† These authors contributed equally.
Academic Editor: Erika Di Zazzo
Introduction: Solitary plasmacytoma (SP) is a rare plasma cell disorder characterized by localized neoplastic proliferation of monoclonal plasma cell. Due to its rarity, further understanding of the spectrum of its clinicopathologic features is needed. Methods: A retrospective analysis of cases from a single institution was conducted. Clinical characteristics of the patients were collected; histopathological and semi-quantitative immunohistochemical analyses were performed. Results: Thirteen cases were identified from our pathology archives, including 4 cases of solitary plasmacytoma of bone (SPB) (30.8%) and 9 extraosseous plasmacytoma (EP) (69.2%). The mean age of EP is a decade older than SPB. There is no gender disparity. The most common sites involved are the vertebrae and nasopharynx. Histologically, the tumors can be classified into two grades based on degree of differentiation. Immunohistochemically the tumor cells express CD38, CD138, MUM-1, and exhibit light chain restriction. Ki-67 proliferation index is 30%. In situ hybridization for Epstein-Barr virus-encoded small RNAs (EBER) is negative in six cases tested. Semi-quantitative immunohistochemical analysis showed decreased integrated optical density (IOD) of CD38 in neoplastic cells. IgH gene rearrangement was identified in two cases. Conclusion: SP is a rare plasmacytoid neoplasm that occurs more frequently in older patients. Diagnosis requires a systematic clinical approach combined with the pathological characteristics of plasmacytoid morphology, immunophenotype and light chain restriction. There are more cases of EP than SPB in our series, which is in contrast to that reported in literature. Results from this study suggest that CD38 is a potential immunohistochemical marker associated with prognosis of SP. Further studies with more cases and longer term follow-up may provide more definitive information on risk of progression from SP to multiple myeloma (MM).
Plasma cell dyscrasia encompasses a spectrum of monoclonally proliferative disorders of plasma cells. At one end of the spectrum lies monoclonal gammopathy of undetermined significance (MGUS) that is indolent, whereas at the other, multiple myeloma (MM), which is a malignancy with aggressive biologic behavior [1]. Within this spectrum, solitary plasmacytomas (SP) occur at a single location with no bone marrow involvement. SP accounts for 1–5% of all plasma cell neoplasms. The patients lack the CRAB features (increased calcium, renal insufficiency, anemia, or multiple bone lesions) and bone marrow involvement [2, 3, 4]. However, SP has a propensity to eventually progress to MM in some patients [5]. SP can be classified into solitary plasmacytoma of bone (SPB) and extraosseous plasmacytoma (EP) depending on the locations. SPB can involve any bone, but more frequently involves bone with hematopoietic elements such as pelvis, skull, ribs, spine, and femur. Approximately 80% of EP arises in the submucosa in the head and neck region. Less common sites such as the gastrointestinal tract, respiratory tract, bladder, thyroid, kidney, pancreas, uterus and the central nervous system have also been reported [6, 7, 8].
Morphologically SP is characterized by tumor cells with round and eccentrically located nuclei, with dense chromatin cluster surrounded by nuclear membrane, similar to mature plasma cells. Plasmablasts with vesicular nuclei and a prominent nucleolus, or bizarre multinucleated cells may also be prominent [9, 10]. Other cytologic variants derive from dysregulated synthesis and secretion of immunoglobulin, leading to intracellular accumulation of intact or partially degraded protein. Based on the criteria described by Bartl et al. [9], six histologic types can be delineated according to cytomorphology, namely, Marschalko type, small cell type, cleaved type, polymorphous type, asynchronous type, and blastic type. These six types have subsequently been combined into three prognostic grades: plasmacytoma (low grade), anaplastic plasmacytoma (intermediate grade) and plasmablastic plasmacytoma (high grade).
