Academic Editor: Eun Sook Hwang
Background: Microplastics (MPs) and Nanoplastics (NPs) are plastic fragments that spread in the environment and accumulate in the human body, so they have been becoming a worldwide environmental concern because of their potential human health effects. The aim of this systematic review was to investigate the prospective impact of MPs and NPs on the inflammatory process. Methods: Electronic article search was performed on PubMed, Scopus and Web of Science international databases from 1 Jan 2012 to 31 Dec 2021. Screenings of titles, abstracts and full texts were performed according to the Preferred Reporting Item for Systematic Review and Meta-analyses (PRISMA). The methodological quality of the studies was checked by the Toxicological data Reliability Assessment Tool. Results: Electronic article search identified 125 records, from which 6 in vitro, 11 in vivo and 2 both in vivo and in vitro studies were included. Both in vivo and in vitro studies have showed an increase ofdifferent inflammatory outcomes (Interleukines, Tumor necrosis factor, Chemokines, Interferones, Transcription factors, Growth factors, Oxydoreductase, Proteins and others), thus it seems to confirm the association withthe exposure to microplastics of different types, sizes, exposure times and exposed species. Conclusions: This systematic review seems to support the relationship between the exposure to MPs and the inflammatory processboth in vivo and in vitro. Greater caution is needed about the role of NPs because ofa very small number of studies. Additional high-quality studies are warranted to confirm these results, especially the research should be focused on NPs being lacking literature.
Microplastics are the consequence of the high production of plastics and of its unmanaged release in the environment. Extreme solidity at room temperature, excellent electrical, thermal, and acoustic insulation, portability, ease of use thanks to the lightweight and cheapness are the main properties of plastic. These properties made it the most widespread and the most used synthetic material worldwide, causing a global pollution [1, 2, 3]. It was found in soil, sea, air, and also in the Arctic sea ice [4, 5].
Plastic is made up of polymers set such as polyethylene (PE), polypropylene (PP), polystyrene (PS), polyvinyl chloride (PVC), polyethylene terephthalate (PET), polycarbonate (PC), polymethyl methacrylate (PMMA), polyurethane (PU), etc. and several classes of additives (plasticizers) are added in formula for improving their performance [1, 6].
The plastic degradation, due to thermo-oxidative degradation processes and/or
mechanical fragmentation and/or biodegradation, produces smaller particles
classified according to the size as: nanoplastics
MPs and NPs size seems to favor their penetration into animal and vegetal tissues and cells besides accumulation in organs [11, 12, 13], causing alterations in physiological process [3]. Some studies highlighted the capable of MPs and NPs to cause toxicity, chronic inflammation and increase risk of neoplasm [7, 14, 15, 16, 17]. The in vivo studies show that both MPs and NPs are absorbed and accumulated in the tissues altering the correct functioning of organs and systems [18, 19, 20].
The human body is continuously exposed to microplastics through digestion, inhalation or dermal contact. Human’s ingestion is considered the simplest form of exposure to MPs and NPs [12, 13, 21]; which could cause an inflammatory response. In vitro test showed that NPs alter the cell membrane surface and trigger the inflammatory process [18]. Also, the exposures to MPs through inhalation and dermal contact were identified as triggers of lung and skin pro-inflammatory responses [17, 22, 23] which can lead to an increased risk of developing cancer [24].
The aim of the present study was to review the in vitro and in vivo studies that evaluated the impacts of exposure to MPs and NPs on inflammatory process.
We conducted this systematic review according to the protocol registered in PROSPERO (CRD348887). The protocol was not published in any peer-reviewed journal. The development of the protocol was guided by the Preferred Reporting Item for Systematic Review and Meta-analyses (PRISMA) Statement [25].
The literature search was performed through Pubmed, Scopus and Web of Science international databases. The keywords and search terms used were (“microplastics” OR “nanoplastics”), AND (“inflammation” OR “inflammatory response”) AND (“health” OR “wellness”).
Articles were initially screened based on the title and abstract according to the scope of this review, the articles that do not include original data on MPs/NPs and inflammatory process and based on the publication type (i.e., reviews, comments, opinion, letters, or abstract) were excluded. We included only studies published in English, from 2012 to 2021 and that report experimental data from in vivo and in vitro controlled exposure studies. Furthermore, a hand search of the reference lists of relevant studies was also performed to check papers that met our selection criteria but missed the keyword search criteria.
Two researchers (M.Fi and E.P.) performed data selection, extraction and quality assessment independently. Any disagreement between the two researchers was resolved through consensus session with a third researcher (M.Fe.).
In the screening phase, this systematic review was split into two main sections:
(a) The first referring exclusively to in vitro models and,
(b) The second referring exclusively to in vivo models,
both for the assessment of the inflammatory capacity of NPs and MPs through the evaluation of the inflammatory biomarkers.
We excluded: (i) studies whose method of inflammatory assessment was not clear, or incompletely described or that do not evaluate, or only evaluate the inflammatory of MPs/NPs qualitatively; (ii) studies that do not evaluate inflammatory through methods specific for MPs/NPs; (iii) studies that only report other health effects (e.g., carcinogenic effect); (iv) studies other than in vitro, e.g., in silico; and (v) studies not reporting statistical data.
The following descriptive and quantitative information was extracted from each of the eligible study for both sections, i.e., in vitro and in vivo studies: authors and year of publication, type and size of MPs/NPs, dose/exposure time, inflammatory biomarkers, animals (in vivo studies)/cell models (in vitro studies), assay(s) and outcomes. Information was summarized and organized in tables and for each table studies can be identified by their listed study details (First–Author name and year of publications). For in vitro and in vivo studies, we created 10 different tables summarizing for specific outcomes (Interleukine, Tumor necrosis factors, Chemochine, Interferon, Transcriptor factors, Growth factors, Oxidoreductase, Proteins and Others).
