Academic Editors: Leonid A. Gavrilov and Thomas Heinbockel
Introduction: L-carnosine has been found to have multimodal activity.
Aim: The aim of this review was to find out the efficacy of L-carnosine
in patients with age-related diseases. Methods: Clinical studies
evaluated the effect of L-carnosine on cancer, cardiovascular disease, diabetes,
and neurodegenerative disorders were searched in electronic bibliographic
databases. The protocol has been registered with PROSPERO (CRD42022314033). The
revised Cochrane risk of bias tool for randomized trials was used to assess all
of the reports for risk of bias. RevMan 5.4 was used to conduct the
meta-analysis. Results: Following the screening process, 14 papers were
selected for systematic review, with 9 of them being qualified for meta-analysis.
Many of the included studies showed that L-carnosine has potential therapeutic
activity in age related diseases. Results from the meta-analysis showed that in
diabetes mellitus, HbA1c [mean difference (MD) 95% CI = –1.25 (–2.49,
–0.022); p = 0.05; p = 0.001; I
Gulevitsch discovered carnosine (
Aging is defined as the irreversible loss of physiological function and ageing processes are described as those that enhance an individual’s susceptibility to conditions that eventually lead to death as they grow older. It’s a complicated multi-factorial process in which numerous components interact at the same time and work at several levels of functional organisation. Aging leads to age-related diseases like cancer, cardiovascular disease, diabetes, and neurological disorders.
Toxicity from chemotherapy is a common and unfortunate side effect of treatment
that can occur even at standard doses. Oral mucositis is a common consequence in
patients who have undergone high-dose chemotherapy and after hematopoietic stem
cell transplantation (HSCT). Oral mucositis is frequently accompanied by pain,
odynophagia, xerostomia, and dysgeusia, and it can lead to malnutrition,
dehydration, and lowers the patients’ quality of life significantly (QoL) [5].
Polaprezinc (PZ), a chelated form of zinc and L-carnosine, inhibits the
production of inflammatory cytokines such as tumour necrosis factor-alpha
(TNF-
Chemotherapy-induced peripheral neuropathy (CIPN) is a typical side effect of neurotoxic chemotherapeutic drugs used to treat cancer patients. It is characterized by inflammation, damage, or degeneration of the peripheral nerve fibres. As a commonly used third-generation platinum analogue, oxaliplatin is responsible for more than 70% of symptomatic neurotoxicity of any severity, which frequently leads to treatment discontinuation [7, 8].
The oxidative stress, inflammatory, and apoptotic pathways are the responsible
pathophysiological factors for Oxaliplatin-induced peripheral neuropathy (OPIN).
The inflammatory cascade includes the up-regulation of the transcriptional factor
NF-κB and its downstream molecule TNF-
Pro-inflammatory cytokines, such as IL-6, and acute-phase proteins, such as C-reactive protein (CRP) are elevated in individuals with myocardial infarction (MI). The role of inflammation in the onset of MI is regarded to be significant [12]. L-carnosine has been shown to reduce the production of IL-6, and thereby it helps in the management of MI [13, 14].
Despite breakthroughs in pharmacological therapy and the introduction of novel
devices, patients with heart failure continue to have a poor QoL and a lower
exercise tolerance. Several factors contribute to the decline in factional
capacity and exercise tolerance, including changes in endothelial and
vasodilatory function, abnormalities in skeletal muscle metabolism, and reduction
in muscle blood flow during exercise [15]. L-carnosine is a key antioxidant that
protects against oxidative damage caused by anaerobic exercise. Lactic acid
production increases during exercise, followed by dissociation into lactate and
H
Type 2 diabetes mellitus (T2DM) accounts for 90% of all diabetes cases. Insulin resistance is a condition in which the body does not fully respond to insulin, causing blood glucose levels to rise. Obesity, hypertension, and dyslipidemia are all common co-morbidities of T2DM [22]. Advanced glycation end products (AGEs) are a group of heterogeneous compounds that form in greater quantities in hyperglycemic conditions. The formation of covalent cross-links with proteins, increased oxidative stress, and up-regulation of inflammation are biological processes for AGEs’ detrimental effects. Increased reactive oxygen species formation and NF-κB targets-gene transcription is caused by AGEs interacting with the receptor for advanced glycation end products (RAGE), resulting in low-grade inflammation, endothelial dysfunction, platelet activation, and insulin resistance [23]. The identification of AGEs and their binding to RAGE contribute to diabetes’ micro- and macro-vascular complications [24]. L-carnosine has antioxidant properties and the ability to protect against the production of AGEs, making it useful in the treatment of T2DM [25, 26, 27].
Hyperglycemia causes aberrant glucose-dependent pathway activation, i.e., the
polyol pathway, hexosamine pathway and protein kinase C pathway. Multiple
components, such as transforming growth factor-beta (TGF-
Skin complications are common in diabetic patients, with over 30% of individuals developing some form of skin involvement during the course of their condition [33]. Xerosis of the skin is found in up to 40% of diabetic patients [34]. In T2DM, topical use of urea 5% combined with arginine and carnosine increases skin hydration and alleviates skin dryness [33].
Individuals aged 55 and up will soon make up a quarter or more of the total population of schizophrenia patients globally. Glutamate dysfunction and dysregulation of the N-methyl-d-aspartate (NMDA) receptor (glutamatergic receptor) are thought to be linked to the pathogenesis of cognitive deficits of schizophrenia [35, 36]. L-carnosine is a known antioxidant that is co-localized at glutamatergic synapses, and it helps people with schizophrenia with their deficient cellular antioxidant defences [37, 38, 39]. Furthermore, L-carnosine has been shown to modulate NMDA receptor activity, lower glutamate levels, and limit glutamate release [40, 41].
Alzheimer’s disease (AD) is the most common type of dementia, accounting for 60 to 70% of all cases. According to Hipkiss’s [42] study, certain areas of the brain are the most sensitive to damage associated with AD when carnosine levels decline with age. Furthermore, heavy metal ions can be found in the brains of people with AD, which is defined by the presence of a pathogenic form of amyloid beta protein and intracellular lesions made up primarily of the cytoskeletal protein tau [43]. L-carnosine binds to metal ions and inhibits amyloid beta formation while also acting as a “scavenger” of free radicals [44].
