Academic Editor: Josef Jampilek
Background: A novel human coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has become the leading threat to global health. An effective antiviral could not only help those still vulnerable to the virus but could be a critical treatment if a virus emerges toward evading coronavirus disease 2019 (COVID-19) vaccines. Despite the significant efforts to test already-approved drugs for their potential to kill the virus, researchers found very few actually worked. Methods: The present report uses the electronic molecular descriptors, the quasi-valence number (AQVN), and the electron-ion interaction potential (EIIP), for the analysis of natural compounds with proven therapeutic activity against the COVID-19. Results: Based on the analysis of the electronic properties of natural compounds which are effective against SARS-CoV-2 virus the simple theoretical criterion for the selection of candidate compounds for the treatment of COVID-19 is proposed. Conclusions: The proposed theoretical criterion can be used for the identification and optimization of new lead compounds for the treatment of the COVID-19 disease and for the selection of the food and food supplements which could have a beneficial effect on COVID-19 patients.
The outbreak of severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) has led to coronavirus disease-19 (COVID-19); a pandemic disease that represents the global health, social and economic threat. Currently, SARS-CoV-2 is spreading worldwide very rapidly and its control is very difficult because there is no available effective vaccine or drugs. As of October, 2021 globally, more than 247 million people have been diagnosed with COVID-19, 223 countries have been affected and more than 5 million deaths have been reported. Therefore, it is an urgent need for development of effective drugs for treatments of the COVID-19 disease.
Natural products have been the primary source of medicines in all cultures with a vast diversity of terrestrial and marine organisms; also, they are the most successful source of drug leads for the treatment of many diseases [1]. Flavonoids are extensively distributed natural products produced by plants having an essential role in plant physiology, with a potential anti-inflammatory, anticancer, antibacterial, antifungal, and antiviral activity [2].
Around ten years of research is needed for the development of de-novo medicines. Thus, the repurposing of natural products could be an efficient strategy against SARS-CoV-2 infection [3].
Some natural compounds have been shown to possess antiviral activities against various viruses (influenza virus, human immunodeficiency virus (HIV), hepatitis C and B viruses, measles-virus, herpes simplex virus, poliovirus and human coronaviruses SARS and MERS) [4, 5, 6, 7, 8, 9, 10, 11]. The potential treatments for viral diseases using natural plant compounds are actively studied worldwide, especially against viruses from the coronavirus group were intensified last year. Furthermore, in recent years, understanding the antiviral mechanisms of complex plant extract and isolated plant-derived compounds have been actively studied. In addition to molecular docking studies, in silico analyses of extracted compounds were used in these studies [12]. To date, numerous Chinese herbs and herbal formulations have been reported to possess antiviral activities [13, 14, 15]. This suggests the natural phyto compounds and medicinal plant-based formulations as a base for the development of novel drugs for the treatment of the COVID-19 disease.
Previously, it was showed that the biological properties of organic molecules are determined by the physical parameters the electron-ion interaction potential (EIIP) and the average quasy-valence number (AQVN) [16]. These electronic molecular descriptors served as a base for the in silico screening of different molecular libraries for candidate drugs against HIV, influenza virus, Ebola virus, Leishmania disease, malaria disease and antibiotic-resistant bacteria [17, 18, 19, 20, 21, 22, 23]. The purpose of this study is to develop the criterion for in silico selection of natural compounds that have promising antiviral effects against SARS-CoV-2 virus.
It has been previously proposed the model of the molecular interactions in
biological systems which encompass two steps [16, 24]. The first step is
determined by the selective long-range forces which allow specific recognition
and targeting between interacting molecules at a distance longer than one linear
dimension of the interacting macromolecules (102–103 Å). These forces
directly influence the number of productive collisions between interacting
molecules. The second step is the chemical binding between interacting molecules
which involves the weak non-covalent forces (van der Waals, hydrogen bonding,
ionic interactions, etc.) which operate over a short distance range (
The number of valence electrons and the electron–ion interaction potential (EIIP), are the molecular descriptors which determine the long-range properties of biological molecules [16]. These physical parameters are derived from the general model pseudopotential [25, 26] and for organic molecules are determined by the following equations.
where Z* is the AQVN determined by
where Z
We will further use these molecular descriptors for analysis of natural compounds with the anti-COVID-19 activity.
