1 Department of Obstetrics and Gynecology, the First Affiliated Hospital of Xi'an Jiaotong University, 710061 Xi'an, Shaanxi, China
2 Department of Clinical Laboratory, Women's Hospital of Nanjing Medical University, Nanjing Women and Children's Health Care Hospital, 210029 Nanjing, Jiangsu, China
Abstract
The prevalence of sperm DNA fragmentation (SDF) is significantly higher in males with infertility, which is often associated with oligozoospermia and hypospermia. It can also occur in patients with infertility who have normal conventional semen indicators. The etiologies involve aberrations in sperm maturation, dysregulated apoptotic processes, and heightened levels of oxidative stress. In this article, we retrieved PubMed, China National Knowledge Infrastructure (CNKI) and Web of Science databases for articles and reviews published before February 28, 2024. Using “sperm DNA fragments; assisted reproductive technology, mechanism, clinical pregnancy outcome” as keywords, and comprehensively reviewed on their basis. Numerous literature sources have reported an increased utilization of SDF testing in the context of male infertility, as there is a negative correlation between SDF levels and the success of natural conception as well as assisted reproductive technologies. To enhance the clinical outcome for individuals experiencing infertility, investigating the prevalence and underlying mechanisms of sperm DNA damage is beneficial. This review article delves into the mechanisms that lead to sperm DNA damage and assesses the impact of DNA fragmentation index (DFI) on pregnancy outcomes in the context of assisted reproductive technologies.
Keywords
- sperm DNA fragments
- clinical pregnancy outcome
- AI-DFI
- underlying mechanism
- assisted reproductive technology
Due to rapid socio-economic development, shifts in social structure, and the
rise in human life expectancy, postponing childbirth has become a prevalent trend
globally. Infertility is a complex reproductive disorder that affects nearly 15%
of couples, with 50% of cases being caused by male factors [1]. Routine semen
analysis is the cornerstone of male laboratory work and the basic examination for
the diagnosis of male infertility. Traditional semen analysis evaluates the
quality of semen based on its microscopic cellular characteristics, including
parameters such as concentration, viability, and morphology. Despite providing
basic information for evaluating male reproductive capacity, conventional semen
analysis has limitations in clinical application. Routine semen parameters may
not accurately assess male reproductive capacity, as about 15% of infertile men
have normal semen parameters [2]. Moreover, the standard reference values for
routine semen parameters do not fully correlate with reproductive ability and
cannot be considered as the minimum threshold for fertility. Relying only on
routine semen analysis may not accurately reflect sperm’s fertilization
potential, limiting assessment of male reproductive capacity. As a valuable
addition to routine semen analysis, sperm DNA fragmentation (SDF) can provide
insight into the integrity of sperm genetic material. The DNA fragmentation index
(DFI) is a crucial indicator for evaluating sperm DNA integrity, measuring the
extent of fragmented DNA produced during sperm generation and maturation as a
result of damage to sperm DNA integrity [3]. In a study conducted by Yang J [4]
in 2020, the relationship between sperm DNA damage and embryo quality, as well as
embryonic developmental potential, was investigated. The research revealed that
8-hydroxy-2′deoxygua-nosine (8-OHdG) was found to be partially co-localized
with testis-specific histone2
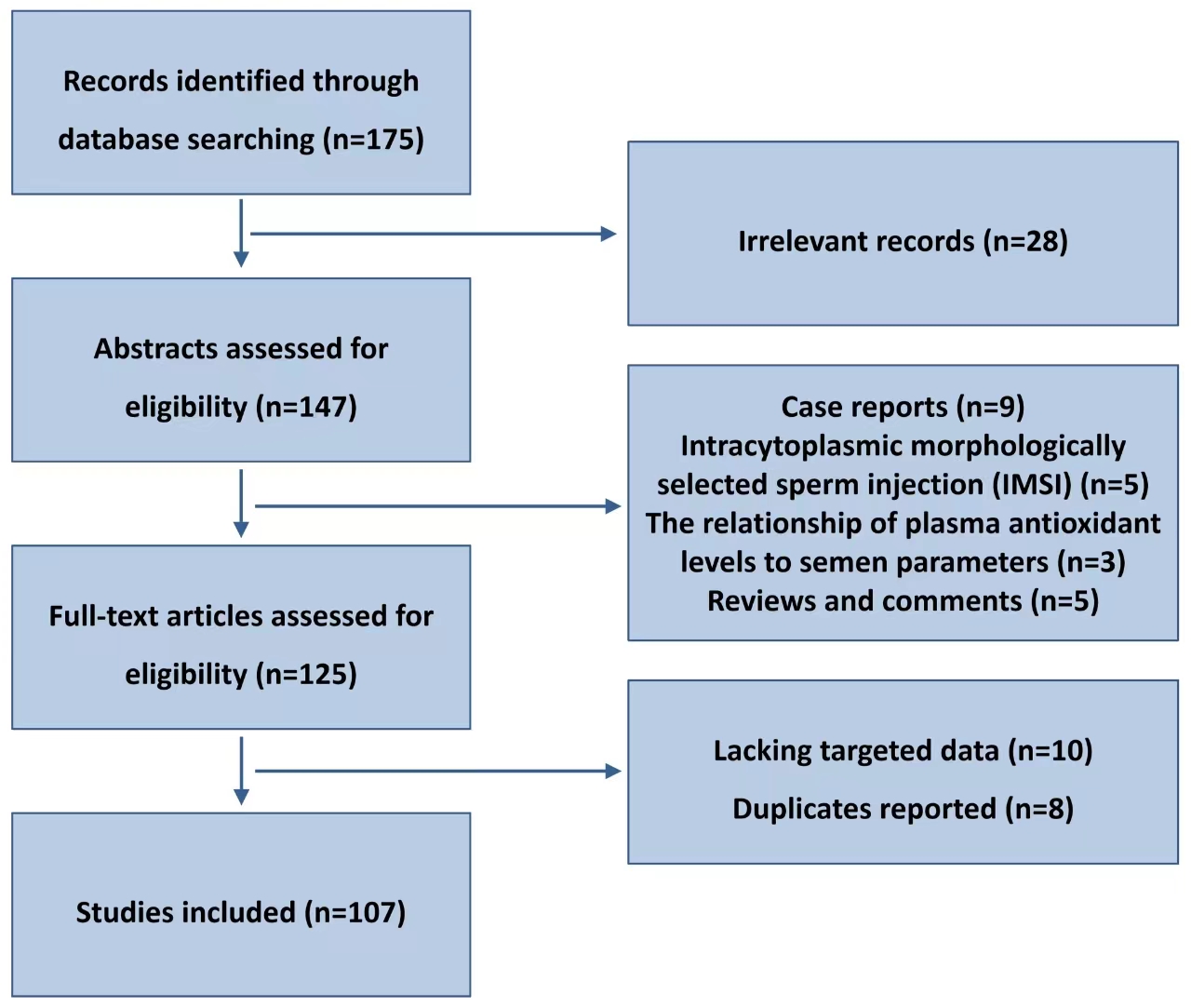
Therefore, it is crucial to conduct additional multi-center research studies and broaden the research cohorts that fulfill the inclusion criteria. This will help in elucidating the effects of DFI on embryo quality, intrauterine insemination, and pregnancy outcomes in ART diagnosis and treatment [3, 4]. An extensive review is provided in this article on the mechanism of sperm DNA damage and the influence of DFI on assisted reproductive technology results. Flow chart of articles identified, included, and excluded is shown in Fig. 1.
