1 Department of Ultrasound, Zhuji People’s Hospital of Zhejiang Province, 311800 Zhuji, Zhejiang, China
2 Department of Cardiology, The First Affiliated Hospital, Zhejiang University School of Medicine, 310000 Hangzhou, Zhejiang, China
3 Cardiovascular Ultrasound Center, The First Affiliated Hospital, Zhejiang University School of Medicine, 310000 Hangzhou, Zhejiang, China
Abstract
Since the decision to proceed with valve re- placement remains controversial due to conflicting prog- nostic evidence, the use of normal-flow low-gradient aor- tic stenosis (NFLGAS) presents a clinical dilemma. Thus, this study aimed to evaluate the clinical utility of two- dimensional strain echocardiography (2D-STE) in distin- guishing therapeutic outcomes between transcatheter aortic valve implantation (TAVI) and conservative management in patients with NFLG AS.
This retrospective cross-sectional study analyzed 97 patients diagnosed with NFLG AS between October 2019 and June 2023. Patients were divided into two groups based on treatment strategy: 34 underwent TAVI, and 63 received conservative management. Clinical data were collected at baseline, discharge, and 6-month follow-up. Key echocardiographic parameters included left ventricular (LV) ejection fraction (LVEF), aortic valve area (AVA), relative wall thickness (RWT), obtained via transthoracic echocardiography (TTE), and LV global longitudinal strain (LVGLS) measured using 2D-STE. Multivariable linear regression models were used to adjust for potential confounding factors. Kaplan–Meier analysis was employed to compare 6-month cardiac event-related readmission rates between the two groups.
Preoperatively, the mean LVGLS was –14.2% ± 1.5%. In the TAVI group, LVGLS significantly improved to –16.7% ± 1.4% at discharge and further to –18.5% ± 1.3% at 6-month follow-up (p < 0.001). After adjusting for potential confounders, the improvement in LVGLS remained significant in the TAVI group (p < 0.001). In contrast, the conservative management group showed no significant changes in LVGLS across the same time points (–14.0% ± 1.8%, –14.2% ± 1.6%, and –14.7% ± 2.2%, respectively; p = 0.118). The TAVI group also exhibited a significantly lower 6-month cardiac event-related readmission rate compared to the conservative group (χ2 = 4.53; p = 0.033; hazard ratio (HR) = 0.47, 95% confidence interval (CI): 0.24–0.94).
These preliminary findings suggest that TAVI may offer significant improvements in LVGLS and reduce short-term cardiac event-related readmissions in symptomatic NFLG AS patients. Nonetheless, further validation in larger, prospective studies is warranted to confirm these potential clinical benefits.
Keywords
- aortic stenosis
- TAVI
- echocardiography
Assessing the severity of aortic stenosis (AS) remains clinically challenging, as discrepancies frequently arise among peak velocity, mean pressure gradient, and aortic valve area (AVA), leading to conflicting severity classifications [1]. A mismatch between transvalvular hemodynamic parameters, specifically, a mean pressure gradient
Contemporary registry data suggest similar all-cause mortality rates among patients with NF-LG AS, LF-LG AS, and high-gradient AS (HG-AS), with no statistically significant differences observed in adjusted survival analyses [5]. Consequently, whether aggressive AVR is appropriate for patients with NF-LG AS remains a topic of debate in current clinical practice.
Although AS contributes to progressive left ventricular (LV) dysfunction, LVEF often lacks the sensitivity to detect early myocardial impairment. In contrast, strain echocardiography (STE), an advanced imaging technique, has demonstrated superior sensitivity over conventional parameters in detecting subclinical myocardial deformation related to AS-induced remodeling [6]. Specifically, LV global longitudinal strain (LVGLS) and layer-specific strain analyses have shown particular value in identifying early myocardial dysfunction in AS patients [7]. Building on this diagnostic capability, we hypothesize that STE can similarly detect functional myocardial abnormalities in patients with NF-LG AS, the focus of this study.
This retrospective study evaluated two-dimensional strain echocardiography (2D-STE)-derived myocardial deformation indices and clinical outcomes in NF-LG AS patients undergoing either transcatheter aortic valve implantation (TAVI) or conservative management. Given the hemodynamic complexity and uncertain prognosis in this patient population, we aimed to determine whether TAVI offers a clinical advantage. By systematically comparing echocardiographic functional parameters and 6-month cardiac event-related readmission rates, this study seeks to provide nuanced evidence to inform optimal management strategies for this challenging patient subset.
