1 Division of Gastroenterology, Department of Internal Medicine, University Hospital of Patras, 26504 Patras, Greece
Abstract
Vitamin D possesses a crucial role in preserving bone health, modulating the immune system responses, and supporting various physiological functions throughout the body. Chronic atrophic autoimmune gastritis (CAAG) constitutes an autoimmune condition marked by inflammation and damage to the stomach cells, often resulting in a decreased ability to absorb certain nutrients, including vitamin B12 and iron. Although, vitamin D is not directly affected by this condition, the sufficiency of this micronutrient seems to have important implications for overall health and management of the disease. The aim of the current review was to assess the incidence and related features of vitamin D deficiency in patients with CAAG and to elucidate the complex regulatory role of this nutrient, in an effort to improve patient outcomes. Vitamin D greatly contributes to the regulation of the immune system. In patients with CAAG, the immune system attacks the stomach lining; thus, the maintenance of a healthy and balanced immune response is important. In autoimmune conditions such as CAAG, where inflammation plays a decisive role in disease progression, vitamin D could potentially exert a role in managing and controlling the associated symptoms. Adequate vitamin D levels may help in regulating the immune response and reducing inflammation. In addition, patients with CAAG are at risk of nutrient deficiencies, including vitamin B12 and iron, which can lead to anemia and bone health issues. As vitamin D is critical for calcium absorption and bone health, assurance of sufficient levels of this micronutrient can be beneficial in preventing or mitigating bone-related complications. In conclusion, regular monitoring of vitamin D levels, among other nutrients, and appropriate supplementation, when necessary, can help improve overall health and well-being in these patients.
Keywords
- chronic atrophic autoimmune gastritis
- autoimmune gastritis
- parietal cell
- vitamin D
- vitamin D receptor
- T cells
- inflammatory response
Chronic atrophic autoimmune gastritis (CAAG), also known as type A gastritis or autoimmune metaplastic atrophic gastritis, is a chronic immune-mediated, inflammatory condition that affects the stomach lining and especially the gastric oxyntic mucosa [1, 2]. The disease is characterized by the auto-immune-induced depletion of gastric parietal cells, resulting in their substitution by atrophic and metaplastic tissues [1, 2]. Specifically, CAAG undergoes histopathological changes across three distinct phases [3, 4]. Initially, there is evidence of sporadic damage to oxyntic glands, caused by the infiltration of plasma cells and lymphocytes. This infiltration is frequently observed in a gradient from top to bottom, accompanied by a pseudo-enlargement of the parietal cells that remain akin to those under proton-pump inhibitor therapy [4, 5]. Subsequently, during the florid phase, the dense infiltration of lymphocytes and plasma cells occurs in the lamina propria, accompanied by evident atrophic glandular alterations and replacement of native parietal cells by muco-secreting epithelia resembling antral glands (termed oxyntic antralization) [6]. The advanced stages of CAAG feature “oxyntic mucosa desertification”, marked by fibrosis of the lamina propria replacing oxyntic glandular units. This stage is also distinguished by foveolar hyperplasia accompanied by microcystic changes underneath, as well as the emergence of inflammatory and hyperplastic polyps. Furthermore, there is a widespread occurrence of intestinal, pancreatic, and pseudopyloric metaplasia, typically accompanied by minimal inflammation [4].
The parietal cells are responsible for producing stomach hydrochloric acid and intrinsic factor, a protein necessary for vitamin B12 absorption [7]. CAAG often leads to micronutrient deficiencies due to the autoimmune-mediated destruction of gastric parietal cells, impairing the absorption of essential nutrients such as vitamin B12 and iron [8]. This can result in symptoms such as fatigue and bone health issues, necessitating careful monitoring and supplementation to address specific deficiencies and prevent complications [9].
Beyond autoimmune inflammation, Helicobacter pylori infection is also acknowledged as the primary etiological contributor for the progression of chronic atrophic gastritis [10]. However, in the autoimmune etiology of gastritis, the inflammation is confined to the glands of the corpus and fundus in the stomach, while the antrum remains unaffected [8]. Inflammation of the oxyntic mucosa and its subsequent atrophic development are distinctive features of CAAG, which are essential for a definitive diagnosis [8]. The incidence of CAAG is approximately estimated to be around 1–2% and is more common in females than in males, with a ratio of 3:1 between women and men, and increasing occurrence with advancing age [11, 12].
Apart from vitamin B12 and iron deficiency, patients with CAAG exhibit deficiencies in various vitamins and micronutrients including vitamin C, vitamin D, and calcium [13, 14, 15]. The underlying pathogenetic mechanism driving these alterations seems to entail either heightened degradation or diminished nutrient absorption in the gastric mucosa, potentially stemming from elevated pH levels and bacterial overgrowth [14]. Vitamin D greatly contributes in regulating the absorption of calcium and upholding bone health by facilitating the deposition of calcium in bones. Additionally, it has important immunomodulatory effects, helping to regulate the immune system and reduce inflammation [16, 17, 18, 19]. In autoimmune diseases, including CAAG, dysregulation of the immune system responses leads to tissue damage. The function of vitamin D to modulate the immune system balance is essential for maintaining immune equilibrium. Insufficient vitamin D levels might worsen immune regulation, potentially contributing to the onset or advancement of CAAG. Additionally, vitamin D deficiency can impair calcium absorption, which may further exacerbate gastric mucosal damage and contribute to CAAG pathology.
The aim of the current review was to assess the occurrence of vitamin D deficiency among CAAG patients and to elucidate the intricate regulatory function of this nutrient. In parallel, we attempted to elucidate the role of vitamin D role in modulating immune dysregulation and improving overall patient outcomes in this setting.
