1 Department of Cardiothoracic Surgery, Istanbul Medipol University, 34718 Istanbul, Türkiye
2 Department of Cardiovascular Surgery, Kartal Kosuyolu High Specialization Training and Research Hospital University of Health Sciences, 34846 Istanbul, Türkiye
Abstract
Transseptal atriotomy provides better exposure to the mitral valve in challenging cases but has conflicting results with postoperative rhythm disturbances. We aimed to investigate long term results of limited transseptal atriotomy in cases with a small left atrium.
From January 2010 through February 2014, 1214 patients underwent mitral valve surgery at the SBÜ Kartal Kosuyolu High Specialization Training and Research Hospital. Left atrium diameter on 2-dimensional (2-D) echocardiography defined in 119 patients who had small left atrium and met the inclusion criteria were enrolled in the study, of which 57 patients (47.9%) underwent transseptal atriotomy (Group TS), while 62 patients (52.1%) underwent a left atriotomy (Group LA). Data was retrospectively collected. Long-term analyses were performed based on survival. The mean follow-up duration was 10.7 ± 4.2 years.
Isolated mitral procedures were performed in 49 patients (41.2%). Concomitant tricuspid valve surgery was performed in 42 patients (35.3%), concomitant aortic valve surgery in 24 patients (20.2%), and concomitant coronary artery bypass grafting in 15 patients (12.6%). The procedure rates were comparable in both Groups (p > 0.05). There was no significant difference in pre-operative variables. Ischemic time and total perfusion time were found to be similar in the Group TS vs. Group LA (87.6 ± 33.5 vs. 77.4 ± 27.8 minutes and 117.2 ± 38.4 vs. 112.3 ± 33.8 minutes respectively; p > 0.05). New-onset arrhythmia was higher in the Group TS but did not reach statistical significance (26.3% vs. 19.4%; p = 0.5). The rate of permanent pacemaker insertion was similar (5.3% vs. 4.8%; p = 0.9). Follow-up was completed in all cases and survival rate was 64.7% (64 ± 7% in Group TS vs. 58 ± 7% in Group LA; p > 0.05). Log rank analyses shows similar survivals (Group TS: 11.7 ± 0.6 years, 95% CI: 10.5–12.9; Group LA: 11.8 ± 0.6 years, 95% CI: 10.6–12.9; p > 0.05). In the multivariate Cox regression analysis, age, obesity, procedure type, and left ventricular dysfunction were found to be independent risk factors for late mortality. Regardless of tricuspid valve surgery, concomitant coronary artery bypass grafting (CABG) had worse survival compared to isolated mitral procedures and concomitant aortic valve replacement (AVR) (12.5 ± 0.5 years for mitral, 11.4 ± 1 years for concomitant AVR, and 8.2 ± 1.2 years for concomitant CABG; p < 0.01).
Limited transseptal atriotomy was not found to be inferior when compared to left atriotomy in cases with a small left atrium undergoing while mitral valve (MV) should be performed when the exposure is challenging.
Keywords
- mitral valve surgery
- transseptal approach
- atriotomy
- small left atrium
Mitral valve disease is the most common valve pathology in the general population and the second most common in the elderly population, with an anticipated increase in prevalence over the next decade [1]. Due to its deep and inferior location, exposure and accessibility of the mitral valve is more challenging than other valves. In the society of thoracic surgery (STS) 2019 database, the mortality rate associated with mitral valve replacement is 5%, which is higher than aortic valve replacement [2]. Although the most commonly preferred incision in mitral valve surgery is a left atriotomy anterior to the left pulmonary veins, a Sondergaard’s Groove incision with an interatrial sulcus dissection is also preferred. However, in patients with a deep chest and a long anteroposterior axis, a history of previous aortic valve surgery and a small left atrium, the exposure is challenging [3, 4]. Reports from the early 2000s concluded that transseptal atriotomies, which provide better exposure for the mitral valve, were associated with complete loss of sinus rhythm with a need for transient pacemaker insertion up to 66% and permanent pacemaker insertion of 10% in the postoperative period [5, 6, 7]. As a result, transseptal atriotomy was infrequently performed in most surgical practices.