This retrospective study was conducted at The First Affiliated Hospital, Zhejiang University School of Medicine, between October 2019 and June 2023. Inclusion criteria were as follows: (1) a diagnosis of NF-LG AS confirmed by multidetector computed tomography (MDCT), transthoracic echocardiography (TTE), and dobutamine stress echocardiography; (2) AVA
Exclusion criteria included: (1) rheumatic valvular disease; (2) severe mitral regurgitation; (3) bicuspid aortic valve; (4) persistent atrial fibrillation; (5) prior percutaneous coronary intervention (PCI); (6) ischemic cardiomyopathy with regional wall motion abnormalities; (7) poor echocardiographic image quality due to inadequate acoustic windows; (8) absence of cardiovascular symptoms; and (9) missing follow-up data within six months, which led to exclusion from the final analysis to ensure data integrity.
A screening flowchart is presented in Fig. 1. The study protocol was approved by the Ethics Committee of the First Affiliated Hospital, Zhejiang University School of Medicine (Approval No. IIT20251364A).
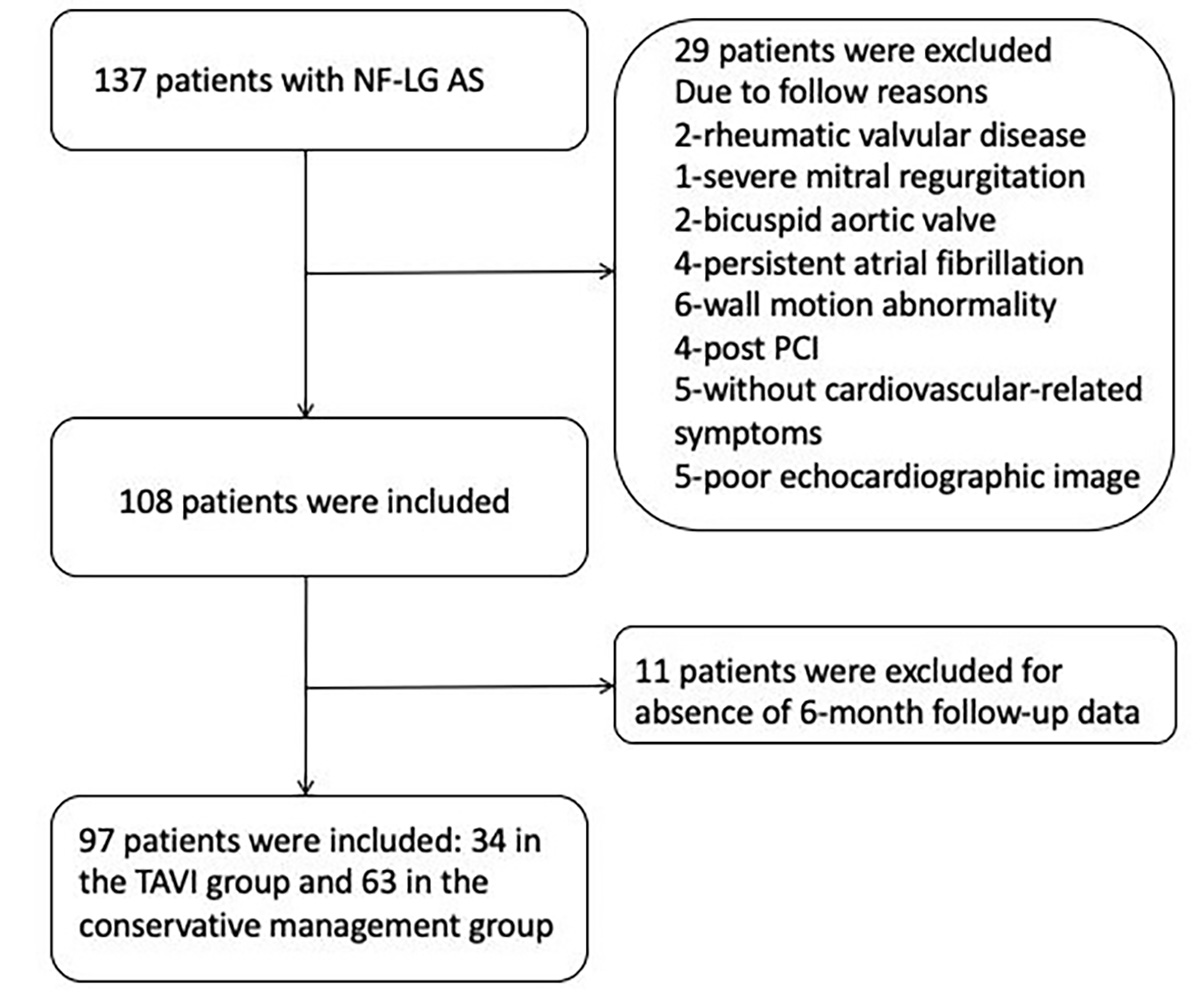 Fig. 1.