The methodological quality of the studies has been checked using the Toxicological data Reliability Assessment Tool (ToxR Tool) guidelines for reporting randomized clinical trials for in vitro and in vivo studies [26]. In particular, two researchers (E.P., M.P.) performed data selection, extraction and quality assessment independently. Any disagreement between the two researchers was resolved by consensus session with a third researcher (M.F.).
The initial search retrieved a total of 125 studies, from which 55 were excluded because of duplicate records. A total of 70 studies were screened based on the title and abstract, from which 28 were excluded, resulting in 42 full-text studies that were considered potentially eligible for inclusion. A total of 23 studies were excluded because of carried out using a mixture of contaminants including MPs and lack of statistical data analysis. Finally, 19 studies (6 in vitro, 11 in vivo and 2 both in vivo and in vitro) were included in this review [27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45].
The full process of article collection, screening, and eligibility assessment is presented in Fig. 1.
The general/methodological information of the included in vitro and/or in vivo studies are described in Table 1 (Ref. [27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45]). In particular, 11 studies (5 in vitro studies [29, 33, 37, 38, 42], 4 in vivo [27, 30, 32, 36] and 2 both [34, 39]) investigated exposure to PS , 3 studies investigated PP exposure (2 in vitro [37, 42, 41] and 1 in vivo [41]), 3 studies investigated PE exposure (1 in vitro [42] and 2 in vivo [28, 31]), 2 studies investigated exposure to MPs (2 in vivo studies [44, 45]) instead of PVC exposure (1 study in seen [3]), polymers (1 in vitro study [43]), LDPE (1 in vivo study [35]), HDPE (1 in vivo study [40]), SFb and LFb (1 in vivo study [41]), sphere (1 in vivo study [45]) and NPs (1 in vivo study [44]) was evaluated in a species-specific study.
| Author, year | Particles Type | Size | Dose/exposure time | Biomarkers | Cell/Animal models | Assay (s) |
| MPs (5 mm–0.1 | ||||||
| NPs ( | ||||||
| In vitro studies | ||||||
| Dong et al., 2020 [29] | PS | 1.72 |
1–1000 |
IL-6, IL-8, HO-1, ROS, AAT | Normal human lung epithelial BEAS-2B cells maintained in LHC-9 medium and incubated at 37 °C in a humidified atmosphere containing 5% of CO |
Cytotoxicity assay, ELISA assay, DCFH-DA assays, Western blot assay, TEER |
| Visalli et al., 2021 [33] | PS | 3 and 10 |
100–1600 particles/mL; 0.5, 1, 2, 3, 4, 5, 6, and 24 h; 7, 14, 21, 28 and 48 days | ROS | Human intestinal cell line HT-29 grown in RPMI 1600 medium with 2 mM L-glutamine, 1 mM sodium pyruvate, 10% (v/v) fetal bovine serum, 100 IU/mL penicillin and 100 |
Viability Assays, Comet Assay, MTT assay |
| Jeon et al., 2021 [37] | PP and PS | 100 |
200 mg/mL; 0.5, 1, 2, 3, 4, 5, 6, and 24 h | In THP-1 cells: IL-6, TNF-a, IL-1 |
Caco-2 cells, HepG2 cells, THP-1 cells | Lactatedehydrogenase (LDH) assay kit, DCFH-DA assay, DCFDA/H2DCFDA-Cellular ROS Assay, Bicinchoninic acid assay |
| Busch et al., 2021 [38] | PVC, PS-NH2, PS | PS: From 59 |
0, 1, 5, 10, 50 |
IL-1 |
Caco-2 cells, HT29-MTX-E12 cells, THP-1 cells. Caco-2 cells were cultured in MEM, the second line in DMEM and the third in RPMI | Lactate dehydrogenase (LDH) assay, Alkaline comet assay, WST-1 assay, ELISA, TEER |
| Hwang et al., 2020 [42] | PS, PP, PE | 0, 46 |
500 |
IL-2, IL-6, IL-10, TNF- |
Human dermal fibroblasts (HDFs), Human peripheral blood mononuclear cells (PBMCs), Human Must cells line (HMC-1) | Hemolysis assay, Histamine assay, ELISA |
| Lehner et al., 2020 [43] | Polymers such as tire wear and polyolefins | 50–500 |
823.5–1380.0 |
IL-8, TNF |
Caco-2 cells, HT29-MTX-E12 cells, MDM cells, MDDC cells. The first one and second one was cultivated at 37 °C under a 5% CO |
Cell viability assay (LDH), ELISA |
| In vivo studies | ||||||
| Hou et al., 2021 [27] | PS | 5 |
100, 1000 10000 |
Nrf2/HO-1, NF-κB, TNF- |
Four to 5 weeks old ICR male mice placed in a pathogen-free animal room (T 22 |
Western blot analysis, qPCR |
| Li et al., 2020 [28] | PE | 10–150 |
2, 20, 200 |
TLR4, AP-1, IRF5, IL-1 |
Male and 5-week-old SPF grade mice C57BL/6. Intestinal tissue was analyzed | Chao1 diversity index, Shannon index and UniFranc beta diversity metrics, Wilcoxon test, Mouse cytokine/Chemokine magnetic bead panel 96-well plate assay |
| Zheng et al., 2021 [30] | PS | 5 |
500 |
IL-1 |
Six weeks old male C57 mice maintained at a T 24 |
ELISA, FITC-dextran intestinal permeability assay |