Accurate diagnosis of SP is critical for proper management. Cell markers are
recommended for confirming the diagnosis. The tumor cells usually express CD79a,
CD138 and CD38, while CD20 is absent [11, 12]. The integral membrane protein CD
79a and CD20 had been identified as B cells markers over 20 years ago [13]. The
CD79a protein is present on surface of B cells throughout their life cycle, while
CD20 is expressed on pre-, naive, and mature B cells [14], as well as the
majority of B-cell lymphomas [15], but not on plasma cells. CD38 is expressed on
normal plasma cells as well as on neoplastic plasma cells in MM and SP. Indeed,
Due to the monoclonal nature, the neoplasm produces either kappa (
To our knowledge, up till 2020, only 11 papers focusing on SP cases had been published in English language literature, mostly from the West [2, 4, 5, 7, 22, 27, 28]. Thus, a systemic review of the clinicopathologic characteristics of the disease from China would have significant value.
In this study, we aimed to examine SP patients from a single medical center in China and assess the clinicopathological characteristics of this disease.
In this retrospective study, cases of SP between January 2015 and April 2021 were retrieved from the pathology archives of our hospital. Clinical history and findings from physical examination, hematological results including complete blood count (CBC), peripheral smear and bone marrow examination were collected from the clinical charts. The diagnoses of SP were made based on the following criteria: (i) histological evidence of monoclonal plasmocytic solitary bone lesion (in case of SPB) or extraosseous mass lesion (in case of EP), (ii) lack of monoclonal plasmocytic infiltration in bone marrow aspirations and biopsies other than the single lesion in SPB, (iii) absence of osteolytic lesions based on skeletal survey of the spine and pelvis, (iv) lack of evidence of end organ damage, such as CRAB [29, 30].
A representative formalin-fixed, paraffin-embedded (FFPE) tissue block from each case was retrieved. A tissue microarray (TMA) was constructed as described previously [31], using MINICORE PLUS (MNC0100005157, Mitogen, UK). The array contains 5 cases of low-grade SP and 3 cases of intermediate-grade SP. As control, 3 cases of inflammatory polyps, each from the uterine cervix, maxillary sinus and eyelid that contain normal plasma cells were included. An index TMA is shown in Fig. 1.
 Fig. 1.
Fig. 1.Schematic map of the index tissue microarray (TMA). There are 8 cases of SP, and 3 cases of mucosal polyps as control for normal plasma cells. SP are grouped according to histologic differentiation into plasmacytoma (n = 5) and anaplasmacytoma (n = 3).
Immunohistochemical stains were performed using a Dako
EnVision detection kit, according to the manufacture’s instructions (DAKO,
Carpinteria, CA). For antigen retrieval, the slides were
steamed for 20 minutes in citrate antigen unmasking buffer. The sections were
then incubated with the primary antibodies against
DNA was extracted in formalin-fixed and paraffin-embedded (FFPE) sections from SP specimens. Immunoglobulin (IG) genes rearrangement analysis was performed with a polymerase chain reaction (PCR)-based technique using BIOMED-2 primer system [33] (Yuanqi Biomedical Technology Inc, China).
The plasmacytoma TMA slides immunohistochemically stained for CD19, CD38, and CD138 were evaluated with the Image. Proplus 6.0 software (Media Cybernetics, Inc., Rockville, MD, USA) and the value of integrated optical density (IOD) was assessed.
All data were analyzed using SPSS 19.0 (SPSS Inc., Chicago, IL, USA) and were
expressed as mean
As shown in Table 1, 13 cases of SP were identified and included in this study, with seven men and six women. The mean age was 54.2 years (ranging from 23 to 78 years). The cases were classified into 4 SPB and 9 EP. The mean ages of SPB and EP were 46.5 and 57.6 years, respectively. As shown in Table 2, a variety of sites involvement were identified, including the head and neck region (6 cases, 16.7% cases of SPB and 83.3% cases of EP), sinonasal or nasopharyngeal area (50%), gastrointestinal tract (2 cases), central nervous system (1 case), and inguinal lymph node (1 case). For SPB, long bones were involved in 50% cases. The most common presentation for SPB is pathological fracture, whereas for EP, soft tissue masses or polyps in the gastrointestinal tract were most common.