Several laboratory and clinical studies have demonstrated remarkable efficacy of different herbal preparations in prevention and treatment of the COVID-19 disease [27, 28, 29, 30, 31]. This especially concerns the traditional Chinese medicine (TCM) which successfully used for clinical treatment of mild, moderate, severe and critical cases and convalescence [32]. However, these studies still leave the question of whether these extraordinary health effects are the consequence of some unique properties of the herbal ingredients in these preparations. In order to answer this question, we analyzed the electronic properties, represented by the molecular descriptors AQVN and EIIP, of chemical ingredients of 22 therapeutic TMC preparations recommended for COVID-19 in China [33]. The molecular descriptors AQVN and EIIP, calculated for 92 essential compounds from these preparations, are given in Table 1 (Ref. [30]) and Fig. 1 (Ref. [30]).
| Compound | Formula | AQVN | EIIP [Ry] |
| kaempferol | C15H10O6 | 3.419 | 0.1339 |
| quercetin | C15H10O7 | 3.500 | 0.1260 |
| luteolin | C15H10O6 | 3.419 | 0.1339 |
| glycyrrhetinic acid | C30H46O4 | 2.375 | 0.0941 |
| indigo | C16H10N2O2 | 3.200 | 0.1092 |
| C29H50O | 2.150 | 0.0578 | |
| naringenin | C15H12O5 | 3.188 | 0.1060 |
| isorhamnetin | C16H12O7 | 3.371 | 0.1341 |
| formononetin | C16H12O4 | 3.125 | 0.0879 |
| isoflavone | C15H10O2 | 3.037 | 0.0577 |
| licochalcone B | C16H14O5 | 3.086 | 0.0750 |
| glyasperin C | C21H24O5 | 2.760 | 0.0435 |
| licochalcone a | C21H22O4 | 2.766 | 0.0416 |
| 3-methoxyglabridin | C21H22O5 | 2.833 | 0.0188 |
| anhydroicaritin | C21H20O6 | 2.979 | 0.0359 |
| stigmasterol | C29H48O | 2.180 | 0.0644 |
| 6-(3-oxoindolin-2-ylidene) indolo (21-b) | C23H13N3O2 | 3.220 | 0.1138 |
| quinazolin-12-one | C14H8N2O2 | 3.308 | 0.1293 |
| bicuculline | C20H17NO6 | 3.136 | 0.0915 |
| physciondiglucoside | C28H32O15 | 3.120 | 0.0863 |
| dihydroverticillatine | C27H41N02 | 2.271 | 0.0820 |
| licoisoflavanone | C20H18O6 | 3.046 | 0.0608 |
| 574’-trihydroxy-8-methoxyflavone | C16H12O6 | 3.294 | 0.1275 |
| acacetin | C16H12O5 | 3.212 | 0.1121 |
| irisolidone | C17H14O6 | 3.189 | 0.1064 |
| wogonin | C15H10O5 | 3.333 | 0.1319 |
| baicalein | C15H10O5 | 3.333 | 0.1319 |