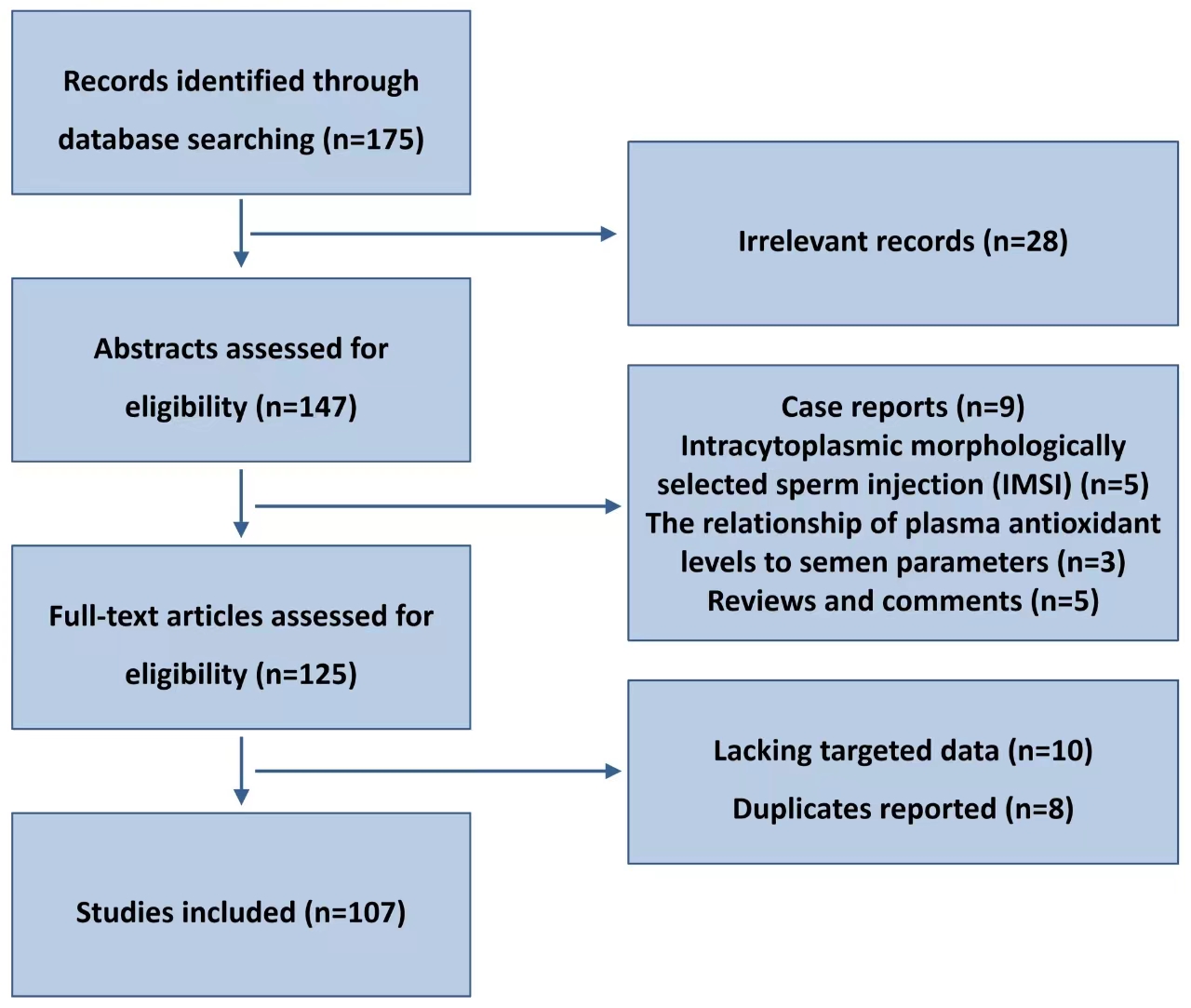 Fig. 1.
Fig. 1.
Flow chart of articles identified, included, and excluded.
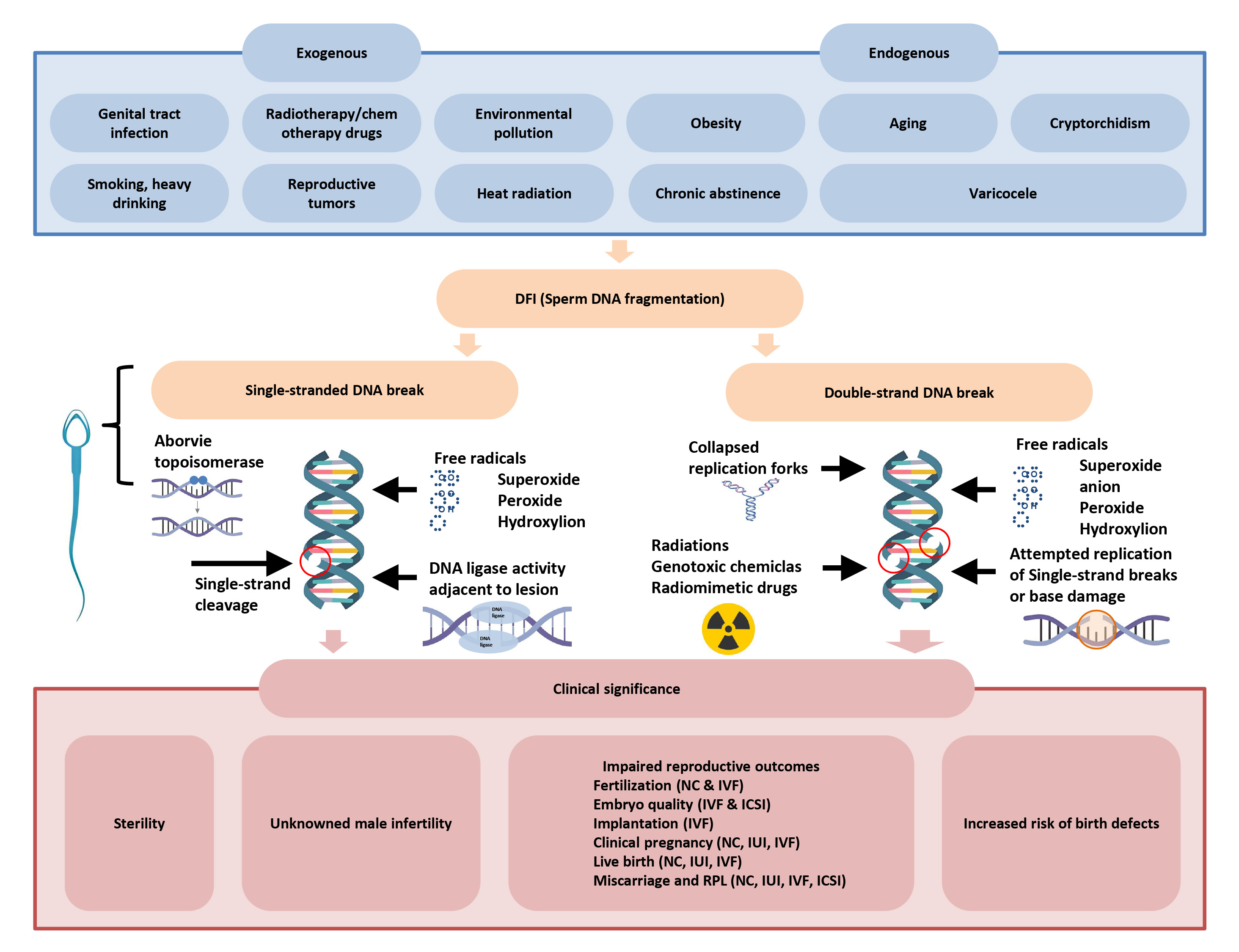
There are three components to the mechanism of sperm DNA damage: sperm abnormalities, oxidative stress damage, and abnormal sperm apoptosis (Fig. 2, Ref. [7]).