| Solitary plasmacytomas | EP | SPB | ||||
| Number of cases | (%) | Number of cases | (%) | Number of cases | (%) | |
| Gender | ||||||
| Male | 7 | 53.8 | 5 | 55.6 | 2 | 50 |
| Female | 6 | 46.2 | 4 | 44.4 | 2 | 50 |
| Total | 13 | 9 | 4 | |||
| Age (years) | ||||||
| 5 | 38.5 | 3 | 33.3 | 2 | 50 | |
| 50–60 | 3 | 23.0 | 2 | 22.2 | 1 | 25 |
| 5 | 38.5 | 4 | 44.4 | 1 | 25 | |
| Mean |
54.2 |
57.6 |
46.5 |
|||
| SD, Standard deviation; EP, extraosseous plasmacytoma; SPB, solitary plasmacytoma of bone. | ||||||
| Case | Age/Gender | Site | Diagnosis | Grade | κ/ |
EBER | IgH gene rearrangement | Follow-up time |
| 1 | 23/F | Femur | SPB | Plasmacytoma | κ | / | / | 6y2m |
| 2 | 46/F | Lymph node | EP | Anaplastic plasmacytoma | / | - | / | 6y |
| 3 | 53/M | Parotid gland | EP | Anaplastic plasmacytoma | κ | / | / | 4y11m |