| glycyrrhizic acid | C42H62O16 | 2.717 | 0.0564 |
| hesperidin | C28H34O15 | 3.065 | 0.0678 |
| hyperoside | C21H20O12 | 3.321 | 0.1308 |
| andrographolide | C20H30O5 | 2.546 | 0.0906 |
| gallic acid | C7H6O5 | 3.556 | 0.1150 |
| rosmarinic acid | C18H16O8 | 3.238 | 0.1179 |
| rutin | C27H30O16 | 3.206 | 0.1105 |
| chlorogenic acid | C16H18O9 | 3.163 | 0.0993 |
| tanshinone II A | C19H18O3 | 2.800 | 0.0304 |
| hydroxysafflor yellow A | C27H32O16 | 3.147 | 0.0946 |
| paeoniflorin | C23H28O11 | 3.000 | 0.0439 |
| chlorogenin | C27H44O4 | 2.347 | 0.0918 |
| 5-Hydroxy-67345-pentamethoxyflavone | C20H20O8 | 3.083 | 0.0742 |
| isokaempferol | C15H10O6 | 3.419 | 0.1339 |
| morin | C15H10O7 | 3.500 | 0.1260 |
| gardenin E | C19H18O9 | 3.217 | 0.1133 |
| artemisetin | C20H20O8 | 3.083 | 0.0742 |
| genistein | C15H10O5 | 3.333 | 0.1319 |
| dryobalanone | C30H50O3 | 2.265 | 0.0810 |
| curcumin | C21H20O6 | 2.979 | 0.0359 |
| elemicin | C12H16O3 | 2.645 | 0.0742 |
| chrysoeriol | C16H12O6 | 3.294 | 0.1275 |
| apigenin | C15H10O5 | 3.333 | 0.1319 |
| scutellarin | C21H18O12 | 3.412 | 0.1342 |
| oroxylin-7-O-glucuronide | C22H20O11 | 3.283 | 0.1259 |
| forsythin | C27H34O11 | 2.889 | 0.0016 |
| forsythiaside E | C20H30O12 | 2.935 | 0.0193 |
| ursodeoxycholic acid | C24H40O4 | 2.353 | 0.0923 |
| chenodeoxycholic acid | C24H40O4 | 2.353 | 0.0923 |
| ophiopogonin D | C44H70O16 | 2.631 | 0.0772 |
| ginsenoside rg 2 | C42H72O14 | 2.531 | 0.0921 |
| methyl ophiopogonanone A | C19H18O6 | 3.023 | 0.0526 |
| ginsenoside Rb2 | C56H92O25 | 2.694 | 0.0626 |
| ginsenoside R0 | C48H76O19 | 2.671 | 0.0682 |
| ophiopogon A | C44H70O18 | 2.682 | 0.0656 |
| sanchinoside rd | C48H82O18 | 2.581 | 0.0860 |
| ophiopogonanone E | C19H20O7 | 3.000 | 0.0439 |
| schisanlactone E | C30H44O4 | 2.410 | 0.0959 |
| N-transferuloyltyramine | C20H23NO5 | 2.816 | 0.0248 |
| angeloylgomisin O | C28H34O8 | 2.771 | 0.0399 |
| gomisin-A | C23H28O7 | 2.793 | 0.0327 |
| gomisin R | C22H24O7 | 2.906 | 0.0079 |
| changnanic acid | C29H44O4 | 2.390 | 0.0985 |
| kadsulactone | C30H44O3 | 2.364 | 0.0932 |
| kadsulignan B | C23H26O7 | 2.857 | 0.0102 |
| ginsenoside rh2 | C36H62O8 | 2.396 | 0.0953 |
| bisindigotin | C32H18N4O2 | 3.178 | 0.1036 |
| irisolidone | C17H14O6 | 3.189 | 0.1064 |