Given this background, we aimed to find out the efficacy of L-carnosine versus placebo, or usual treatment, or other pharmacological/non-pharmacological interventions in patients with age-related diseases such as cancer, cardiovascular disease, diabetes, and neurodegenerative disorders. We, therefore, conducted a systematic review and meta-analysis of clinical studies, which will raise awareness of the clinical usage of L-carnosine for the aforementioned disorders among healthcare professionals, researchers, patients, and the general public.
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed in this systematic review [45]. The protocol for this systematic review was registered into PROSPERO, an international database of prospectively registered systematic reviews in health and social care, with the registration number CRD42022314033.
The population, intervention, comparison, outcomes, and study design (PICOS) approach was used to rate studies for eligibility [46]. Table 1 shows the PICOS criteria for inclusion and exclusion. Papers published before 2011 and articles written in languages other than English were excluded from the study.
| Category | Inclusion criteria | Exclusion criteria |
|---|---|---|
| Population | Patients with age-related diseases such as cancer, cardiovascular disease, diabetes, and neurodegenerative disorders, any gender, ethnicity, geographic area, comorbidities, or medication use | Studies involving pediatrics or animal sciences |
| Intervention | L-carnosine supplemented in any dose, any dosage form, either alone or in combination with other intervention/s, of any dosage and for any duration | Studies without L-carnosine |
| Comparator | Placebo, usual care, before-after/pre-post comparison, or other pharmacological or non-pharmacological interventions | The comparison of only one group without considering before and after |
| Outcome | Effect of L-carnosine for the management of age-related diseases such as cancer, cardiovascular disease, diabetes, and neurodegenerative disorders | Studies without well-defined outcome parameter |
| Study design | Randomized Controlled Trials | Non-randomized controlled trials |
Before defining our research question, we ran a preliminary search in PROSPERO
to see if there were any current systematic reviews or meta-analyses on the same
topic. PubMed, Science Direct, Proquest, Scopus, Cochrane Library, Google
Scholar, Clinical Trials Registry India (CTRI), and clinical trials.org were used
by two investigators (KSur and KSri) to perform an independent literature search.
Various combinations and phrases of major Medical Subject Heading (MeSH) terms
and non-MeSH terms, such as L-carnosine, Carnosine,
The EndNote reference manager was used to remove duplicates, and a final duplicate removal was done. Disagreements over the study selection were settled through discussion among the reviewers. To validate eligibility, the full texts of the selected publications were extracted and independently examined by the three reviewers (KSri, KSur, MD). In the literature search, lists of references to important articles were also checked for intelligibility. Any discrepancies were handled through consensus; with the help of a third author (KU).
One reviewer (KSur) conducted the primary literature search. The identified papers’ titles, abstracts, keywords, authors, author affiliations, year of publication, volume, issue, and DOI were exported to Microsoft Office Excel ver. 2019 (Redmond, Washington, United States). Furthermore, two reviewers (RMG and VTM) independently examined the titles and abstracts of the retrieved records to find studies that would fit the aforementioned inclusion criteria. Full-text screening was performed on an article with confusing qualifying criteria. The authors of the papers that might have an impact on the review but whose full text couldn’t be found were contacted via email and asked for the full-text article. Following that, two review team members (RMG and VTM) independently reviewed the complete texts of these possibly eligible papers for eligibility. Any differences between the RMG and VTM over the eligibility of certain studies were resolved by discussion and consensus with a third reviewer (KU). After a month, the searches were conducted again.
In a specially designed data extraction form, two independent investigators (KSri and KSur) extracted key data from all of the included publications. A discussion with the third independent investigator (MD) addressed the discrepancy amongst the reviewers, and data accuracy was validated. For each article, we extracted data on authors, year of publication, a number of participants, age, gender, history of diseases, comorbidities, clinical parameters, route of administration, dose and therapeutic regimen of the intervention and control groups, study follow-up time, randomization, concealment of allocation, blindness scheme, presence of an intention-to-treat analysis, loss of follow-up, early termination, expected results and results.
Two independent reviewers (MD and KSur) assessed the risk of bias (RoB) in the included papers using the revised Cochrane tool to assess the risk of bias, consisting of five domains: randomization procedure, deviation from intended intervention, missing outcome data, measurement of the outcome and selection of the reported result [47]. Finally, any discrepancies were resolved through discussion until an agreement was reached or by consulting a third reviewer (KS).
Among the studies that reported similar outcomes, the meta-analysis was
conducted using RevMan 5.4 software (Cochrane Training, New York, NY, USA). If continuous data had baseline and endpoint scores, we looked at the
change from baseline to endpoint and estimated mean difference and standard error
for continuous exposure. The mean difference (MD) and 95% CI were provided as
the final pooled result. A forest plot was used to show the results and study
level estimations. A p-value of 0.05 was used to determine statistical
significance. In each of the included studies’ analyses, the I
Cohen’s kappa statistics were used to verify the inter-rater reliability of the reviewers. The Kappa test was used to determine the level of agreement and likelihood of inconsistency among the reviewers. Between MD and KSur, a Kappa test was conducted, yielding a value of kappa = 0.654 (standard error (SE) = 0.171; 95% CI: 0.321–1.000; weighted kappa = 0.774), indicating substantial agreement. The third reviewer (KSri) did not participate in the title, abstract, or full-text screening, but did analyses and explained inconsistencies between MD and KSur in the quality assessment. However, kappa tests were conducted across all four reviewers to confirm their consistency, revealing high agreement. RMG and KSri had a Kappa score of 0.641 (SE of kappa = 0.148; 95% CI: 0.421–0.994; weighted kappa = 0.499), while RMG and KSri had a Kappa test score of 0.632 (SE of kappa = 0.157; 95% CI: 0.263–0.969; weighted kappa = 0.625).
An electronic database search yielded a total of 116 studies. The titles and abstracts of 105 potentially relevant articles were evaluated after duplicate studies were removed. Based on title and abstract screening, 86 articles were eliminated. The full-text screening included the remaining 21 studies. As shown in the PRISMA flow diagram (Fig. 1), 5 studies were excluded for the following reasons: unavailable data, inappropriate study design, and unrelated outcomes. At the end of the selection process, 14 studies [5, 22, 33, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58] meeting the inclusion criteria were eventually selected for systematic review and meta-analysis. The PRISMA checklist is presented as a Supplementary File.
 Fig. 1.
Fig. 1.PRISMA flow chart illustrating the selection process for all included and excluded studies.