| 4 | 40/F | Rectum | EP | Plasmacytoma | - | + | 4y10m | |
| 5 | 70/F | Nasal cavity | EP | Anaplastic plasmacytoma | / | - | / | 4y6m |
| 6 | 67/M | Colon | EP | Plasmacytoma | - | / | + | 4y3m |
| 7 | 57/M | Humerus | SPB | Plasmacytoma | / | / | 4y3m | |
| 8 | 65/M | Thigh | EP | Anaplastic plasmacytoma | - | / | 4y2m | |
| 9 | 55/M | Parasagittal | EP | Anaplastic plasmacytoma | κ | / | / | 4y1m |
| 10 | 28/M | Vertebral | SPB | Plasmacytoma | κ | / | / | 3y8m |
| 11 | 47/F | Nasal cavity | EP | Plasmacytoma | - | / | 2y11m | |
| 12 | 75/M | Nasal septum | EP | Plasmacytoma | / | - | / | 1y4m |
| 13 | 78/F | Skull | SPB | Plasmacytoma | / | / | 8m |
As morphological reference, normal plasma cells are 14 to 20
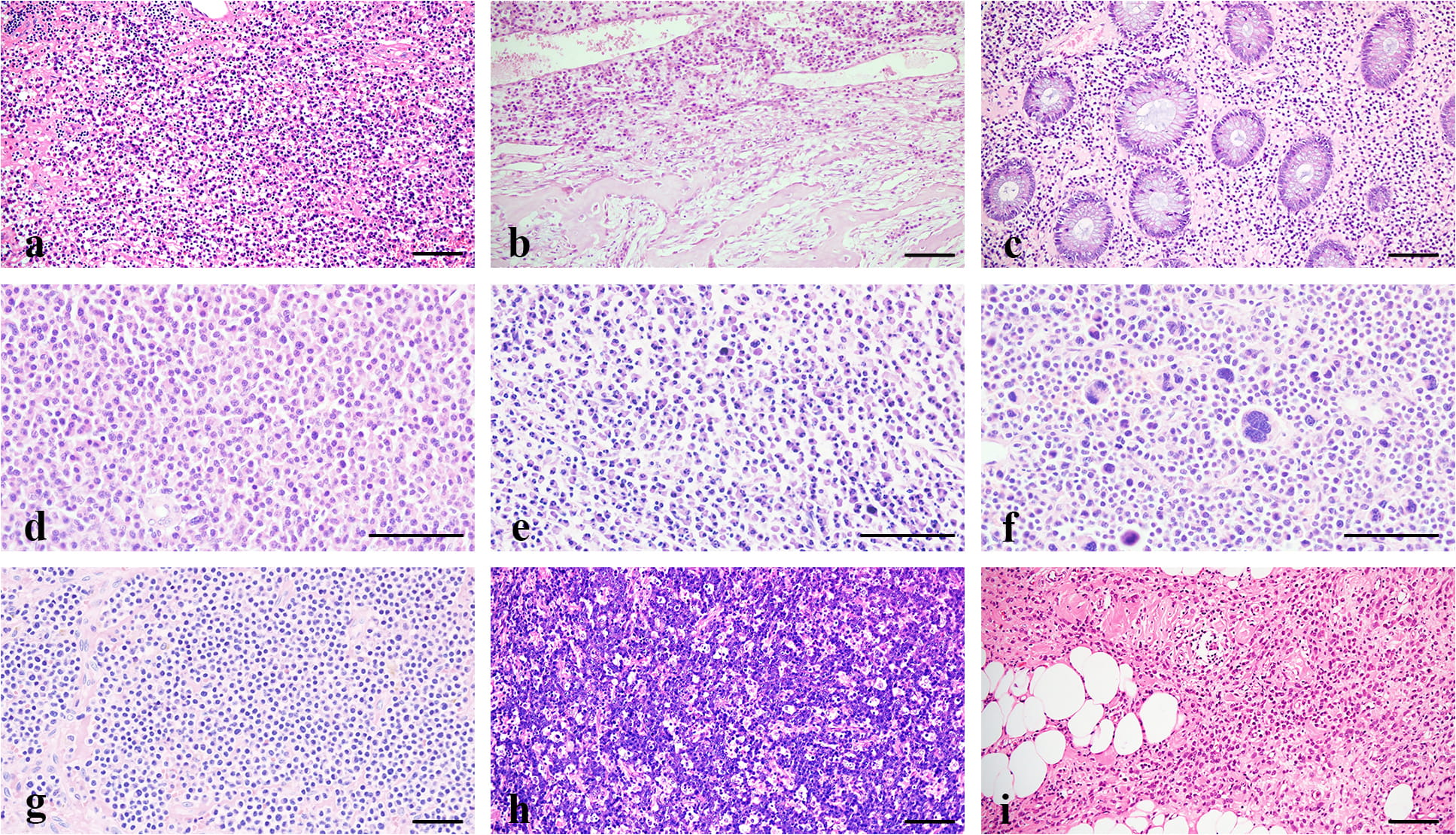 Fig. 2.