| 8-isopentenyl-kaempferol | C20H18O6 | 3.046 | 0.0608 |
| neobaicalein | C19H18O8 | 3.156 | 0.0972 |
| dihydrooroxylin A | C16H14O5 | 3.086 | 0.0750 |
| chrysin-5-methylether | C16H12O4 | 3.125 | 0.0879 |
| catechin | C15H14O6 | 3.143 | 0.0934 |
| 72-dihydroxy-58-dime thoxyflavone | C17H14O6 | 3.189 | 0.1064 |
| 7-hydroxy-58-dimethoxy-2-phenylchromone | C17HH14O5 | 3.054 | 0.0639 |
| 57-dihydroxy-8-methoxy-2-(2-methoxyphenyl)chromone | C17H14O7 | 3.263 | 0.1227 |
| formononetin | C16H12O4 | 3.125 | 0.0879 |
| Isoglabrolide | C30H44O4 | 2.410 | 0.0959 |
| glabrolide | C30H44O4 | 2.410 | 0.0959 |
| ebeiedinone | C27H43NO | 2.250 | 0.0784 |
| peimisine | C27H41NO3 | 2.389 | 0.0949 |
| verticinone | C27H43NO3 | 2.351 | 0.0922 |
| imperialine | C27H43NO3 | 2.351 | 0.0922 |
| ussuriedinone | C27H35NO3 | 2.515 | 0.0935 |
| euchrenone A5 | C25H26O4 | 2.727 | 0.0534 |
| glycyrol | C21H18O6 | 3.067 | 0.0684 |
| indirubin | C16H10N2O2 | 3.200 | 0.1092 |
| acacetin | C16H12O5 | 3.212 | 0.1121 |
| syrigin | C17H24O9 | 2.920 | 0.0134 |
| emodin | C15H10O5 | 3.333 | 0.1319 |
| 7-Omethylisomucronulatol | C18H20O5 | 2.837 | 0.0174 |
| formononetin | C16H12O4 | 3.125 | 0.0879 |
| ellagic acid | C14H6O8 | 3.929 | 0.0416 |
| 39-di-O-methylnissolin | C17H16O5 | 3.000 | 0.0439 |
Recently, 105 herbs from 24 TCM prescriptions that are highlighted in the guidelines for the treatment of COVID-19, were analyzed [34]. The results of this analysis showed that the combination of Amygdalus Communis Vas (ACV) and Ephedra sinica Stapf (ESS) is the best for the treatment of patients in almost all the stages of COVID-19, so ACV and ESS (AE) were selected as the most important herbal pair [34]. The molecular descriptors AQVN and EIIP, calculated for 26 active ingredients of the herbal pair AE is given in Table 2 (Ref. [31]) and Fig. 2 (Ref. [31]).
 Fig. 2.
Fig. 2.
The distribution of electron donors (n
| Compound | Formula | AQVN | EIIP [Ry] |
| Quercetin | C15H10O7 | 3.500 | 0.1260 |
| Kaempferol | C15H10O6 | 3.419 | 0.1339 |
| Luteolin | C15H10O6 | 3.419 | 0.1339 |
| C29H50O | 2.150 | 0.0578 | |
| Naringenin | C15H12O5 | 3.188 | 0.1060 |
| Stigmasterol | C29H48O | 2.180 | 0.0644 |
| Herbacetin | C15H10O7 | 3.500 | 0.1260 |