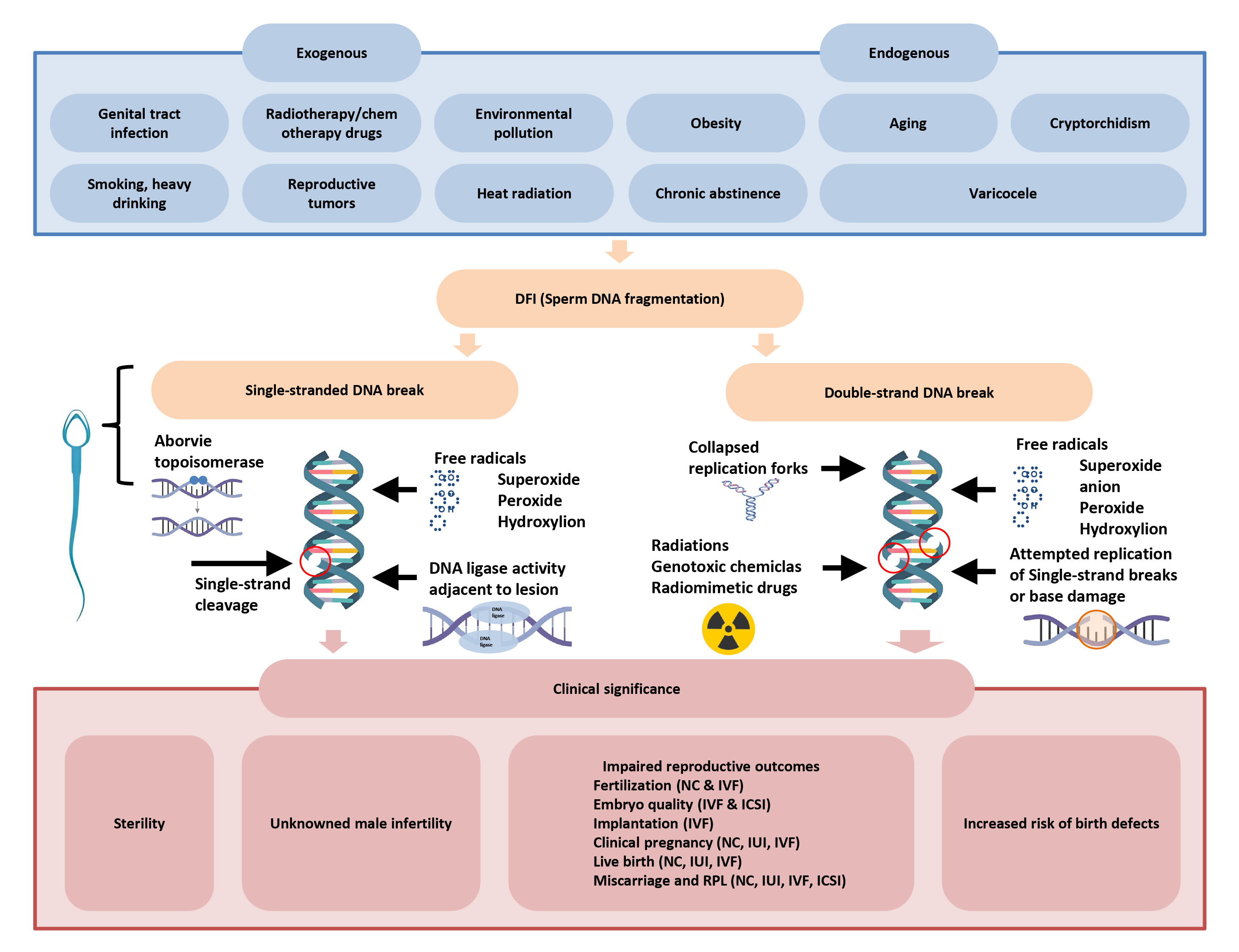 Fig. 2.
Fig. 2.
Risk factors, molecular mechanisms and clinical significance of sperm DNA breakage [7]. DFI, DNA fragmentation index; NC, natural conception; IVF, in vitro fertilization; IUI, intrauterine insemination; ICSI, intracytoplasmic sperm injection.
Spermatogenesis starts with immature spermatogonia developing in the testis
seminiferous tubules. Through divisions, the spermatogonia increase in size and
eventually transform into spermatocytes. Spermatocytes divide to produce haploid
spermatocytes, which mature into sperm. Protamines help compact DNA in sperm
heads [8]. Topoisomerase II
DNA methylation silences genes, while testicular cells have regions of DNA with low methylation levels. Changes in DNA methylation in infertile men may result in a higher occurrence of ART-related complications [11]. The methylation of imprinting genes (BLCAP, DIRAS3, FAM50B, GNAS, MEST, TSPAN32, H19, SNRPN) is often associated with sperm DNA damage [12]. One possible explanation for male infertility could be attributed to MEST hypermethylation, leading to SDF. This is supported by the significant difference in MEST methylation levels between infertile males and fertile men [13]. Alterations in H19 gene methylation have been reported in published studies to be connected with male infertility phenotypes such as oligozoospermia and teratozoospermia [14]. Likewise, the regions linked to non-imprinting genes (PSMA8, SYCP1, and TEX12) exhibit high levels of methylation in cases with elevated SDF. Lack of PSMA8 methylation in mice spermatocytes leads to mitotic abnormalities, SYCP1 degradation, and abnormal sperm production. Methylation of the MLH1 promoter may result in reduced MLH1 expression and increased susceptibility to sperm DNA damage [10, 13]. Genetic barrier-to-autointegration factor-like protein (BAF-L) deficiency in mice affects the transition of histones to protamines in germ cells. BAF-L mutations in human sperm may lead to the retention of more histones or the inability to acquire functional protamines after in vitro fertilization (IVF), leading to male infertility [10].
In 2023, Zhu et al. [15] found 352 genes with differential expression in normal and high DFI sperm (189 upregulated, 163 downregulated). Through SWATH technique, they detected 252 differentially expressed proteins (124 upregulated and 128 downregulated). Notably, DFFA, a DNA damage and repair gene, was highly expressed in the high DFI sperm group, suggesting its potential role in repairing sperm DNA damage [15]. Enrichment analysis revealed significant links between the two groups in Adenosine triphosphatease (ATPase) activity, reactive oxygen species response, P53 signaling pathway, and glycolysis/gluconeogenesis. This indicates that these biological processes and pathways could be crucial in the mechanism of sperm DNA damage and repair [15].
Reactive oxygen species (ROS) play a crucial role in controlling sperm capacitation, acrosome reaction, and sperm-egg binding when present in normal levels. Sperm cells are particularly vulnerable to ROS because of the high concentration of polyunsaturated fatty acids in their membranes and their location in the cytoplasm [16]. ROS damage to single and double strands of sperm DNA is caused by disruption of the sperm cell membrane structure, which exposes sperm DNA directly to oxidizing agents in seminal plasma. Disruption of the sperm cell membrane structure is what causes ROS damage to single and double strands of sperm DNA, exposing the DNA directly to oxidizing agents in seminal plasma [17].
While physiological levels of ROS can enhance sperm binding to the acrosome and zona pellucida, excess ROS can lead to oxidative stress reactions. This can result in sperm membrane immobility, loss of integrity, and damage to mitochondrial DNA and sperm nuclear DNA, ultimately causing abnormal sperm. In infertile males, ROS can cause genetic information loss by binding to DNA bases in mitochondria and breaking single- or double-stranded DNA [18]. The expression of superoxide dismutase genes is altered due to lipid peroxidation caused by ROS, leading to an increase in the DFI [19]. These harmful effects of ROS can impact the biochemical quality of semen and raise the risk of male infertility [20].
