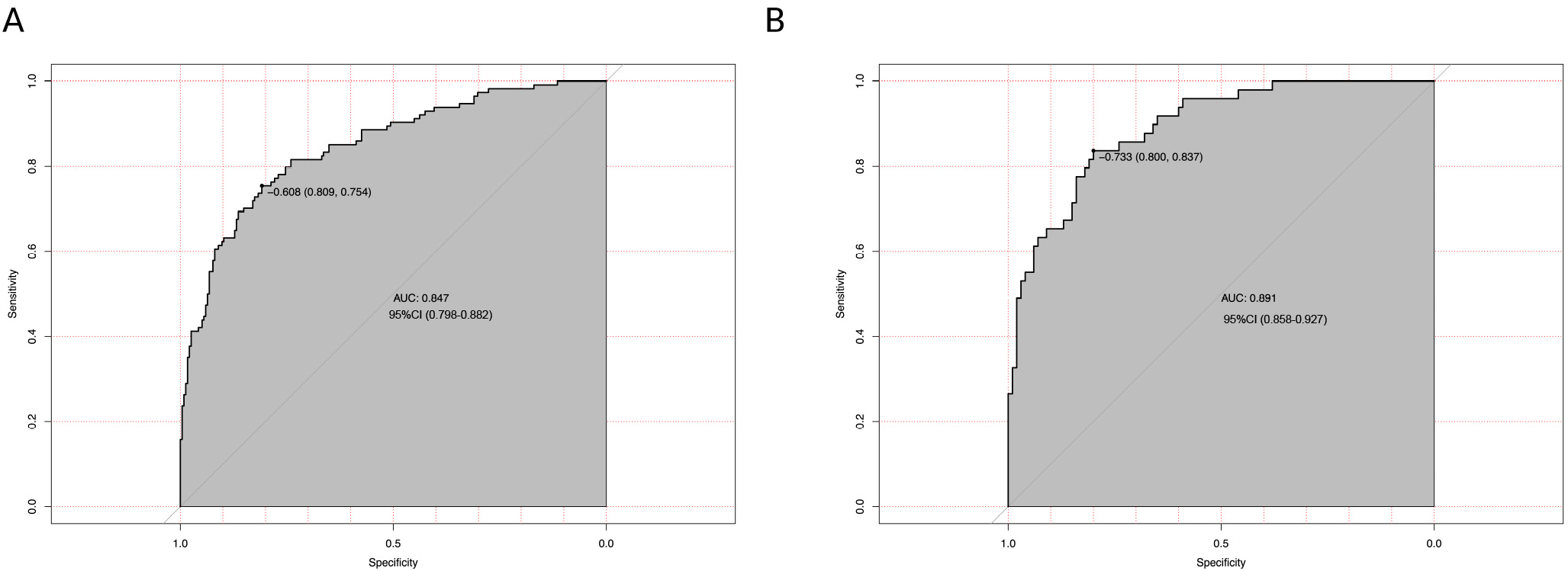 Fig. 4.
Fig. 4. Receiver operating characteristic (ROC) curves evaluating the discriminatory performance of the prediction model for prolonged mechanical ventilation (PMV). (A) In the derivation cohort (training set, n = 349), the model achieved an area under the curve (AUC) of 0.847 (95% CI: 0.798–0.882), indicating excellent discrimination between patients requiring PMV and those extubated within 48 hours. (B) In the internal validation cohort (n = 149), model performance remained high, with an AUC of 0.891 (95% CI: 0.858–0.927). The diagonal reference line indicates chance-level prediction. PMV was defined as mechanical ventilation lasting
DCA was used to quantitatively assess the clinical utility of the PMV prediction model. As shown in Fig. 5, the model demonstrated superior net benefit compared with the strategies of treating all or no patients across a wide range of clinically relevant threshold probabilities. This suggests that applying the model to guide clinical decisions—such as targeted ICU resource allocation or early preventive measures for high-risk individuals—offers greater clinical value than indiscriminate or absent intervention.
