†These authors contributed equally.
Academic Editor: Graham Pawelec
Background: Reactive oxygen species (ROS) accumulation plays a pivotal
role in the onset of cell damage induced by hyperglycemia and represents one of
the major factors in the pathogenesis of diabetic retinopathy. In this study, we
tested the antioxidants cyanidin-3-O-glucoside (C3G) and verbascoside (Verb) in
the protection of retinal endothelium against glucose toxicity “in vitro”. Methods: Increasing amounts (5–50
Diabetic retinopathy (DR) is a common retinal microvascular complication
occurring in diabetic patients, often leading to vision loss and blindness [1, 2].
Clinical manifestations of DR are classified in two main stages: the earliest
stage being non-proliferative diabetic retinopathy (NPDR) and the more advanced
stage being proliferative diabetic retinopathy (PDR). A prolonged poor glycemic
control triggers NPDR characterized by increased vascular permeability, capillary
occlusion, microaneurysms, hemorrhages and hard exudates [3]. The progressive
breakdown of the inner blood-retinal barrier (iBRB) can contribute to the
formation of diabetic macular edema (DME), which is the most common cause of
vision impairment in DR, through subretinal and intraretinal accumulation of
fluid leading to the swelling of the macula [4]. The production of new abnormal
vessels due to neovascularization processes represents the main clinical hallmark
of PDR that worsens DR symptoms by bleeding into the vitreous and inducing
retinal detachment and severe vision impairment [5]. Chronic exposure to
hyperglycemia drives the early microvascular damage through an excessive
production of advanced glycation end products (AGEs) and the aberrant activation
of polyol, hexosamine and protein kinase C (PKC) pathways [6, 7]. These activated
pathways lead to increased levels of oxidative stress in retinal cells resulting
from an altered balance between reactive oxygen species (ROS) production and the
cellular antioxidant defense system. This includes enzymes, such as superoxide
dismutase (SOD), heme oxygenase, and catalase [8, 9], as well as nonenzymatic
antioxidant agents such as uric acid, bilirubin and glutathione including
vitamins (E and C) and polyphenols, all aimed at protecting cells through a
direct scavenging activity of free radicals [10]. However, hyperglycemia-induced
metabolic changes trigger the generation of an overwhelming amount of ROS from
several pathways including the mitochondrial electron transportation chain, and
the increased activity of NADH oxidase, cytochrome P450 and xanthine oxidase
[11, 12, 13]. Moreover, the excessive consumption of NADPH produced by the activation
of the polyol pathway decreases glutathione (GSH) synthesis, contributing to the
alteration of the homeostatic redox system and the increase of intracellular ROS
[11, 14, 15]. Oxidative damage induced by ROS accumulation underlies multiple
pathological processes in DR such as lipid peroxidation and mitochondrial
dysfunction, finally leading to apoptosis and progressive endothelial
abnormalities [16, 17, 18, 19]. ROS were shown to promote inflammatory processes through
the production of cytokines such as tumor necrosis factor-alpha (TNF-
Anthocyanins were proposed as promising nutraceutical molecules for the prevention of DR via their antioxidant and anti-inflammatory properties [28, 29]. Among anthocyanins, cyanidin-3-O-glucoside (C3G) represents a powerful natural antioxidant with beneficial effects in cases of increased oxidative stress, and at pharmacological concentrations it has been shown to be able to decrease tissue damage occurring in myocardial ischemia and reperfusion [30]. C3G also prevented the hyperglycemia-induced hepatic oxidative damage by activating GSH synthesis against ROS production [31]. Moreover, C3G showed a protective scavenging activity against endothelial dysfunction and vascular failure induced by peroxynitrite [32]. Besides the antioxidant activity, C3G could enhance vascular eNOS activity, thus contributing to improve vascular endothelial function [33].
Verbascoside (Verb, a.k.a. acteoside), a molecule belonging to the phenylpropanoid family, was discovered in extracts from Verbascum sinuatum L. in 1950, and found to be largely diffused in other dietary plants and fruits, such as pigmented oranges that are very common in the Mediterranean diet [34, 35]. The antioxidant capacity of Verb is related to its free radical scavenging ability, leading to anti-hypertensive, anti-cancer and neuroprotective effects [36, 37, 38, 39, 40]. Verb reduced platelet aggregation in patients with cardiovascular risk factors and enhanced the anti-aggregating activity of aspirin after adenosine diphosphate (ADP) stimulation [41, 42], thus improving blood perfusion.