Minimally invasive techniques are gaining popularity in cardiac surgery but the conventional left atriotomy poses significant limitations in patients who require concomitant procedures. In the last decade, there have been successful reports of concomitant cardiac operations including multiple valve procedures and minimally invasive direct coronary artery bypass (MIDCAB) plus mitral valve procedures using minimally invasive approaches, which have contributed to a resurgence in the popularity of transseptal atriotomies [8, 9]. In previous studies that reporting unfavourable results, the incision was extended to the dome of the left atrium and concomitant valve procedures were performed more frequently in the Group TS. Meta-analyses have demonstrated that these factors are significant risk factors for postoperative arrhythmias. In addition, studies comparing transseptal atriotomy with conventional left atriotomy have not excluded surgical selection bias and have not conducted analyses on diverse patient Groups [7, 10, 11, 12].
We aimed to investigate the long-term outcomes of limited transseptal atriotomy during mitral valve surgery in patients with isolated small left atria.
The study was designed as a retrospective cohort. Patient data undergoing mitral valve surgery at the SBÜ Kartal Kosuyolu High Specialization Training and Research Hospital between January 2010 and February 2014 were analyzed. 1214 patients were extracted from the hospital database. Informed consents was obtained from all patients. The ethical approval number is E-10840098-202.3.02-596. Pre-operative echocardiographic evaluations were performed routinely in all patients. Left atrial (LA) diameter was derived from the parasternal long-axis B-mode view on 2-D echocardiography. The operations were performed by 4 different surgical teams, and two of them performed a left atriotomy in all cases. One surgical team decided the incision intra-operatively and performed transseptal incisions in patients with challenging mitral valve exposure. A 4th surgical team routinely performed transseptal incisions in cases with an LA diameter below 4.5 cm. A left atrial diameter measured at 4.5 cm or below on two-dimensional echocardiography was classified as small left atrium. Emergency indications, infective endocarditis, critical preoperative conditions, pre-operatively implanted permanent pacemaker, and concomitant rhythm ablation procedures were excluded. Additionally, patients with a history of cancer, cirrhosis, or those receiving immunosuppressive medication, which could influence long-term survival, were excluded from the study to ensure accurate survival analysis. 119 patients with a small left atrium and meeting the inclusion criteria were enrolled in the study. Of these, 57 patients (47.9%) underwent a transseptal atriotomy (Group TS), while 62 patients (52.1%) underwent a left atriotomy (Group LA).
In all patients a post-operative electrocardiography (ECG) was performed shortly after transfer to the intensive care unit (ICU). An ECG was recorded in all patients and was repeated daily until discharge. A-V block other than Type 1, junctional rhythm, atrial fibrillation-flutter, amiodarone or electro-cardioversion therapies were defined as arrhythmias. In case of new-onset atrial fibrillation/flutter an electro-cardioversion was performed as the first stage therapy and treatment was continued with iv-amiodarone. An anticoagulation regimen was started on postoperative day 1 with low molecule weight heparins (LMWHs) and warfarin after the removal of all drainage catheters. The international normalized ratio (INR) goal was 2–3 in the repair group and 2.5–3.5 in the mitral mechanical prosthesis group. The anticoagulation therapy was decided based on the patients CHA2DS2-VASc score after 3 months; and warfarin was preferred over non-vitamine K oral anti-coagulants (NOACs). Post-operative evaluations were performed routinely at 1st week, 1st month, 1st year and 2nd year after discharge. 3 points major adverse cardiac events (MACE) rates at 2 years were obtained from the hospital database. However, further evaluation of MACE could not be performed due to inadequate contact addresses and telephones. Survival datas for all patients were obtained from the National population administration system (MERNIS).