| Sun et al., 2021 [31] | PE | 1–10 |
0.002, 0.2 |
IL-8, IL-6, IL-10, IL-1 |
Eight weeks old female ICR mice kept in a cage with a cycle of 12/12 light and dark hours. Mice’ colon tissue and feces were analyzed | Biochemical assay |
| Chen et al., 2020 [32] | PS | 0.05, 0.50, 6.00 |
0.1, 1 × 10 |
CYP1A1, BMP4, GATA4, NKx2.5, FGF8, JAK, IL6, CCL11, SOD, NF-κB | Four months old Oryziasmelastigma maintained in aerated 30‰ artificial seawater at 6.0 |
RT - qPCR assay |
| Capó et al., 2021 [35] | LDPE | 100–500 |
250–690 |
CAT, SOD, GRd, GST, MPO, MDA | Seven months old Sparusaurata (length 11.8 |
Biochemical assay |
| Zhang et al., 2021 [36] | PS | 2, 10 and 200 |
10, 000 |
IL-1 |
Eight months old Oryziasmela stigma placed in an artificial seawater (salinity 30‰) under a 14 h/10 h light and dark cycle. T was 28 |
Gut microbiota assay, LDA Effect Size (LEfSe) and MetaStat assay, Mrna expression with 18s Rrna |
| von Moos et al., 2012 [40] | HDPE | 0−80 |
2.5 g HDPE-fluff/L, 96 h | ROS | Mytilusedulis L. placed in an artificial seawater (31‰ salinity) at 15 °C. HDPE particles were taken up into the stomach and transported into digestive gland where they accumulated in the lysosomal system | Lysosomal Membrane Stability (LMS) assay |
| Zhao et al., 2021 [41] | PP, SFb L-H, LFb L-H | 20 |
20, 000 |
ROS, SOD, IL-1 |
18 weeks old Daniorerio placed in a culture water at 28 °C, 14/10 light and dark cycle, Ph 7.4 |
Enzymatic assay, Imaging assay, ELISA |
| Xie et al, 2021 [44] | MPs and NPs | 8 |
10 |
IL-6, IL-8, IL-10, IL-1 |
Daniorerio placed in a culture water at 28 °C, 14/10 light and dark cycle. The zebrafish’ intestinal microbial community and the intestinal tissue were analyzed | RT- qPCRs assay |
| Lu et al., 2021 [45] | Microplastics and sphere | 1–5 |
300 |
TNF- |
6–8 weeks old female BALB/c mice with a 12/12 light and dark cycle. The respiratory system was analyzed, in particular lungs in asthmatic and not asthmatic mice | ELISA |
| In vitro and in vivo studies | ||||||
| Wang et al., 2021 [34] | PS | 2 |
25, 50, 100, 200, 400, 800 |
ROS, Bad, Bcl2, LC3, MAPK, p38, ERK1/2, JNK, AKT-mTOR, IRE1 |
HK-2 cells, C57BL/6 mice. HK-2 cells were incubated at 37 °C with 5% CO |
Sulforhodamine B (SRB) assay, ELISA, Western Blot assay, Immunostaining Assay |
| Jin et al., 2021 [39] | PS | 0.5 |
100 |
TNF- |
6 weeks old BALB/C Mice, GC-1 cells, Leydig cells, Sertoli cells. Testicular tissue was analyzed | qRT-PCR assay, ELISA, Western blotting |
| Notes: r, Pearson correlation coefficient; IL-6, Interleukin 6; IL-8,
Interleukin 8; HO-1, Heme oxygenase-1; ROS, Reactive oxygen species; AAT, alpha-1
antitrypsin; ZO-1, Zonula occludens-1; TEER, trans-epithelial electrical
resistance; TNF- | ||||||
Changes in levels of all investigated outcomes (Interleukines, Tumor necrosis factor, Chemokines, Interferons, Transcription factors, Growth factors, Oxydoreductase, Proteins and others) have been summarised in Tables 2–10.
Below we have summarized the main results for each individual study included in the review by outcomes specifying different type of plastic exposure and study design.
Table 2 (Ref. [27, 28, 29, 30, 31, 32, 36, 37, 38, 39, 41, 42, 43, 44, 45]) shows an increase in interleukins 6, 8 and
1
| Author, year | Particles type | Outcomes | ||||||||
| IL-2 | IL-4 | IL-5 | IL-6 | IL-8 | IL-9 | IL-10 | IL-1 |
IL-1 | ||
| In vitro studies | ||||||||||
| Dong et al., 2020 [29] | PS | |||||||||
| Jeon et al., 2021 [37] | PP | |||||||||
| PS | ||||||||||
| Busch et al., 2021 [38] | PVC | |||||||||
| PS-NH2 | ||||||||||
| PS | ||||||||||
| Hwang et al., 2020 [42] | PS | |||||||||
| PP | ||||||||||
| PE | ||||||||||
| Lehner et al., 2020 [43] | Polymers | |||||||||
| In vivo studies | ||||||||||
| Hou et al., 2021 [27] | PS | |||||||||
| Li et al., 2020 [28] | PE | |||||||||
| Zheng et al., 2021 [30] | PS | |||||||||
| Sun et al., 2021 [31] | PE | |||||||||
| Chen et al., 2020 [32] | PS | |||||||||
| Zhang et al., 2021 [36] | PS | |||||||||
| Zhao et al., 2021 [41] | PP | |||||||||
| SFb | ||||||||||
| LFb | ||||||||||
| Xie et al, 2021 [44] | MPs | |||||||||
| NPs | ||||||||||
| Lu et al., 2021 [45] | MPs | |||||||||
| In vitro and in vivo study | ||||||||||
| Jin et al., 2021 [39] | PS | |||||||||
| TOTAL | 1 |
1 |
1 |
9 |
5 |
1 |
2 |
1 |
11 | |
| 3 |
18 N.A. | 1 |
7 |
1 |
18 N.A. | 5 |
18 N.A. | 4 | ||
| 17 N.A. | 17 N.A. | 9 N.A. | 13 N.A. | 15 N.A. | 10 N.A. | |||||
| We escluded Visalli et al., 2021 [33], Capó et al., 2021
[35], von Moos et al., 2012 [40], Wang et al., 2021 [34], from
Table 2 due to none of these outcomes studied.