Recently, it has been hypothesized that an enhanced antioxidant activity can be achieved through a cooperative interaction among different antioxidant constituents as they may exert multi-target effects activating or modulating different pathways involved in redox homeostasis [43, 44]. For instance, the association of C3G and Verb has been shown to protect the retina from different types of insults and oxidative stress. In a rat model of light-induced photooxidative retinal damage, oral pretreatment with a mixture containing C3G, Verb, lutein and zinc protected photoreceptor cells from death by preventing oxidative stress, inflammation, gliotic and apoptotic responses. The better efficacy of the mixture on the dysfunctional electroretinogram was also demonstrated by the improved rod and cone photoreceptor responses [45]. More pertinent to the goal of this study is the efficacy shown by the oral administration of the association of C3G, verbascoside and zinc on the diabetic retinopathy induced in rats by streptozotocin treatment [46]. This in vivo preclinical study reported a dose-dependent inhibition of oxidative stress-related and inflammation-related mechanisms in the retina of diabetic rats, with a significant reduction of DR-associated vasculopathy and its related retinal damage. Electroretinography under photopic and scotopic conditions also demonstrated the preventive efficacy of the compound on dysfunctional a-waves and b-waves.
Therefore, based on this preliminary evidence, we set out to evaluate in this study the antioxidant and protective effects of C3G and Verb, either alone or in association, on the dysfunction of the endothelial barrier triggered by high glucose in an in vitro model of DR obtained by treating human retinal endothelial cell (HREC) monolayers with high concentrations of glucose (25 mM).
Cyanidin-3-glucoside (Black Rice Extract 20, Bionap, Italy) and verbascoside (Verbalief, Bionap, Italy) were kindly donated by ‘La Sorgente del Benessere’ (Fiuggi, Italy). Antibodies against tight junction proteins were: rabbit polyclonal ZO-1 antibody (Thermo Fisher Scientific, Waltham, MA, USA); rabbit monoclonal VE-cadherin antibody (Cell Signaling Technology, Danvers, MA, USA). Reagents for cell cultures were from Invitrogen Life Technologies (Carlsbad, CA, USA).
Primary human retinal endothelial cells (HREC) were purchased from Innoprot
(Derio-Bizkaia, Spain) and cultured with endothelial cell medium (ECM)
supplemented with 5% fetal bovine serum (FBS), 1% endothelial cell growth
supplement (ECGS), 100 U/mL penicillin and 100
 Fig. 1.
Fig. 1.Von Willebrand staining. HREC at passage 3 (A) and 9 (B) were immunostained for the endothelial molecular marker von Willebrand factor, showing similar morphologies and staining intensity, indicating a reliable stability of the endothelial phenotype.
Cell viability was determined by the metabolic MTT colorimetric assay (Cell
Counting Kit 8 (CCK8) reagent) (Sigma-Aldrich, St. Louis, MO, USA), based on the reduction of a
yellow tetrazolium salt (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide or MTT) to purple formazan crystals by metabolically active cells. HREC
were seeded at 1.5
Reactive oxygen species (ROS) were measured by means of the DCFDA Cellular ROS
Detection Assay Kit (ab113851, Abcam Cambridge, UK), according to the
manufacturer’s protocol. After treatments with HG or NG, HREC were incubated with
25
HREC were plated in transwells (Costar, 3412) with
The diffusion of sodium fluorescein (Na-F, Santa Cruz Biotechnology, cat. number
sc206026) across HREC monolayers was determined as previously described [49].
HREC confluent monolayers were grown in transwells until reaching barrier
properties and then were treated with NG or with HG in 2.5% FBS ECM medium with
or without C3G (50
Where C
The correlation coefficient between TEER values and Pe in the different experimental conditions was calculated with GraphPad Prism 7 for Windows using the function linear fit dose-response interpolation.
Immunostaining of HREC with rabbit polyclonal antibodies against Von Willebrand
Factor (Abcam, Cambridge, UK; catalog num. ab6994) was done on cells at passage 3
or 9. Cells were seeded on poly-L-lysine precoated glass coverslips in a 24-well
plate, at 2
After treatments under different conditions with NG or HG, cellular monolayers
were washed twice with PBS and harvested mechanically, using a cell scraper.