The distribution of continuous variables were evaluated both visually (using histograms) and statistically (using the Kolmogorov-Smirnov or Shapiro-Wilk tests). For numerical variables, statistical comparisons were performed using the independent samples t-test if the data were normally distributed, and the Mann-Whitney U test if the data were not normally distributed. For categorical variables, the Chi-square and Fisher’s exact tests were used. Survival analyses were conducted using the Kaplan-Meier method and Cox regression tests.
The significance level was set at p
Operations were performed via a full median sternotomy. In redo cases, femoral cannulation was performed prior to sternotomy, followed by median sternotomy using an oscillating redo saw. The operations were conducted under moderate hypothermia at 32 °C, with myocardial protection achieved via antegrade and retrograde isothermic blood cardioplegia. In the left atriotomy group (Group LA), the incision was made using the standard method without an interatrial groove dissection. In the transseptal group (Group TS), the right atriotomy was performed parallel to the atrioventricular groove then the fossa ovalis was incised vertically. The septostomy was not extended to the left atrial dome. An extrended transseptal incision was performed by only one surgical team in 4 patients who met the inclusion criteria. These 4 patients were not included in the study. For left atrial venting, a suction cannula was placed through the right superior pulmonary vein (RSPV) in Group LA and through the transseptal incision in Group TS. Mitral valve repairs were performed with a valvuloplasty and ring annuloplasty, while mechanical valves were preferred for both mitral and aortic valve replacements. Tricuspid annuloplasty was performed using the De Vega suture annuloplasty technique. Isolated mitral procedures were performed in 49 patients (41.2%). Concomitant tricuspid valve surgery was performed in 42 patients (35.3%), concomitant aortic valve surgery in 24 patients (20.2%), and concomitant coronary artery bypass grafting in 15 patients (12.6%). Although concomitant procedures were more frequently performed in Group TS, the difference was not statistically significant. The mean perfusion time and the ischemic time were found to be similar in both Groups (Table 1).
| Group TS | Group LA | p | ||
| Isolated MV procedure | 22 (38.6%) | 27 (43.5%) | 0.7 | |
| MV repair | 12 (21.1%) | 24 (38.7%) | 0.052 | |
| Concomitant procedure | ||||
| TV annuloplasty | 23 (40.4%) | 19 (30.6%) | 0.3 | |
| AV replacement | 16 (28.1%) | 8 (12.9%) | 0.07 | |
| CABG | 8 (14.1%) | 7 (11.3%) | 0.7 | |
| Total perfusion time (min.) | 117.2 | 112.3 | 0.4 | |
| Ischemic time (min.) | 87.6 | 77.4 | 0.07 | |
MV, mitral valve; AV, aortic valve; TV, tricuspid valve; CABG, coronary artery bypass grafting; TS, transseptal; LA, left atriotomy.