Notes: IL-2, Interleukin 2; IL-4, Interleukin 4; IL-5, Interleukin 5; IL-6, Interleukin 6; IL-8, Interleukin 8; IL-9, Interleukin 9; IL-10, Interleukin 10; IL-1 * p a, only for HepG2 cells statistical significance; b, only for THP-1 cells statistical significance; c, only for 2 and 200 um; d, only for 1000 | ||||||||||
Table 3 (Ref. [27, 30, 36, 37, 38, 39, 42, 43, 44, 45]) shows the trend of TNF-a, the only member
of the TNF category that have been taken into consideration in the studies. In
particular, exposure to PP (evaluated in two in vitro studies [37, 42])
did not show significant differences. Whereas 7 studies (three in vitro
studies [37, 38, 42], three in vivo studies [27, 30, 36], one both [39])
assessed PS exposure, noting an increase in TNF-
| Author, year | Particles type | Outcome (TNF- |
| In vitro studies | ||
| Jeon et al., 2021 [37] | PP | |
| PS | ||
| Busch et al., 2021 [38] | PVC | |
| PS-NH2 | ||
| PS | ||
| Hwang et al., 2020 [42] | PS | |
| PP | ||
| PE | ||
| Lehner et al., 2020 [43] | polymers | |
| In vivo studies | ||
| Hou et al., 2021 [27] | PS | |
| Zheng et al., 2021 [30] | PS | |
| Zhang et al., 2021 [36] | PS | |
| Xie et al., 2021 [44] | MPs | |
| NPs | ||
| Lu et al., 2021 [45] | MPs | |
| In vitro and in vivo study | ||
| Jin et al., 2021 [39] | PS | |
| TOTAL | 7 | |
| 9 | ||
| 9 N.A. | ||
| We escluded Dong et al., 2020 [29], Visalli et al., 2021 [33],
Li et al., 2020 [28], Sun et al., 2021 [31], Chen et al.,
2020 [32], Capó et al., 2021 [35], von Moos et al., 2012
[40], Zhao et al., 2021 [41], Wang et al., 2021 [34], from Table
3 due to none of these outcomes studied.
Notes: TNF- * p a, only at high dose; b, only for 1000 | ||
Table 4 (Ref. [28, 32, 37, 39]) shows the trend of different chemokines
following exposure to PP (one study in vitro [37]), PS (three studies
respectively one in vitro [37], one study in vivo [32] and one
both [39] and PE (one study in vivo [28]). Only one study [37] reported
an increase in MIP-1
| Author, year | Particles type | Outcomes | ||||
| MIP-1 |
IP-10 | RANTES | CCL11 | CXCL10 | ||
| In vitro study | ||||||
| Jeon et al., 2021 [37] | PP | |||||
| PS | ||||||
| In vivo studies | ||||||
| Li et al., 2020 [28] | PE | |||||
| Chen et al., 2020 [32] | PS | |||||
| In vitro and in vivo study | ||||||
| Jin et al., 2021 [39] | PS | |||||
| TOTAL | 1 |
1 |
1 |
1 |
1 | |
| 18 N.A. | 18 N.A. | 18 N.A. | 18 N.A. | 18 N.A. | ||
| We escluded Dong et al., 2020 [29], Visalli et al., 2021 [33],
Busch et al., 2021 [38], Hwang et al., 2020 [42], Lehner
et al., 2020 [43], Hou et al., 2021 [27], Zheng et al.,
2021 [30], Sun et al., 2021 [31], Capó et al., 2021 [35],
Zhang et al., 2021 [36], von Moos et al., 2012 [40], Zhao
et al., 2021 [41], Xie et al., 2021 [44], Lu et al., 2021
[45], Wang et al., 2021 [34] from Table 4 due to none of these outcomes
studied.
Notes: MIP-1 * p N.A., Not applicable. | ||||||
In Table 5 (Ref. [30, 44, 45]) we have reported the trend of INF levels following exposure to MPs (two in vivo studies [44, 45]), NPs (one in vivo study [44]) and PS (one in vivo study [30]). Both exposure to MPs and NPs did not change the trend of IFN-y [45] and INFPHI1 [44] levels. Finally, only exposure to PS was associated with an increase in IFN-y [30] (Table 5).
| Author, year | Particles type | Outcomes | |
| IFN- |
IFNPHI1 | ||
| In vivo studies | |||
| Zheng et al., 2021 [30] | PS | ||
| Xie et al., 2021 [44] | MPs | ||
| NPs | |||
| Lu et al., 2021 [45] | MPs | ||
| TOTAL | 1 |
1 | |
| 1 |
18 N.A. | ||
| 17 N.A. | |||
| We escluded Dong et al., 2020 [29], Visalli et al., 2021 [33],
Jeon et al., 2021 [37], Busch et al., 2021 [38], Hwang et
al., 2020 [42], Lehner et al., 2020 [43], Hou et al., 2021 [27],
Li et al., 2020 [28], Sun et al., 2021 [31], Chen et al.,
2020 [32], Capó et al., 2021 [35], Zhang et al., 2021 [36],
von Moos et al., 2012 [40], Zhao et al., 2021 [41], Wang
et al., 2021 [34], Jin et al., 2021 [39], fromtable 5 due to none
of these outcomes studied.