| Genkwanin | C16H12O5 | 3.212 | 0.1121 |
| Taxifolin | C15H12O7 | 3.352 | 0.1333 |
| Pectolinarigenin | C17H14O6 | 3.189 | 0.1064 |
| (+)-catechin | C15H14O6 | 3.143 | 0.0934 |
| Diosmetin | C16H12O6 | 3.294 | 0.1275 |
| Eriodictyol | C15H12O6 | 3.273 | 0.1243 |
| Truflex OBP | C20H30O4 | 2.482 | 0.0956 |
| Leucopelargonidin | C15H14O6 | 3.143 | 0.0934 |
| Resivit | C15H14O7 | 3.222 | 0.1144 |
| (+)-Leucocyanidin | C15H14O7 | 3.222 | 0.1144 |
| Mandenol | C20H36O2 | 2.207 | 0.0702 |
| 24-Ethylcholest-4-en-3-one | C29H48O | 2.180 | 0.0644 |
| poriferast-5-en-3beta-ol | C29H50O | 2.150 | 0.0578 |
| campest-5-en-3beta-ol | C28H48O | 2.156 | 0.0591 |
| Stigmasterol | C29H48O | 2.180 | 0.0644 |
| l-SPD | C19H21NO4 | 2.800 | 0.0304 |
| Estrone | C18H22O2 | 2.534 | 0.0928 |
| Glabridin | C20H20O4 | 2.818 | 0.0242 |
| Machiline | C17H19NO3 | 2.750 | 0.0466 |
| Licochalcone | C21H22O4 | 2.766 | 0.0416 |
| Phaseol | C20H16O5 | 3.073 | 0.0707 |
| (+)-catechin | C15H14O6 | 3.143 | 0.0934 |
| Glycyrol | C3H8O3 | 2.714 | 0.0570 |
| Liquiritin | C21H22O9 | 3.077 | 0.0769 |
| CLR | C27H46O | 2.162 | 0.0606 |
| Sitosterol | C29H50O | 2.150 | 0.0578 |
| Spinasterol | C29H48O | 2.180 | 0.0644 |
| gondoic acid | C20H38O2 | 2.167 | 0.0616 |
| 11,14-eicosadienoic acid | C20H36O2 | 2.207 | 0.0702 |
| Mairin | C22H22O8 | 3.038 | 0.0582 |
Natural polyphenols, playing a relevant role in reducing inflammation and preventing the onset of serious chronic diseases, have been recently reviewed as promising agents to fight COVID-19 [35]. The AQVN and EIIP values calculated for 50 natural polyphenols with demonstrated anti-COVID-19 activity [35] are given in Table 3 (Ref. [32]) and Fig. 3 (Ref. [32]). The molecular descriptors AQVN and EIIP were also calculated for hypericin which is nathodyanthrone and not a typical phenolic compound [36].
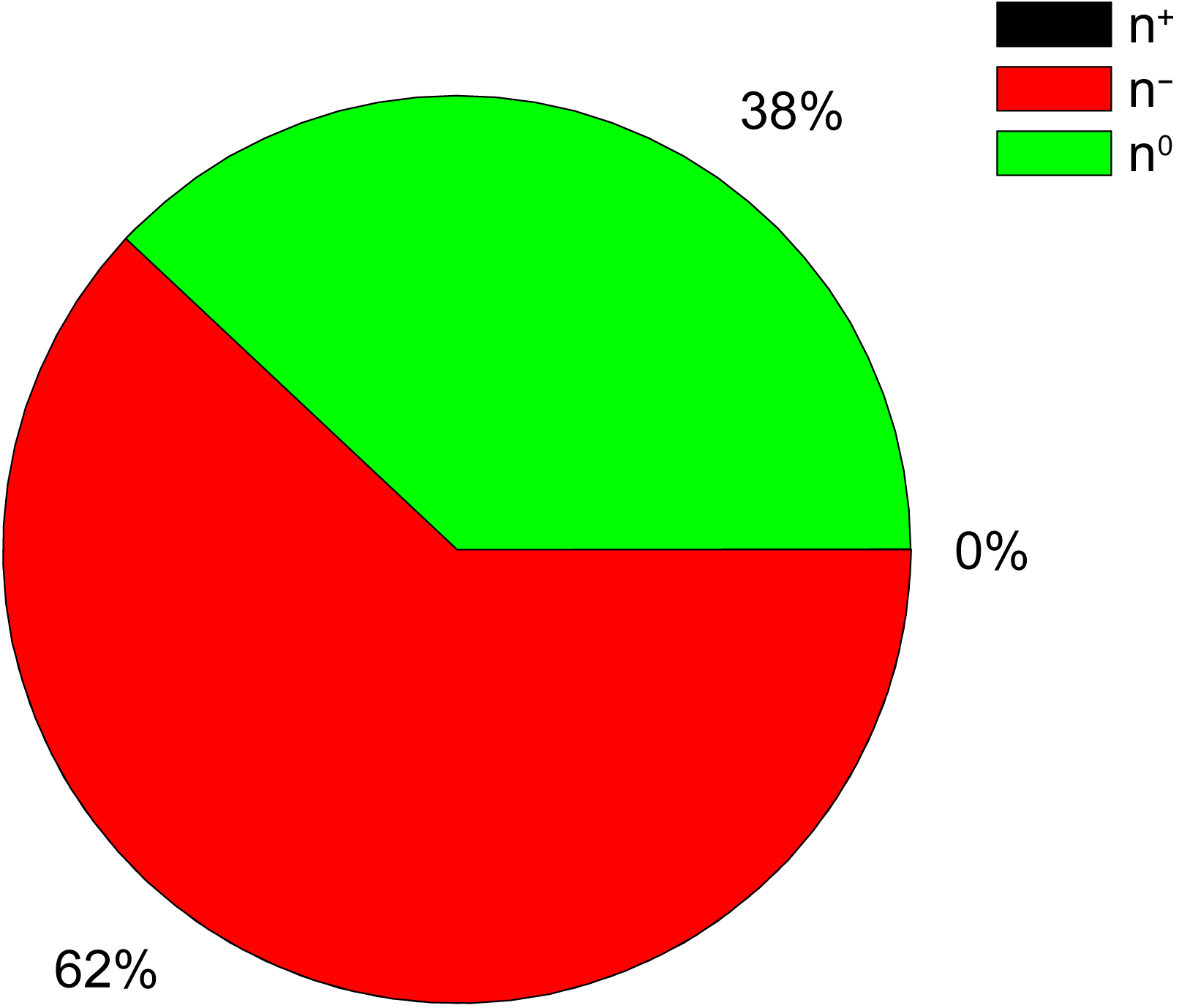 Fig. 3.
Fig. 3.
The distribution of electron donors (n
| Compound | Formula | AQVN | EIIP [Ry] |
| Cyanidin | C15H11O6 | 3.344 | 0.1327 |
| Daidzein | C15H10O4 | 3.241 | 0.1185 |
| Dieckol | C36H22O18 | 3.605 | 0.1016 |
| Genistein | C15H10O5 | 3.333 | 0.1319 |
| Mearnsitrin | C22H22O12 | 3.250 | 0.1202 |
| Myricitrin | C21H20O12 | 3.321 | 0.1307 |