Fig. 1. Patient screening flowchart. NF-LG AS, normal-flow low-gradient aortic stenosis; PCI, percutaneous coronary intervention; TAVI, transcatheter aortic valve implantation.
Baseline demographic and clinical parameters, including New York Heart Association (NYHA) functional class, comorbidities (hypertension, diabetes mellitus, coronary artery disease, and chronic heart failure), and brain natriuretic peptide (BNP) levels, were systematically extracted from institutional electronic medical records using predefined data abstraction protocols.
Serial echocardiographic evaluations were performed at three time points: initial hospitalization, post-treatment discharge, and 6-month follow-up. Standard TTE included measurements of LVEF, assessed using the biplane Simpson’s method; relative wall thickness (RWT), calculated as (interventricular septal thickness + LV posterior wall thickness) divided by LV internal dimension at end-diastole; AVA; and mean aortic valve pressure gradient.
LVGLS was assessed using a Philips EPIQ7 ultrasound system (manufacturer: Philips Healthcare, Amsterdam, Netherlands) equipped with a 2.5–5 MHz phased-array transducer. Standard apical 4-chamber (A4C), 2-chamber (A2C), and 3-chamber (A3C) views were obtained at end-expiration, with frame rates maintained between 40–80 frames per second. LVGLS was quantified via speckle-tracking STE using QLab Cardiac Motion Quantification software (v11.0, Philips Ultrasound, Bothell, Washington, USA), by analyzing myocardial deformation across the three apical views. The final LVGLS value was calculated as the arithmetic mean of the strain measurements from A4C, A2C, and A3C views. Manual adjustment of region-of-interest boundaries was performed to ensure complete myocardial wall coverage (Fig. 2).
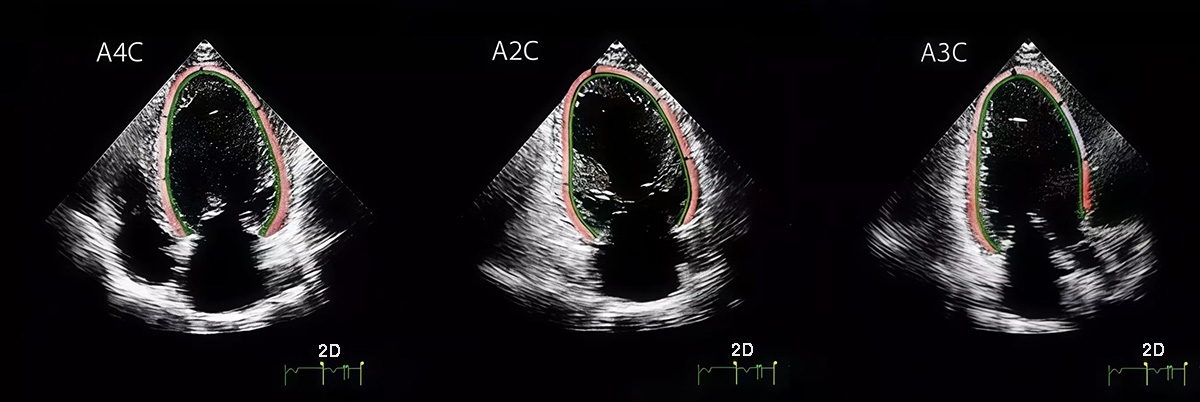 Fig. 2.
Fig. 2. LVGLS was obtained by analyzing standard A4C, A2C, and A3C views using Qlab strain analysis and calculated from these three views. LVGLS, LV global longitudinal strain; A4C, apical 4-chamber; A2C, apical 2-chamber; A3C, apical 3-chamber. The red and green lines represent the LVGLS sampling lines along which data were collected.
All echocardiographic measurements were performed in accordance with the guidelines of the American Society of Echocardiography (ASE) and independently validated by a senior cardiac sonographer [8].
All statistical analyses were performed using R (version 4.3.1; R Foundation for Statistical Computing, Vienna, Austria) and GraphPad Prism (version 7.0; GraphPad Software, San Diego, CA, USA), with two-tailed significance set at p
LVEF, RWT, and LVGLS were assessed across three time points (admission, discharge, and 6-month follow-up) using repeated-measures analysis of variance (ANOVA) to evaluate longitudinal changes within each treatment group.
To compare outcomes between the two treatment groups, we utilized multivariable linear regression models for continuous variables, thoroughly adjusting for potential confounders such as age, weight, BNP, AVA, and other relevant factors.
Kaplan-Meier survival analysis was conducted to compare 6-month cardiac event-related readmission rates between the TAVI and conservative management groups. Cardiac events were predefined as heart failure exacerbation, arrhythmias, myocardial infarction, or valve-related complications.