Cells were centrifuged at 1000 rpm for 5 min at 25 °C, and pellets were
lysed in RIPA buffer (Calbiochem-Merck, 20188, Darmstadt, Germany) supplemented with protease and
phosphatase inhibitor cocktails (Protease Inhibitor Cocktail Set III EDTA-Free,
Calbiochem-Merck, 539134; Phosphatase Inhibitor Cocktail 2, Sigma Aldrich, P5726,
USA; Phosphatase Inhibitor Cocktail 3, Sigma Aldrich, P0044, USA) by incubation
for 30 min on ice. Extracts were clarified by centrifugation at 13,000 rpm for 20
min at 4 °C, and proteins in the supernatant quantitated by the BCA
protein assay (BCA kit assay, Santa Cruz biotechnology, 10410, Dallas, TX, USA). For Western
blot analysis, 40
Each experiment was carried out three times, each time in triplicate (n = 3).
Data are reported as mean
The effects of increasing amounts of C3G and Verb, or their association were
evaluated on HREC cell viability cultured for 48 h under NG (5 mM) or HG (25 mM)
conditions. Initially, cell viability was tested under NG conditions in order to
evaluate the tolerability of the antioxidant compounds at the concentration of 5,
10 and 50
 Fig. 2.
Fig. 2.Effects of C3G and Verbascoside on HG-induced damage on
HREC. Cell viability was evaluated by the MTT assay in cells after 48 h of
growth in cell culture medium with normal glucose (NG, 5 mM: white bars) or with
high glucose (HG, 25 mM: grey bars). NG or HG media also contained increasing
amounts (5, 10 and 50
Endothelial damage induced by the presence of HG follows the intracellular
accumulation of ROS within HREC. Consistently with the decrease in cell
viability, HG more than doubled the amount of intracellular ROS in HREC (Fig. 3
and Table 1). The concomitant presence of C3G or Verb had a dose-dependent effect