The mean age was 52.8
| Group TS | Group LA | p | ||
| Age | 50.8 | 57.8 | 0.07 | |
| Obesity | 8 (14.1%) | 14 (22.6%) | 0.3 | |
| Gender (male) | 22 (38.6%) | 23 (37.1%) | 0.8 | |
| EuroSCORE II* | 2.38 | 2.33 | 0.3 | |
| Previous cardiac surgery | 7 (12.3%) | 6 (9.7%) | 0.6 | |
| NYHA class** | 0.2 | |||
| II | 23 (40.4%) | 30 (48.4%) | ||
| III | 28 (49.1%) | 30 (48.4%) | ||
| IV | 6 (10.5%) | 2 (3.2%) | ||
| LVEF | 53.6 | 53.5 | 0.5 | |
| LV systolic dysfunction | 14 (24.6%) | 14 (22.6%) | 0.8 | |
| Mitral stenosis | 22 (38.6%) | 16 (25.8%) | 0.1 | |
| Secondary MR | 11 (19.3%) | 9 (14.5%) | 0.1 | |
| Left atrial diameter (cm) | 4.2 | 4.3 | 0.7 | |
| Normal sinus rhythm | 42 (73.7%) | 46 (74.2%) | 0.9 | |
*Median
**Statistical analyse was performed with Mann-Whitney U Test.
Postoperative variables are summarized in Table 3, and both Groups have similar outcomes. Surgical mortality occurred in 4 patients (3.4%). One patient who underwent isolated MVR died due to an atrioventricular groove rupture, two patients who underwent aortic valve replacement (AVR) plus MVR died in the early postoperative period due to low cardiac output syndrome (LCOS), and one patient who underwent CABG plus MVr died due to mediastinitis and sepsis. Permanent pacemaker implantation was required in 6 patients (5.2%) and no statistically significant differences were observed between the Groups (p
| Group TS | Group LA | p | |
| LCOS | 12 (21.1%) | 22 (35.5%) | 0.08 |
| IABP | 0 | 4 (6.5%) | 0.052 |
| ECMO | 1 (1.8%) | 0 | 0.3 |
| Respiratory insufficiency | 3 (5.3%) | 4 (6.5%) | 0.7 |
| Bleeding-revision | 5 (8.8%) | 3 (4.8%) | 0.3 |
| AKI | 5 (8.8%) | 4 (6.5%) | 0.6 |
| Haemodialysis | 1 (1.8%) | 1 (1.6%) | 0.9 |
| New onset arrhythmia | 15 (26.3%) | 12 (19.4%) | 0.5 |
| Atrial fibrillation flutter | 4 (7.1%) | 5 (8.1%) | 0.7 |
| Type 2–3 A-V Block | 1 (1.7%) | 1 (1.6%) | 0.9 |
| Junctional Rhythm | 10 (17.5%) | 6 (9.7%) | 0.09 |
| Persistent arrhythmia | 7 (12.3%) | 7 (11.3%) | 0.9 |
| Transient pacemaker | 12 (21.1%) | 7 (11.3%) | 0.06 |
| Pacemaker implantation | 3.0 (5.3%) | 3.0 (4.8%) | 0.9 |
| NSR at discharge | 35 (65.5%) | 42 (70%) | 0.7 |
| CVE | 2 (3.6%) | 0 | 0.1 |
| Wound infection | 2 (3.5%) | 3 (4.8%) | 0.7 |
| ICU stay (days) | 2 | 2 | 0.8 |
| Discharge (days) | 8 | 7 | 0.2 |
| In-hospital mortality | 2 (3.5%) | 2 (3.2%) | 0.9 |
LCOS, low cardiac output syndrome; AKI, acute kidney injury; NSR, normal sinus rhythm; CVE, cerebrovascular event; ICU, intensive care unit; IABP, intra-aortic balloon pump; ECMO, extracorporeal membrane oxygenation.
The mean follow-up duration was 10.7
| Group TS | Group LA | p | |
| 1st year survival | 95 | 92 | 0.8 |
| 5th year survival | 86 | 89 | 0.6 |
| 10th year survival | 70 | 69 | 0.6 |
| 14th year survival | 64 | 58 | 0.5 |
| 1st year MACE free survival | 52 (91.2%) | 56 (90.3%) | 0.8 |
| 2nd year MACE free survival | 49 (85.9%) | 55 (88.7%) | 0.6 |
MACE, major adverse cardiac events.