A total of 97 patients were included in this study. Of these, 34 patients underwent TAVI, with a mean age of 73.9
Hypertension was the most common comorbidity in both groups, with a prevalence of 73.5% in the TAVI group and 74.6% in the conservative management group. Hypertension was defined according to Chinese diagnostic criteria as systolic blood pressure
There were no significant between-group differences in BNP levels (TAVI: 461.6
| TAVI (n = 34) | CM (n = 63) | p-value | ||
| Age (years) | 73.2 | 74.1 | 0.409 | |
| Male sex | 23 (67.7%) | 41 (65.1%) | 0.799 | |
| Height (cm) | 167.7 | 168.4 | 0.510 | |
| Weight (kg) | 64.9 | 65.3 | 0.554 | |
| Body surface area (m2) | 1.74 | 1.74 | 0.814 | |
| NYHA class III–IV | 22 (64.7%) | 37 (58.7%) | 0.565 | |
| AVA (cm2) | 0.76 | 0.78 | 0.095 | |
| AVA index (cm2/m2) | 0.446 | 0.450 | 0.456 | |
| BNP (pg/mL) | 461.6 | 434.5 | 0.452 | |
| Comorbidities: | ||||
| Hypertension | 25 (73.5%) | 47 (74.6%) | 0.908 | |
| Diabetes mellitus | 13 (38.2%) | 26 (41.3%) | 0.771 | |
| Coronary artery disease | 15 (44.1%) | 28 (44.4%) | 0.975 | |
| Chronic heart failure | 10 (29.4%) | 19 (30.2%) | 0.939 | |
TAVI, transcatheter aortic valve implantation; NYHA, New York Heart Association; AVA, aortic valve area; BNP, brain natriuretic peptide.
All patients in the TAVI cohort voluntarily consented to undergo the procedure due to symptoms secondary to severe aortic stenosis and provided written informed consent. All interventions were performed via the transfemoral approach, with successful closure of the vascular access site in every case. Importantly, no patients required surgical repair for vascular access complications. All TAVI procedures were completed successfully without intraoperative complications, and no cases of severe aortic regurgitation were observed post-procedure. Additionally, there were no instances of perioperative mortality during the index hospitalization.
Longitudinal echocardiographic assessments demonstrated comparable temporal patterns of cardiac function across both treatment groups. In the TAVI cohort, LVEF remained stable over time (admission: 61.4
Similarly, in the conservative management group, LVEF remained stable throughout the follow-up period (admission: 60.7
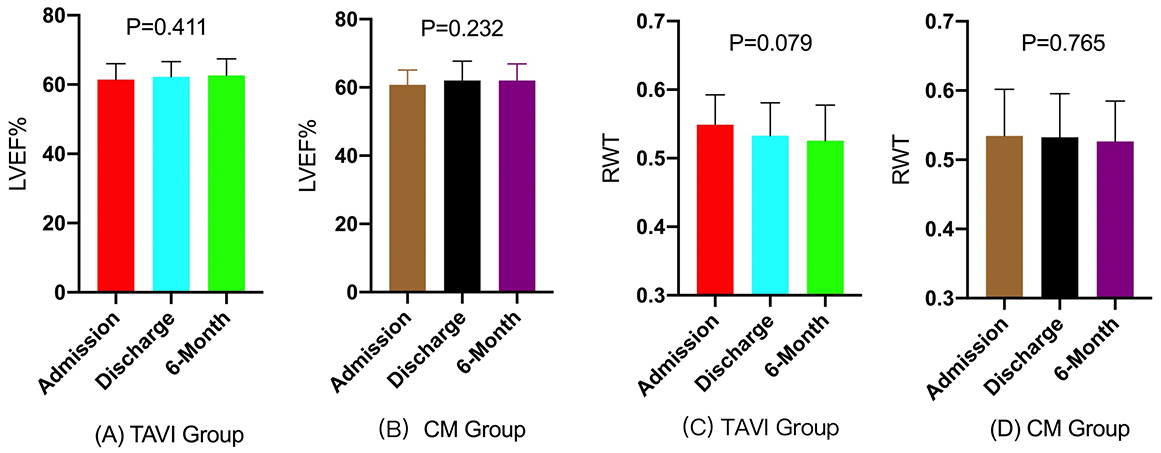 Fig. 3.