Testicular germ cell apoptosis is a natural cell death process triggered by specific factors activating pre-existing death mechanisms [21]. Supporting cells ensure an optimal ratio of germ cells by regulating germ cell apoptosis during spermatogenesis. Through the Fas and Fas ligand (FasL) system, supporting cells induce apoptosis in 50%–60% of germ cells that enter meiosis [22]. With the increasing use of intracytoplasmic sperm injection (ICSI), there is a growing risk of sperm with apoptotic markers, abnormal morphology, or nuclear DNA damage passing on that damage to their descendants [10]. The miR-17-92 cluster is responsible for inhibiting germ cell apoptosis during meiosis by suppressing E2f1 expression, while miR-18, predominantly found in spermatocytes, governs the development of the Hsf2 regulatory factor in male mice germ cells, thereby impacting sperm development [23]. Sperm with damaged DNA can still be involved in fertilization during both natural conception and assisted reproductive technologies, potentially impacting the normal development of embryos or fetuses. Apoptosis decreases sperm motility and leads to DNA fragmentation, which in turn reduces the ability of abnormal sperm to bind to oocytes. Oxidative stress triggers apoptosis by causing mitochondrial dysfunction, release of cytochrome c, activation of caspases, and DNA damage [24]. Double-strand breaks can result in chromosomal abnormalities during the morula stage, which can trigger the apoptotic pathway and impact the formation of the blastocyst [7].
Metabolic processes and environmental factors can cause sperm DNA damage, categorized as endogenous and exogenous factors.
The DNA repair mechanism maintains genetic material integrity but cannot fix all types of damage. Mature sperm have a lower capacity to repair DNA damage compared to other cells [25], leading to age-related mutations due to reduced DNA repair ability in germ cells as individuals age [26]. Additionally, top-notch oocytes have the capability to mend certain sperm DNA damage [27]. Similar to the sperm DNA damage repair mechanism, the capacity of oocytes to repair sperm DNA damage diminishes as individuals age [28]. However, Salehi et al.’s [29] study found that even fully developed immature oocytes generated in vitro were incapable of repairing substantial levels of sperm DNA damage. The unrepaired DNA damage that persists could potentially have adverse effects under certain circumstances [30].
Varicocele is the leading cause of male infertility, responsible for 40% of infertile men. In patients with varicocele, three components (epididymal cells, pampiniform plexus endothelial cells, and testicular cells) release ROS under high temperature and low oxygen conditions [31]. Excessive ROS is linked to SDF and can cause reduced sperm function and infertility in individuals with varicocele. This is due to lipid membrane peroxidation, oxidative damage to DNA and sperm proteins, epigenetic alterations, and the apoptosis of specialized germ cells [32]. The link between oxidative stress and SDF is a key factor in varicocele-related infertility [33]. Elevated ROS levels and reduced glutathione S-transferase (GST) activity may lead to sperm membrane damage and SDF. Increased ROS levels are linked to male accessory gland infections and can lead to oxidative damage in sperm, increasing sperm DNA fragmentation [34, 35].
The oxidation-reduction potential has been found to be linked to SDF levels in the semen of varicocele patients [36]. Varicocelectomy is considered the best treatment for reducing testicular oxidative stress and improving semen parameters and the DNA fragmentation index in individuals with varicocele [37].
In a study of 150 semen samples from healthy non-smoking men in Europe, Rubes
et al. [38] found a correlation between increasing age and increasing
DFI, which frequently led to an increase in DFI. A retrospective study conducted
in China in 2020 analyzed 18,441 semen samples and found that as the male
patient’s age increased, there was a decrease in semen volume, total sperm count,
viability, and high DNA stainability (HDS). Conversely, sperm concentration and
DFI showed an increase [39]. Specifically, semen volume and total sperm count
decreased after the age of 35, while sperm motility and HDS started to decline at
30 years old. Despite whether semen parameters are normal or abnormal, the
likelihood of DFI abnormality significantly increases with age [39]. This trend
was also observed in the research conducted by Li et al. [40], where DFI
levels showed a negative correlation with sperm concentration, motility, and
forward motility percentage. In their study on the impact of semen parameters on
pregnancy outcomes during intrauterine insemination (IUI), it was found that the
group with sperm DFI
This may have a connection to oxidative stress. With age comes the accumulation of reactive oxygen species in the body, leading to an imbalance with antioxidants that results in elevated oxidative stress and harm to sperm DNA [42]. A study conducted in 2020 by Vaughan DA et al. [43] on 16945 semen samples from the years 2010 to 2018 also concluded that reactive oxygen species are responsible for increased fragmentation of sperm DNA. The analysis of the data revealed a significant association between age and sperm DFI (DFI), indicating that oxidative stress compounds in the body increase with age and DFI. Younger patients with higher sperm DFI were found to be more likely to have oxidative stress compared to older patients [43]. Furthermore, the study showed that the longer the duration of abstinence, the higher the SDF rate [44], which is notably higher in older males compared to younger males. This could be a key factor contributing to the increase in DFI with age.
In men, inflammation, particularly from genital tract infections, is a frequent cause of sperm DNA breaks. Sperm DNA breaks are triggered by male genital tract infections through the rise in leukocyte count and the escalation of oxidative stress in semen [45]. The rise in inflammatory mediators like leukocytes directly impacts the integrity of sperm DNA. Bacterial infection, on the other hand, primarily affects sperm chromatin condensation and the ratio of ichthyoglobin, leading to a negative impact on sperm DNA integrity [46]. The damage bacteria can inflict on sperm cells ranges from DNA breaks and peroxidation of the cell membrane to acrosome damage. This harm can be attributed to toxins and metabolites produced by bacteria, as well as to bacteria physically attaching to sperm cells and activating signaling pathways associated with oxidative stress, apoptosis, and inflammation [47].
Along with bacterial infections, research has indicated that Mycoplasma and Chlamydia trachomatis infections in the male genital tract may cause prolonged semen liquefaction time, decreased sperm quality, and ultimately, male infertility [48]. Furthermore, infections worsen the harm to sperm DNA breaks. Recent research suggests that this effect is likely due to infection-induced oxidative stress, as oxidative stress not only impacts chromatin assembly and changes protein expression, but also results in modifications to cellular ion channels [49].
Fraczek et al. [50] discovered that the presence of Staphylococcus haemolyticus, Bifidobacterium lysodeoxidans, and/or C. albicans in semen in vitro significantly increased the percentage of Tunel test positive spermatozoa. This indicates that bacteriospermatosis and leukocytospermia could potentially result in sperm DNA damage, ultimately impacting male fertility. Zeyad et al. [51] discovered that among the 120 infertile men they studied, 36 of them had semen samples infected with oxidative stress, which could potentially affect the cellular ion channels. Within these samples, 30% were found to have bacterial infections, with nine different species of bacteria identified from five genera including Staphylococcus, Escherichia, Streptococcus, Enterococcus, and Klebsiella. As a result, the sperm concentration, motility, nucleus ichthyoglobin P1/P2 ratio, and DNA integrity were all significantly decreased [51]. Farahani L conducted a meta-analysis on 55 studies and retrospectively analyzed 51,299 data. The results showed that bacterial spermatosis has a negative impact on sperm concentration, motility, and DNA integrity, whereas Lactobacillus casei was found to protect sperm quality [52].