in decreasing the amount of ROS, respectively by 44% and 25%. The association
of the two molecules at 10 and 50
 Fig. 3.
Fig. 3.Effects of C3G and Verbascoside on HG-induced ROS
accumulation within HREC. Relative ROS levels were quantitated by the H2DCFDA
assay after 48 h of growth with normal glucose (NG, 5 mM) or with high glucose
(HG, 25 mM). NG or HG media also contained increasing amounts (5, 10 and 50
| Treatment | NG (O.D.) | HG (O.D.) |
| Vehicle | 2.87 |
7.31 |
| C3G 5 |
2.50 |
6.20 |
| C3G 10 |
2.47 |
5.34 |
| C3G 50 |
2.46 |
4.13 |
| Verb 5 |
2.70 |
7.23 |
| Verb 10 |
2.99 |
6.36 |
| Verb 50 |
2.91 |
5.48 |
| C3G + Verb 5 |
2.69 |
4.75 |
| C3G + Verb 10 |
3.12 |
4.52 |
| C3G + Verb 50 |
2.40 |
3.43 |
| Reactive oxygen species (ROS) levels measured through the H2DCFDA assay in HREC
treated with normal glucose (NG, 5 mM) or with high glucose (HG, 25 mM) for 48 h.
NG or HG media were supplemented with increasing amounts (5, 10 and 50 | ||
HG conditions may disrupt the endothelial barrier, causing leakage and edema. In
order to evaluate the protective effect of C3G and Verb on the barrier integrity
of HREC monolayers upon HG-treatment, we measured the Trans Endothelial
Electrical Resistance (TEER). This parameter usually provides a good in vitro estimation of blood-retinal barrier (BRB) integrity [48]. As shown
in Fig. 4, shifting the HREC confluent monolayer (at time 0) to HG conditions
causes a progressive decrease of TEER by 40% and 50% of the original value at
48 and 72 h of treatment, as compared to NG conditions, which guarantee a
stability of the barrier effect. Treatment with 50
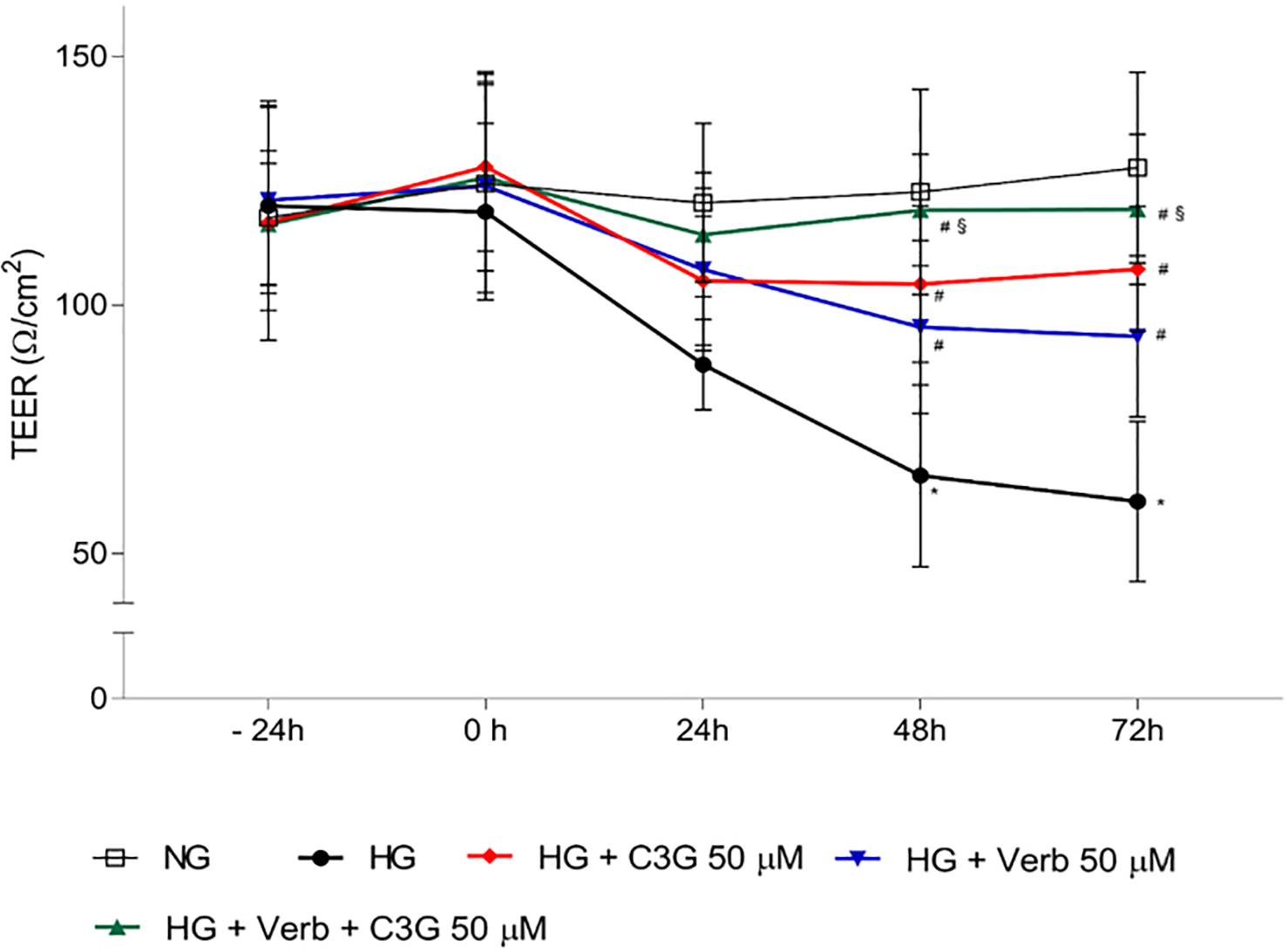 Fig. 4.