The pathophysiological processes of autoimmune gastritis entail a multifaceted interplay of genetic, environmental, and immunological parameters [20]. The stimulation of autoreactive T cells that identify and attack parietal cells and intrinsic factor is a key player in CAAG pathogenesis. Stimulation of T cells initiates the destruction of parietal cells, which occurs as a consequence of the activity of anti-parietal cell antibodies (PCAs); PCAs identify subunits of the proton pump, H+/K+ ATPase, a distinctive feature found exclusively in parietal cells [8]. In vitro studies have shown that PCAs bind to the proton pump, leading to direct cellular damage of parietal cells through complement-dependent cytotoxic activity [21]. The destruction of parietal cells leads to a decrease in the secretion of hydrochloric acid, higher pH of the stomach, and reduction in intrinsic factor, which in turn, results into vitamin B12 and iron malabsorption, causing pernicious anemia and iron deficiency anemia, respectively [8]. These conditions constitute prevalent clinical manifestations of CAAG; specifically, iron deficiency anemia is observed in approximately 25–50% of CAAG patients [22, 23], whereas pernicious anemia may be identified in up to 15–25% of patients [22, 24].
Vitamin B12, an indispensable nutrient crucial for various physiological processes such as development of red blood cells, synthesis of DNA, and function of the nervous system faces reduced absorption in this scenario [1]. Autoantibodies against intrinsic factor (IFAs) targets intrinsic factor, a protein secreted by parietal cells necessary for vitamin B12 absorption [25]. The reduced absorption of vitamin B12 presents with various symptoms, such as anemia and neurological issues, which could escalate if left untreated.
However, PCAs in the circulation are not likely to present a direct pathogenic role in CAAG [8]. CAAG patients have been found to be PCA-negative, and a considerable number of individuals diagnosed with common variable immunodeficiency also experience CAAG, with the majority testing negative for PCA antibodies [26, 27]. In such cases, cell-mediated immunity appears to greatly contribute to CAAG development, and the diagnosis is confirmed through histological examination. IgG4-secreting plasma cells have been noted in the mucosal tissue of patients with CAAG, specifically in individuals with pernicious anemia, yet their presence does not confirm a potential pathogenic effect [28]. Thus, although PCAs and IFAs are typically viewed as indicators of autoimmunity in CAAG, they do not directly influence the apoptosis of parietal cells and are not consistently detected in CAAG [8].
Genetic predisposition is a major contributor to the onset of CAAG. Specific alleles of the human leukocyte antigen (HLA), notably HLA–DR isotype (HLA-DR) and HLA–DQ isotype (HLA-DQ) have been linked to an elevated risk of developing this condition [29]. Additionally, genetic factors associated with impaired immune regulation and autoimmunity, including specific gene polymorphisms, may further contribute to the susceptibility of this disease [30]. In individuals with a genetic predisposition, exposure to specific dietary components or H. pylori infection can trigger the autoimmune response. The involvement of infectious agents as triggers for the disease occurrence has been debated. There are data indicating that H. pylori infection serves as a catalyst for the disease in individuals with genetic susceptibility, based on the theory of molecular mimicry between H. pylori and the H+/K+ ATPase of parietal cells [31]. Specifically, microbial antigens resembling self-antigens are able to stimulate autoreactive immune cells that target the gastric mucosa [31, 32, 33, 34, 35].
The primary pathogenic role in CAAG is attributed to autoreactive CD4
Patients with CAAG may progress to intestinal metaplasia. Moreover, the clinical manifestations of CAAG are intricate due to its common correlation with various autoimmune conditions, particularly Hashimoto’s thyroiditis [38]. Additionally, there exists an increased likelihood of developing gastric adenocarcinoma and type 1 gastric neuroendocrine tumors associated with CAAG [27, 29, 39]. Autoimmune gastritis involves diverse molecular and cellular mechanisms, encompassing genetic susceptibility, autoantibodies, and the development of a pro-inflammatory microenvironment [8]; however, the comprehensive pathophysiological mechanism remains unclear.