Fig. 3. Bar graphs of longitudinal changes in LVEF and RWT. (A–D) Longitudinal Changes in LVEF and RWT in TAVI and Conservative management (CM) Groups, Demonstrating No Significant Statistical Differences Across Three Time Points. Normality testing confirmed all continuous variables were suitable for parametric analysis (Shapiro-Wilk p
| Group | Admission | Discharge | 6-Month | p-value | |
| LVEF (%) | TAVI | 61.4 | 62.2 | 62.6 | 0.411 |
| LVEF (%) | CM | 60.7 | 62.1 | 62.0 | 0.232 |
| RWT | TAVI | 0.549 | 0.533 | 0.526 | 0.079 |
| RWT | CM | 0.534 | 0.532 | 0.527 | 0.765 |
All continuous variables were confirmed to be normally distributed based on the Shapiro-Wilk test (p
Preoperative LVGLS in the TAVI group demonstrated moderate impairment, with a mean value of –14.2%
| Group | Admission | Discharge | 6-Month | p-value | |
| LVGLS (%) | TAVI (n = 34) | –14.2 | –16.7 | –18.5 | |
| LVGLS (%) | CM (n = 63) | –14.0 | –14.2 | –14.7 | 0.118 |
All continuous variables were confirmed to be normally distributed based on the Shapiro-Wilk test (p
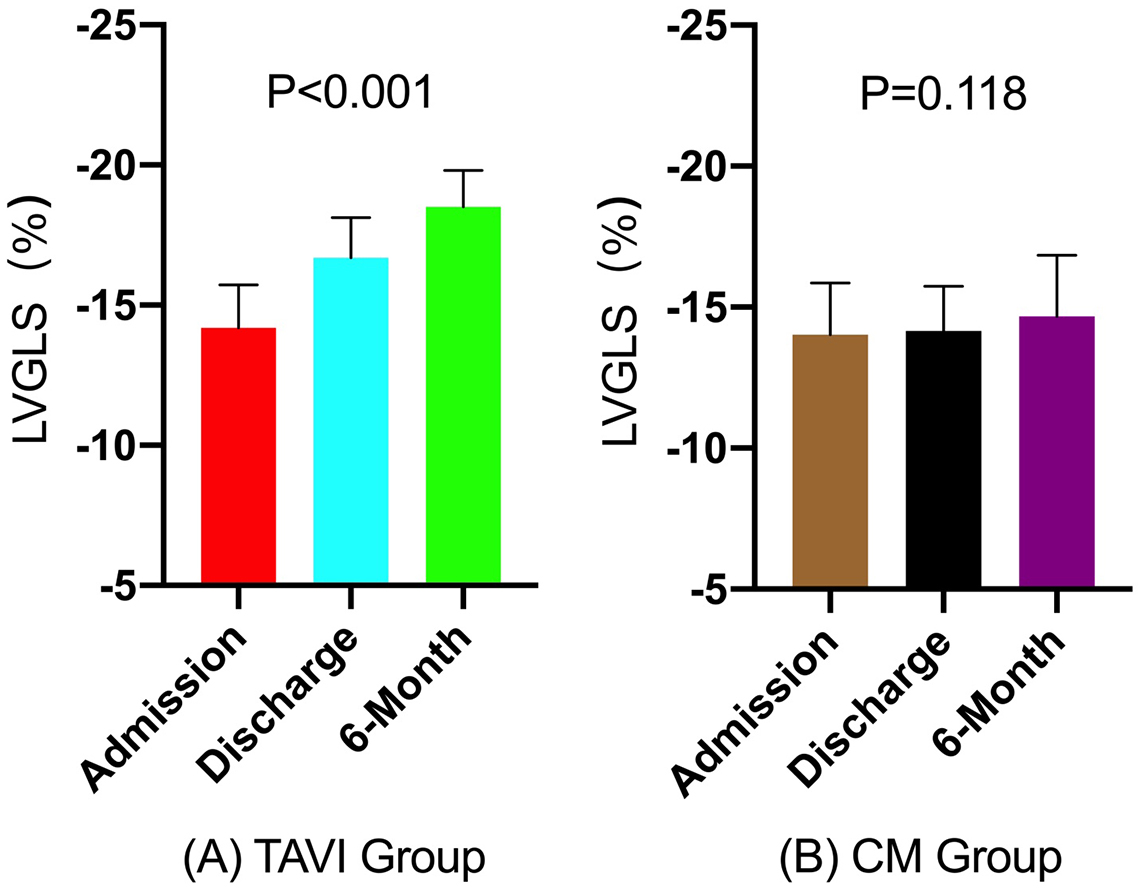 Fig. 4.