Fig. 4.Effects of C3G and Verbascoside on Trans Endothelial Electrical
Resistance (TEER) of HREC monolayers grown under NG or HG conditions with or
without C3G and verbascoside. Stable cell monolayers were obtained by culturing
the cells in appropriate insert-wells for 5 days. Then, cell monolayers were
treated up to 72 h with HG with or without 50
Increased paracellular permeability of the retinal endothelium represents one of
the main hallmarks of the HG-induced cell damage in vitro,
which mimics the pathological increase in retinal vascular leakage in
hyperglycemic conditions [51]. Given the reduction of TEER reported in Fig. 4,
likely due to a decrease in tight junctions between endothelial cells, we would
expect to also see an increase of paracellular permeability in HREC monolayers
after shifting to HG conditions, and a preventive effect of C3G and Verb. We used
sodium fluorescein added to the upper well of a transwell plate as a marker to
follow leakage from the upper to the lower well. Fig. 5A shows the fluorescein
permeation kinetics through an intact endothelial monolayer maintained under NG
or shifted to HG conditions, showing a dramatic progressive increase induced by
HG throughout the recorded times. The presence of Verb or C3G at 50
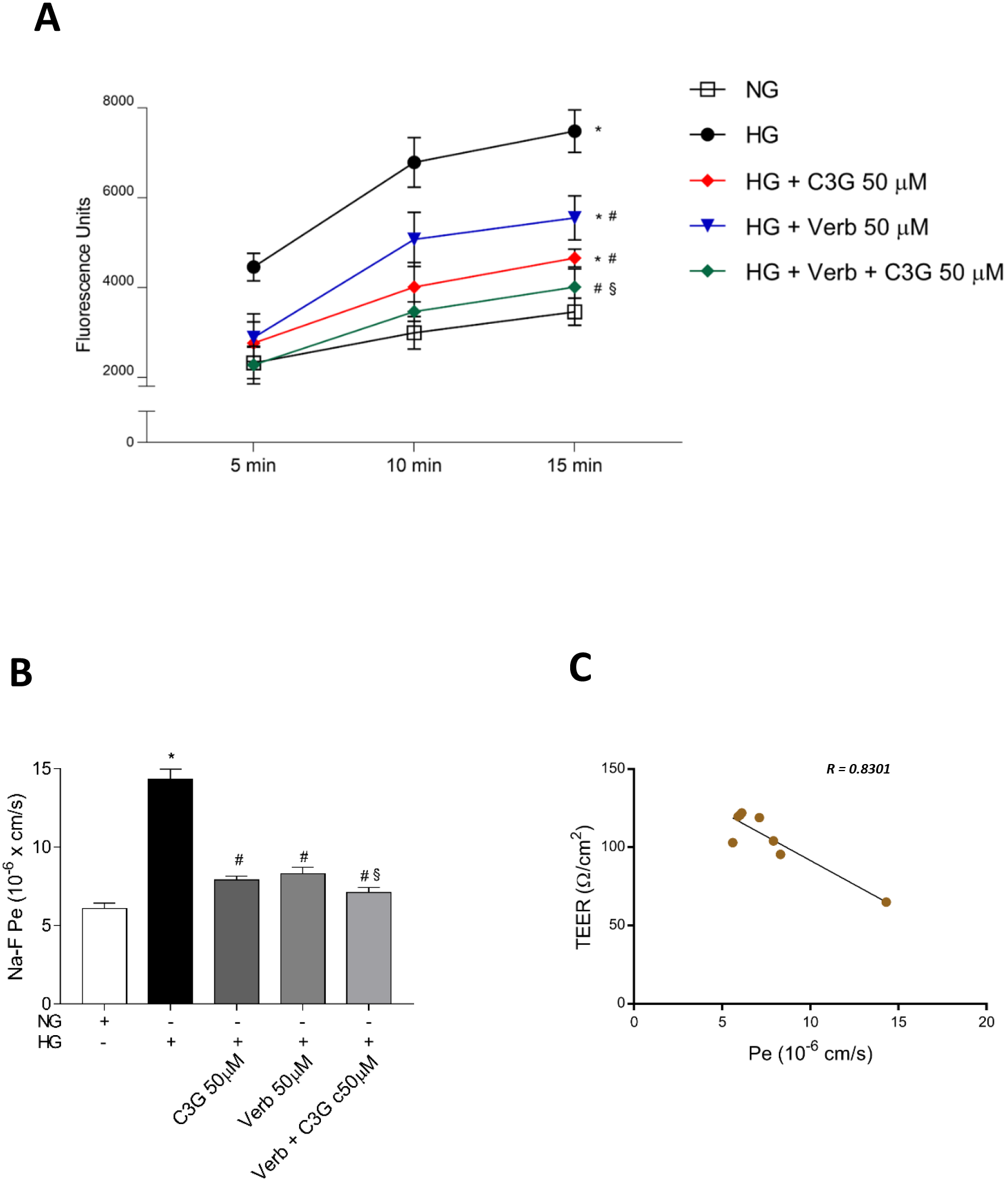 Fig. 5.