In addition, viruses also affect sperm DNA integrity. Exposure to hepatitis B
virus, human papillomavirus and HIV has been reported to reduce sperm DNA quality
[53, 54]. Tangal et al. [55] discovered that out of 117 males who had
experienced two or more ICSI failures, 7.7% had HPV-positive semen. Within the
HPV-positive group, 82.9% had elevated sperm DFI (
Bisphenol A, phthalic acid, lead, cadmium, and polychlorinated biphenyls (PCBs) are substances in the environment that have reproductive toxicity and can cause damage to sperm DNA. Bisphenol A, utilized in the production of numerous medical devices, is a chemical commonly found in epoxy resin plastics [58]. Increased levels of urinary BPA in men have been linked to higher levels of sperm DNA damage and abnormal semen parameters. Studies have demonstrated that phthalic acid can result in elevated sperm ROS production and is associated with fragmented sperm DNA levels [59]. Furthermore, elevated levels of lead and cadmium in semen can exacerbate sperm DNA fragmentation [60]. The exposure to PCBs, known as persistent organochlorine pollutants (POPs) with the potential to disrupt the endocrine system, may harm the integrity of sperm DNA [61]. Unhealthy lifestyle habits such as alcohol consumption, smoking, excessive sitting (cadmium found in tobacco smoke can inhibit the OGG1 enzyme), and staying up late are major factors contributing to sperm DNA damage. Previous research has demonstrated that excessive smoking speeds up cell apoptosis, resulting in increased oxidative damage to sperm DNA.
Nicotine negatively affects male reproductive function by increasing SDF through excessive production of ROS, although the exact mechanism remains unclear [62]. Secondhand smoke exposure can also lead to increased ROS levels in tissues, causing DNA damage [62]. Recent studies have indicated that smokers exhibit a decrease in the expression of checkpoint kinase 1 (Chk1), which is associated with sperm DNA damage and apoptosis. This decrease in Chk1 may result in a reduction in the repair mechanism for sperm damage and an increase in sperm apoptosis [63].
There is solid evidence in published research indicating a strong correlation between increased ROS levels and alterations in both sperm parameters and diabetes [51]. Obesity can increase testicular metabolism, leading to reduced ATP production and alterations in the mitochondrial membrane potential of germ cells. These modifications can trigger apoptotic pathways mediated by caspase-8, subsequently activating caspase-3, 6, and 7 [7]. The JAK/STAT signaling pathway influences male reproductive processes, including sexual development and sperm maturation. This pathway is also a primary cellular route activated by the buildup of intracellular ROS. PLK1, a crucial regulator of the DNA damage checkpoint, plays a vital role in preserving the stability of the genome while DNA replication occurs [64]. When p53 is overexpressed due to DNA damage and PLK1 is suppressed, it can result in cell death and a significant decrease in the antioxidant capacity of germ cells [20]. Human disorders like cancer and premature aging can result from oxidative clustered DNA lesions (OCDL) caused by the buildup of double-strand DNA damage [65].
Some scholars believe that cancer treatment may have adverse effects on male fertility. Radiation and chemotherapy can harm germ cells, leading to reduced sperm survival and increased sperm DNA damage in male infertility patients [66]. Cancer itself can also damage sperm DNA. Prolonged radiation exposure may cause mutations in the hMSH5 gene, disrupting the natural repair process of sperm DNA and increasing the sperm DFI [12]. More research is needed to determine the risks of using fresh sperm after therapy or frozen sperm before treatment in infertile male patients with fertility-demanding malignancies [67].
Various methods have been reported for detecting SDF [68], with direct and indirect methods being the two main classifications based on their approach.
The reported methods are divided into direct methods for the direct detection of DNA breaks (Comet assay-Comet, terminal deoxynucleotidyl transferase-mediat-ed deoxyuridine triphosphate (dUTP) nick end labeling method-TUNEL) and indirect methods for the indirect detection of DNA breaks (sperm chromatin structure analysis-SCSA, sperm chromatin diffusion-SCD method) by detecting the sensitivity of the denaturation of double-stranded DNA to single-stranded (Table 1, Ref. [69, 70, 71, 72, 73]). Nevertheless, the current methods for detecting SDF have their limitations. The detection of SDF may not always have clinical significance, as small amounts of single-stranded DNA breaks can be repaired and replicated by intact DNA strands. Moreover, it is difficult to detect breaks at specific important DNA loci. Lastly, there is a lack of research evidence to either support or oppose the described SDF detection method [74].
| Detection methods | Principle | Detection of fragment types | Advantages/Disadvantages |
| Comet (Direct Detection Method) | Sperm nuclei were depolymerized under alkali denaturation and neutral conditions, and single sperm electrophoresis was performed. Broken DNA formed tails, while intact DNA was located at the head of the sperm without forming tails. | Alkaline conditions detect both single-stranded DNA (ssDNA) and double-stranded DNA (dsDNA) breaks, while neutral conditions primarily detect dsDNA breaks. | High sensitivity and reproducibility, specificity, and a small number of cells can also be used. It has a highly subjective nature and can vary widely among analysts. |
| ISNT (Direct Detection Method) | Fluorescently labeled nucleotides are incorporated into gaps or free 3′OH ends using DNA polymerase I. | ssDNA breakage | Simple, reliable, and accurate with little inter-observer variability. The large inter-room variation requires uniform standards between different laboratories. |
| TUNEL (Direct Detection Method) | Fluorescent dUTP is incorporated into the 3’OH ends of ssDNA and dsDNA breaks by TdT binding. | ssDNA and dsDNA breaks | Sensitivity and reliability are high, with low operator error and low requirements for sperm count. The large inter-room variation requires uniform standards between different laboratories. |
| SCSA (Indirect Detection Method) | Mildly acid-denatured DNA is used for this method. Undenatured dsDNA binds to AO and emits green fluorescence, while denatured ssDNA binds to AO and emits red fluorescence. | ssDNA and dsDNA breaks | Sensitive, standardized, with low inter-laboratory variability, and capable of detecting a large number of sperm cells/Flowcytometry is required. |
| DBD-FISH (Indirect Detection Method) | Quantification of single-cell DNA breaks and base susceptibility degeneration sites after denatured DNA deletion using genome-wide probes bound to ssDNA. | ssDNA and dsDNA breaks | Be used for site-specific detection in sperm cells/Subcellular site-specific detection, complex and high. |
| AI-DFI (Artificial intelligence) | Featuring parallel data analysis with multi-dimensional signal-specific recognition, and self-learning capabilities, such as detection accuracy that improves with cumulative detection volume (imaging system with built-in AI analysis program directly recognizes fluorescently labeled sperm). | Single-stranded A and double-stranded DNA breaks | Reliable technology and accuracy make this method particularly effective in assessing the problem of DFI in extreme samples (e.g., high-impurity samples such as low-concentration semen, bacterial-infected semen, etc.). |
| SCD | Detecting fragmented DNA dispersion after acid denaturation. | After acid denaturation, sperm with fragmented DNA do not create the halo of dispersed DNA loops that sperm with non-fragmented DNA do. | A simple test with easy availability of commercial kits can be performed on neat and washed seminal samples. This indirect assay is time-consuming and may result in inter-observer variations. |
Note: SDF, sperm DNA fragmentation; SCD, Sperm Chromatin Dispersion assay; AO, Acridine Orange; DBD-FISH, DNA Break Detection-Fluorescence In Situ Hybridization; TUNEL, Terminal deoxynucleotidyl transferase mediated dUTP nick end labeling; dUTP, Deoxyuridine triphosphate; dsDNA, Double-stranded DNA; ssDNA, Single-stranded DNA; Comet, Comet assay; ISNT, In situ nick translation; SCSA, Sperm Chromatin Structure Analysis.
SCD and SCSA procedures are the most commonly utilized in China, with the latter often considered the gold standard approach for DFI detection.
The field of medical detection has seen a rise in interest in AI image analysis due to recent advancements in AI technology. The capabilities of AI technology encompass high accuracy, self-learning, multi-dimensional signal specific identification, and parallel data analysis. Therefore, AI image recognition technology could potentially assist in detecting extreme samples with high impurities in DFI, like semen with low concentration and bacterial infections. These samples are challenging to evaluate accurately using the traditional flow SCSA method [61]. This would enable DFI detection to align more closely with real clinical requirements.