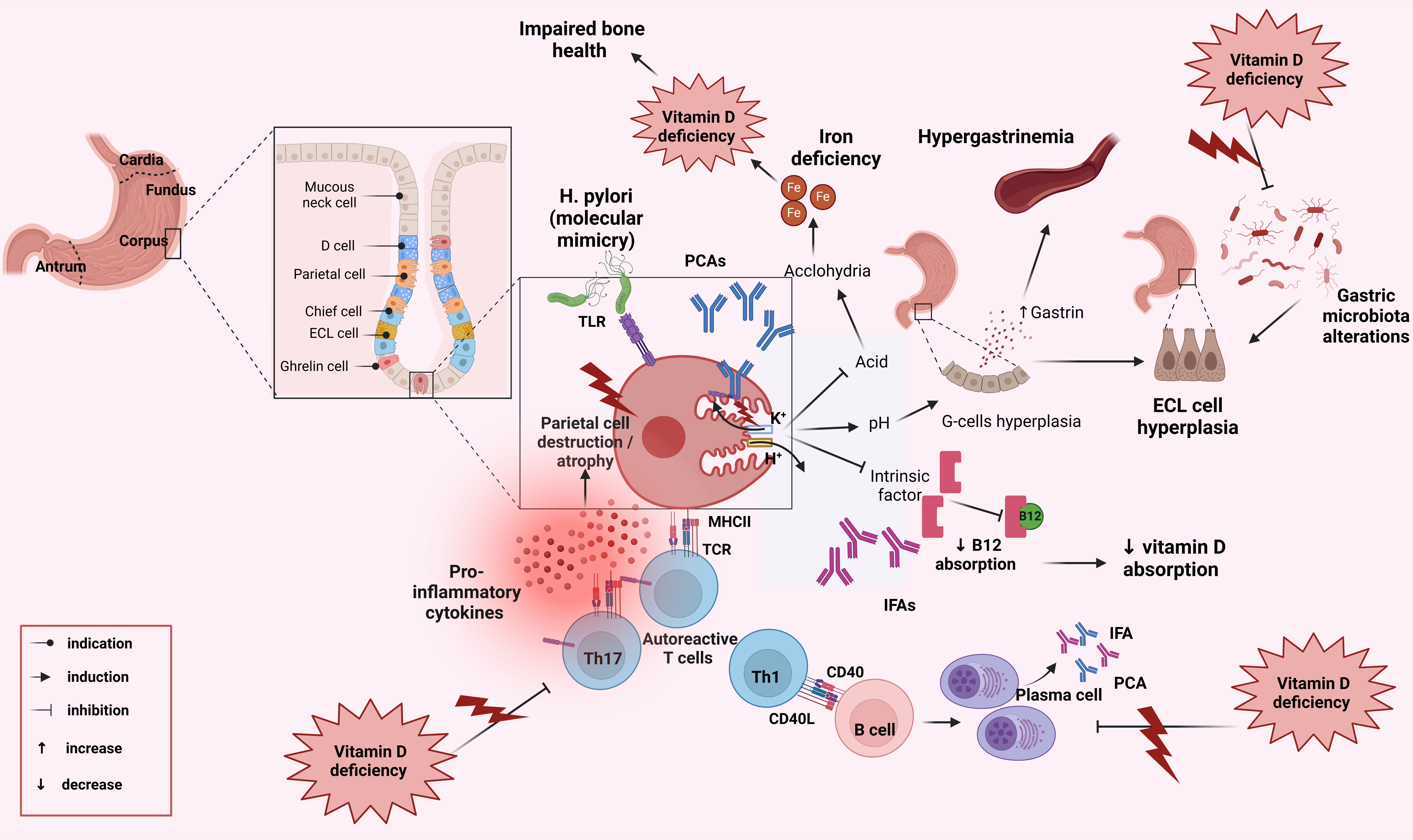
The widely discussed potential influence of vitamin D in hindering the progression of inflammatory diseases, stems from the emerging recognition of its antiproliferative and anti-inflammatory properties. The role of vitamin D in autoimmune gastritis is a subject of ongoing research, and while the precise mechanisms are not fully elucidated, there is evidence suggesting a potential association (Fig. 1).
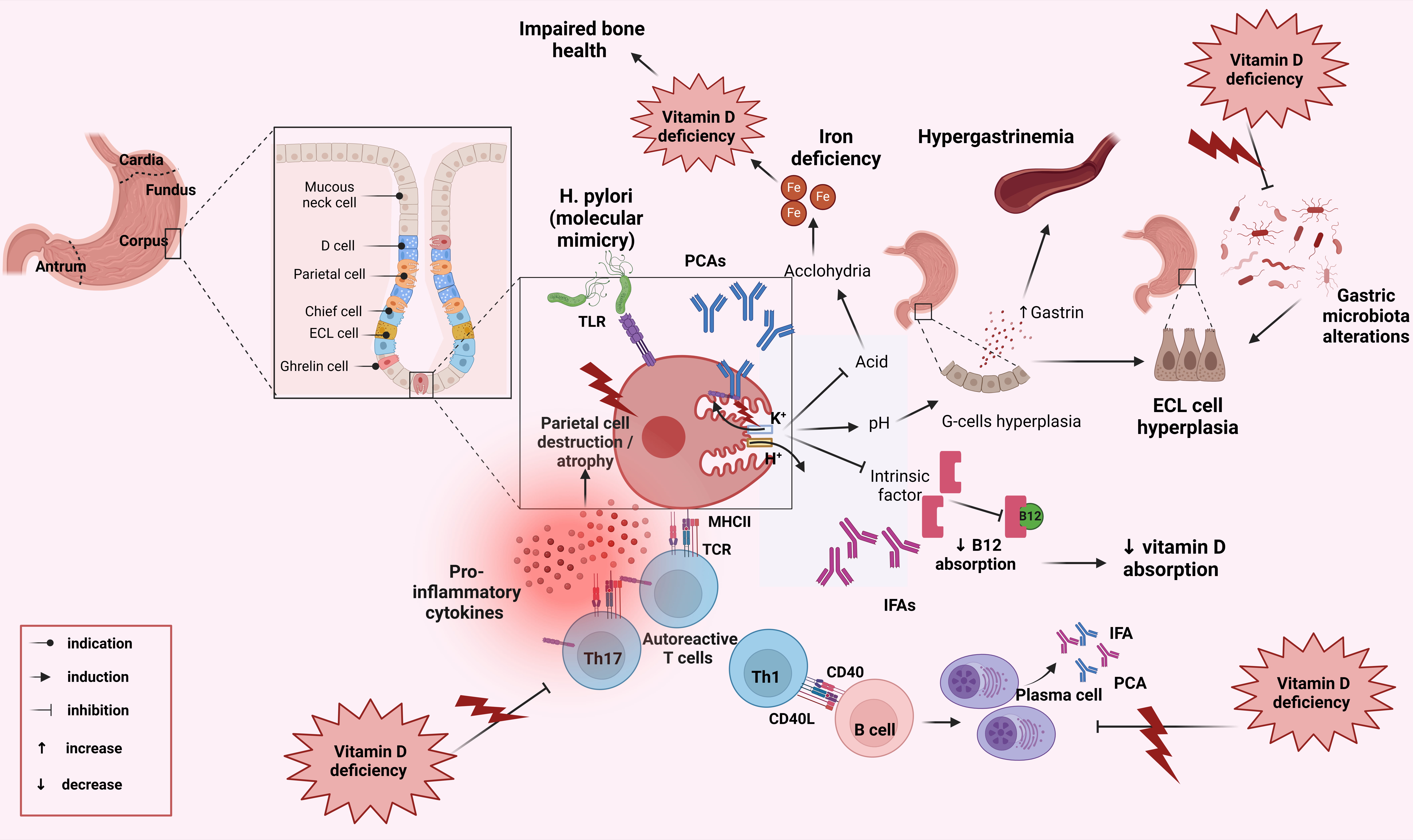 Fig. 1.