Notes: IFN- * p N.A., Not applicable. | |||
Table 6 (Ref. [27, 28, 29, 30, 31, 32, 34]) summarizes the different transcription factors
investigated in the studies included in the review that considered PS exposure
(one in vitro study [29], three in vivo studies [27, 30, 32]
and one both [34]) and PE (two in vivo studies [28, 31]). Only ERK1 [31, 34], TLR 4 [28, 31], NFKb [31, 32] and Nrf2-HO1 [27, 29] were investigated in two
studies whereas for all other transcription factors we found one study only
(Table 6). In particular, exposure to PS showed an increase in IRE1a, MAPK, ERK
1-2, p-INK [34], JAK [32], and PPAR-
| Author, year | Particles type | Outcomes | ||||||||||||||
| IRE1 |
ATF6 | JAK | MAPK | ERK2 | TLR4 | AP-1 | IRF-5 | NFKB | PPAR |
ERK1 | GATA4 | p-JNK | AKT-MTOR | Nrf2-HO1 | ||
| In vitro study | ||||||||||||||||
| Dong et al., 2020 [29] | PS | |||||||||||||||
| In vivo studies | ||||||||||||||||
| Hou et al., 2021 [27] | PS | |||||||||||||||
| Li et al., 2020 [28] | PE | |||||||||||||||
| Zheng et al., 2021 [30] | PS | |||||||||||||||
| Sun et al., 2021 [31] | PE | |||||||||||||||
| Chen et al., 2020 [32] | PS | |||||||||||||||
| In vitro and in vivo study | ||||||||||||||||
| Wang et al., 2021 [34] | PS | |||||||||||||||
| TOTAL | 1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 | |
| 18 N.A. | 18 N.A. | 18 N.A. | 18 N.A. | 18 N.A. | 1 |
18 N.A. | 18 N.A. | 1 |
18 N.A. | 1 |
18 N.A. | 18 N.A. | 18 N.A. | 1 | ||
| 17 N.A. | 17 N.A. | 17 N.A. | 17 N.A. | |||||||||||||
| We escluded Visalli et al., 2021 [33], Jeon et al., 2021 [37],
Busch et al., 2021 [38], Hwang et al., 2020 [42], Lehner
et al., 2020 [43], Capó et al., 2021 [35], Zhang et
al., 2021 [36], von Moos et al., 2012 [40], Zhao et al., 2021
[41], Xie et al., 2021 [44], Lu et al., 2021 [45], Jin et
al., 2021 [39], from table 6 due to none of these outcomes studied.
Notes: IRE1 * p N.A., Not applicable. | ||||||||||||||||
Table 7 (Ref. [28, 32]) shows the trend of growth factors after exposure to PE (one study in vivo [28]) and PS (one study in vivo [32]). There was an increase in GCSF [28] after exposure to PE whereas exposure to PS did not modify the trend of FGF8 [32] (Table 7).
| Author, year | Particles type | Outcomes | |
| GCSF | FGF8 | ||
| In vivo studies | |||
| Li et al., 2020 [27] | PE | ||
| Chen et al., 2020 [31] | PS | ||
| TOTAL | 1 |
1 | |
| 18 N.A. | 18 N.A. | ||
| We escluded Dong et al., 2020 [29], Visalli et al., 2021 [33],
Jeon et al., 2021 [37], Busch et al., 2021 [38], Hwang et
al., 2020 [42], Lehner et al., 2020 [43], Hou et al., 2021 [27],
Zheng et al., 2021 [30], Sun et al., 2021 [31], Capó
et al., 2021 [35], Zhang et al., 2021 [36], von Moos et
al., 2012 [40], Zhao et al., 2021 [41], Xie et al., 2021 [44], Lu
et al., 2021 [45], Wang et al., 2021 [34], Jin et al.,
2021 [39] from table 7 due to none of these outcomes studied.
Notes: GCSF, Granulocyte - Colony Stimulating Factor; FGF8, Fibroblast growth factor 8; PE, polyethylene; PS, Polystyrene. ** p N.A., Notapplicable. | |||
Table 8 (Ref. [32, 35, 36, 41]) shows the enzymes with antioxidant action investigated by the studies included in the review. In general, exposure to PS (two in vivo studies [32, 36], PP (one in vivo study [41], LDPE (one in vivo study [35] and SFB, LFB (one in vivo study [41] showed an increase in all the enzymes investigated. In particular, after exposure to PS the values of CAT [36], GPx [36] were increased, whereas the trend of SOD (two studies [32, 36]) was detected in increase in only one [36] of the two studies. Finally, exposure to LDPE [35] reported an increase in CAT, GRd and GST and no change in values for GPx and SOD, whereas the study by Zhao which investigated the exposure to PP, SFb and LFb showed an increase in SOD only following exposure to LFB [41] (Table 8).
| Author, year | Particles type | Outcomes | ||||
| SOD | CAT | GRD | GST | GPX | ||
| In vivo studies | ||||||
| Chen et al., 2020 [32] | PS | |||||
| Capóet al., 2021 [35] | LDPE | |||||
| Zhang et al., 2021 [36] | PS | |||||
| Zhao et al., 2021 [41] | PP | |||||
| SFb | ||||||
| LFb | ||||||
| TOTAL | 2 |
2 |
1 |
1 |
1 | |
| 2 |
17 N.A. | 18 N.A. | 18 N.A. | 1 | ||
| 15 N.A. | 17 N.A. | |||||
| We escluded Dong et al., 2020 [29], Visalli et al., 2021 [33],
Jeon et al., 2021 [37], Busch et al., 2021 [38], Hwang et
al., 2020 [42], Lehner et al., 2020 [43], Hou et al., 2021 [27],
Li et al., 2020 [28], Zheng et al., 2021 [30], Sun et al.,
2021 [31], von Moos et al., 2012 [40], Xie et al., 2021 [44], Lu
et al., 2021 [45], Wang et al., 2021 [34], Jin et al.,
2021 [39], from table 8 due to none of these outcomes studied.