Fig. 5.Effects of C3G and Verbascoside on the HG-induced
increase of the paracellular permeability in a confluent monolayer of HREC
evaluated by the sodium fluorescein (Na-F) assay. Stable cell monolayers were
obtained by culturing the cells in appropriate insert-wells for 5 days. (A) Na-F
assays were performed in HREC monolayers treated for 48 h with HG with or without
50
The effects of HG on the integrity loss of the endothelial barrier had been
previously related to changes in the expression and/or the degradation of
junction proteins, which may explain the increased leakage under hyperglycemic
conditions [52, 53]. We show here (Fig. 6A) the immunocytochemical analysis
illustrating the abundance and the integrity of the tight junction proteins ZO-1
and VE-cadherin in a confluent HREC monolayer under NG conditions, and their
disarrangement and decrease after 48 h with HG. The simultaneous presence under
HG conditions of the association of C3G and Verb (50
 Fig. 6.
Fig. 6.Effect of C3G and Verbascoside on the HG-induced
down-regulation of endothelial tight junction proteins evaluated by
immunocytochemical analysis. Confluent monolayers of HREC were treated for 48 h
with HG with or without 50
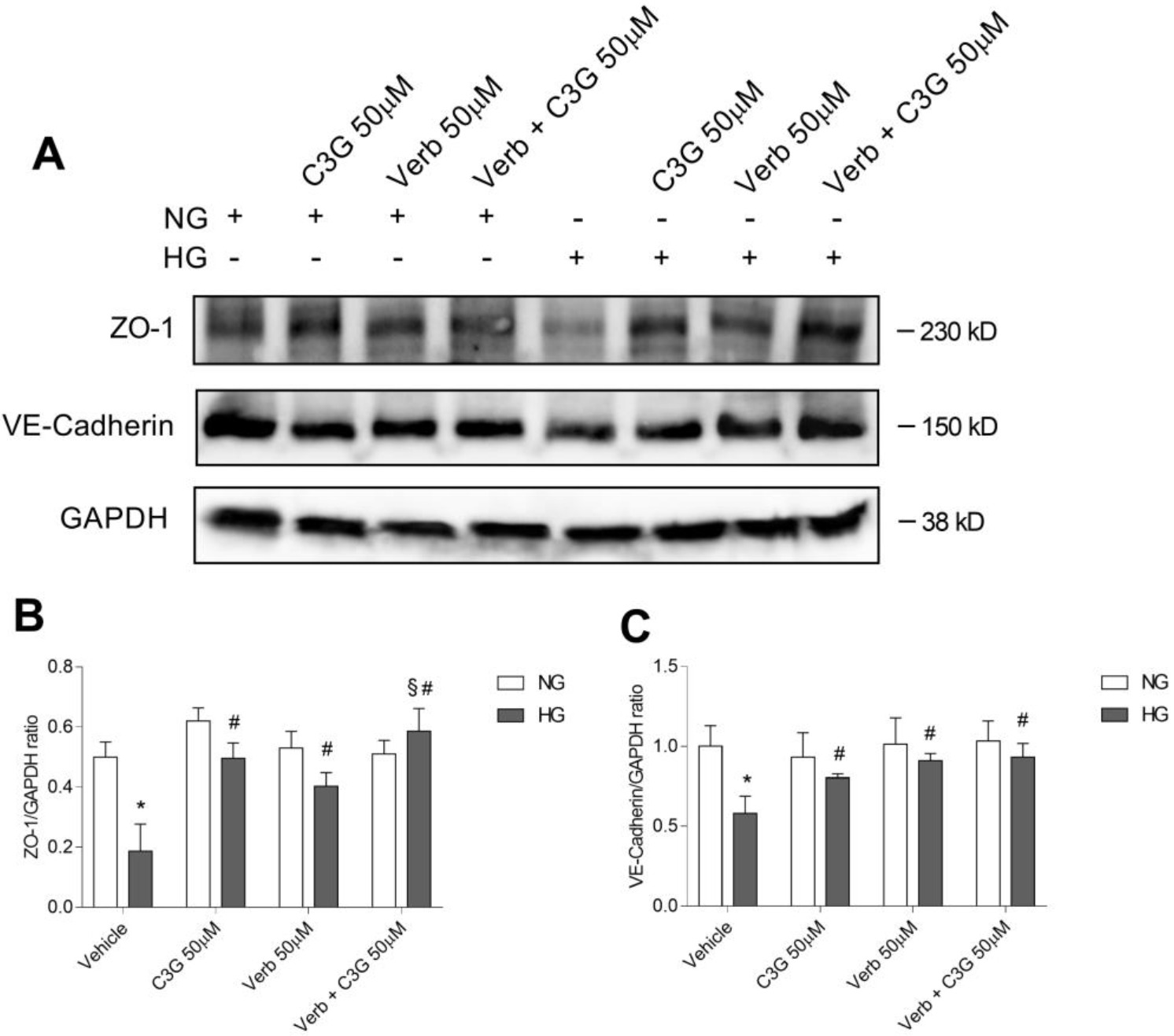 Fig. 7.