Various methods of detecting SDF may vary in their diagnostic specificity and sensitivity, yet they still show a high correlation with each other [69, 70, 71, 72, 73].
The consequences of elevated sperm DFI on IUI outcomes are mainly seen in reduced pregnancy rates and heightened miscarriage rates. A study on IUI revealed that out of 23 male patients with DFI exceeding 27%, only one achieved a successful pregnancy with their partner [75]. In addition, Bungum et al.’s [76] research revealed that couples with a male DFI greater than 30% had decreased chances of achieving successful biochemical and clinical pregnancy. Sugihara et al. [77] included three studies comprising 917 IUI cycles and reported that low DFI was associated with a higher clinical pregnancy rate (relative risk (RR): 3.30, 95% confidence interval (CI): 1.16–9.39). Liu’s study [78] found that the DNA fragmentation index (DFI) was significantly lower in the pregnancy group compared to the non-pregnancy group, even after controlling for variables like patient age and infertility duration. However, the findings of Rex AS et al. [75]and Yang H et al. [79] did not align , possibly due to differences in the sperm density gradient centrifugation technique used during IUI .
Furthermore, Yang HY and team [79] found that high levels of DFI in semen increase the risk of early miscarriage after IUI. This may be due to the presence of sperm with high DNA damage in semen with high DFI levels. The sperm with damaged DNA may have the ability to form pronuclei during fertilization, leading to the development of non-dominant embryos and resulting in spontaneous miscarriage [80].
The predictive value of sperm DFI in determining the outcomes of IVF and ICSI has been a matter of debate. A meta-analysis investigating the relationship between DFI and IVF/ICSI results concluded that DFI does not have the ability to predict the outcomes of these procedures [81]. Likewise, Cissen et al. [82] came to similar conclusions, though the constraints of the studies hinder a definitive dismissal of the potential correlation between DFI and pregnancy outcomes.
Dar et al. [83] carried out a study utilizing the SCSA method to
explore the clinical outcomes of 150 ICSI cycles in 98 couples, which included
114 cycles from the high DFI group (
Chinese studies found a weak negative correlation between DFI and IVF/ICSI
fertilization and optimal embryo rates, but no significant differences in clinical pregnancy were observed [85].
According to recent research conducted by Zha et al. [86] and Sun
et al. [87], there were no notable clinical variances in IVF/ICSI
fertilization rate, cleavage rate, good embryo rate, and clinical pregnancy rate
across various sperm DFI groups. However, Simon et al. [88] revealed in
a meta-analysis that higher sperm DFI could potentially reduce clinical pregnancy
rates in IVF/ICSI. Deng et al. [89] also reported that the clinical
pregnancy rate was significantly lower in the high DFI than in the low DFI (OR =
1.92, 95% CI: 1.33–2.77, p = 0.0005) by evaluating 2130 IVF cycles
from 7 studies. Ribas-Maynou et al. [90] found that DNA fragmentation
reduces clinical pregnancy and implantation rates in IVF/ICSI, but does not
affect live birth rates significantly. Oleszczuk K et al. [91] found
that high sperm DFI is associated with lower fertilization rates, reduced
likelihood of high-quality embryos in IVF cycles when DFI exceeds 20%, decreased
live birth rates, and increased risk of miscarriage when DFI exceeds 40% in
IVF/ICSI cycles. In ICSI cycles, the impact of high DFI on live birth rates is
greater than in IVF cycles [92]. Giwercman et al. [93] used the SCSA
method to investigate sperm DFI in 127 infertile men and 137 fertile men and
found that the odds ratio for infertility was 2.5 (95% CI: 1.0–6.1) for sperm
DFI between 10% and 20% and 8.4 (95% CI: 3.0–23) for sperm DFI greater than
20%. In case of abnormal semen parameters, the infertility preponderance ratio
was 16 (95% CI: 4.2–60) for sperm DFI
A study in China found a higher rate of miscarriage with a DFI exceeding 30%, but it did not affect IVF treatment outcomes. The differences in results may be due to factors such as improved sperm quality after processing, selection of high-quality embryos for transfer, and the repair capacity of damaged sperm by oocytes being overlooked [3].
It is currently unknown whether offspring born through ART are at risk of DNA fragmentation due to a lack of relevant research. Therefore, further follow-up studies involving multiple centers and large sample sizes are necessary. Research has suggested that the extent of SDF does not influence the length of pregnancy or the birth weight of the infants [3]. However, the harm to sperm DNA could cause epigenetic alterations, potentially leading to diseases in future generations.
Li F et al. [3] demonstrated that a rise in sperm DFI can lead to a higher likelihood of low birth weight infants during frozen-thawed embryo transfer. Additionally, the rate of birth defects in babies conceived through ICSI is slightly elevated. Moreover, other studies [92, 95] have suggested that sperm DNA damage might lead to genetic mutations in descendants, potentially causing male infertility, childhood tumors, and diseases related to imprinting defects. In 2017, Olszewska M et al. [96] found that sperm DNA damage can lead to changes in sperm epigenetics, increasing the risk of systemic diseases like cardiovascular disease in offspring.
Recent studies have shown that SDF can have different effects on the outcomes of IVF and ICSI [97, 98]. ICSI is more successful for patients with damaged sperm DNA because it involves selecting the healthiest sperm, leading to lower DFI levels [99]. Hence, ICSI is the recommended course of action for individuals with DNA damage. This suggestion is in line with the recent literature review by Simon L et al. [88], which indicates that ICSI treatment yields higher pregnancy rates in patients with elevated DFI levels compared to IVF.
Bungum et al. [76] investigated the impact of SDF on assisted reproduction through the identification of SDF levels using the SCSA method. Their study included a total of 131 cases in IUI, 109 cases in IVF, and 66 cases in ICSI. Throughout the IUI cycle, 23% of male participants saw a rise in SDF levels, leading to a mere 4% pregnancy rate for their partners. Couples in the IUI group with a DFI of less than 27% experienced significantly higher pregnancy and live birth rates compared to those with a DFI greater than 27%. However, no statistically significant variances in clinical outcomes (such as biochemical pregnancy rate, clinical pregnancy rate, and live birth rate) were found between IVF and ICSI when DFI was below 27% [76]. When sperm DFI was greater than 27%, in vitro fertilization (IVF) showed poorer clinical outcomes compared to ICSI, suggesting that sperm DFI levels can serve as a predictor for assisted reproductive outcomes. High levels of sperm DFI were found to have a significant negative impact on the fertilization success rate for male partners. Subsequently, Bungum et al. [76] expanded the sample size to 637 couples and 998 cycles. They discovered that when DFI exceeded 30%, couples undergoing IUI experienced notably lower chances of biochemical pregnancy, clinical pregnancy, and live birth compared to couples with DFI below 30%. Conversely, when DFI was below 30%, no significant differences in clinical outcomes were observed between ICSI and IVF. Despite this, when the DFI was greater than 30%, the clinical outcome of ICSI proved to be significantly better than that of IVF. These findings suggest that DFI can be used as a stand-alone predictor of fertility in couples undergoing IUI, and ICSI should be the preferred treatment option when DFI levels exceed 30% [76].