Table 2 (Ref. [5, 22, 33, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58]) and Supplementary Table 1 list the basic characteristics of the studies that were included. These studies were published between 2011 and 2022. Studies in neurodegenerative disorders were found in 43% (6 out of 14) of the included studies [48, 49, 50, 51, 53, 56]. Diabetes mellitus was found in 29% (4 out of 14) of the studies [22, 33, 52, 57]. Two of the remaining four studies were on cancer [5, 55], while the other two were on cardiovascular diseases [54, 58]. Both genders were included in the majority of the studies. In 8 of the 14 studies, researchers looked at safety measures and reported on adverse drug reactions [5, 33, 49, 50, 52, 54, 56, 57]. Five of the studies included were from Japan [5, 51, 53, 56, 58], two from Iran [22, 50] and Italy [33, 54] and one from Poland [48], USA [49], Greece [52], Egypt [55], and Thailand [57].
| Author, year | Age | Gender | Drug regimen | L-carnosine Dose &route | Duration | Other supplements | Outcome measures | ADR | Result | Conclusion | ROB | ||||
| Control | Intervention | Control (M/FM) | Intervention (M/FM) | Control | Intervention | Primary | Secondary | ||||||||
| Studies in cancer patients | |||||||||||||||
| Magdy R et al. (2018) [55] | 45.9 |
45.6 |
13/18 | 16/14 | FLOFOX-6 | Control drug regimen plus L-carnosine | 500 mg/day, orally | Patients were assessed on baseline and 3 months after treatment | Nil | Neuropathy grade assessment according to NCI-CTCAE-v4 and analysis of oxidative (Nrf-2, MDA, NF-κB), /inflammatory (TNF- |
Bio-chemical markers (BUN, AST, ALT, T.Bil, Hb, PLT and TLC) | Not mentioned | In both arms a significant correlation was only evident between TNF- |
L-carnosine proved to improve OIPN by amelioration of the pathophysiological triad of inflammation, oxidative stress and apoptosis. These results led to the recommendation of the safe add-on therapy of carnosine to chemotherapeutic agents, especially those associated with CIPN | Low risk |
| Kitagawa J et al. (2020) [5] | 58 (29–72)* | 48 (18–67)* | 24/23 | 24/17 | Ara-C/CPA |
Control drug regimen plus PZ lozenges | 18.75 mg/ four times a day, orally | Patients were assessed daily from beginning of chemotherapy until 35 days after transplantation | Zinc | Incidence of Grade |
Incidence and severity of oral mucositis, xerostomia and taste disturbance. The incidence of other non-haematological AEs, the median time of engraftment and rate of engraftment | Nausea, Vomiting, Constipation, Diarrhoea, Peripheral neuropathy, Skin disorder, sCr, increased, Febrile neutropenia, WBC decreased, Anaemia, PLT decreased | The incidence of Grade |
The incidence of Grade |
Low risk |
| Studies in cardiovascular disease patients | |||||||||||||||
| Lombardi C et al. (2014) Italy [54] | 62.3 |
61.2 |
22/3 | 22/3 | ACE inhibitors, ARB, |
Control drug regimen plus L-carnosine | 500 mg/day orally before food at morning | Patients were evaluated at baseline and after 6 months | Nil | The changes from baseline in exercise parameters (distance at 6-MWT and cardiopulmonary exercise test) and the effects on QoL (EQ-5D and VAS) | Echocardiographic parameters, LVEF, haematological and biochemical assays | Nil | After 6 months of treatment with orodispersible L-carnosine was associated with an improvement in 6MWT distance (419 |
L-carnosine, added to conventional therapy, may improve exercise performance and QoL in ambulatory patients with stable CHF | Low risk |
| Yoshikawa F et al. (2019) [58] | 69.2 |
61 |
18/8 | 23/1 | 300 mg clopidogrel and 200 mg aspirin pre-PCI. Heparin was administered in the catheterization laboratory. After PCI, patients continued to take 100 mg aspirin and 75 mg clopidogrel daily until the follow-up of the study | Control drug regimen plus PZ 75 mg (Zinc and L-carnosine) | 75 mg twice a day | Patients were evaluated at baseline and after 9 months | Zinc | zinc concentration, cardiac enzymes, and the level of inflammation markers (including high-sensitive CRP and IL-6) at 8 days after administration of PZ | Cardiac function in terms of the EF, LVDd, and LVDs, echocardiography was performed upon admission to the hospital and at 9 months post-AMI | Not mentioned | The mean IL-6/maximal CPK level was significantly reduced in the PZ group (0.024 [0.003–0.066] vs. 0.076 [0.015–0.212]; p = 0.045).A significant increase was detected in the ejection fraction of the PZ group at the 2 time points (54 [51–57] vs. 62 [55–71], respectively; p |
PZ was reported to improve cardiac function post-AMI. This effect may be due to anti-inflammatory processes through the suppression of IL-6. | Low risk |
| Studies in diabetes mellitus patients | |||||||||||||||
| Federici A et al. (2015) [33] | 61 |
62 |
12/13 | 8/17 | SEC | UC | carnosine (0.01%), 4.5 finger-tip units (2.5 g of cream), Both study treatments were applied on the feet (dorsal and plantar regions) and on the distal third of the legs, twice daily | Patients were evaluated at baseline and after 4, 12, and 32 weeks for primary end point and at baseline and after 32 weeksfor secondary end point | Urea (5%) and arginine (0.5%) | 9 point grading XAS score(from 0 to 8) and of a 4 point grading OCS (from 0 to 3) | skin hydration and desquamation | Nil | UC induced greater hydration than SEC (p = 0.001) with a 91% reduction at week 32 in XAS score vs. baseline. After 4 weeks, compared with the SEC treated group, the XAS score in the UC treated group was significantly lower. OCS was reduced by 27% from baseline to end of the study in the UC group, and increased by 8% in the SEC group (p = 0.02; between groups) | UC based cream increases skin hydration and alleviates the condition of severe skin dryness in T2DM patients in comparison with a control SEC product, with a greater efficacy observed as early as 4 weeks into treatment. In addition, this approach is effective in improving significantly severe foot xerosis and fissuring | Low risk |
| Houjeghani S et al. (2017) [22] | 40.4 |
43.0 |
12/9 | 10/13 | Microcrystalline cellulose with anti-diabetic medications | Control drug regimen plus L-carnosine | Two capsules of 500 mg (1 g/day) after each meal | Patients were evaluated at baseline and after 12 week | Nil | FBS, TC, HDL, LDL, HbA1c, fasting insulin, HOMA-IR, HOMA- |
Nil | Not mentioned | L-carnosine supplementation resulted in significant decrease in fat mass and an increase in fat free mass in the intervention group compared to the placebo group (1.5% and 1.7% respectively) (p |