| Psoralidin | C20H16O5 | 3.073 | 0.0707 |
| Quercetin 3-O-D-glucoside | C21H19O12 | 3.365 | 0.1339 |
| Rutin | C27H30O16 | 3.205 | 0.1105 |
| Xanthoangelol E | C21H22O6 | 2.898 | 0.0050 |
| Benzoic acid | C7H6O2 | 3.067 | 0.0684 |
| Ellagic acid | C14H6O8 | 3.929 | 0.0416 |
| Gallic acid | C7H6O5 | 3.556 | 0.1150 |
| Kaempferol 3-O-rutinoside | C27H30O15 | 3.167 | 0.1004 |
| Naringenin | C15H12O5 | 3.188 | 0.1060 |
| Oleuropein | C25H32O13 | 3.000 | 0.0439 |
| Quercetin | C15H10O7 | 3.500 | 0.1260 |
| Quercetin 3-O-rutinoside | C27H30O16 | 3.206 | 0.1105 |
| Resveratrol | C14H12O3 | 2.966 | 0.0308 |
| Scutellarein | C15H10O6 | 3.419 | 0.1339 |
| Cyanidin 3-O-glucoside | C21H21O11 | 3.226 | 0.1154 |
| Epigallocatechin | C22H18O11 | 3.373 | 0.1342 |
| Epigallocatechin gallate | C22H18O11 | 3.373 | 0.1342 |
| Hypericin | C30H16O8 | 3.407 | 0.1343 |
| Kaempferol | C15H10O6 | 3.419 | 0.1339 |
| Cryptotanshinone | C19H20O3 | 2.714 | 0.0570 |
| Luteolin | C15H10O6 | 3.419 | 0.1339 |
| Afzelin | C21H20O10 | 3.216 | 0.1130 |
| Apigenin | C15H10O5 | 3.333 | 0.1319 |
| Baicalin | C21H18O11 | 3.360 | 0.1337 |
| Biorobin | C27H30O15 | 3.167 | 0.1004 |
| Caffeic acid | C9H8O4 | 3.238 | 0.1179 |
| Catechin | C15H14O6 | 3.143 | 0.0934 |
| Chlorogenic acid | C16H18O9 | 3.163 | 0.0993 |
| Chrysin | C15H10O4 | 3.241 | 0.1185 |
| Curcumin | C21H20O6 | 2.979 | 0.0359 |
| Delphinidin | C15H11O7 | 3.424 | 0.1337 |
| Ferulic acid | C10H10O4 | 3.083 | 0.0742 |
| Galangin | C15H10O5 | 3.333 | 0.1319 |
| Hesperetin | C16H14O6 | 3.167 | 0.1004 |
| Isoferulic acid | C10H10O4 | 3.083 | 0.0742 |
| Nobiletin | C21H22O8 | 3.020 | 0.0513 |
| Nympholide A | C30H26O15 | 3.324 | 0.1310 |
| Pinocembrin | C15H12O4 | 3.100 | 0.0787 |
| Rhoifolin | C27H30O14 | 3.127 | 0.0885 |
| Rutin | C27H30O16 | 3.206 | 0.1105 |
| Taiwanhomoflavone A | C33H24O10 | 3.224 | 0.1148 |
| Tangeretin | C20H20O7 | 3.021 | 0.0519 |
| Viniferin | C28H22O6 | 3.036 | 0.0572 |
| Artepillin C | C19H24O3 | 2.565 | 0.0882 |