Fig. 7.Effects of C3G and Verbascoside on the HG-induced
down-regulation of endothelial tight junction proteins evaluated by western blot
analysis. (A) Representative immunoblot performed using specific antibodies
against ZO-1 and VE-cadherin on lysates from confluent monolayers of human
retinal endothelial cells (HREC) treated for 48 h with HG with or without 50
The pathological manifestations of DR are triggered by the overwhelming oxidative stress occurring further to the failure of glycemic control, and mainly involve the microvascular endothelial system. Therefore, the therapeutic approach to prevent or treat DR is based primarily on the control of the glycemic index, because maintaining the glycosylated hemoglobin (HbA1c) level below 7% may decrease the risk of DR development and progression [56]. However, despite the known benefits of improved glycemic control, a large number of diabetic patients do not manage to keep safe blood glucose levels, which together with an adequate surveillance program for the monitoring of hyperglycemia-induced damage, could potentially spare diabetic complications to an elevated number of patients [57].
In their advanced stages, PDR and DME are treated by invasive procedures, such as vitrectomy, intravitreal injections of corticosteroids or anti-VEGF and laser photocoagulation. Such treatments are not curative, and at their best they are aimed at preserving the residual visual ability of the patient [57]. Moreover, several patients are not responders to intravitreal therapies [58]. Hence, there is an urgent medical need to develop new and less invasive therapeutic approaches for the prevention or treatment of DR in order to try and reduce the incidence and progression of this disease, starting from its early stages. Such new therapeutic strategies are not necessarily alternative, but may be associated with surgical treatments in order to complement and improve their efficacy. Therefore, since ROS overproduction in mitochondria is caused by diabetic hyperglycemic stress [59], the attenuation of these events may play a pivotal role in the treatment of DR, preventing or delaying its progression towards the more advanced stage of the disease.
Currently, the International Classification of Diabetic Retinopathy and Diabetic Macular Edema considers the use of antioxidants as potentially effective in the treatment of mild forms of NPDR and DME, while no indications are given for their use in the treatment of PDR [60]. Studies from in vitro and in vivo models suggest that nutraceuticals may relieve the pathophysiological complications of DR such as inflammation and neurodegeneration, by an enhancement of the antioxidant defense systems and the consequent reduction of ROS accumulation [61, 62]. The main dietary antioxidants are found in colored foods, and are represented by polyphenols and carotenoids (Fig. 8). Flavonoids belong to a larger group of natural substances with different phenolic structures, which can be found in different kinds of fruits and vegetables, and their derivatives. They are endowed with anti-inflammatory and antioxidant effects, so that they are nowadays considered indispensable components in several nutraceutical, pharmaceutical, medicinal and cosmetic applications [63]. In several different preclinical and clinical studies, the different classes of flavonoids have shown good efficacy in counteracting the deleterious effects of oxidative stress and inflammation on the retina of diabetic subjects [64, 65]. For instance, in HREC stimulated by human retinoblastoma cell line conditioned medium or VEGF, the protective role played by quercetin in counteracting the pro-angiogenic stimulus has been demonstrated [66]. Consistently, data reported in this paper show the protective effects on the BRB of two polyphenolic antioxidants, belonging to different families: cyanidin-3-glucoside is an anthocyanin, and verbascoside is a phenylpropanoid glucoside, a family of molecules mainly found in medicinal plants. We used a primary human retinal endothelial cell line (HREC) to test the protective effects of C3G and Verb when the cells were shifted to HG growth conditions. It was previously shown that HG affects both macro and microvascular endothelial cells, altering their metabolism, finally resulting in the typical vascular dysfunctions of diabetes and DR [67]. In this respect, BRB efficiency depends on the expression by endothelial cells of junction proteins such as VE-Cadherin and ZO-1, which are downregulated in the presence of high glucose conditions [68]. Therefore, strategies have been described to counteract such a decrease and maintain BRB properties. For instance, erythropoietin and tumor necrosis factor ligand-related molecule 1A (TL1A) have been described as factors able to preserve and restore VE-cadherin expression and BRB integrity in the presence of high glucose levels [69, 70]. Along this line, Behl [65] and Matos [64] reported that two different polyphenols, scutellarin and curcumin, could contrast the effects on growth and apoptosis of HG conditions, increase the expression of junction proteins and decrease the formation of branched tubular structures. More recently, HREC grown under HG conditions were used to show the effects of fluorometholone (a glucocorticoid drug used in inflammatory and allergic disorders of the eye) in blunting the release of inflammatory cytokines and VEGF, and inhibiting the HG-induced cellular senescence mediated by the Akt pathway [71].