L-carnosine resulted in a decrease of fat mass and an increase in fat free mass, better control of FBS, HbA1c, and decreased levels of TG, CML and TNF- |
Low risk |
| Karkabounas S et al. (2018) [52] | 57 |
35/47 | Placebo | ALA, carnosine, thiamine | 6 mg/kg three times a day orally | Patients were evaluated after overnight fast before and after the 8 weeks supplementation | ALA (7 mg/kg) and thiamine (1 mg/kg) | Glycaemic profile, Lipid profile, Kidney function, Liver function, Tissue damage, Thyroid function, Cancer markers, Electrolytes, Minerals and Oxidative Stress, ADP, PAF, Arachidonic acid, Epinephrine, Thrombin and Collagen | Nil | Nil | Glucose and HbA1c levels were significantly reduced after supplementation (135.7–19.5 mg/dL vs. 126.5–16.8 mg/dL and 8.3%–0.3% vs. 6.03%–0.58%, respectively, p |
Intake of an individualized ALA, carnosine, and thiamine supplement effectively reduced glucose concentration in T2DM patients, by potentially increasing insulin production from the pancreas. The supplement had only a minor effect on insulin sensitivity since the steep increase in insulin levels was accompanied by a small but significant drop in glucose level | Low risk | ||
| Siriwattanasit N et al. (2021) [57] | 57.0 |
55.6 |
4/16 | 11/9 | Anti-hypertensive drugs, anti-glycaemic drugs with Placebo (starch) | Control drug regimen plus L-carnosine | 2 g/day orally. The dose was split into 2 × 500 mg taken after breakfast and dinner | Patients were evaluated after overnight fast before and after the 8 weeks supplementation | Nil | The change of urinary TGF- |
The improving level of UACR | Nil | Urinary TGF- |
The study showed that oral carnosine supplementation could reduce urinary TGF- |
Low risk |
| Studies in neurodegenerative disorder patients | |||||||||||||||
| Chengappa KN et al. (2012) [49] | 46.5 |
46.6 |
23/14 | 21/12 | Placebo (rice powder and magnesium stearate) | L-carnosine | 500 mg, Titration of L-carnosine (or placebo) 500 mg/day began during the first week, increasing to 500 mg/p.o. twice daily in the second week, 1500 mg/day in the third week and finally to 1000 mg/per oral twice daily during the fourth week | Patients were assessed for cognitive domains at baseline, 4 and 12 weeks, along with psychopathology ratings and safety parameter | Nil | (i) Neurocognition: Set shifting test: basal reaction time, Start imitation reaction time, End imitation reaction time, End imitation reaction time, End reversal reaction time, End reversal reaction time and Reversal errors; (ii) Strategic target detection test: Strategic efficiency and Perseverative errors | Psychopathology, QoLI and SF-36 scales: QoLI, and the SF-36 survey including the PCS and MCS summary score | (i) Cardiovascular: Increased blood pressure; (ii) Digestive: Dry mouth, Diarrhoea, Constipation, Dyspepsia and Nausea; (iii) Metabolic and nutritional: Decreased appetite, Weight loss, Weight gain and Hyperglycaemia; (iv) Dermatological: Skin rash, Dry skin and Pruritus; (v) Musculo-skeletal: Foot pain, Join pain; (vi) Nervous: Dizziness, Sleepiness, Paraesthesia’s, Slurred speech and Lethargy; (vii) Psychiatric: Worsening psychosis Vivid dreams; (viii) Respiratory: Pneumonia | On the strategic target detection test, the L-carnosine group displayed significantly improved strategic efficiency and made fewer perseverative errors compared with placebo. Other cognitive tests showed no significant differences between treatments. | These preliminary findings suggest that L-carnosine merits further consideration as adjunctive treatment to improve executive dysfunction in persons with schizophrenia | Low risk |
| Budzen S et al. (2014) [48] | 80.5 |
81 |
13/13 | 12/13 | Placebo (hydrolysate obtained after chicken meat enzymatic treatment) | CME containing 40% of CRC components (2:1 ratio of anserine to carnosine) was prepared following breast meat extraction with water (cold and at 80 °C) and spray drying | CME were administered 2.5 g per day, which allowed to reach the level of 1 g CRC in dipeptide supplements re-suspended in soup | Patients were assessed for cognitive functioning and physical capacity at baseline and 13 week follow-up supplementation | Anserine | (i) The cognitive function, presence of depressive symptoms, physical capacity, body measurements, BP and HR; (ii) Behavioural measurements: The MMSE and STMS to assess cognitive functions, GDS to assess the presence of depressive symptoms, CDR to evaluate the severity of dementia | Nil | Not mentioned | The groups differed significantly at baseline according to certain clinical and physical parameters, such as HR before exercise (p |
Anserine and carnosine supplementation in the elderly may bring promising effects on cognitive functioning and physical capacity | Low risk |
| Hisatsune T et al. (2016) [51] | 70.6 |
67.8 |
9/10 | 8/11 | Placebo (43 mg/day L-lysine) and L-histidine (150 mg/day) | Anserine and carnosine | 1.0 g of an anserine/carnosine (3:1) formula daily | Patients were evaluated at baseline and after 3 months | Anserine | (i) A dietary survey was conducted using a semi-quantitative method as reported. Inventory of food intake during the 3-month test Period; (ii) Cognitive testing: WMS-LM1, WMS-LM2, ADAScog, BDI, SF-36 MCS and SF-36 PCS; (iii) Blood samples and immunoassays: Concentrations of 27 cytokines in serum samples collected from the volunteers at the baseline and during the follow-up; (iv) Microarray analysis: PBMCs from volunteers were used for total RNA extraction. The total RNA quality was assessed; (v) Acquisition of human MRI data and MRI dataanalysis | Nil | Not mentioned | Among the tests, delayed recall verbal memory assessed by theWMS-LM showed significant preservation in the ACS group, compared to the placebo group (p = 0.0128). Blood analysis revealed a decreased secretion of inflammatory cytokines, including CCL-2 and IL-8, in the ACS group. MRI analysis using arterial spin labeling showed a suppression in the age-related decline in brain blood flow in the posterior cingulate cortex area in the ACS group, compared to the placebo group (p = 0.0248). In another RCT, delayed recall verbal memory showed significant preservation in the ACS group, compared to the placebo group (p = 0.0202) | The survey results indicated that the anserine and carnosine consumption from the diet was equivalent between the ACS and placebo groups in this study, a better method is needed to estimate their levels more accurately | Low risk |