The previous analysis of 45,010,644 compounds randomly selected from the PubChem database [37] showed that 90.5% of these compounds have EIIP and AQVN values in the intervals (0.00–0.10 Ry) and (2.4–3.2), respectively (Fig. 4, Ref. [33]) [24]. This part of the EIIP/AQVN space, encompassing the majority of analyzed compounds, was referred as the “basic chemical space” (BCS) [24]. The small fraction (4.3%) of analyzed compounds from the PubChem, representing the strong electron donors and are located left of BCS. The compounds with the strong electron-acceptor properties (5.3% of analyzed compounds from the PubChem) are in the domain right of BCS. Results presented in Table 4 show that the percentage of strong electron-acceptors and electron-donors in preparations with anti-COVID-19 activity is significantly higher in the comparison with compounds in the PubChem [37], approved drugs [38], natural compounds [39] and collection of small molecules that are relevant to biological systems (KEEG) [40].
 Fig. 4.
Fig. 4.
The distribution of electron donors (n
| Source of compound | Number of compounds | n |
n |
n |
| TMC | 93 | 18 | 29 | 47 |
| AE | 37 | 27 | 32 | 59 |
| Polyphenols | 50 | 0 | 29 | 29 |
| Approved drugs | 1469 | 8 | 9 | 17 |
| KEEG | 7638 | 8 | 9 | 17 |
| Natural compounds | 4668 | 11 | 16 | 25 |
| PubChem | 4,5010,644 | 4 | 5 | 9 |
The COVID-19 pandemic is one of the greatest challenges modern medicine has ever faced. Doctors and scientists are trying to find treatments and drugs that can cure and prevent this disease. Unfortunately, nearly two years into the pandemic there is no cure yet for COVID-19 disease. A handful of therapies—remdesivir, monoclonal antibodies and the steroid dexamethasone—have improved the care of COVID patients, but these drugs are not cure-alls and they are not for everyone. On the other hand, efforts to repurpose other drugs, or discover new ones, have not had much success.
At the beginning of the COVID-19 pandemic the National Health Commission of the People’s Republic of China released the Dignosis and treatment protocol for COVID-19 which includes the TCM treatment [41]. TCM’s preventing and curative effect was outstanding. As of March 2020 new cases of COVID-19 in China have significantly decreased and more than 70,000 people were cured of COVID-19 and discharged from the hospital with the total treatment time of approximately 2 months [42]. China has today the lowest number of the daily new cases and the lowest COVID-19 mortality in the world [43].