Fig. 4. Bar graphs of longitudinal changes in LVGLS. (A) TAVI group, (B) Conservative management group (CM). LVGLS in the TAVI group significantly improved at discharge and 6-month follow-up compared to baseline (p
At admission, LVGLS measurements were comparable between the TAVI and CM groups across all analytical models (all p
| Model 1 | Model 2 | Model 3 | Model 4 | |||||
| p | p | p | p | |||||
| Group | ||||||||
| CM | Ref. | Ref. | Ref. | Ref. | ||||
| TAVI | –0.17 (–0.90 to 0.55) | 0.640 | –0.17 (–0.91 to 0.57) | 0.655 | –0.19 (–0.96 to 0.57) | 0.624 | –0.36 (–1.11 to 0.38) | 0.341 |
Model 1: Crude model; Model 2: Adjusted for sex, age, height, and weight; Model 3: Additionally, adjusted for hypertension, coronary artery disease, diabetes mellitus, heart failure, and NYHA class; Model 4: Further adjusted for BNP, AVA, and AVA index.
At discharge, the TAVI group exhibited more favorable changes in LVGLS compared to the CM group. This finding was confirmed in the fully adjusted Model 4 (
| Model 1 | Model 2 | Model 3 | Model 4 | |||||
| p | p | p | p | |||||
| Group | ||||||||
| CM | Ref. | Ref. | Ref. | Ref. | ||||
| TAVI | –2.54 (–3.19 to –1.90) | –2.54 (–3.20 to –1.88) | –2.56 (–3.24 to –1.88) | –2.72 (–3.37 to –2.07) | ||||
Model 1: Crude model; Model 2: Adjusted for sex, age, height, and weight; Model 3: Further adjusted for hypertension, coronary artery disease, diabetes mellitus, heart failure, and NYHA; Model 4: Additionally, adjusted for BNP, AVA, and AVA index.
The observed treatment benefit remained statistically significant at the 6-month follow-up (p
| Model 1 | Model 2 | Model 3 | Model 4 | |||||
| p | p | p | p | |||||
| Group | ||||||||
| CM | Ref. | Ref. | Ref. | Ref. | ||||
| TAVI | –3.83 (–4.63 to –3.04) | –3.82 (–4.63 to –3.01) | –3.84 (–4.68 to –3.01) | –4.07 (–4.87 to –3.26) | ||||
Model 1: Crude model; Model 2: Adjusted for sex, age, height, and weight; Model 3: Further adjusted for hypertension, coronary artery disease, diabetes mellitus, heart failure, and NYHA; Model 4: Additionally, adjusted for BNP, AVA, and AVA index.
During the 6-month follow-up period, no mortality was observed in either cohort. However, there were 8 cardiac event-related readmissions in the TAVI group, compared to 26 in the conservative management group. Kaplan-Meier analysis with log-rank test confirmed a significantly lower cumulative incidence of cardiac event-related readmission in the TAVI group compared to the CM group (
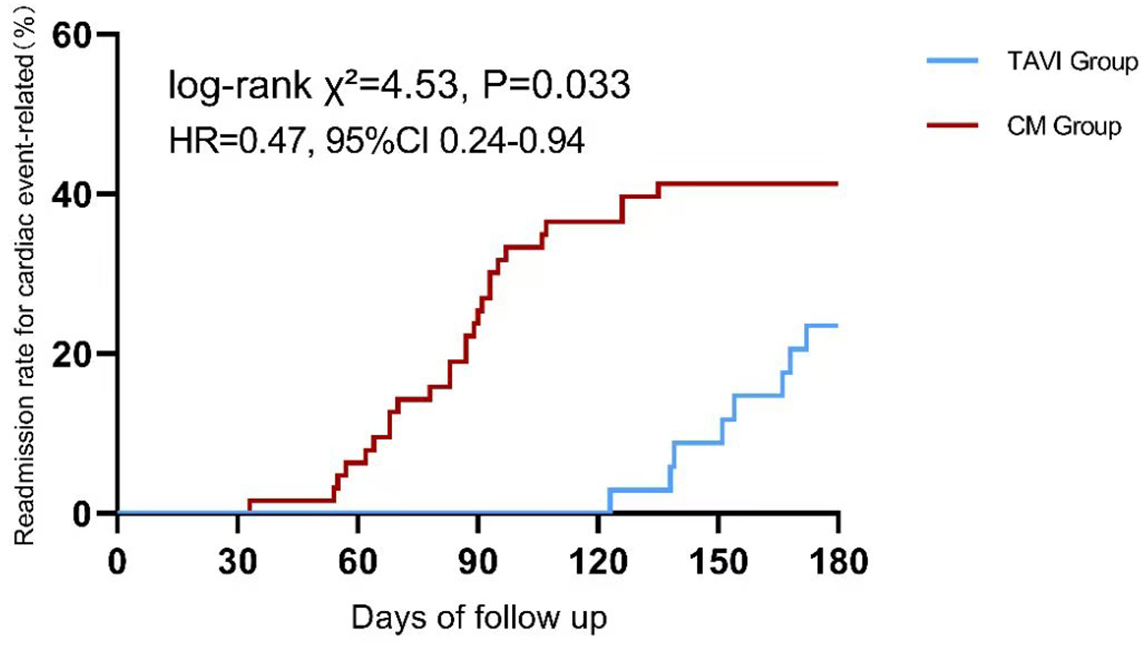 Fig. 5.