Fig. 1.CAAG impacts the corpus and fundus, which results in atrophy of the mucosal tissue. The antrum remains unaffected. The parietal cells, which constitute the primary targets in CAAG, are exclusively situated in the oxyntic mucosa and are responsible for the production of hydrochloric acid and intrinsic factor. Alongside parietal cells, oxyntic mucosa involves many other cells including mucous neck cells, chief cells, enterochromaffin-like cells, ghrelin cells, and D cells. CAAG is mainly characterized by the secretion of autoantibodies, particularly anti-PCAs and anti-IFAs. These antibodies target specific proteins expressed by parietal cells within the gastric mucosa. Parietal cells greatly contribute to gastric acid secretion through the action of the proton pump (H+/K+ ATPase). The autoantibodies produced in CAAG target components of this proton pump, leading to atrophy or destruction of parietal cells. This results in decreased production of hydrochloric acid and intrinsic factor, which are essential for digestion and absorption of nutrients such as B12 and iron, causing pernicious anemia and iron deficiency anemia, respectively. These conditions may lead to impaired absorption of vitamin D and thus vitamin D deficiency. The increased secretion of gastrin by G-cells leads to hypergastrinemia. In parallel, elevated gastrin levels stimulate ECL proliferation, leading to hyperplasia and subsequently to the development of gastric neoplastic lesions. Increased pH leads to ECL hyperplasia due to increased gastrin levels and to alterations in the gastric microbiota composition and favors adverse outcomes. The composition of the gastric microbiota might also be influenced by the occurrence of vitamin D