Fig. 2.Comparative histology of SP and controls. (a,
Our cases were distinguished from the following differential diagnosis: (1) MM,
although the histological changes of SP overlap with that of MM (Fig. 2g), the
latter shows multiple bone destructions, CRAB symptoms, and increasing serum
Immunohistochemically, both normal and neoplastic plasma cells are negative for
CD20 (Fig. 3a), as expected. Most cells express cytoplasmic CD79a and MUM-1 (Fig. 3b,c), while tumor cells from SP exhibit diffuse membranous CD38 and CD138
expression (Fig. 3d,e). SP cells show light chain restriction, as shown in Fig. 3f,g a case of SPB being kappa positive and lambda negative. The tumors are
negative for EBER that differential diagnosis with plasmablastic lymphoma (Fig. 3h), and negative for cytoplasmic P120 in contrast to carcinomas
with plasmacytoid features (Fig. 3i). All the cases are negative for Cyclin D1 as
well. Eleven cases express MUM-1, and less than 30% express CD56. Eight cases
tested for immunoglobulin light-chain production, 5 of which
(62.5%) are
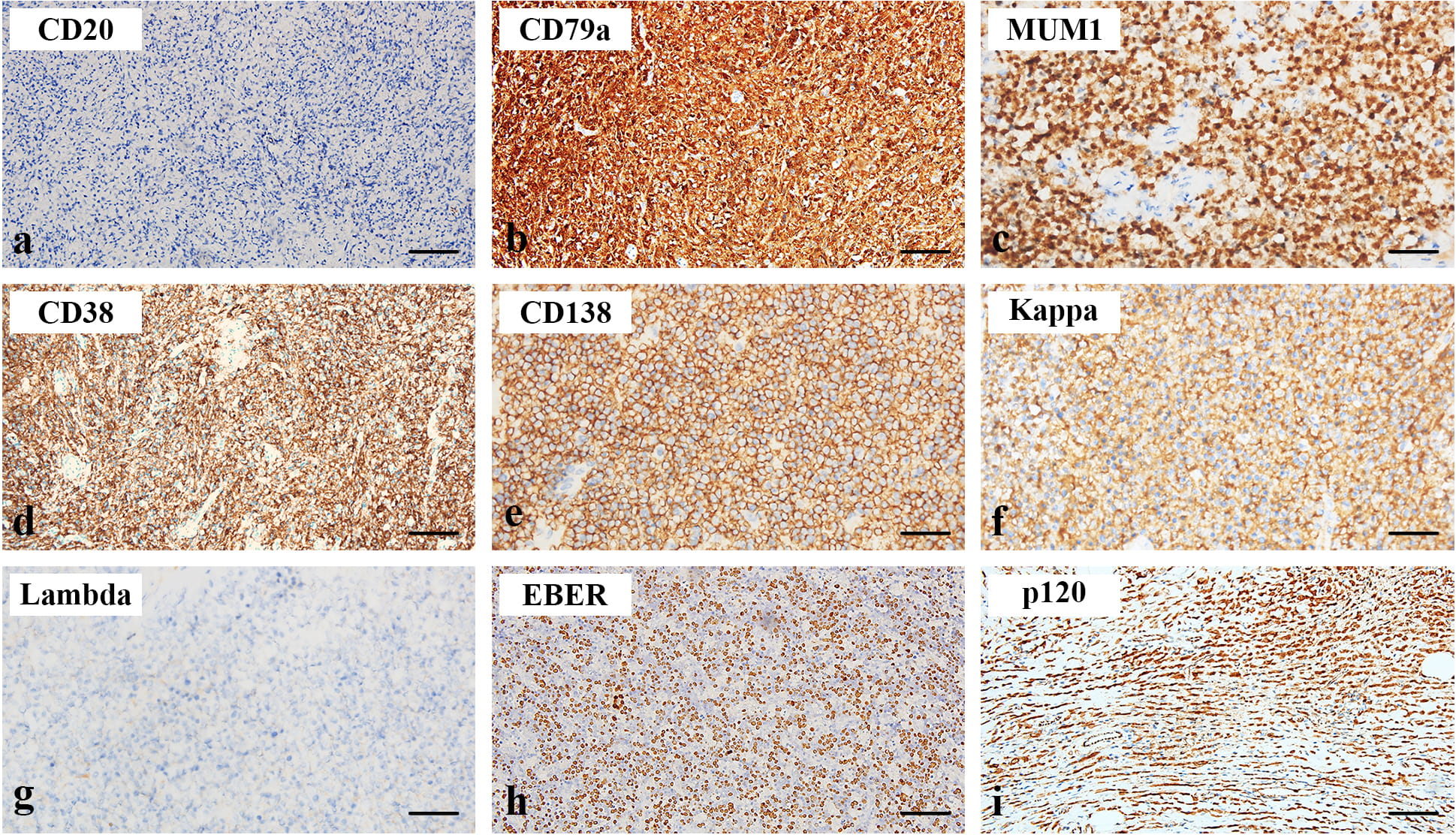 Fig. 3.