 Fig. 8.
Fig. 8.Molecular structure of the compounds used in this study. The generic structure of flavonoids is illustrated (A), together with the structure of anthocyanins (B), which include the cyanidins such as C3G, and verbascoside (C).
Under our experimental conditions, HREC monolayers shifted to HG conditions (25 mM) for 48 h showing a dramatic increase of intracellular ROS, concomitant with growth inhibition, a two-fold decrease of TEER correlating with an increased paracellular permeability and a decreased expression of tight junction proteins. All these effects could be efficiently dampened in a dose-dependent way by C3G and Verb or - better - by the combination of the two. Accordingly, C3G has been shown to be able to prevent the disruption of the barrier function of retinal pigmented epithelial cells (RPE) caused by blue light irradiation, besides activating the Nrf2 pathway, thus enhancing endogenous antioxidant protection and promoting RPE survival [72]. In a more comprehensive study addressing DR, C3G suppressed migration, invasion and angiogenesis of HREC and blunted the activation of microglial BV2 cells stimulated in vitro by HG [66]; moreover, in diabetic mice induced by streptozotocin the oral treatment with C3G decreased inflammation, microglial activation and angiogenesis in the retina [73]. Moreover, C3G could protect pancreatic beta-cell dysfunction induced by palmitic acid treatment, conserving their secretory function and alleviating apoptosis [74]. Finally, C3G could prevent diabetic cataract formation as suggested by its protective activity on lens epithelial cells in vitro exposed to HG conditions [75].
The phenylethanoid glycoside verbascoside/acteoside is a widespread polyphenolic plant compound, showing several biological properties including strong antioxidant, anti-inflammatory and neuroprotective activities. In fact, it is an efficient ROS scavenger and inhibitor of lipid peroxidation. It also inhibits the inducible NO synthase (iNOS) in the CNS, prevents the activation of COX2 in glioma cells, and protects from inflammatory events by downregulating the activation of the pro-inflammatory transcription factor NFkB, while activating the Nrf2 pathway leading to the endogenous expression of antioxidants, such as HO1 [76]. Similar to what has previously been described for C3G, also Verb can protect pancreatic beta cells from endoplasmic reticulum (ER)-stress mediated dysfunctions, acting on the protein kinase RNA-like endoplasmic reticulum kinase (PERK) branch of the unfolded protein response and enhancing mitochondrial dynamics. As a consequence, beta cells showed increased viability, mitochondrial function and insulin content [77].
For the first time ever, we have shown in an in vitro model system with human microvascular retinal endothelial cells stressed by HG treatment, that the association of C3G and Verb is more efficient than each single molecule in preserving the cells from death and the loss of barrier function, in line with the idea that a pleiotropic action obtained by the combination of different molecules can be more effective than the protection given by the single components [78].
In conclusion, we have shown here in an in vitro model system with human microvascular retinal endothelial cells that the association of two polyphenols (C3G and verbascoside), belonging to different families, may efficiently prevent the endothelial barrier dysfunction that usually follows diabetic hyperglycemic stress, and is the cause of diabetic retinopathy progression towards the most dangerous proliferative neovascular stage. These data confirm and further support from a biological point of view what had already been shown in an in vivo model system of early-stage diabetic retinopathy [45, 46].
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conceptualization—DR, GG, CDA, GL; investigation—GG, AL, AC, AA; data curation—GG, CDA and GL; writing - original draft preparation—DR, GG, CDA, GL; writing - review and editing—DR, GG.
Not applicable.
The Authors thank prof. Antony Bridgewood for critical English proofreading of the manuscript.
This research received no external funding.
DR and GL are serving as Editorial Board members of this journal. We declare that DR and GL had no involvement in the peer review of this article and had no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to GP. DR is a full-time employee of Fidia Pharmaceuticals, a pharmaceutical company that commercializes a food supplement containing C3G and verbascoside.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