| Katakura Y et al. (2017) [53] | 65.3 |
60.4 |
10/20 | 10/20 | Placebo (43 mg/day L-lysine) and L- histidine (150 mg/day) | Anserine and carnosine | 1.0 g of an anserine/carnosine (3:1) formula daily | Patients were evaluated at baseline and after 3 months | Anserine | (i) A dietary survey wasconducted using a semi-quantitative method as reported. Inventory of food intake during the 3-month test period; (ii) Cognitive testing: WMS-LM1, WMS-LM2, ADAScog, BDI, SF-36 MCS and SF-36 PCS; (iii) Microarray analysis: PBMCs from volunteers were used for total RNA extraction. The total RNA quality was assessed; (iv) qRT-PCR: Gene expression levels were normalized to the corresponding |
Nil | Not mentioned | A microarray analysis and subsequent qRT-PCR analysis of PBMCs showed decreased expression of CCL24, an inflammatory chemokine (p |
ACS may preserve verbal episodic memory, probably owing to CCL24 suppression in the blood, especially in elderly participants. | Low risk |
| Ghajar A et al. (2018) [50] | 24.93 |
22.00 |
28/2 | 26/4 | Risperidone (up to 6 mg/day) + Placebo. Patients with sleep problems received 1 mg lorazepam every night for the first week | Control drug regimen plus L-carnosine | 2 g/day, participants received 500 mg/day of L-carnosine for 5 days; on day 6, the dose of L- carnosine was increased to 1000 mg/day in two divided doses for 5 days. On day 11, patients received the maximum fixed dose of 2 g/day in 2 divided dose | Patients were evaluated at baseline and 2, 4, 6 and 8 week | Nil | The difference in PANSS negative subscale score reduction | (i) The difference of change in the PANSS subscale scores and the PANSS and ESRS total scores (at each visit); (ii) HDRS to assess changes in depressive symptoms; (iii) ADRs | Headache, Dry mouth, Nausea, Day time drowsiness and sweating | L-carnosine resulted in greater improvement of negative scores as well as total PANSS scores but not positive subscale scores compared to placebo. HDRS scores and its changes did not differ between the two groups. Both groups demonstrated a constant ESRS score during the trial course. Frequency of other side effects was not significantly different between the two groups. In a multiple regression analysis model (controlled for positive, general psychopathology, depressive and extrapyramidal symptoms, as well as other variables), the treatment group significantly predicted changes in primary negative symptoms | L-carnosine as an add-on to risperidone showed good tolerability and significant beneficial effects on negative symptoms of patients with chronic stable schizophrenia | Low risk |
| Masuoka N et al. (2019) [56] | 73.6 |
72.9 |
12/13 | 12/13 | Placebo | Anserine and carnosine | 1 g of ACS (250 mg of carnosine) | Patients were evaluated at baseline and 4, 8 and 12 week | Anserine (750 mg) | (i) Inventory of Anserine and Carnosine in the Normal Diet; (ii) Blood Analysis: APOE4 Genotyping and ELISA for Amyloid Beta 42; (iii) Cognitive Testing: MMSE, gloCDR, CDRsob, WMS-1, WMS-2, ADAS and GDS; (iv) EEG Recording; (v) Adverse Events and Safety | Nil | Nil | The score improvement in the gloCDR was superior in the active group than placebo (p = 0.023). No beneficial effect in the active group was detected in the other psychometric tests including the MMSE, WMS and ADAS. When APOE4 positive (APOE4 (+)) or negative (APOE4 (–)) subjects were separately analyzed, beneficial change in the APOE4 (+) subjects was observed in MMSE (p = 0.025) as well as in gloCDR (p = 0.026) | The results did not indicate that ACS has significant efficacy on cognitive change of MCI subjects but might suggest that beneficial effect in APOE4 (+) MCI subjects exists, and thereby, ACS might help to prevent a transition from MCI to AD as a secondary effect in APOE4 (+) MCI subjects. Furthermore, changes in sNAT scores, which are based on the EEG analysis, may be a useful biomarker for assessing these benefits | Low risk |
| Age-data expressed as mean | |||||||||||||||
All of the studies are RCT that provide clinical evidence for the efficacy of L-carnosine as an individual supplement or in combination with other supplements. Of 14 studies, 4 studies used anserine as an add-on supplement with L-carnosine in neurodegenerative disorders [48, 51, 53, 56], 2 studies used zinc as an additional supplement with L-carnosine [5, 58], and 6 studies did not use any supplements with L-carnosine [22, 49, 50, 54, 55, 57]. Federici et al. [33], study used urea and arginine as supplements along with L-carnosine. Karkabounas et al. [52], study used α-Linolenic acid (ALA) and thiamine as a supplement to L-carnosine for diabetes mellitus. As an intervention group regimen for L-carnosine supplementation, one of the included studies used chicken meat extract (CME) containing 40% carnosine related compounds (CRC) [48]. The dose and mode of administration, on the other hand, differed significantly between the included studies.
Across the studies that were considered, a wide range of outcome measures were
observed. L-carnosine has been shown in cancer studies to minimize
treatment-related toxicities [5, 55]. In addition, Magdy et al. [55],
examined neuropathy grade and rated it using the National Cancer Institute Common
Terminology Criteria for Adverse Events-version 4 (NCI-CTCAE-v4) system,
revealing that L-carnosine improved OIPN by reducing the pathogenic triad of
inflammation, oxidative stress, and apoptosis. The Kitagawa et al. [5],
study looked at the incidence of Grade
Lombardi et al. [54], study had the primary outcome measure to evaluate the exercise parameters [distance at 6-minute walk test (6-MWT)] and cardiopulmonary exercise test) and the effects on QoL [EuroQol-5D (EQ-5D) and visual analogue scale (VAS)] among congestive heart failure (CHF) patients and concluded that L-carnosine added to conventional therapy may improve exercise performance and QoL. The zinc concentration, cardiac enzymes, and the level of inflammation markers in blood and urine samples were analysed in the Yoshikawa et al. [58], study, and it was found that it improves cardiac function post-acute myocardial infarction (AMI), and that this effect may be due to anti-inflammatory processes through the suppression of IL-6. Ejection fraction was a common outcome in cardiovascular diseases between these 2 studies [54, 58].