Fig. 3.Immunophenotypes of SP. The neoplastic cells are negative for
CD20 (a,
| IHC maker | Positive | Negative | Not tested |
| CD20 | 0 | 13 | 0 |
| Mum1 | 11 | 1 | 1 |
| CD56 | 1 | 4 | 7 |
| CD38 | 13 | 0 | 0 |
| CD138 | 13 | 0 | 0 |
| EBER | 0 | 6 | 7 |
A TMA was constructed as described in Material and Method in
order to examine the immunophenotypic features in normal and neoplastic plasma
cells. The array contain 2 groups of specimens (normal plasma cell group, n = 3;
neoplastic plasma cell group, n = 8). It showed positive CD19 in normal plasma
cells, but negative in neoplastic plasma cells (Fig. 4a). Both
normal and neoplastic plasma cells expressed CD38 and CD138 (Fig. 4b,c).
Quantitative analysis showed no significant difference in CD138 expression
between the normal (50.66
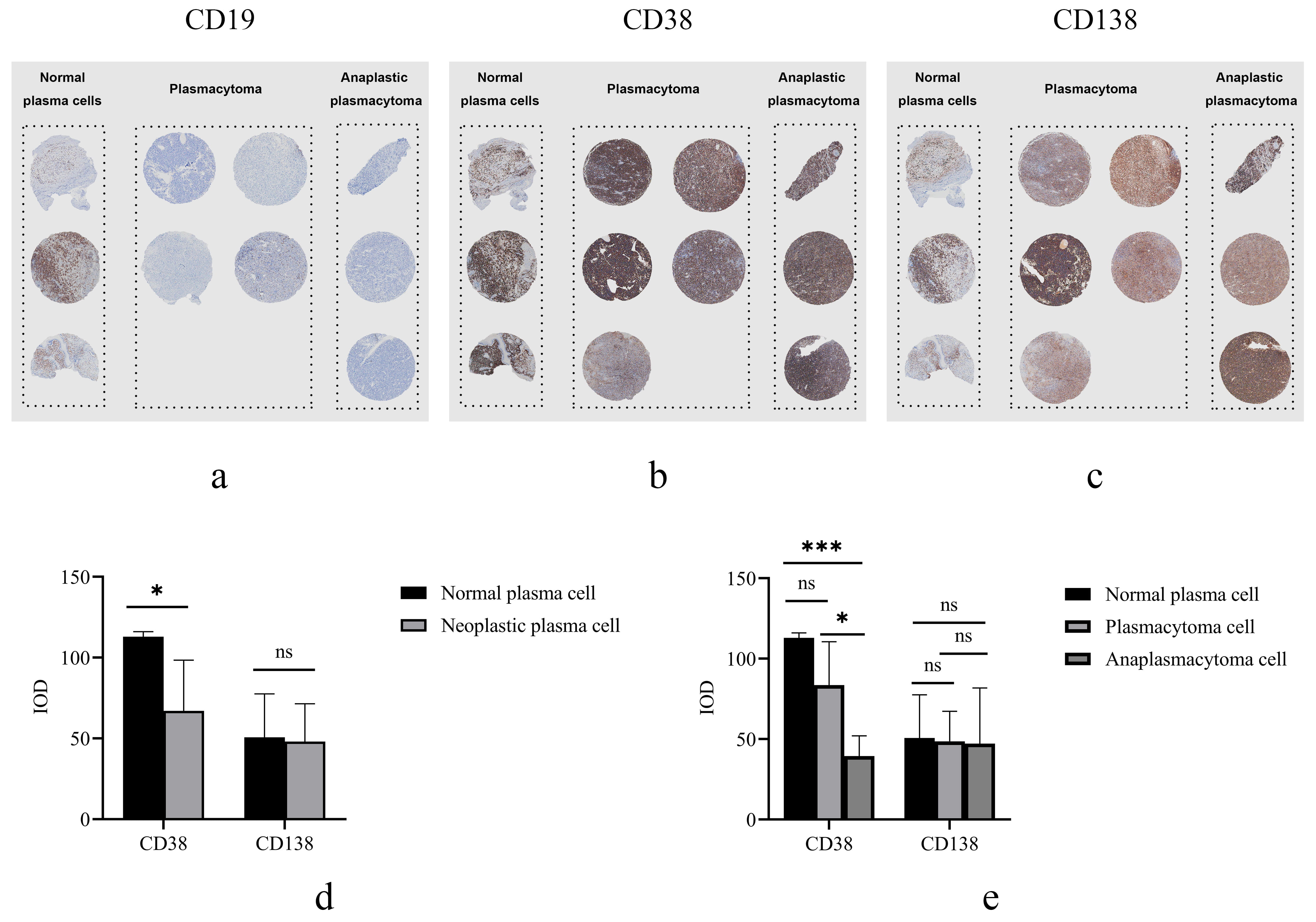 Fig. 4.