Notes: SOD, Superoxide dismutase; CAT, catalase; GRd, Glutathione reductase; GST, Glutathione-s-transferase; GPx, Glutathione peroxidase; PS, Polystyrene; LDPE, Low density Polyethylene; PP, Polyethylene; SFb, Shortmicroplastic fibers; LFb, Long microplastic fibers. * p N.A., Not applicable. | ||||||
Table 9 (Ref. [29, 31, 32, 34, 35, 36, 39, 41]) shows the proteins and enzymes investigated following exposure to PS (one in vitro study [29], two in vivo studies [32, 36], two both [34, 39]), PE (one study in vivo [31]), LDPE (one in vivo study [35]), PP, SFB and LFB (one in vivo study [41]). As for the exposure to PS, an increase in BMP [32], COX1-2 [36], D-lac, Bad, LC3, p38, cPLA2 [35], MCP-1 [39] was highlighted, conversely no differences were found in CIP1A1, NRX2.5 [32] and p62 [34]. In addition, a decrease in AAT [29] and ZO-1 [29, 39] levels was noted. Exposure to PE did not change the performance of GPR41, GPR43 and MyD88 [31]. Finally, an increase in MPO [35] was highlighted for exposure to LDPE, whereas following exposure to PP, SFb and LFb there was a decrease in D-Lac [41] (Table 9).
| Author, year | Particles type | Outcomes | ||||||||||||||
| AAT | GPR41 | MyD88 | CYP1A1 | BMP | NKX2.5 | MPO | COX1 | D-lac | Bad | LC3 | p38 | cPLA2 | MCP-1 | ZO-1 | ||
| GPR43 | COX2 | p62 | ||||||||||||||
| In vitro study | ||||||||||||||||
| Dong et al., 2020 [29] | PS | |||||||||||||||
| In vivo studies | ||||||||||||||||
| Sun et al., 2021 [31] | PE | |||||||||||||||
| Chen et al., 2020 [32] | PS | |||||||||||||||
| Capóet al., 2021 [35] | LDPE | |||||||||||||||
| Zhang et al., 2021 [36] | PS | |||||||||||||||
| Zhao et al., 2021 [41] | PP | |||||||||||||||
| SFb | ||||||||||||||||
| LFb | ||||||||||||||||
| In vivo and in vitro studies | ||||||||||||||||
| Wang et al., 2021 [34] | PS | |||||||||||||||
| Jinet al., 2021[39] | PS | |||||||||||||||
| TOTAL | 1 |
2 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
2 | |
| 18 N.A. | 36 N.A. | 18 N.A. | 18 N.A. | 18 N.A. | 18 N.A. | 18 N.A. | 18 N.A. | 1 |
18 N.A. | 18 N.A. | 1 |
18 N.A. | 18 N.A. | 17 N.A. | ||
| 17 N.A. | 36 N.A. | |||||||||||||||
| We escluded Visalli et al., 2021 [33], Jeon et al., 2021 [37],
Busch et al., 2021 [38], Hwang et al., 2020 [42], Lehner
et al., 2020 [43], Hou et al., 2021 [27], Li et al., 2020
[28], Zheng et al., 2021 [30], von Moos et al., 2012 [40], Xie
et al., 2021 [44], Lu et al., 2021 [45], from table 9 due to none
of these outcomes studied.
Notes: AAT, alpha-1 antitrypsin; ZO-1, Zonula occludens-1; GPR 41, mammalian G protein receptors 41; GPR 43, mammalian G protein receptors 43; MyD88, Myeloid differentiation primary response 88; CYP1A1, Cytochrome P450 Family 1 Subfamily A Member 1; BMP4, Bone morphogenetic protein 4; NKX2.5, Homeobox protein Nkx-2.5; MPO, Myeloperoxidase; COX2, Cyclooxygenase-2; COX1, cyclooxygenase-1; D-Lac, D-lactate; Bad, BCL2 Associated Agonist Of Cell Death; LC3, Microtubule-associated proteins 1A/1B light chain 3B; p-38, mitogen-activated protein kinases 38; cPLA2, Cytosolic phospholipase A2; p62, ubiquitin-binding protein 62; MCP-1, Monocyte chemoattractan protein-1; PS, Polystyrene; PE, Polyethylene; LDPE, Low-density Polyethylene; SFb, Short microplastic fibers; LFb, Long microplastic fibers. * p N.A., Not applicable. a, only for COX2. | ||||||||||||||||
In Table 10 (Ref. [29, 30, 33, 34, 35, 37, 40, 41, 45]) we have reported the
trend of the remaining outcomes, which we named “others” (ROS, TG, MDA, IgG1,
IgE, P-EIF2
| Author, year | Particles type | Outcomes | |||||
| ROS | TG | MDA | IgG1 | IgE | P-EIF2 | ||
| In vitro studies | |||||||
| Dong et al., 2020 [29] | PS | ||||||
| Visalliet al., 2021 [33] | PS | ||||||
| Jeon et al., 2021 [37] | PP | ||||||
| PS | |||||||
| In vivo studies | |||||||
| Zheng et al., 2021 [30] | PS | ||||||
| Capóet al., 2021 [35] | LDPE | ||||||
| von Moos et al., 2012 [40] | HDPE | ||||||
| Zhao et al., 2021 [41] | PP | ||||||
| SFb | |||||||
| LFb | |||||||
| Lu et al., 2021 [45] | MPs | ||||||
| In vivo and in vitro study | |||||||
| Wang et al., 2021 [34] | PS | ||||||
| TOTAL | 8 |
1 |
1 |
1 |
1 |
1 | |
| 11 N.A | 18 N.A | 1 |
18 N.A | 18 N.A. | 18 N.A. | ||
| 17 N.A | |||||||
| We escluded Busch et al., 2021 [38] , Hwang et al., 2020 [42],
Lehner et al., 2020 [43], Hou et al., 2021 [27], Li et
al., 2020 [28], Sun et al., 2021 [31], Chen et al., 2020 [32],
Zhang et al., 2021 [36], Xie et al., 2021 [44], Jin et
al., 2021 [39] from table 10 due to none of these outcomes studied.
Notes: ROS, Reactive oxygen species; TG, liver triglyceride; MDA, Malondialdehyde; IgG1, Immunoglobulin G1; IgE, Immunoglobulin E; p-EIF2 * p N.A., Not applicable. a, only for 1000 ug/cm | |||||||
The results of the quality assessment of the studies are reported in Figs. 2,3 and Supplementary Tables 1, 2. In particular, only two in vitro studies didn’t report information on the source/origin of the test system [29, 39]. Only one author did not report the necessary information on test system properties, and on conditions of cultivation and maintenance. Only one study reported the number of replicates [34].