Survival analyses revealed no significant differences between the Groups (Group TS: 11.7
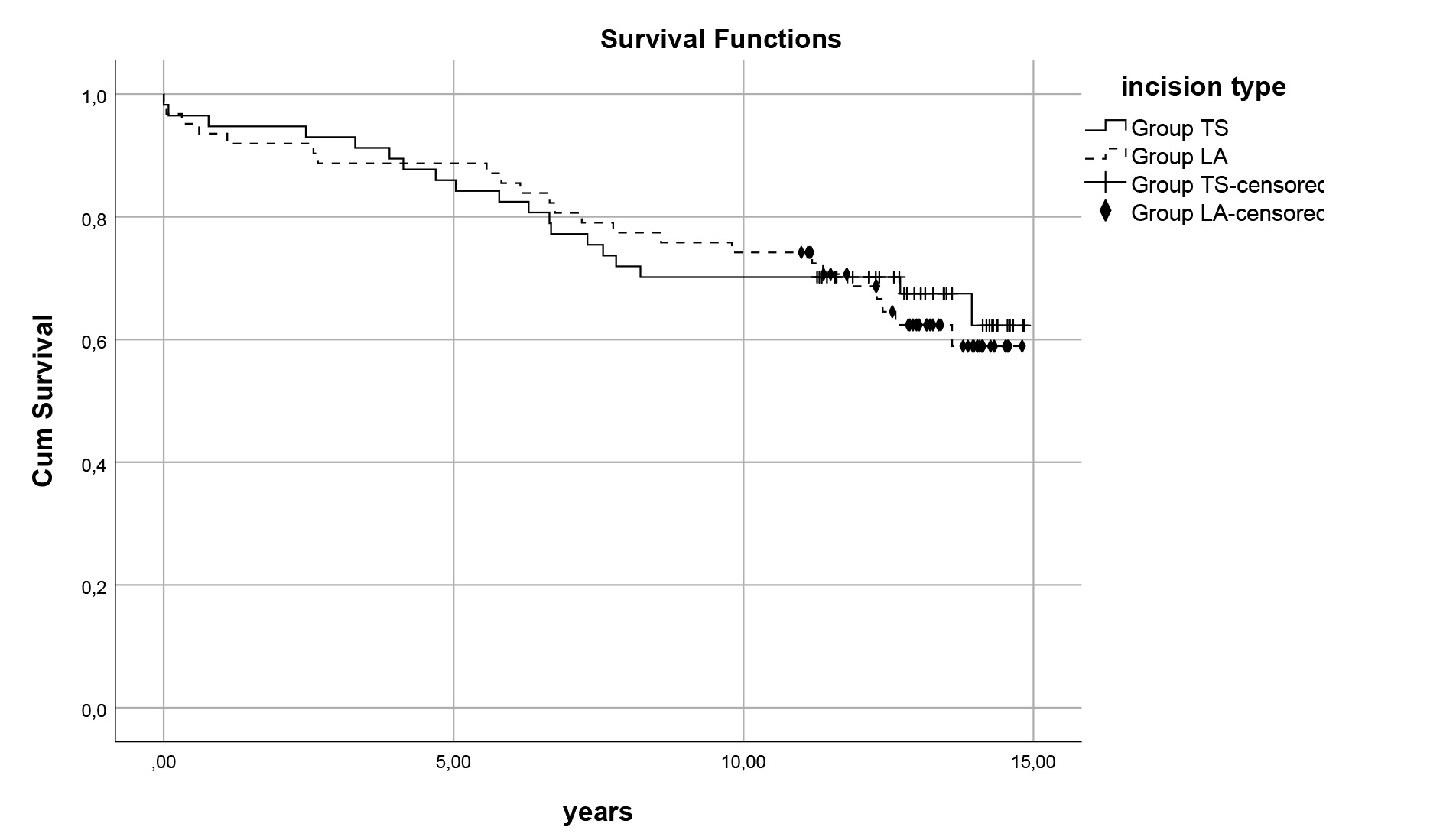 Fig. 1.