Fig. 4.Representative images and quantitative analysis of a
standardized TMA. Images of TMA stained immunohistochemically shown at lower
magnification (
Solitary plasmacytoma (SP) is defined as a localized mass of neoplastic, monoclonal plasma cells. Although SP is morphologically indistinguishable from multiple myeloma (MM), both location and clinical behavior are significantly different between the two entities. Among SP, SPB is reported to be about 40% more frequent than EP [29]. However, more EP is encountered in our cohort (69%), indicating that in the central region of China, distribution of SP is different from that described in the literature from the West. Otherwise, the tumor locations of our cases are similar to those from the literature, with SPB most frequently involving the vertebra, and EP, nasopharynx. In addition, we describe an unusual site of EP in lymph nodes, the latter had been rarely reported [8].
Several studies had shown mean age of SP patients at diagnosis ranging from 55 to 60 and a gender disparity for EP [2, 4]. In our study, no gender disparity is observed. The mean age of EP at diagnosis in our series is 57.6 years which is one decade later than SPB, possibly due to the small number of patients studied. According to the literature, age at diagnosis has a significant role in determining prognosis [29].
Histological evaluation of biopsies is mandatory for diagnosis of SP, with exclusion of other bone lesions and lymphoma [8]. The tumor cells include mature, normal appearing, immature, plasmablastic, and pleomorphic types [9]. About 10% cases have plasmablastic morphology. Multinucleated, multilobed, pleomorphic plasma cells are prominent in some cases [9]. Although low grade tumors are easily recognized histologically, high grade tumors with poorly differentiated cells such as plasmablastic or anaplastic types require special studies. In our series, all cases of SPB are of low grade, while 5 cases of EP are anaplastic plasmacytoma. Testing for monoclonal light chain expression helps for diagnosis. Particularly, kappa or lambda light chain restriction is helpful in differentiating SP from an inflammatory process with enrichment of plasma cells, and lymphomas with prominent plasmocytic differentiation, such as extranodal marginal zone lymphoma (MZL) of mucosa-associated lymphoid tissue (MALT lymphoma). The latter is positive for CD20, while SP is typically CD20 negative. Immunophenotype feature can also distinguish other plasmocytoid tumors, such as some poorly differentiated carcinomas.
SP is usually treated with radiation, surgery or a combination of both. EP is highly radiosensitive. Radiation therapy is currently the first line of treatment for EP with a local control rate reaching 90%. The 10-year disease-free and overall-survival rates range from 50% to 80% [27, 28]. EP in the lower respiratory tract is managed by radiation therapy alone, while EP in the upper respiratory tract may be treated with both radiation and surgery. EP in the gastrointestinal tract requires surgical excision [5, 34]. Follow-up time for our cases ranged from 8 to 74 months, with an average of 47.8 months. Up to the end of our study, the patients were either cured or progressed to MM.