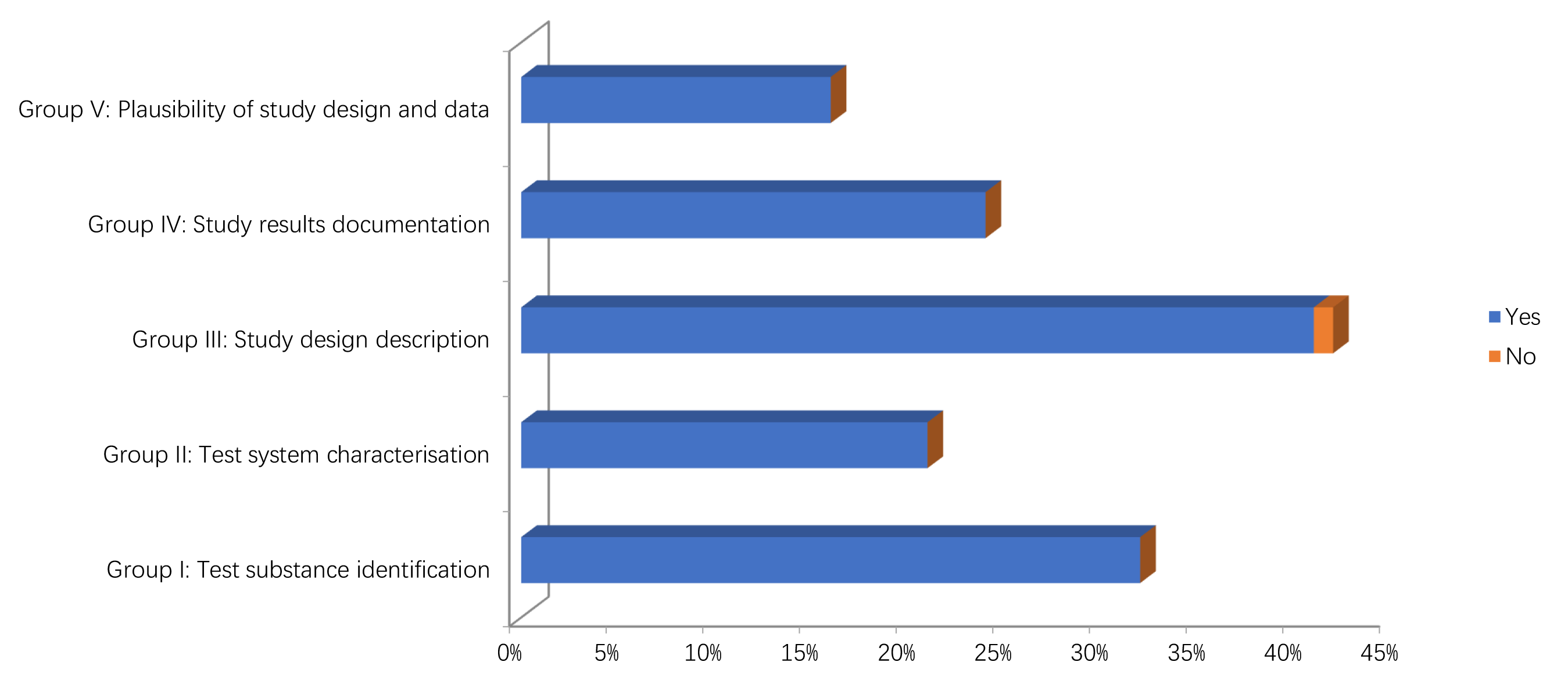 Fig. 2.
Fig. 2.Methodological quality assessment of in vitro studies.
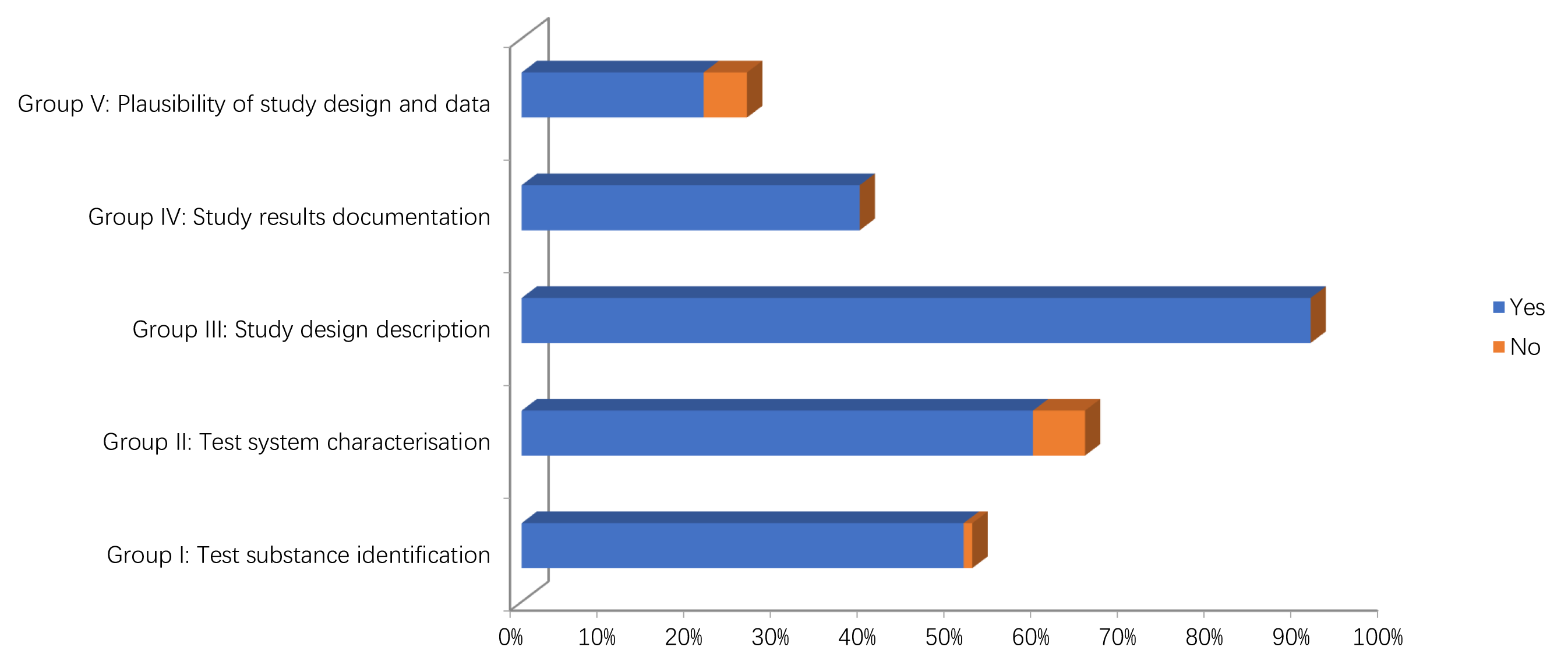 Fig. 3.