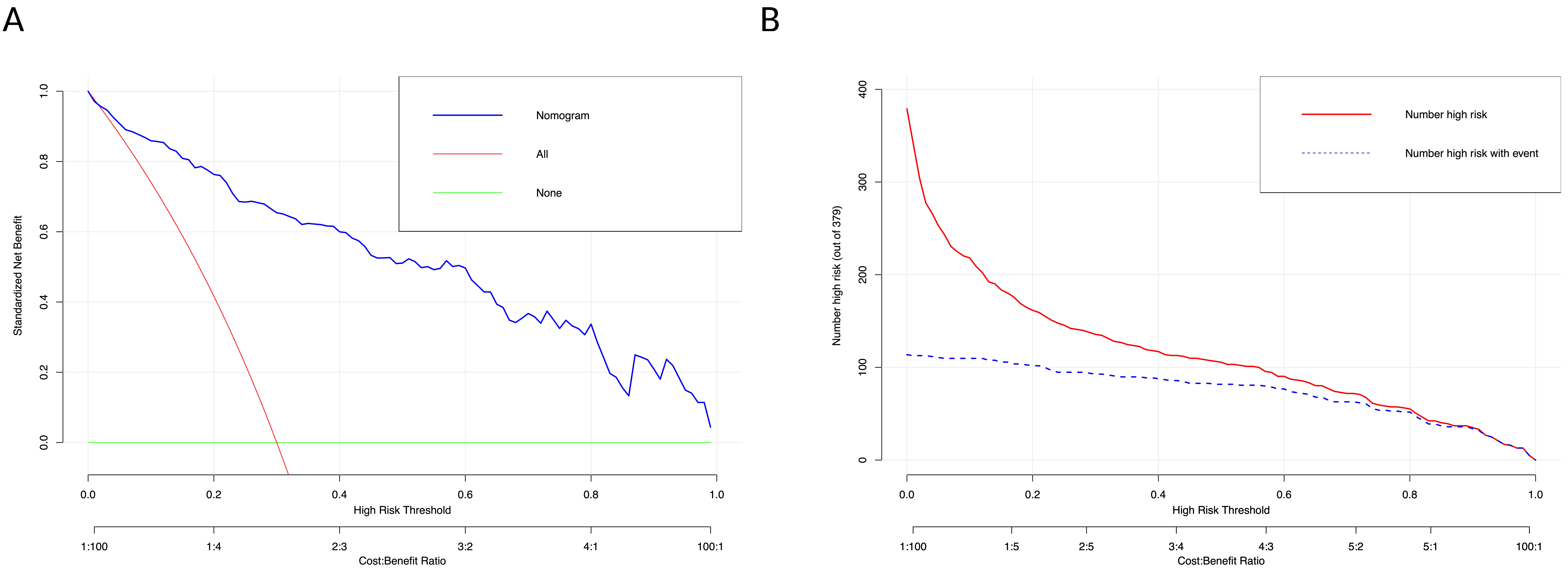 Fig. 5.
Fig. 5. Clinical utility of the prediction model for prolonged mechanical ventilation (PMV), assessed using decision curve analysis (DCA) and clinical impact curves (CIC). (A) DCA quantifies the net clinical benefit of the predictive nomogram (red curve) compared with treating all or no patients across a range of threshold probabilities (0–100%). The model shows superior clinical utility across nearly all thresholds range, with cost-benefit ratios indicated on the x-axis. (B) CIC evaluates the number of patients classified as high-risk (red curve) versus the number of actual PMV events (blue curve) at each threshold probability. Below the 40% threshold (dashed vertical line), high-risk classifications exceed observed PMV cases, indicating increased false positives. At the prespecified 40% cutoff, the model achieves optimal alignment between predicted and observed outcomes, maximizing true-positive identification while maintaining an acceptable false-positive rate. These findings support the model’s application in targeted ICU resource allocation. PMV was defined as mechanical ventilation lasting
Further assessment using a clinical impact curve revealed that, at thresholds below 40%, the number of false-positive classifications (i.e., patients identified as high-risk who did not experience PMV) increased disproportionately relative to true positives (Fig. 5). This underscores the need for careful threshold selection to balance the benefits of intervention with the risk of overtreatment. Calibration-guided threshold analysis identified a predicted risk probability of 0.40 as the optimal cutoff. At this threshold, true-positive identification was maximized while maintaining an acceptable false-positive rate, supporting its use for targeted postoperative respiratory monitoring. Overall, both DCA and the clinical impact curve confirmed that predicted probability thresholds between 40% and 100% yielded a meaningful clinical benefit. Within this range, the model achieved an optimal trade-off between minimizing false negatives and avoiding false positives. These findings support the model’s utility in enhancing perioperative decision-making and ICU resource allocation for patients undergoing valve surgery.