Zini et al. [100] found that sperm DNA damage has a greater impact on embryo quality during ICSI compared to IVF, possibly due to different repair mechanisms of oocytes. Natural selection during IVF may decrease the likelihood of high-DNA-fragmentation sperm binding to oocytes. Semen samples used for ICSI are typically of lower quality than those used for IVF. However, Bungum et al. [76] argued that semen samples with higher levels of DNA fragmentation are more likely to result in pregnancy with ICSI. This is due to the low frequency of ROS exposure of oocytes in ICSI, as the procedure bypasses the naturally selected fertilization process. Additionally, male factor infertility is the primary cause of ICSI, which explains the discrepancy between the results of Zini et al. [100] and Bungum et al. [76]. The inconsistencies in the studies by Zini A and Bungum M may be due to differences in sample inclusion criteria, natural selection and oxidative damage on sperm during IVF fertilization, and the inability to compare these factors accurately. Additionally, the impact of female factors and oocyte repair ability on sperm fragmentation and pregnancy outcomes was not assessed [101, 102]. It is still uncertain whether sperm with high DNA fragmentation are selected. Moreover, certain research has indicated that DFI does not have a varying effect on the two techniques. Nevertheless, more studies are needed to establish whether ICSI is the preferable choice for semen samples with elevated DFI [103]. The analyses on impact of SDF on pregnancy outcome in ART are presented in Table 2 (Ref. [3, 73, 76, 77, 83, 84, 88, 89, 90, 91]).
| Authors | Method for DFI detection | ART | n/cycles | Results |
| Bungum et al. [73] | SCSA | IVF | 109 | CP (36.6% with DFI |
| ICSI | 66 | CP (41.5% with DFI | ||
| Bungum et al. [76] | SCSA | IVF | 5388 | CP (33.7% with DFI |
| ICSI | 5223 | CP (37.3% with DFI | ||
| Sugihara et al. [77] | SCSA | IUI | 3 studies, 917 cycles | Low DFI associated with higher clinical pregnancy rate (RR = 3.30, 95% CI: 1.16–9.39) |
| SCD | ||||
| Dar et al. [83] | SCSA | ICSI | 150 | There was no significant variance in fertilization rate and clinical pregnancy rate between the two groups, the high DFI group exhibited a tendency towards a higher miscarriage rate |
| Boe-Hansen et al. [84] | SCSA | IVF | 139 | CP (29% with DFI |
| ICSI | 47 | CP (27.6% with DFI | ||
| Simon et al. [88] | SCSA | IVF | 16 studies, 3734 cycles | Significant negative effect of DFI on clinical pregnancy after IVF (OR = 1.92, 95% CI: 1.33–2.77, p = 0.0005) |
| SCD | ||||
| Deng et al. [89] | SCSA | IVF | 7 studies, 2130 cycles | The clinical pregnancy rate was significantly lower in the high DFI than in the low DFI (RR = 0.77, 95% CI: 0.59–1.00, p = 0.05) |
| SCD | ||||
| Comet | ||||
| Ribas-Maynou et al. [90] | SCSA SCD | ICSI | 9 studies, 3017 cycles | No significant relationship between DNA damage and live-birth rate (RR = 0.92, 95% CI: 0.67–1.27, p = 0.62) |
| SCSA SCD | IVF | 15 studies, 3711 cycles | Significant negative association between sperm DNA damage and pregnancy rate (RR = 0.72, 95% CI: 0.55–0.95, p = 0.02) | |
| SCSA | ICSI | 24 studies, 2282 cycles | Significant negative effect of DFI on clinical pregnancy after ICSI (OR = 1.49, 95% CI: 1.11–2.01, p = 0.0075) | |
| SCD | ||||
| Oleszczuk K et al. [91] | SCSA | IVFI | 1663 | High DFI is associated with lower FR, reduced likelihood of high-quality embryos in IVF cycles when DFI |
| ICSI | ||||
| Li F et al. [3] | SCSA | IVFI | 6330 | The incidences of miscarriage rates in IVF/ICSI groups with DFI |
| ICSI |
Note: DFI, DNA fragmentation index; ART, assisted reproduction techniques; ICSI, intracytoplasmic sperm injection; IVF, in vitro fertilisation; CP, clinical pregnancy; CI, Confidence interval; RR, relative risk; OR, odds ratio; IR, implantation rate; FR, fertilization rate.
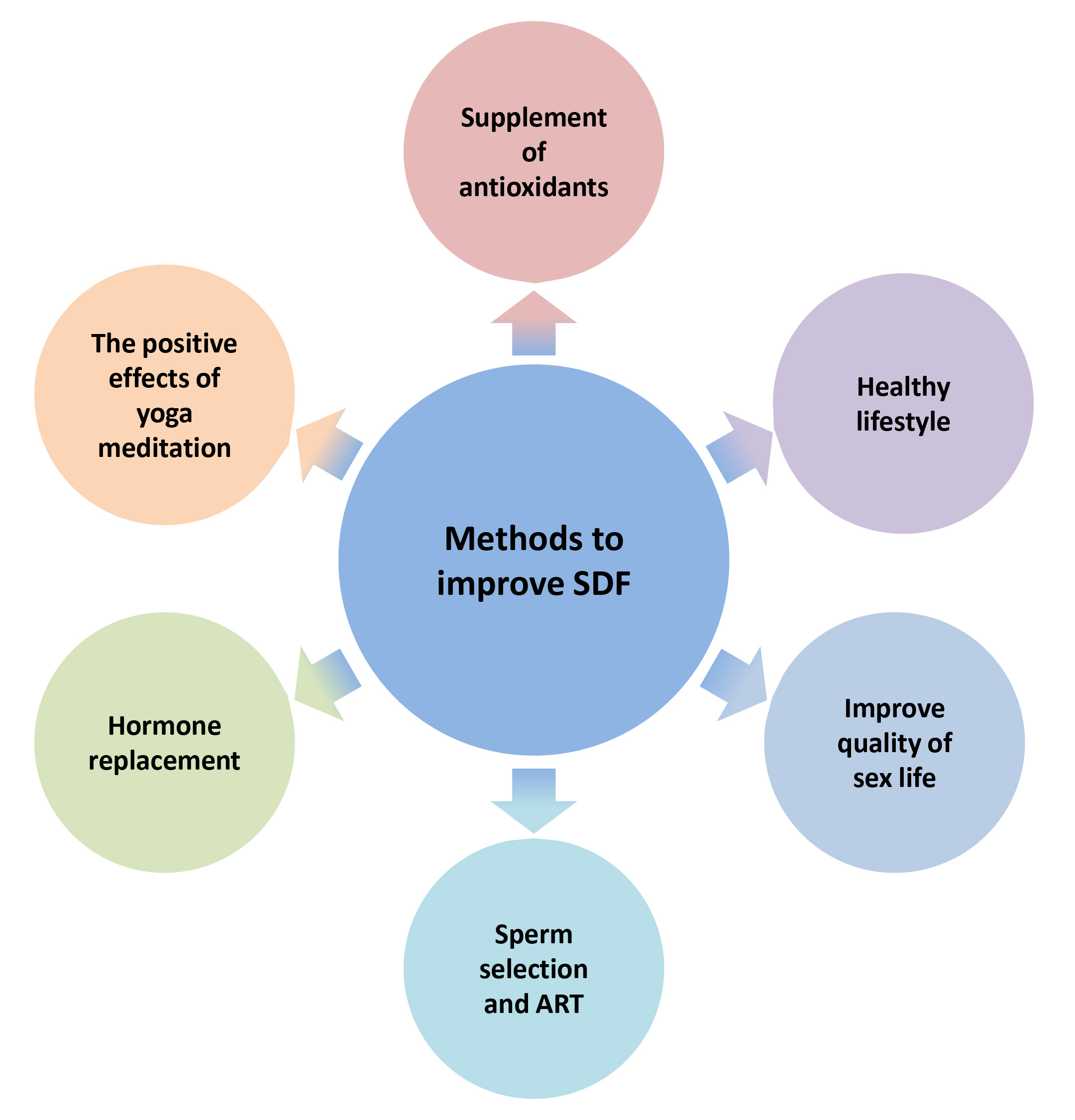
Enhancing the integrity of sperm DNA has emerged as a significant issue for medical experts, as it could enhance the chances of natural conception in infertile couples undergoing IUI (Fig. 3). Lifestyle changes like quitting smoking, reducing alcohol intake, maintaining a consistent sleep schedule, exercising regularly, eating a diet rich in fruits and vegetables, and avoiding high-triglyceride foods can improve sperm DNA quality [3]. Other effective methods include shortening ejaculation abstinence, using proper sperm handling techniques, treating varicocele, and addressing infections and inflammation [3]. Recent studies show that yoga, meditation, and antioxidant supplementation can improve sperm DNA integrity by reducing oxidative stress, DNA damage, and inflammation. Several studies have shown that moderate supplementation of antioxidants like vitamin E, carnitine, vitamin C, and folic acid can reduce DNA fragmentation in male sperm [104]. Maintaining a healthy frequency of sexual activity can help improve DNA integrity by reducing the time sperm spends in the epididymis and decreasing free radical damage. It is important to intervene promptly in patients with conditions that can affect sperm quality, such as varicocele and urogenital infections. In severe male infertility with high sperm DNA damage, testicular sperm may be a better option due to lower DNA damage compared to epididymal and ejaculated sperm. Additionally, traditional Chinese medicine has shown promise in improving sperm DNA integrity. In 2022, Deng [105] reported that a combination of Chaihu Jia Longgu Muli decoction and alpha-lipoic acid can effectively improve sperm DNA damage and reduce the fragmentation index. The combined traditional and Western medicine group showed more significant effects compared to the Western medicine group alone.