Fig. 1. Survival curves for atirotomies. Comparison of late survival 11.7
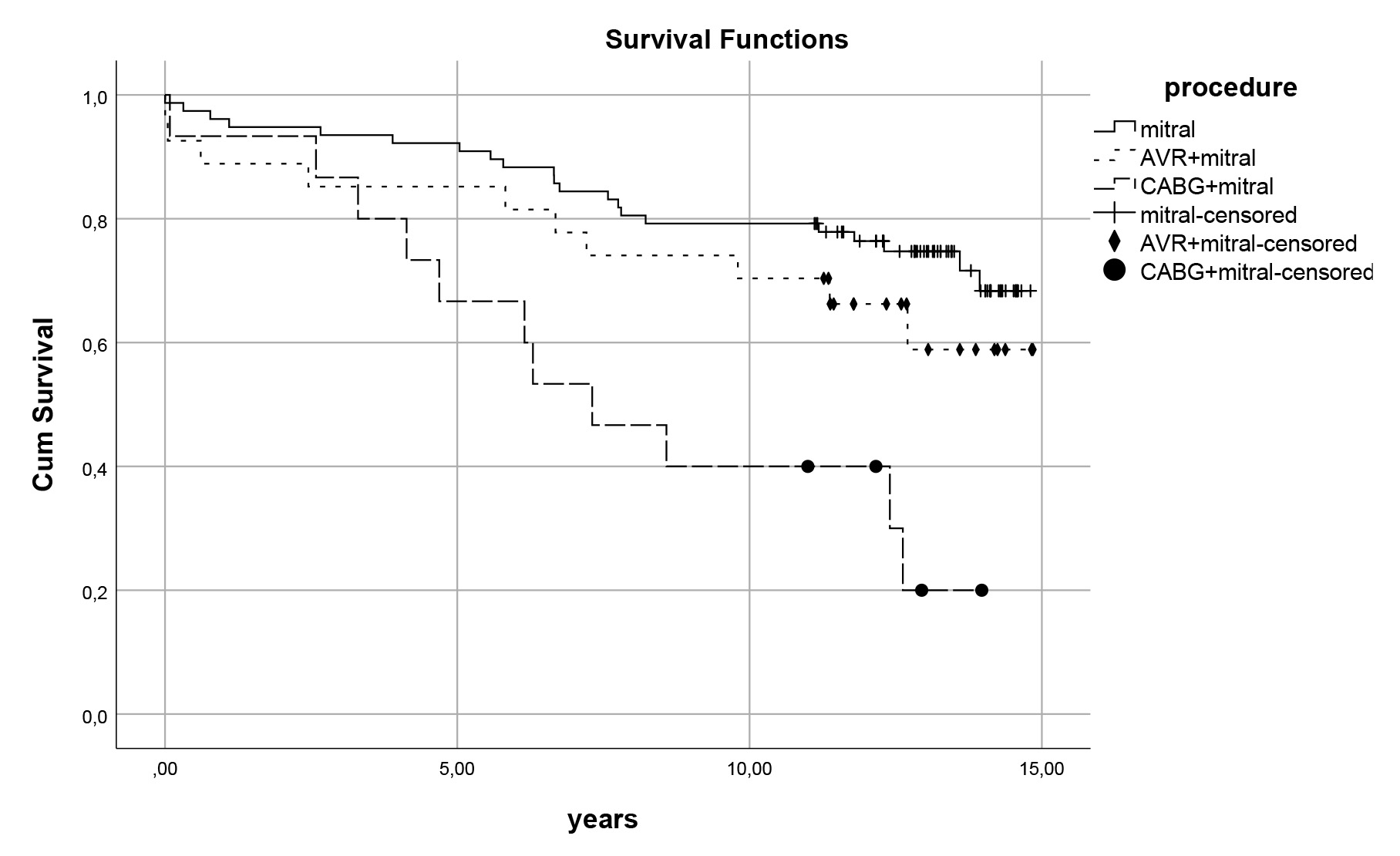 Fig. 2.