Federici et al. [33], study examined the evolution of a 9-point grading
xerosis assessment scale (XAS) score and a 4-point grading overall cutaneous
score (OCS) score in T2DM patients, which enhances skin hydration and alleviates
the state of severe skin dryness. Fasting blood sugar (FBS), total cholestrol
(TC), high- density lipoprotein cholesterol (HDL-c), low-density lipoprotein
cholesterol (LDL-c), Glycated hemoglobin (HbA1c), fasting insulin, homeostatic
model assessment of insulin resistance (HOMA-IR), homeostatic model assessment of
insulin secretion (HOMA-
The glycemic profile, lipid profile, kidney function, liver function, tissue damage, thyroid function, cancer markers, electrolytes, minerals, oxidative stress, platelet activating factor (PAF), arachidonic acid, epinephrine, thrombin, and collagen were all examined in the Karkabounas et al. [52], study, which found that L-carnosine as an added supplement reduced glucose concentration in T2DM patients by potentially increasing insulin production from the pancreas.
Siriwattanasit et al. [57], looked at the change in urine TGF-
Budzen et al. [48], study investigated cognitive function, the existence of depressive symptoms, physical capacity, body measures, blood pressure (BP), and heart rate (HR) in neurodegenerative disorder patients. They also looked at the mini mental state examination (MMSE) and short test of mental status (STMS) to test cognitive functions, the geriatric depression scale (GDS) to examine the presence of depressive symptoms, and the clinical dementia rating (CDR) to assess dementia severity, and found that L-carnosine supplementation in the elderly could have positive effects on cognitive functioning and physical capacity.
Based on the results of a neurocognition set-shifting test and a strategic target detection test, L-carnosine was found to be an additional treatment to ameliorate executive dysfunction in people with schizophrenia [49]. The difference in positive and negative syndrome scale (PANSS) negative subscale score reduction and hamliton depression rating scale (HDRS) was used to assess changes in depressive symptoms, and L-carnosine as an add-on to risperidone was found to have good tolerability and considerable favourable effects on negative symptoms in individuals with chronic stable schizophrenia [50].
The outcome measured in the Hisatsune et al. [51], study included a semi-quantitative dietary survey; cognitive testing: Wechsler memory scale-revised logical memory immediate recall (WMS-LM1), Wechsler memory scale-revised logical memory delayed recall (WMS-LM2), Alzheimer’s disease assessment scale (ADAScog), beck depression inventory (BDI), study, 36-item d physical health component summary (SF-36 MCS), and study, 36-item d physical health component summary (SF-36 PCS); blood samples and immunoassays; microarray analysis and acquisition of human Magnetic Resonance Imaging (MRI) data and MRI data analysis; and microarray analysis and acquisition of human MRI data. The Anserine and Carnosine (ACS) and placebo groups in this trial consumed the same amount of anserine and carnosine from their diets, according to the survey results.
Katakura et al. [53], evaluated dietary survey outcome measures using a semi-quantitative method; cognitive testing: WMS-LM1, WMS-LM2, ADAScog, BDI, SF-36 MCS and SF-36 PCS; microarray analysis and real-time quantitative reverse transcription polymerase chain reaction (qRT-PCR), which found that ACS may preserve verbal episodic memory, likely due to CCL24 suppression in the blood, especially in elderly participants.
Inventory of anserine and carnosine in the Normal Diet; Blood Analysis; Cognitive Testing: MMSE, gloCDR, CDRsob, WMS-1, WMS-2, ADAS and GDS; and electroencephalogram (EEG) recording were used in the Masuoka et al. [56], study, which showed that the results did not indicate that ACS has significant efficacy on cognitive change in mild cognitive impairment (MCI) subjects but might suggest that ACS has a beneficial effect in APOE4 (+) MCI subjects exists, and thereby, ACS might help to prevent a transition from MCI to AD as a secondary effect in APOE4 (+) MCI subjects.
WMS-1, WMS-2, and ADAS [51, 53, 56] were the most commonly used outcome measures in neurodegenerative disorder studies, followed by BDI, SF-36 MCS, and SF-36 PCS [51, 53], GDS [48, 51, 56] and MMSE [48, 56].
Supplementary Table 2 shows the RoB assessment for each of the studies. All reports were critically examined using the standard checklist for the revised Cochrane RoB tool. The method of randomization is rarely described, despite the fact that randomization is an important tool to reduce bias. The method of randomization was not reported in any of the included trials, or there was insufficient information. Fourteen RCTs were analyzed for the effect of L-carnosine in various age-related diseases such as cancer [5, 55], cardiovascular disease [54, 58], diabetes mellitus [22, 33, 52, 57] and neurodegenerative disorder [48, 49, 50, 51, 53, 56].
The bias arising from randomization technique was “low risk” for all 14 included studies in a quality assessment using the Revised Cochrane Risk of Bias Tool for RCT, whereas bias due to deviation from intended intervention had “some concerns” for 5 studies [5, 33, 54, 55, 58] and low risk for the other 9 [22, 48, 49, 50, 51, 52, 53, 56, 57].
Bias for missing outcome data was found to be “low risk” in all of the included studies. Only one study [33] found a “low risk” of bias in outcome measurement, whereas the remaining 13 studies had “some concerns” about outcome measuring. All of the studies that were considered demonstrated a “low risk” of selecting the reported result. All of the studies were evaluated separately, and the aggregate assessment had a “low risk” of bias (Supplementary Table 2).
Only four studies [5, 33, 55, 57] identified a “high risk” of allocation concealment in the selection bias (Fig. 2), and one study identified an “unclear risk” of allocation concealment in the selection bias [54]. Only six studies had a “high risk” of performance bias due to participant and personnel blinding [5, 33, 54, 55, 57, 58]. However, in one trial, the outcome assessors were blinded [33]. The random sequence generation in selection bias was addressed in all of the studies. All of the papers were evaluated to see if the research question, study population, and L-carnosine indices were well described. Other causes of bias, such as medication pooling, dropouts, unit-of-analysis mistakes, design-specific bias, and conflicts of interest, were not mentioned in these studies.
 Fig. 2.
Fig. 2.Methodological quality of the included studies based on the revised Cochrane risk of bias tool for randomized trials. (A) Overall risk of bias. Each item is presented as a percentage based on all included studies. (B) Individual risk of bias. Each item is scored as ‘weak’, ‘moderate’, or ‘strong’.