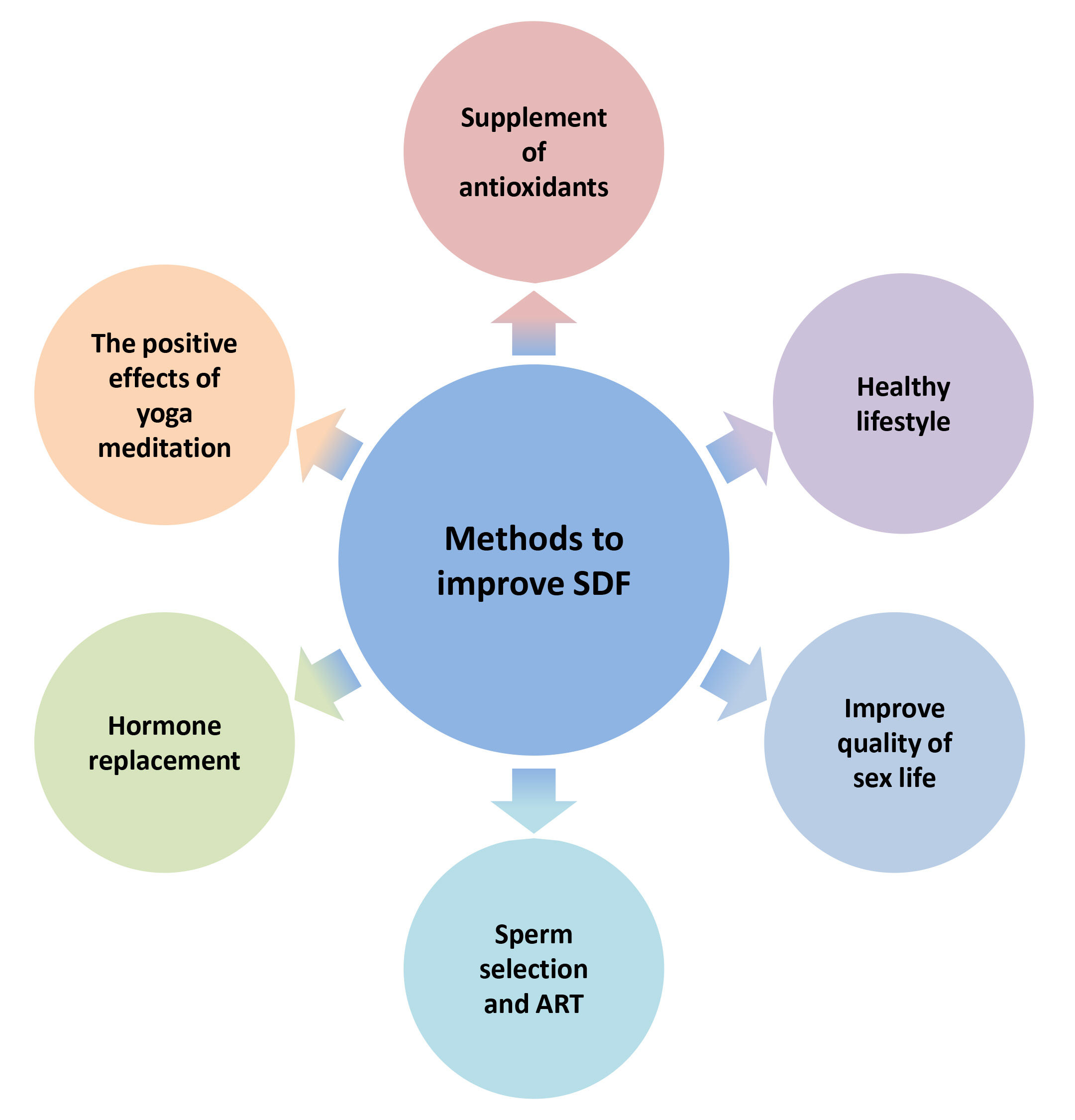 Fig. 3.
Fig. 3.
Methods to improve sperm DNA integrity.
As the carrier of paternal genetic information, the integrity of sperm DNA is fundamental to maintaining normal pregnancy and plays a significant role in embryonic development and pregnancy outcomes. Yet, the impact of sperm DNA integrity on the results of in vitro fertilization-embryo transfer (IVF-ET) and ICSI remains unclear based on current research. This may be due to several reasons. There is no consensus among experts on the threshold for determining sperm DNA damage and detection methods. Sample heterogeneity and factors from infertile couples, especially eggs, need to be considered. Current clinical testing technology cannot accurately determine SDF in single sperm in ART. The molecular mechanism affecting sperm DNA damage and its impact on ART outcomes are not definitive. The direct effect of SDF on ART pregnancy outcomes needs further research with a larger sample size. Despite limited predictive value, SDF analysis provides additional information beyond traditional semen analysis and can aid in diagnosing male infertility. The American Urological Association (AUA) and the European Urology Association (EWAU) have acknowledged the significance of DFI parameters sooner than the American Society for Reproductive Medicine.
In the field of clinical practice, it is imperative to incorporate DFI testing as a regular practice. Timely intervention and management of elevated DFI levels in males can significantly contribute to improving outcomes of ART pregnancies [6]. In general, it is believed that having a SDF level higher than 40% can be a risk factor for miscarriages after in vitro fertilization. Furthermore, an SDF level surpassing 30% is connected with a decreased chance of successfully achieving conception with assisted reproductive techniques [106]. When SDF levels go beyond 80%, it can have a detrimental effect on fertilization and embryo development, leading to the recommendation of exploring better sperm selection methods and considering Preimplantation Genetic Diagnosis (PGD)/Preimplantation Genetic Screening (PGS) in these situations [107].
DFI, DNA fragmentation index; ART, Assisted reproduction technology; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; ACE2, angiotensin-converting enzyme 2; AUA, American Urological Association; EWAU, European Urological Association; TOP2B, topoisomerase II
KSL, YJC and RFA contributed to the study design; KSL and YJC wrote the manuscript. All authors reviewed drafts and approved the final version of the manuscript. All authors have participated sufficiently in the work to take public responsibility for appropriate portions of the content and agreed to be accountable for all aspects of the work in ensuring that questions related to its accuracy or integrity. All authors contributed to editorial changes in the manuscript.
Not applicable.
Not applicable
This research received no external funding.
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.