Fig. 2. Survival curves for procedures. Late survival curves for procedure types regardless of TV repair 12.5
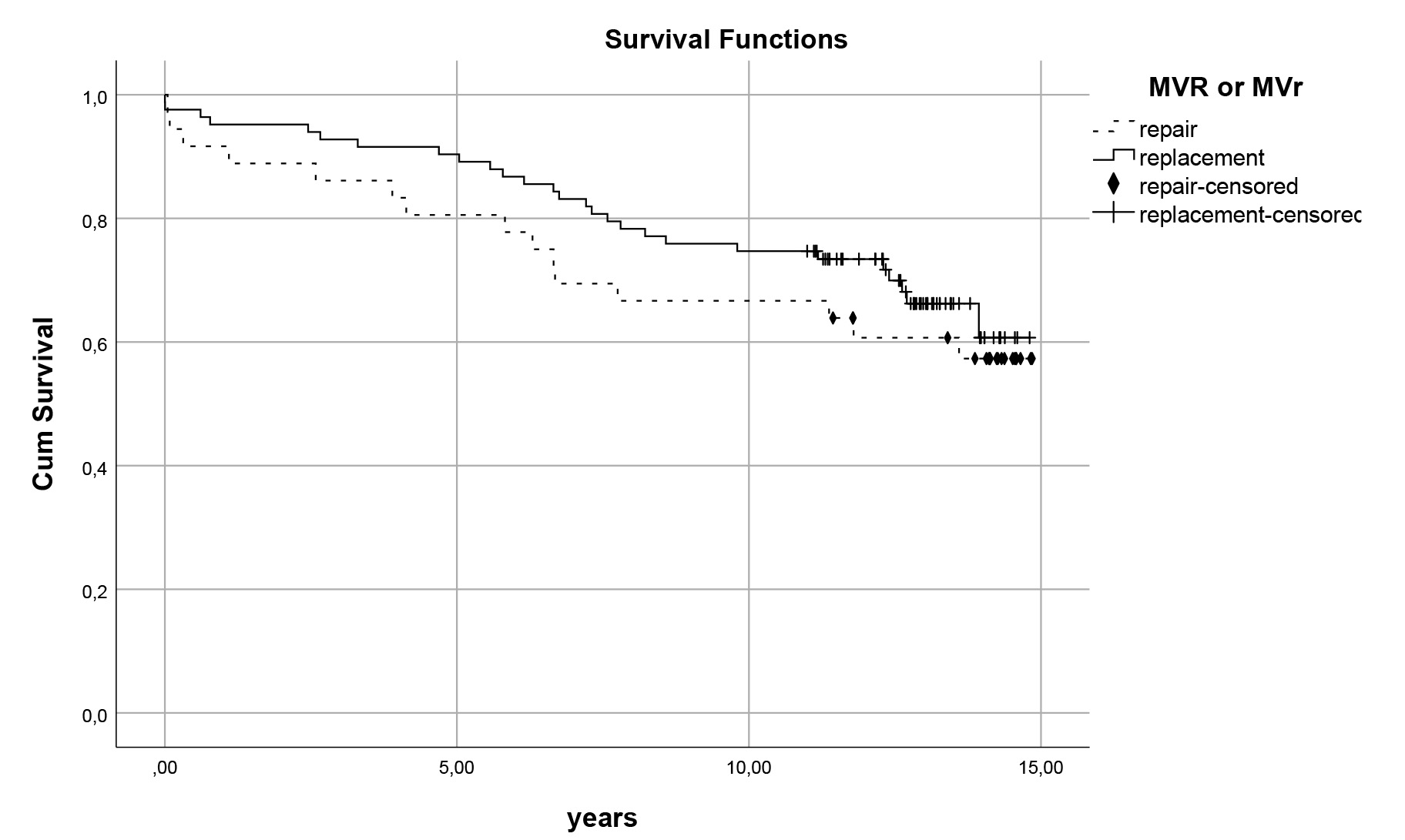 Fig. 3.