Fig. 3.Methodological quality assessment of in vivo studies.
Concerning in the vivo studies, only one study didn’t report information on the source/origin of the test system [41]. Four studies did not give the sex of the test organism [32, 35, 40, 44]. One study did not give age or body weight of the test organisms at the start of the study [40]. One study did not give information on the housing or feeding conditions in case of repeated dose toxicity studies [39].
The in vitro [29, 37, 38, 42, 43]and in vivo [27, 28, 36]studies
included in this review seem to confirm an association between the increase in
pro-inflammatory interleukins Il-6, IL-8 and IL-1b and the exposure to
microplastics of different types, sizes, exposure times and exposed species,
whereas the interpretation of the results, relating to the other interleukins
investigated, requires more caution because there are only few heterogeneous
studies(42 in vitro, 28 and 45 in vivo) in literature to date.
In particular, it is already known that these pro-inflammatory interleukins take
part in the acute inflammatory response [46], whereas IL-6 acts as an
anti-inflammatory myokine too [47]. Furthermore, the results of the studies seem
to confirm that the persistence of acute inflammation can become chronic up to
result in a further systemic inflammatory action, inducing COPD (Chronic
Obstructive Pulmonary Disease), asthma [48] and Inflammatory Bowel Diseases (IBD)
[31, 38]. This process could be caused by the reduction of TEER and by the
expression of the ZO-1 protein, this leads to a loss of epithelial integrity of
the barrier cells [29, 39, 49]. This reduction in the integrity of the barrier
cells has been confirmed for microplastics of 3
The proinflammatory action of TNF-
Levels related to the chemokine MIP1
A few in vivo studies quantifying the levels of IFNPHI-1,
IFN-
The results concerning transcription factors, investigated both through
in vitro [29, 34] and in vivo studies [28, 30, 32, 34] do not
allow us to reach a conclusion because, like other studies already mentioned,
they are very heterogeneous. In particular, the results of NF
Finally, as regards the other outcomes investigated, not classifiable in the aforementioned categories, a potential association emerged between exposure to microplastics of different type, size and exposure time and ROS both in vitro [29, 33, 37] and in vivo studies [30, 35, 40, 41, 45]. Although ROS are known to cause chronic oxidative stress including inflammation, alteration of permeability and histopathological damage [56, 57, 58], it would underline that their formation may also depend on the surface of microplastics. This is supported by the fact that the experiment carried out with NAC-coated (N-acetylcysteine) nanoparticles reduce toxicity and oxidants, subsequently reducing the toxic effect on THP-1 macrophages [37].
The studies included in the review have various limitations regarding, for example, the use of different animal and cell models, size and type of particles investigated, doses and exposure time or conditions, quantified outcomes and tests used for their quantification. Moreover, it should be noted that most of the authors summarize the results through graphs in which is difficult to obtain numerical data comparable to each other and to estimate the quality. Furthermore, none of the in vivo studies included in the review exposed male and female mice to microplastics at the same time, this may be a limitation as the influence of sex has already been demonstrated for pollutants exposure as for example to metals [59, 60]. Another limitation of the studies concerns the lack of studies on NPs, in fact, only 2 studies included in this review investigated NPs. Moreover, only one study took into consideration the limits related to methodology [43]. In particular, it has been discussed the difficulties of the particles to translocate across the epithelium to the basolateral side in the membrane of 12-well insert, whereas only one study focused on methods to detect MPs in tissues are not appropriate [39]. Surprisingly six authors showed no limitation in their studies [30, 32, 34, 37, 41, 44].
Finally, we have included only English language articles in this review, and it was not possible to compare the results with those of other reviews as to the authors’ knowledge there are no reviews like this one in the literature to date. Although the limitations, the results of this review may be useful for the organization of future studies. In particular, they provide information on potential outcomes that could help confirm the hypothesis of an association between exposure to various types of microplastics and the inflammatory process.
In conclusion, this review seems to support the association between the MPs exposure and the inflammation response both in vivo and in vitro. Conversely greater caution is needed regarding the role of NPs due to the very small number of studies in literature. Additional high-quality studies are warranted to confirm these results, especially the research should be focused on NPs being lacking literature.
EP and GOC performed the research and writing original draft preparation. MFe—Supervision. NB, CF, AC and EA—datacuration. MP and MFi—writing - review and editing. MFi—project administration. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
Not applicable.
Not applicable.
Funding for this study was provided by the Interdepartmental Research Plan (PIAno di inCEntivi per la Ricerca di Ateneo) 2020/2022 of Department of Medical, Surgical and Advanced Technologies, University of Catania, grant number: 6C722202112.
The authors declare no conflict of interest. GOC is serving as one of the Editorial Board members of this journal. We declare that GOC had no involvement in the peer review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to ESH.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.