deficiency, further aggravating these effects. The autoimmune attack on parietal cells triggers an inflammatory response in the gastric mucosa. Activated autoreactive T cells including Th1 and Th17 cells release pro-inflammatory cytokines, which further contribute to tissue damage and inflammation. Chronic inflammation and damage to the gastric mucosa result in tissue remodeling including thinning of the mucosal layer and loss of gastric glandular architecture. Vitamin D deficiency hampers the ability of this micronutrient to repress the Th1 and Th17 immune responses and the cytokine production, further worsening the inflammatory microenvironment. H. pylori may contribute to the autoimmune process via molecular mimicry, leading to an autoimmune response, particularly in patients carrying specific TLRs. Created with BioRender.com. Fig. 1 abbreviations: CAAG, chronic atrophic autoimmune gastritis; ECLs, enterochromaffin-like cells; PCAs, parietal cell antibodies; TLRs, Toll-like receptors; MHC, major histocompatibility complex; TCR, T cell receptor; Th cell, T helper cell; CD, cluster of differentiation; IFAs, intrinsic factor antibodies.
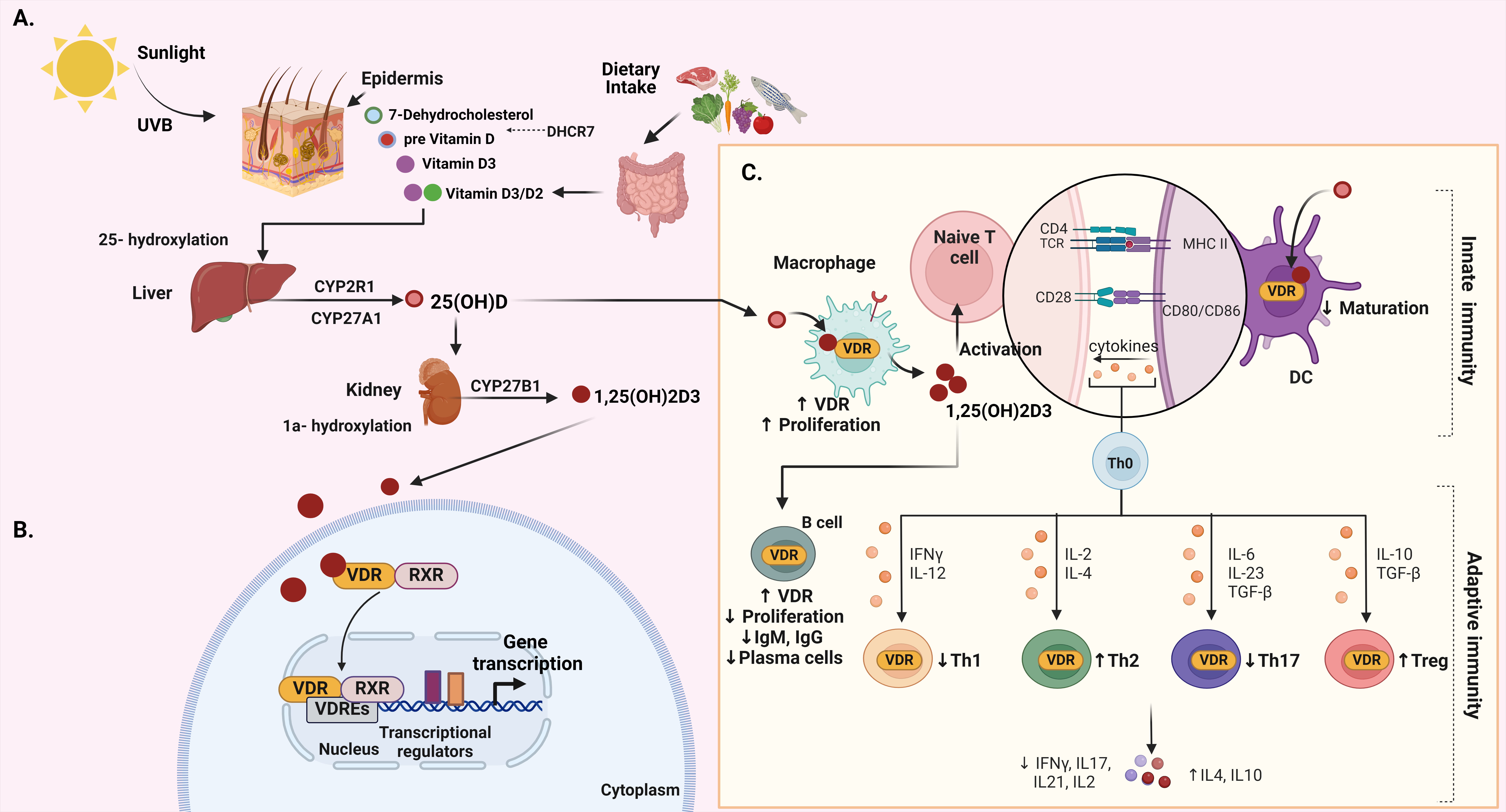
Vitamin D, a fat-soluble vitamin, plays a major role in various functions in the human body. Its main role is in regulating calcium and phosphorus metabolism, vital for bone health [40]. Additionally, vitamin D is pivotal in modulating the immune system, as well as influencing cell growth and differentiation [40]. These physiological effects of this nutrient are orchestrated through a series of metabolic transformations, primarily occurring in the skin, hepatic tissue, and kidneys [40] (Fig. 2A).
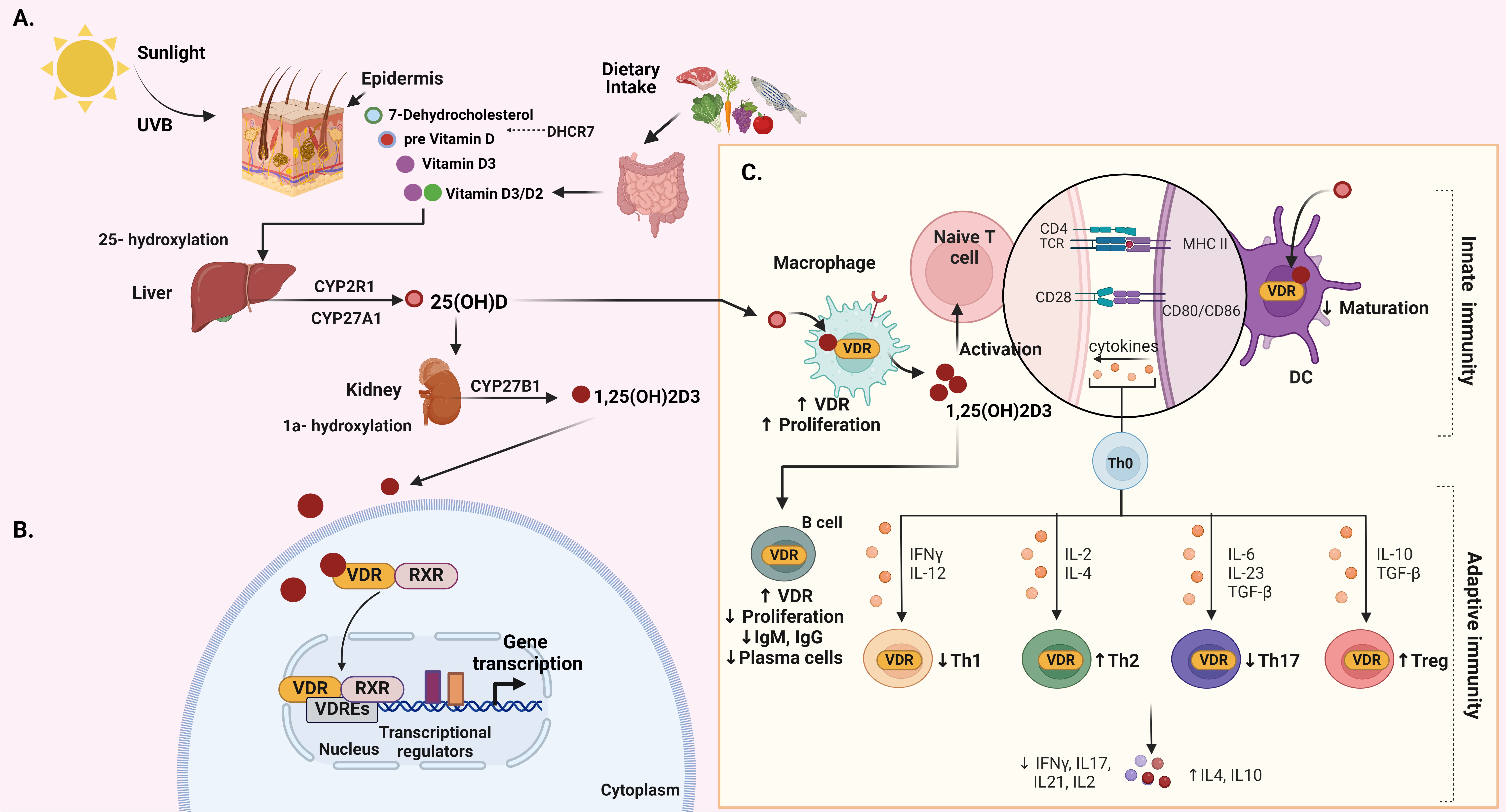 Fig. 2.