Fig. 3. Survival curves for mitral repair vs. replacement. Comparison of late survival of patients who had mitral valve repair (MVr) versus replacement (MVR) (p
In univariate analyses, several variables were identified as risk factors for long-term survival: age, obesity, chronic obstructive pulmonary disease (COPD), left ventricular systolic dysfunction, procedure type, and EuroSCORE II (p
| HR | 95% CI | Sig | ||
| Age (per years) | 1.05 | 1.02–1.08 | ||
| Obesity | 3.9 | 1.7–8.6 | ||
| Pre-operative cardiac failure | 2.6 | 1.2–5.1 | ||
| Procedure type | ||||
| Concomitant AVR | 1.8 | 0.8–4.3 | ||
| Concomitant CABG | 3 | 1.3–6.9 | ||
AVR, aortic valve replacement; CABG, coronary artery bypass graft; Sig, significance.
Transseptal atriotomies preferred for its better exposure in challenging mitral valve cases. However, it has been associated with a high incidence of postoperative sinus node dysfunction in studies conducted between 1990 and 2000 and an incidence of permanent pacemaker insertion [5, 6, 13]. This pathology was primarily attributed to the transection of the sinus node artery during the extension of the transatrial incision to the left atrial dome. In a previous report, the superior vena cava was also transected to enhance exposure, resulting in a ten-fold increase rate of permanent pacemaker implantation in the Group TS [14]. Large-scale meta-analyses have shown that concomitant valve procedures increase the need for pacemaker implantation by up to 2.7-fold independent of the incision type [11, 12]. Studies reporting higher rates of postoperative transient and permanent pacemaker requirements in the Group TS also noted a significantly higher prevalence of redo cases and concomitant tricuspid and aortic valve interventions in this Group [7, 8]. In our study, concomitant procedures were similar in both Groups (61.4% in Group TS vs. 53.5% in Group LA; p
The mitral valve is more difficult to access and expose compared to the other valves. Studies reported that isolated mitral valve procedures have an operative mortality rate between 1.1% and 1.4%. However, real-world population data show that this rate can rise to 5%, and the mortality rate for mitral valve replacement (MVR) is twice as high as that for aortic valve replacement (AVR) [2, 15, 16]. In our study, the overall operative mortality rate was 3.4%, and comparable in both Groups (3.5% in Group TS vs. 3.2% in Group LA; p
In mitral valve surgery, mitral valve repair has demonstrated a significant improvement in both early postoperative outcomes and long-term survival, reducing mortality by 50% [2, 18]. However, the use of biological valves in the mitral position negatively impacts survival in patients under 70 years of age [19]. In our study, mechanical valves were chosen as the prosthetic valve, and mitral repair did not show a long-term survival advantage over replacement. This may be attributed to the significantly higher prevalence of secondary mitral regurgitation (MR) in the repair group (44.4% in MVr vs. 4.8% in MVR; p
This is a single centre, retrospective study, which introduces an element of selection bias. The lack of a multi-centre study reduces its generalizability to the other centers. The extended transseptal incision is a valuable technique for patients with challenging mitral exposure; however our study only included patients undergoing limited transseptal incision. We report long-term survival data but quality of life is also an important outcome after cardiac interventions.
Transseptal atriotomy, when performed without extending the incision to the left atrial dome, yields similar early and long-term outcomes compared to conventional left atriotomy. In our study, no adverse effects on postoperative arrhythmias were observed with this approach.
The datasets analyzed during the current study are not publicly available because they were extracted from the hospital database. But they are available from the corresponding author on reasonable request.
DG designed and drafted the research study. MET and DG made reviewing the study critically for important intellectual content. The interpretation of the data for the work was conducted by DG and MET. Both authors made the final approval of the version to be published. Both authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
The Institutional Ethical Committee of Istanbul Medipol University approved the study protocol (date: 17 January 2025/reference number: E-10840098-202.3.02-596. Preoperative informed consent was obtained from all patients who underwent surgery. The study was conducted in accordance with the Declaration of Helsinki.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.