This study developed and internally validated a predictive nomogram incorporating six readily available clinical variables—age, BMI, COPD severity, FEV1%, LVEF%, and CPB time—to stratify the risk of PMV (
Our findings align with previously identified predictors for PMV [4, 9, 11, 16, 17], including advanced age, impaired cardiac function (reduced LVEF%), and prolonged CPB duration. These factors reflect established pathophysiologic mechanisms linking cardiopulmonary stress to postoperative ventilatory dependency [18]. However, our model improves upon prior approaches in several ways. First, unlike models such as the Intensive Care Unit Respiratory Support Score (ICURSS), which rely solely on static preoperative variables, or the Simplified Acute Physiology Score II (SAPS II), which focuses on ICU parameters [19, 20], our nomogram incorporates intraoperative data (i.e., CPB duration), thereby improving temporal relevance to surgical insult. Second, the model is procedure-specific, targeting valve surgery rather than mixed cohorts dominated by coronary artery bypass grafting [1, 3, 7, 8], allowing greater sensitivity to the unique intraoperative and postoperative risks of valvular procedures. Third, the use of a
Beyond risk prediction, this model offers a platform for real-time, actionable clinical decision-making. Patients identified as high-risk (
This study has several limitations that merit consideration. Its retrospective, single-center design introduces potential selection bias and limits generalizability to centers with different surgical practices or case mixes. Although internal validation was rigorous, external verification in multicenter populations—particularly those with higher extracorporeal membrane oxygenation or urgent procedures—is needed. The exclusion of dynamic postoperative data (e.g., serial blood gases or sedation depth) limits the model’s capacity for recalibration, which could be addressed in future iterations using machine learning algorithms. While the nomogram is simple and clinically accessible, its real-world impact depends on integration into hospital informatics systems for automated risk scoring. Future studies should assess whether model-based preoperative interventions (e.g., preoperative physiotherapy for high-risk patients) translate into reduced PMV incidence and better resource utilization. Despite these limitations, the current model provides a physiologically informed, clinically actionable tool for preoperative risk stratification and resource allocation in heart valve surgery.
This study developed and internally validated a predictive model for PMV (
Prolonged mechanical ventilation (PMV
The datasets supporting this study are included in the article. To preserve participant confidentiality, raw datasets containing identifiable information remain restricted. De-identified data may be accessed by qualified researchers contingent upon ethical approval from their institution and authorization by our ethics committee. Requests must be submitted to the corresponding author, requiring submission of a methodologically sound proposal and institutional review board certification. Approved applicants will receive anonymized data in alignment with GDPR/regional data protection laws, contingent on executed data use agreements. Full compliance with institutional privacy protocols and collaborative governance frameworks is mandated for data utilization.
YQW (First Author): Conceptualization, Methodology, Software, Investigation, Resources, Supervision, Formal Analysis, Validation Writing — Original Draft. QYZ, HDT, LC and LWC: Data Curation, Visualization, Funding Acquisition, Investigation, Writing — Original Draft and Writing — Review & Editing. XYC (Corresponding Author): Conceptualization, Funding Acquisition, Resources, Supervision, Writing — Review & Editing. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
All authors confirm accountability for study integrity, including thorough investigation of any data accuracy concerns. The protocol received ethical approval under the Declaration of Helsinki (2013 revision) and approved by the Institutional Review Board (IRB) of Zhangzhou Affiliated Hospital of Fujian Medical University (Approval No. 2025LWB257), which waived the requirement for informed consent due to the retrospective study design, with written informed consent obtained from participants for data publication and image usage. To ensure ethical rigor, explicit reconsent was secured from participants or legal proxies for secondary data utilization in this research. No conflicts of interest were reported by investigators. The study protocol posed no physical or psychological risks, as verified by an independent institutional review board. Methodological adherence to privacy standards and risk mitigation protocols was maintained throughout data collection and analysis.
Not applicable.
This work was supported by the Startup Fund for Scientific Research, Fujian Medical University (grant number 2022QH1273).
No financial interests or institutional conflicts of interest were declared by the authors (Yueqiong Wang, Qiuyan Zhao, Huadong Tang, Ling Chen, Liangwan Chen and Xiaoyun Chen). The manuscript, including text, tables and figures, represents original work free from third-party intellectual property infringement. All visual and textual content has not been previously published or submitted elsewhere. Ethical standards for originality and attribution were rigorously maintained throughout the research and publication process.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.