Primarily, we analysed whether or not L-carnosine supplementation had a positive impact on age-related diseases such as cancer, cardiovascular disease, diabetes mellitus, and neurodegenerative disorders. We used 14 studies in our qualitative analysis [5, 22, 33, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58]. Five studies were excluded from the quantitative synthesis [5, 33, 49, 50, 55] because they did not correlate with the outcome variables and did not report any data with the other included studies under each age-related disease.
Two studies [54, 58] looked at the effects of L-carnosine on patients with
cardiovascular disease. On the ejection fraction, there was no statistically
significant difference between the L-carnosine and control groups [MD 95% CI =
0.82 (–2.38, 4.01); p = 0.62; p = 0.56; I
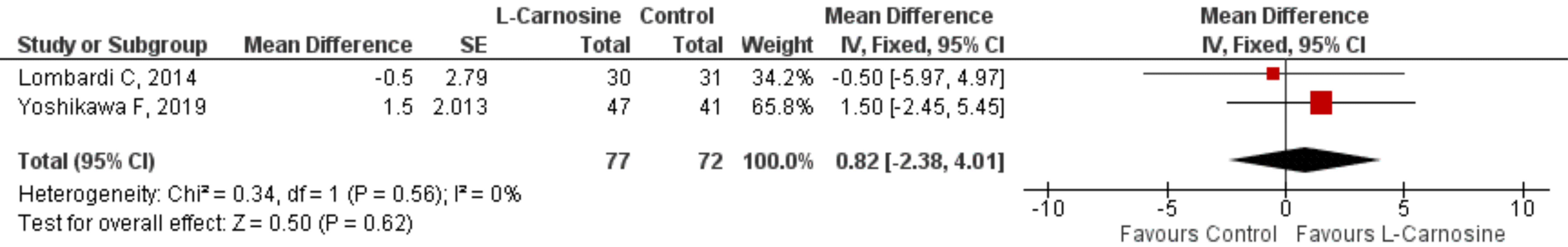 Fig. 3.
Fig. 3.Effect of L-carnosine in cardiovascular disease patients. Effect of L-carnosine treated versus control on measures of ejection fraction in patients with cardiovascular disorders. CI, confidence interval; df, degrees of freedom; IV, inverse variance; SE, standard error.
HbA1c, FBS, LDL-c, HDL-c, TGs, TCs, and serum Cr. were used to study the effect
of L-carnosine in diabetes mellitus patients. Three studies (n = 248) [22, 52, 57] showed a statistically significant difference between the L-carnosine group
and the control group on HbA1c [MD 95% CI = –1.25 (–2.49, –0.022); p
= 0.05; p = 0.001; I
 Fig. 4.
Fig. 4.Effect of L-carnosine in diabetes mellitus patients. (A) Effect of L-carnosine treated versus control on measures of HbA1c in patients with diabetes mellitus. (B) Effect of L-carnosine treated versus control on measures of FBS in patients with diabetes mellitus. (C) Effect of L-carnosine treated versus control on measures of LDL-c in patients with diabetes mellitus. (D) Effect of L-carnosine treated versus control on measures of HDL-c in patients with diabetes mellitus. (E) Effect of L-carnosine treated versus control on measures of TGs in patients with diabetes mellitus. (F) Effect of L-carnosine treated versus control on measures of TCs in patients with diabetes mellitus. (G) Effect of L-carnosine treated versus control on measures of serum Cr. in patients with diabetes mellitus. HbA1c, glycated hemoglobin; FBS, fasting blood sugar; LDL-c, low-density lipoprotein cholesterol; HDL-c, high-density lipoprotein cholesterol; TGs, triglycerides; TCs, total cholesterol; Cr, creatinine; CI, confidence interval; df, degrees of freedom; IV, inverse variance; SE standard error.
On the other hand, there was no statistically significant difference between the
L-carnosine and control groups on the LDL-c [MD 95% CI = –4.70 (–13.33, 3.94);
p = 0.29; p = 0.36; I
Variables such as WMS-1, WMS-2, ADAS, BDI, SF-36 MCS, SF-36 PCS, MMSE, and GDS
were used to find the effect of L-carnosine in patients with neurodegenerative
disorder. On WMS-1, the association between the three studies (n = 149) [51, 53, 56] revealed no statistically significant difference between the L-carnosine and
control groups [MD 95% CI = 0.26 (–0.79, 0.26); p = 0.33; p =
0.75; I
 Fig. 5.
Fig. 5.Effect of L-carnosine in neurodegenerative disorder patients. (A) Effect of L-carnosine treated versus control on measures of WMS-1 in patients with neurodegenerative disorder. (B) Effect of L-carnosine treated versus control on measures of WMS-2 in patients with neurodegenerative disorder. (C) Effect of L-carnosine treated versus control on measures of ADAS in patients with neurodegenerative disorder. (D) Effect of L-carnosine treated versus control on measures of BDI in patients with neurodegenerative disorder. (E) Effect of L-carnosine treated versus control on measures of SF-36 MCS in patients with neurodegenerative disorder. (F) Effect of L-carnosine treated versus control on measures of SF-36 PCS in patients with neurodegenerative disorder. (G) Effect of L-carnosine treated versus control on measures of MMSE in patients with neurodegenerative disorder. (H) Effect of L-carnosine treated versus control on measures of GDS in patients with neurodegenerative disorder. GDS, geriatric depression scale; WMS-1, wechsler memory scale-revised logical memory immediate recall; WMS-2, wechsler memory scale-revised logical memory delayed recall; ADAS, Alzheimer’s disease assessment scale; BDI, beck depression inventory; SF-36 MCS, 36-item short form survey for mental-health component summary scores; SF-36 PCS, 36-item short form survey for physical-health component summary scores; MMSE, mini mental state examination; CI, confidence interval; df, degrees of freedom; IV, inverse variance; SE, standard error.