CD 38 is a membrane-bound protein of 45 kDa first identified in 1980 [35]. It is a single-chain transmembrane type II glycoprotein encoded by a gene mapped to chromosome 4 [36]. CD38 was historically considered a surface marker for T cells [37]. Recent studies have revealed that it is the main nicotinamide dinucleotide (NAD+) catabolic enzyme, related to NAD+ metabolism [38, 39, 40, 41]. As a coenzyme, nicotinamide adenine dinucleotide (NAD+) can directly and indirectly influence many key cellular functions, including metabolic pathways, DNA repair, chromatin remodeling, cellular senescence and immune cell function [42, 43]. CD38 also modulates extracellular NAD+ precursors, and causes a substantial decline in cellular NAD+ levels [41], thus altering the availability of substrates for enzymes regulating cellular homeostasis. In our study CD38 is found to be uniformly expressed at high levels in plasmacytoma, more strongly in low grade plasmacytomas than intermediate grade ones. The prognostic relevance of the histologic categories had been confirmed in a previous study [9]. Our findings suggest that CD38 immunohistochemistry is a clinically useful method of predicting prognosis for SP. Nevertheless, more cases need to be studied to confirm this finding; additionally it may be important to analysis NAD+ level in different tumor grades, to further reveal the role of CD38 as a key modulator for NAD+ metabolism in the context of solitary plasmacytoma.
Existing reports on SP are mainly from the West and India. In this study, we aimed to examine SP patients from China and assess their clinical characteristics, survival, and pathological feature. Most significantly, we have analyzed immunohistochemical characteristics by TMA and shown that CD38 may be a marker related to prognosis of SP. However, duo to the small sample size and short follow-up time, a future study with more samples and longer follow-up time is necessary to confirm this finding.
Clinicopathological features of 13 cases of SP are described in this study, with EP more frequently encountered than SPB. Patients with SPB present at an earlier age than EP. Immunophenotypically, CD38 is more strongly expressed in plasmacytoma than in anaplastic plasmacytoma. Although histopathological examination remains the most important method in diagnosing these lesions, a systematic approach is required for definitive diagnoses. Treatment of SP is either radiotherapy, surgery or a combination of both. Since it takes years for plasmacytoma to progress to multiple myeloma, a longer follow-up is needed to provide more confirmative information on prognostic value of CD38.
Strengths of this study:
(1) A descriptive study examining SP from China, while existing reports about SP are mainly from the West
(2) Comprehensive assessment of clinical features, survival, and pathological parameters of SP, with semiquantitative immunohistochemical analysis using TMA from the cases
(3) Findings suggesting that CD38 as a potential prognostic marker for SP
Weakness of this study:
(1) Small sample size and short follow-up time
(2) Lack of in depth analysis of NAD+ level in fresh tumor tissues to further elucidate the role of CD38 in modulating NAD+ metabolism in the context of solitary plasmacytoma biology
CBC, complete blood count; EBER, Epstein-Barr virus-encoded small RNAs; EP, extraosseous plasmacytoma; H&E, haematoxylin and eosin; MGUS, monoclonal gammopathy of undetermined significance; MM, multiple myeloma; SP, Solitary plasmacytomas; SPB, solitary plasmacytoma of bone.
Design, review cases, data analysis and writing of manuscript—YL and JG, Design of study, editing and proof of manuscript—SYX, Review cases, data analysis—CY and JX, Editing and proof of manuscript—PM.
This study was approved by the Ethics Committee of Zhongnan Hospital for discarded diagnostic material (no consent required).
There is no financial conflict of interest to report. We thank Bei Qi for excellent secretarial assistance, and Huan Liu for technical assistance.
Department of Science and Technology, Hubei Provincial People’s Government (2020CFB343).
The authors declare no conflict of interest.
Data and materials from this study will be available upon request.