Fig. 2.Integrated overview of vitamin D: biosynthesis, metabolism, VDR
signaling, and immune function. (A) The biosynthesis and metabolism of vitamin
D. (B) The signaling of vitamin D–VDR. (C) The function of vitamin D in both
innate and adaptive immunity. Created with BioRender.com. Fig. 2 abbreviations:
UVB, ultraviolet B; DHCR7, 7-dehydrocholesterol reductase; CYP2R1, cytochrome
P450, family 2, subfamily R, polypeptide 1; CYP27A1, cytochrome P450, family 27,
subfamily A, polypeptide 1; CYP27B1, cytochrome P450, family 27, subfamily B,
polypeptide 1; VDR, vitamin D receptor; RXR, retinoid X receptor; VDRE, vitamin D
response element; TCR, T cell receptor; MHC, major histocompatibility complex;
CD, cluster of differentiation; DC, dendritic cell; Th, T helper; IL-2,
interleukin 2; IFN-
Two primary forms of vitamin D exist, each with distinct chemical structures:
either as vitamin D2, also known as ergocalciferol, or as vitamin D3, also
referred to as cholecalciferol [41, 42]. The synthesis of vitamin D initiates in
the skin when exposure to ultraviolet B sunlight triggers the conversion of
7-dehydrocholesterol into pre-vitamin D3 [41, 42]. Following this, pre-vitamin D3
undergoes thermal isomerization, leading to its conversion into vitamin D3.
Alternatively, vitamin D can be obtained from dietary sources; in this case, the
small intestine absorbs this form in conjunction with chylomicrons. After this,
vitamin D is conveyed to the liver, bound to vitamin D-binding protein. Upon
synthesis or ingestion, the enzyme 25-hydroxylase (CYP2R1) in the liver,
transforms vitamin D into 25-hydroxyvitamin D [25(OH)D], which is the primary
circulating form of the vitamin [42]. Following that, 25(OH)D undergoes further
hydroxylation in the kidneys, facilitated by the enzyme 1
Vitamin D insufficiency/deficiency is a significant health concern worldwide and
a common feature not only among elderly people and patients with chronic diseases
but also in the general population, affecting individuals of all ages and
demographic groups. Its prevalence can vary based on factors such as geographical
location, lifestyle, age, and skin pigmentation [45]. About 1 billion individuals
have insufficient serum levels of 25(OH)D [46]. Cui et al. [47],
assessed the prevalence of vitamin D deficiency both globally and regionally
among individuals aged 1 year or older from 2000 until 2022. The global
prevalence was estimated at 15.7% in patients with 25(OH)D
The physiological range of vitamin D levels has been a subject of debate. It is
suggested that combining both 25(OH)D2 and 25(OH)D3 to determine the total
25(OH)D serum levels is an indicator of vitamin D status. In general, diagnostic
thresholds defining serum 25(OH)D concentrations are outlined as follows: 25(OH)D
levels ranging from
Calcitriol exerts its influence by binding to the vitamin D receptor (VDR), which is found in various tissues in the human body [50]. Encoded by the VDR gene and classified within the nuclear receptor superfamily, the VDR function relies on its molecular composition and structure. In the majority of cases, VDR serves as a transcription factor, impacting the expression of genes associated with diverse biological processes in reaction to vitamin D [51]. Vitamin D–VDR complex has the potential to promote various biological actions through both genomic and nongenomic mechanisms [52, 53] (Fig. 2B). Upon activation, the VDR establishes a complex with the retinoid X receptor (RXR), and this composite binds to vitamin D response elements (VDREs) found in the promoter regions of target genes [52, 53]. In this manner, the activated VDR assumes a crucial role in the transcriptional activation or suppression of diverse target genes by a vitamin D-mediated signaling or by heterogeneous mechanisms, which involve interplay with transcription factors [54]. Target genes encompass calcium channels and transient receptor potential vanilloid member 6 (TRPV6) [55], which are crucial for triggering capsaicin-dependent apoptosis in gastric cancer cells [56]. Moreover, various vitamin D target tissues have been revealed in the gastrointestinal tract including colon, stomach, and small intestine, in various animal species [44]. Furthermore, VDR is expressed in the gastrointestinal tract of humans, rats, and mice [57, 58, 59]. The functions of vitamin D and VDR in the intestine have been extensively investigated. Vitamin D target genes like intestinal plasma membrane calcium ATPase and TRPV6 play crucial role in maintaining adequate calcium absorption in the intestinal tissue [60].
Beyond the gastrointestinal tract, the expression of VDR is extensive across various tissues, encompassing kidneys, bones, and immune cells, emphasizing its participation in a range of physiological processes [61, 62]. Notably, VDR is expressed in various immune cells engaged in gastrointestinal immune response, such as in stimulated T cells, B cells, cytotoxic natural killer (NK) cells, dendritic cells (DCs), monocytes, and macrophages [61, 63, 64, 65, 66, 67] (Fig. 2C). Scattered cells located in the pyloric antrum and the isthmus within the corpus have been identified as target tissues for 3H-1,25(OH)2D3 [68]. However, the precise role of vitamin D in the normal stomach function remains to be investigated.