Fig. 5. Kaplan-Meier curve comparing cardiac event-related readmission rates between TAVI and conservative management group (CM) at 6-month follow-up.
Our study observed that symptomatic patients with NF-LG AS who underwent TAVI exhibited sustained improvements in LVGLS and experienced lower 6-month readmission rates compared to those managed conservatively. These findings are consistent with previous observations in HG-AS populations following TAVI, suggesting that valve replacement may confer hemodynamic benefits across AS subtypes, despite differing transvalvular pressure gradients [11]. Recent studies have begun to challenge traditional management paradigms for NF-LG AS. Specifically, emerging data indicate that patients with NF-LG AS may have long-term mortality rates comparable to those with HG-AS, despite markedly different hemodynamic profiles at baseline [12]. Notably, a single-center retrospective analysis of 860 patients with aortic stenosis, 28.5% of whom were classified as NF-LG AS, reported that the one-year mortality rate in NF-LG AS patients was significantly higher than in the most prevalent HG-AS subgroup, contradicting conventional clinical assumptions [13].
The diagnostic complexity of NF-LG AS likely contributes to delayed intervention in routine practice. Although preserved LVEF may give the false impression of normal myocardial function, mounting evidence indicates that subclinical ventricular dysfunction, driven by progressive myocardial fibrosis, occurs well before overt LVEF deterioration [14]. This pathophysiological trajectory is particularly problematic in NF-LG AS, where diagnostic ambiguity hinders timely and appropriate management.
TTE, although recommended as the first-line imaging modality by the ASE, presents technical limitations in this context [15]. In particular, TTE relies on the geometric assumption that the LV outflow tract (LVOT) is circular, when it is often elliptical. This mismatch introduces systematic error in AVA calculation, potentially leading to misclassification of AS severity [16]. MDCT offers more accurate anatomical assessment, including sex-specific Agatston unit (AU) thresholds for severe AS (
In addition to the diagnostic complexities of NF-LG AS, assessing the presence of LV myocardial dysfunction poses a further challenge, as LVEF often remains within the normal range in this patient population. Our findings reinforce the emerging utility of STE in detecting early, subclinical myocardial dysfunction. Unlike LVEF, LVGLS offers a more sensitive measure of subtle contractile impairment. The helically oriented subendocardial myofibers are particularly vulnerable to early microstructural damage from ischemia, fibrosis, or inflammation. By tracking the spatial displacement of natural acoustic markers within the myocardium, LVGLS quantifies the percentage of longitudinal shortening during systole, thereby directly reflecting tissue-level mechanical deformation [22, 23].
In our study, the early and sustained improvement in LVGLS following TAVI suggests that strain-derived indices may serve as dynamic biomarkers of reverse ventricular remodeling. These parameters potentially offer superior sensitivity to early functional recovery compared to traditional structural measures. Persistent RWT deviations observed in our cohort (p = 0.079 for the 6-month trend, approaching but not reaching statistical significance) may reflect the limited duration of post-TAVI follow-up. This finding highlights the nonlinear nature of ventricular remodeling, wherein early improvements in myocardial function (as captured by LVGLS) may precede structural normalization. This temporal dissociation suggests that distinct biological mechanisms may govern functional adaptation and anatomical recovery [24]. Similar observations have been reported in CMR studies, where changes in myocardial strain often precede visible structural remodeling, supporting the concept of decoupled functional and anatomical recovery in the context of myocardial repair [25].
TAVI has become an established and guideline-recommended treatment for patients with severe aortic stenosis. Its advantages, including minimal invasiveness, avoidance of cardiopulmonary bypass, and faster postoperative recovery, make it a particularly attractive therapeutic option [26]. The growing clinical adoption of TAVI has enhanced the recognition and treatment of diverse AS hemodynamic phenotypes with adverse prognostic implications, including LF-LG, paradoxical LF-LG, and NF-LG AS. Preliminary clinical investigations have already begun exploring the role of TAVI in NF-LG AS patients, further supporting the need to refine diagnostic and treatment approaches for this complex subgroup [27].
The pathophysiology of AS extends beyond isolated valvular obstruction, exerting widespread effects on cardiac structure and function through complex and multifactorial mechanisms. Persistent pressure overload caused by the stenotic valve induces compensatory LV hypertrophy in an effort to normalize wall stress and preserve systolic function. However, subendocardial myocytes, particularly those aligned longitudinally, are highly susceptible to ischemia due to reduced coronary perfusion. This vulnerability contributes to early impairment in longitudinal function [28]. Over time, chronic pressure overload leads to irreversible myocardial fibrosis and dysfunction, particularly in the longitudinally oriented fibers. This explains why global LV afterload, LV mass, and myocardial replacement fibrosis independently correlate with impaired LVGLS in AS patients [29].