Presented results show that most essential ingredients of analyzed TCM preparations (Table 1) can be represented by the specific groups of organic compounds according to their basic electronic properties AQVN and EIIP, and these differ from the vast majority of known chemicals which are located in BCS. These ingredients are in the domains of the AQVN/EIIP space that are situated left and right of BCS. The distribution of the essential ingredients of the important herbal pair AE (Table 2) in the AQVN/EIIP space is similar with distribution of ingredients of other analyzed TCM preparations with anti-COVID-19 activity. Previously, we reported that the domains left and right outside BCS contain the strong electron-donors and electron-acceptors, respectively [23, 24].
Polyphenols have been recently suggested as promising agents to fight COVID-19, and some clinical trials have already been approved with polyphenols to treat COVID-19. It was suggested that these compounds possess a potential immunomodulatory therapeutic value against SARS-CoV-2-induced immune response dysregulation [35]. In Table 1, the authors also analyzed emodin, a precursor of nathodyanthrones fagopyrin characterized by antiviral potential [44].
Results presented in Table 3 show that the main fraction (58%) of analyzed polyphenols with anti-COVID-19 activity are the strong electron-acceptors which are located in the AQVN/EIIP space right of BCS. Of note also is that ingredients with the electron-acceptors properties dominate in the analyzed TCM, compared to the electron-donor compounds.
Knowledge of the possible active mechanisms of TCM preparations in the treatment of COVID-19 may provide meaningful information for further study to investigate the mechanisms of TCMs as a therapeutic approach to overcoming COVID-19. TMC could treat COVID-19 through multiple components, multiple targets, and multiple pathways. Polyphenols are most abundant ingredients in all TCM preparations with the anti-COVID-19 activity. Among these polyphenols kaempferol, quercetin, baicalein and luteolin are the main components of Chinese patent medicine representing an indispensable part of TCM [33]. These flavonoids also are within natural polyphenols with demonstrated anti-COVID-19 activity [35] (Table 3).
Previously, to identify human proteins that represent the most probable
candidates for interactors with flavonoids, we analyzed human proteins from the
UniProt database [45], using the informational spectrum method (ISM) [46].
Results of this analysis suggest that among the human proteins NF-kB represents
one of the more likely targets for flavonoids. The role of NF-kB pathway in the
COVID-19 pathogenesis was confirmed in numerous studies. The investigation of the
host-virus interaction provides evidence that SARS-CoV-2 modulates NF-
Quercetin represents a most investigated natural compound with the anti-COVID-19 activities. Antiviral, immunomodulatory, and anticoagulant effects of quercetin and its derivatives are recently reviewed and its potential role in prevention and management of COVID-19 were suggested [54]. It is also suggested that quercetin could prevent the cell-to-cell transmission of SARS-CoV-2 [55]. Currently, therapeutic effect of quercetin is investigated in 14 clinical studies [56].
The simple EIIP/AQVN criterion described in this paper can be used for powerful and rapid in silico screening of natural compounds that are active against SARS-CoV-2 virus and for identification and optimization of new lead compounds for treatment of the COVID-19 disease. These results, together with data from the USDA Food Database [57] (http://www.nal.usda.gov/fnic/) also can be used for selection of food and the food supplements which could have a beneficial effect for COVID-19 patients.
VV and SP designed the concept. VV, MV and SG done formal analysis. VP, MV and SG performed data curation. VV and SP prepared the original draft. VV, VP, SG, MV and SP wrote and reviewed the manuscript. VP implemented the software. VV and VP done the visualization, VV and VP designed the methodology. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
Not applicable.
This work was supported partially by John S. Dunn Endowment to SP and through Cooperative Agreement Number U01AI151801 between the National Institute of Allergy and Infectious Diseases (NIAD) and University of Texas Medical Branch/West African Center for Emerging Infectious Diseases.
This research was supported by the Ministry of Education, Science and Technological Development of the Republic of Serbia and Biomed Protection Galveston Texas USA.
The authors declare no conflict of interest.