VDR is expressed in low levels in gastric cancer [69] and Barrett’s esophagus [57]. Interestingly, VDR expression has been found higher in premalignant gastric tissues with moderate differentiation compared to those exhibited poor differentiation [57, 69]. Moreover, vitamin D deficiency has been related to other gastrointestinal diseases such as inflammatory bowel disease [70, 71], colorectal cancer [72], and H. pylori infection [73].
Vitamin D has been linked to the modulation of bone metabolism; however, growing
evidence suggests that it also greatly contributes to numerous biological
processes governing immune responses [61, 62, 74]. Interest in the potential
immunomodulatory effects of vitamin D has been growing since the discovery of VDR
in monocytes [74, 75], followed by their presence in DCs and activated T cells
[76, 77, 78] (Fig. 2C). VDR agonists selectively hamper exaggerated type 1 Th1-driven
inflammatory reactions and limit subsequent Th cell differentiation towards a
pro-inflammatory state [79]. Vitamin D suppresses the pro-inflammatory activity
of Th1 cells and the secretion of proinflammatory cytokines such as IL-2,
IFN-
To date, there are limited data on the relation between CAAG and vitamin D deficiency. Studies evaluating vitamin D levels in patients with CAAG are presented in Table 1 (Ref. [92, 93, 94, 95, 96, 97]).
| Author (ref) | Date | Study population (n) | Age | M/F | Vitamin D deficiency | Vitamin D levels (ng/mL) | Design | Other outcomes | Exclusion criteria |
| Eastell et al. [92] | 1992 | 21 pernicious anemia vs 24 controls | 68 |
0/21 vs. 0/24 | NA | 19.78 |
NR | Patients with pernicious anemia |
Osteoporosis-causing conditions |
| Antico et al. [93] | 2012 | 62 CAAG vs. 54 NSLG vs. 21 HPG vs 212 controls | 37–80 vs. 20–80 vs. 20–80 vs. 20–80 | NR | NA | 9.8 |
Case control study | NR | |
| Massironi et al. [94] | 2013 | 107 CAAG | 57.4 (21–88) | 21/86 | 22.96 ng/mL in CAAG with PHPT | 14.1 (2.9–33.7) | Monocentric prospective study | ||
| 8.86 ng/mL in CAAG with SHPT | |||||||||
| Massironi et al. [95] | 2017 | 138 NENs | 63 (26–90) | 77/61 | 94 of 138 (68%) |
12.9 (2–32) | Monocentric study | ||
| 46 of 138 (33%) |
|||||||||
| Massironi et al. [96] | 2018 | 87 CAAG vs. 1232 controls | 63.5 |
16/71 vs. 276/976 | 57 out of 87 (66%) vs. 438 of 1232 (36%) |
18.8 |
Monocentric prospective study | ||
| 27 of 87 (31%) vs. 160 out of 1232 (13%) |
|||||||||
| Zilli et al. [97] | 2019 | 122 CAAG | 65 (25–90) | 22/100 | 76 of 122 (62%) | 18 (4–35) | Monocentric observational study | ||
Footnotes: Data are presented as n (%) or median (range) or
mean
Abbreviations: Ref, reference; n, number; M/F, male/female; NA, not assessed; NR, not reported; CAAG, chronic atrophic autoimmune gastritis; NSLG, nonspecific lymphocytic gastritis; Th1, T helper 1; HPG, H. pylori gastritis; PHPT, primary hyperparathyroidism; SHPT, secondary hyperparathyroidism; PPIs, proton pump inhibitors; PTH, parathyroid hormone; NENs, neuroendocrine neoplasms; OS, overall survival; PFS, progression-free survival; rs, spearman’s rank correlation coefficient.
The assessment of vitamin D levels in postmenopausal women with pernicious
anemia as a potential risk factor for osteoporosis has been first explored in
1992 [92]. This study revealed no correlation between vitamin D and the presence
of pernicious anemia in a cohort of 21 patients compared to controls. However,
the small sample size and the inclusion of postmenopausal women may restrict the
reliability of the results [92]. In a subsequent study, significantly lower
levels of 25-hydroxy vitamin D were observed in CAAG patients compared to those
with nonspecific gastritis or healthy controls [93]. In CAAG subjects, the mean
vitamin D levels were 9.8
In addition, the vitamin D role has been assessed in the setting of
gastro-entero-pancreatic neuroendocrine neoplasms (NENs) [95]. The results showed
25(OH)D median levels at 12.9 ng/mL (ranging from 2 to 32 ng/mL) [95].
Specifically, 94 patients (68%) exhibited levels
Both vitamin D and calcium are critical for maintaining bone health, and their deficiency consist of an essential risk factor for osteoporosis development [98]. Although there is no evidence on the prevalence of osteoporosis within CAAG patients, there are data reporting a strong correlation between chronic atrophic gastritis and osteoporosis in a study of 401 postmenopausal women [99]. Moreover, since vitamin D is critical for absorbing calcium, a lack of vitamin D could also explain a decline in calcium levels in CAAG patients [15].
In a mechanistic study, the role of cluster of differentiation 24 (CD24), an antigen that has been used as a marker to indicate tumor invasiveness in gastric cancer [100], has been explored in patients with chronic atrophic gastritis [101]. The study indicated a gradual increase in CD24 expression across samples obtained from normal gastric mucosa, non-atrophic chronic gastritis, chronic atrophic gastritis, chronic atrophic gastritis with intestinal metaplasia, dysplasia, and gastric cancer [101]. Moreover, the data demonstrated that CD24 knockdown resulted in significantly increased expression of E-cadherin and VDR in gastric cancer cells, whereas CD24 overexpression led to converse effects [101]. VDR has been found to mediate E-cadherin expression, whereas the receptor expression has been eliminated during the progression of colon cancer [102, 103]. Thus, VDR-mediated reduction in E-cadherin expression indicates a pivotal stage in tumor metastasis and represents a key occurrence in the epithelial–mesenchymal transition (EMT) [104]. The EMT may be considered a pathological mechanism that takes part in tumor progression, particularly during the invasion and metastasis of tumor cells [105]. Consequently, CD24 might stimulate invasion by controlling the VDR-mediated onset of EMT.