Following TAVI, the rapid improvement in LVGLS reflects a series of intricate physiological adaptations beyond simple hemodynamic relief. While afterload reduction is central, concurrent microvascular and cellular-level changes also play pivotal roles. Early improvements in strain are partially attributable to restored myocardial perfusion, especially in patients with preserved ejection fraction, through enhancement of coronary flow reserve [30]. Additionally, the reduction in mechanical stress triggers neurohormonal modulation that gradually reverses interstitial fibrosis, a process that typically becomes evident only months after the procedure [31]. At the subcellular level, normalization of calcium handling and mitochondrial function in previously pressure-overloaded cardiomyocytes further contributes to myocardial recovery, although these mechanisms require further investigation [32]. This stepwise recovery, encompassing functional, microvascular, structural, and cellular remodeling, underlies the longitudinal improvement in LVGLS observed after TAVI.
LVGLS is increasingly recognized as a robust prognostic marker in patients undergoing TAVI. Meta-analytic evidence indicates that each 1% decrease in absolute LVGLS (i.e., a less negative value) is associated with a 6% increase in all-cause post-TAVI mortality and an 8% higher risk of major adverse cardiovascular events (MACE), with a hazard ratio (HR) of 1.08 for the latter [33]. Given the complex myocardial remodeling that often accompanies AS, LVGLS serves not only as a sensitive marker of LV function but also as a valuable tool in risk stratification. Its clinical relevance extends beyond predicting perioperative outcomes to informing individualized treatment planning and postoperative management. Routine preoperative assessment of LVGLS should be incorporated into clinical practice for patients undergoing TAVI, as it enables identification of high-risk individuals and supports the implementation of more tailored therapeutic strategies.
This study has several important limitations that warrant careful consideration. First, the retrospective observational design inherently limits the ability to establish causal relationships, as residual confounding may persist despite multivariable adjustments. Additionally, being a single-center, cross-sectional study with a relatively small sample size, the findings are susceptible to selection bias, particularly due to the exclusion of asymptomatic NF-LG AS patients, which may limit the generalizability of the results. The limited cohort size also restricted our ability to conduct meaningful analyses of all-cause mortality outcomes. Moreover, COVID-19-related restrictions likely affected data completeness, particularly post-discharge follow-up, potentially underestimating cardiac readmissions and introducing attrition bias. These limitations highlight the preliminary nature of our findings and warrant cautious interpretation. A large-scale, prospective randomized ̵controlled ̵trial (RCT) with long-term follow-up is needed to more accurately compare outcomes between TAVI and conservative management in NF-LG AS. Additionally, integrating STE with biomarkers and advanced imaging may improve risk stratification and support personalized care. Where RCTs are unfeasible, well-designed observational studies using techniques like propensity score matching can help mitigate bias and provide valuable clinical insights.
Symptomatic NF-LG AS is a clinically diverse condition often marked by underestimated LV dysfunction. Our findings indicate frequent LVGLS impairment in these patients and suggest that TAVI is associated with improved myocardial deformation. These results support the potential use of LVGLS for risk stratification and highlight the need for further studies to assess its prognostic value and role in guiding early intervention decisions.
AVA, aortic valve area; AVR, aortic valve replacement; BNP, brain natriuretic peptide; CHF, chronic heart failure; CM, conservative management; CMR, cardiac magnetic resonance; HFpEF, heart failure with preserved ejection fraction; LV, left ventricular; LVEF, LV ejection fraction; LVGLS, LV global longitudinal strain; LVOT, LV outflow tract; MDCT, multidetector computed tomography; NF-LG, normal flow low-gradient; PCI, percutaneous coronary intervention; RWT, relative wall thickness; RCT, randomized controlled trial; STE, strain echocardiography; SVI, stroke volume index; TAVI, transcatheter aortic valve implantation.
The data that support the findings of this study are available on request from the corresponding author.
LW and XYC designed the research study; LW and XYC participated in data collection and analysis, and writing of the manuscript; FY and ZLZ contributed to the acquisition, interpretation of data and writing some of the manuscript. All authors contributed to critical revision of the manuscript for important intellectual content. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
The study was conducted in accordance with the Declaration of Helsinki (2013) and approved by the Ethics Committee of the First Affiliated Hospital, Zhejiang University School of Medicine (Protocol No. IIT20251364A). Written informed consent was obtained from all participants. We followed all relevant guidelines and regulations during the study.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.