On the ADAS, a statistically significant difference was observed between the
L-carnosine and control groups of the three studies (n = 149) [51, 53, 56] [MD
95% CI = 0.98 (–1.55, –0.42); p = 0.0007; p = 0.86; I
Pooling analysis of the SF-36 MCS [MD 95% CI = –0.25 (–1.29, 0.79); p
= 0.64; p = 0.55; I
Meta-analysis of data from three studies (n = 140) [48, 51, 53] showed no
statistically significant difference in MMSE score between the L-carnosine and
control groups [MD 95% CI = 0.50 (–0.60, 1.60); p = 0.37; p =
0.97; I
The purpose of this review is to assess the effect of L-carnosine in the context of age-related illnesses and makeweight in the literature, as well as to convey the relevance of existing data as evidence for clinical practise and future research. Clinical studies on the efficacy of L-carnosine have been widely published in the last decade, indicating a growing interest among health care professionals in the use of L-carnosine for the treatment of age-related illnesses. Egypt, Japan, Italy, Iran, Greece, Thailand, the United States, and Poland have all conducted studies that look at the preference for dietary/therapeutic intervention in L-carnosine, particularly in oriental medicine, in these countries.
L-carnosine has been found to have multimodal activity. Its impact on age-related diseases such cancer, cardiovascular disease, diabetes, and neurological problems is comprehensively reviewed in this paper. Fig. 6 depicts the mode of action of L-carnosine in the aforementioned illnesses. Apart from the selected 14 RCTs, the therapeutic potential of L-carnosine was studied in pre-clinical and other clinical (non-RCTs) studies. The anti-cancer effect of L-carnosine has been investigated in animals for various cancer types such as ovarian cancer [59], HER2/neu cancer [60], bladder cancer [61], gastric cancer [62, 63], hepatic cancer [64] and colon cancer [65]. L-carnosine has also been demonstrated to help with chemotherapy-induced toxicities [66, 67, 68]. L-carnosine has clinical evidence for its action against chemotherapy-induced toxicities [69, 70], but no research has been conducted on its anticancer activity. Pre-clinical evidence from cardiovascular research suggests that L-carnosine improves functional capability in ischemia circumstances [71, 72, 73, 74, 75, 76, 77, 78]. Similarly, pre-clinical evidence from diabetes studies showed that L-carnosine had an effect on diabetes [79, 80] and its complications [81, 82, 83]. Pre-clinical and clinical (other than RCTs) studies from neurodegenerative disorders explored the effect of L-carnosine on cognitive decline and neurophysiological effects [84, 85, 86, 87, 88].
 Fig. 6.
Fig. 6.Mechanism of L-carnosine in age-related disorders (cancer, cardiovascular disease, diabetes, and neurodegenerative disorders).
This systematic review and meta-analysis of the literature presents data using the PRISMA guidelines and the PICOS approach. The 14 RCTs included in the study recruited a total of 794 individuals. Neurodegenerative studies had 329 (41%) participants, diabetes studies had 216 (27%) participants, cancer studies had 149 (18%) participants, and cardiovascular studies had 100 (12.5%) participants.
When we looked at all of the studies on the effects of L-carnosine supplementation on age-related disorders, we found that just 9 of them provided adequate data to make a quantitative comparison of variables. In the remaining 5 studies, 2 of the cancer studies [5, 55] investigated the mechanism of dissimilar toxicities induced by chemotherapy, and 1 of the diabetes studies [33] investigated L-carnosine on severe xerosis, which is one of a skin complication of diabetes, where they measured skin hydration, so it wasn’t eligible for quantitative comparison, and the other 2 studies [49, 50] looked at neurodegenerative diseases and weren’t eligible because of their dissimilar assessments.
The majority of the studies included in this meta-analysis used a combination of anserine and L-carnosine. Anserine is a natural derivative of carnosine that is commonly used because human carnosinase does not cleave it. Anserine and carnosine have been shown to have similar physiological activities [89]. Just a few of the studies employed PZ, a synthetic carnosine derivative in which zinc and carnosine are linked in a one-to-one ratio to form a polymeric structure. Several companies are currently marketing this product as a zinc dietary supplement with “additional value for gastric health” [90]. For a better understanding of L-carnosine’s therapeutic potential against age-related disorders, future clinical research focusing on L-carnosine should attempt L-carnosine alone.
Our meta-analysis of diabetes studies [22, 52, 57] suggests that the intervention group (L-carnosine alone or in combination with the control group regimen, or with or without add-on supplements) can improve FBS and HbA1c levels in diabetes patients. Despite the fact that individual studies showed increases in exercise capacity and functional capacity, statistical significance was not found in the intervention group compared to the control group in cardiovascular studies [54, 58]. Neurodegenerative studies [51, 53, 56] suggest that L-carnosine improves memory deficiencies among elderly populations accordance with WMS-2 scale but other than that failed to show significant results for other tools. It’s likely that L-carnosine affects specific cognitive functions, which may be observed in the clinical setting using more specialised techniques rather than a broad assessment of cognitive status [91].
According to the Caruso et al. [91], study T2DM is known to be a risk factor for cognitive impairment and AD, and different neurobiological links have been identified between T2DM and AD, such as insulin resistance, low-grade inflammation, increased oxidative stress, and AGE accumulation. In accordance with Caruso et al. [91], study and our meta-analysis, L-carnosine has anti-diabetic and cognitive properties. To validate the therapeutic potential of L-carnosine, more double-blind, randomised, placebo-controlled trials on age-related illnesses are needed.
The diversity in measures (for example, different scales for cognitive function) used across studies made meta-analysis challenging, and as a result, not all studies included in the systematic review could be included in the meta-analysis, resulting in lower statistical power and potential overestimation of the results.
The findings of the review indisputably demonstrated a significant beneficial effect of L-carnosine only for diabetes mellitus and cognitive impairment, but not for cardiovascular illnesses. L-carnosine research in cancer was solely focused on chemotherapy-induced toxicity. Its anti-cancer abilities have not been investigated. Future research should use preclinical evidence to look at anticancer potential of L-carnosine in a variety of cancer patients to fill this gap in the literature. To establish the therapeutic potential of L-carnosine in various age-related disorders, more RCTs with larger sample size are needed.
PROSPERO, The International Prospective Register of Systematic Reviews; PICO, population, intervention, comparison, outcomes, and study design; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analysis; RevMan, Revenue manager; HbA1c, Glycated haemoglobin; FBS, Fasting blood sugar; WMS-LM2, Beck Depression Inventory; ADAS, Alzheimer’s disease Assessment Scale; BDI, Beck Depression Inventory.
KSur, MD, KSri, KU, RMG contributed to the database search, data collection; KSur, MD, KWG, KU, VTM, and RMG contributed to data extraction, data analysis; KSur, KWG, KU, VTM, CA, LCM, RMG contributed to writing and revising of the manuscript. The topic was conceptualized by VTM, CA and RMG. All authors read and approved the final manuscript.
Not applicable.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