Vitamin D has emerged as a significant factor in various autoimmune diseases, as indicated by numerous epidemiological studies. Its ability to bind to VDR and act as a transcriptional factor allows it to modulate gene expression and influence the immune cells through its immunoregulatory role. Emerging data suggest a close association among VDR polymorphisms, serum vitamin D levels, and the risk of autoimmune diseases such as multiple sclerosis, type 1 diabetes mellitus, and systemic lupus erythematosus [106, 107]. Thus, disruptions in vitamin D signaling or impaired vitamin D intake, influenced by genetic and environmental factors may be a contributing factor for initiation and progression of autoimmune conditions. Given the high prevalence of vitamin D deficiency observed in patients with autoimmune conditions, vitamin D supplementation has been considered a potential therapeutic approach for this setting. The findings of our study confirm this hypothesis to some extent. First, all of the aforementioned studies presented in the current review report vitamin D deficiency in patients with CAAG, proposing a potential pathogenetic effect of vitamin D deficiency in the initiation and progression of CAAG. Antico et al. [93] supported the notion that hypovitaminosis D could potentially serve as a risk factor for the onset of CAAG, as vitamin D levels in patients with CAAG were notably lower compared to patients with non-specific gastritis or healthy individuals. The study explored the impact of vitamin D in the gastro-entero-pancreatic NENs showed ameliorated clinical outcomes for patients who received vitamin D supplements, enhancing the theory on the antiproliferative role of vitamin D supplementation on NENs [95]. The evidence on the role of vitamin D supplements in patients with CAAG remain scarce; however, our findings support the regular assessment of vitamin D and provide supplementation when necessary. However, specific recommendations should be made on a case-by-case basis by healthcare professionals, considering individual patient needs and medical history to conclusively establish the effectiveness of vitamin D supplementation in preventing and alleviating CAAG.
Vitamin D has been greatly associated with various autoimmune diseases in multiple epidemiological studies. Vitamin D–VDR binding enables this complex to act as a transcriptional factor, regulating gene expression and exerting immunomodulatory effects on immune cells. Research has demonstrated the potential of vitamin D to repress Th17 cytokine secretion, promote Treg activity, stimulate NKT cell action, inhibit Th1 responses, and induce Th2 cytokine secretion, thereby influencing T cell polarization towards a Th2 phenotype. Moreover, growing data suggest a close association between serum vitamin D levels, and polymorphisms in the VDR gene with the risk of autoimmune diseases. Consequently, dysregulated vitamin D signaling or impaired vitamin D intake may contribute to the initiation and progression of autoimmunity.
In the setting of CAAG, the current body of literature lacks sufficient evidence to definitively establish a direct correlation between vitamin D status and the pathogenesis or prognosis of disease. It is plausible that vitamin D deficiency could either be a secondary manifestation of the disease or contribute to impaired tissue responsiveness to both physiological and pathological signals. Moreover, to date, the underlying mechanism of vitamin D deficiency in patients with CAAG remains unclear. However, similar to other micronutrients [14], it is plausible to consider a reduced absorption or increased degradation of vitamin D in the gastrointestinal tissue due to hypochlorhydria and overgrowth of bacterial population. The existing evidence suggests a direct link between the extent of atrophy in the mucosal tissue and vitamin D concentrations. A hypothesis could be that in individuals with mild atrophy, the remaining gastric acid secretion may sustain adequate absorption of vitamin D, which becomes insufficient as the disease progresses to more advanced stages. Moreover, vitamin D deficiency in patients with NENs [95, 96] enhances the potential role of hypovitaminosis D in the development of such neoplasms. Additionally, there are data on the expression of VDR in neuroendocrine cells. Moreover, vitamin D analogue demonstrated the ability to induce cell cycle arrest in the G0/G1 phase and apoptosis in a murine insulinoma cell line [108]. The precise mechanism underlying this correlation remains incompletely understood. Nevertheless, vitamin D has demonstrated involvement in various cellular processes including growth regulation, immune modulation, differentiation, adhesion, angiogenesis, apoptosis, and metastasis in epithelial tissues [109].
In light of the heightened occurrence of vitamin D deficiency among individuals with autoimmune conditions, there is growing interest in exploring vitamin D supplementation as a potential treatment in autoimmune conditions. Based on the data examined in the current review, while the precise mechanisms underlying the role of vitamin D in CAAG remain to be fully elucidated, accumulating evidence suggests that vitamin D may affect immune function and gastric mucosal integrity, making it a promising avenue for further research and potential therapeutic intervention. Thus, the routine evaluation of vitamin D levels in patients with CAAG is essential. Moreover, vitamin D supplementation in CAAG individuals suffering from vitamin D deficiency before the progression of disease may be a crucial preventive factor [110]. However, the lack of large, randomized controlled trials necessitates further well-organized studies with robust methodological design to demonstrate the causality of vitamin D in CAAG patients and thus validate the efficacy of vitamin D in preventing CAAG and determine if vitamin D supplementation can restrict the damage of gastric parietal cells.
IA designed the article, collected the data, wrote the article; CK collected the data, wrote the article; CT designed the article, revised the article critically for important intellectual content. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work to take public responsibility for appropriate portions of the content and agreed to be accountable for all aspects of the work in ensuring that questions related to its accuracy or integrity.
Not applicable.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.