Frontiers in Bioscience-Landmark (FBL) is published by IMR Press from Volume 26 Issue 5 (2021). Previous articles were published by another publisher on a subscription basis, and they are hosted by IMR Press on imrpress.com as a courtesy and upon agreement with Frontiers in Bioscience.
1 Departamento de Biologia de la Reproduccion, DCBS, Universidad Autonoma Metropolitana-Iztapalapa, CDMX, Mexico
2 Doctorado en Ciencias Biologicas, DCBS, Universidad Autonoma Metropolitana, CDMX, Mexico
Abstract
In industrialized countries, the use of Cadmium (Cd) produces a form of anthropogenic pollution. Hence, exposure by human populations is becoming a public health problem. With a half-life of up to 40 years, cadmium is now a topic of great interest due to its role as an endocrine disruptor and its effects on male reproduction. Cd’s diverse toxic mechanisms are based on its capacity to mimic divalent ions –calcium, zinc, iron– that participate in physiological processes. It alters the mitochondrial function and generates the production of free radicals that can induce apoptosis. In male reproduction, Cd alters the precise coordination of the hypothalamic-hypophysis-testis axis (HHT), resulting in the loss of testicular functions like steroidogenesis, spermatogenesis and the onset of puberty, sexual maturity, sexual behavior and fertility. Exposure to Cd may even cause changes in the immune system that are associated with the reproductive system. This review analyses the state of the question regarding Cd’s cellular and physiological mechanisms and the effects of this heavy metal on the neuroendocrine regulation of male reproduction.
Keywords
- Cadmium
- Male Reproduction
- Fertility
- Testicle
- Epididymis
- Prostate
- Semen quality
- Immune system
- Review
In industrialized countries, Cd contamination has become a serious problem for exposed populations due to the severity of its effects, which can cause critical environmental problems and health damage by accumulating in various organs. In 2019, the Metropolitan Area of Mexico City experienced an alarming atmospheric contingency when the concentrations of suspended particles measuring 2.5 micrometers or less (PM 2.5) reached historic levels, as did several other contaminants, such as sulfur dioxide (SO2), carbon monoxide (CO), nitrogen dioxide (NO2) and ozone (O3). If we add pollutants like heavy metals –mercury (Hg), arsenic (As), lead (Pb), cadmium (Cd) and chrome (Cr)– then the outlook for Mexico and other industrialized countries is bleak, indeed. Unfortunately, studies conducted to date on these problems are sorely insufficient (1). The effects of these metal contaminants, especially Cd, on the reproductive health of male and female mammals have been explored to some extent, so we know that they can impact the entire reproductive process (2, 3). However, they do not affect different tissues and systems in precisely the same way because these vary in their degrees of susceptibility (4, 5).
Human activities have increased Cd concentrations in the environment by, for example, using fossil fuels, copper alloys, and coverings to protect iron and steel from corrosion, but this heavy metal is also an ingredient in paints used with ceramics and plastics, and is utilized to manufacture nickel-Cd hydroxide batteries (Ni-Cd) for the automobile industry. In agriculture, Cd is an element in phosphorus-based fertilizers and pesticides, but is also found in leachates from garbage dumps, run-off from cultivated fields, and mining residues; thus, it is easily incorporated into animal feed and foods for human consumption, mainly in the form of cadmium oxide (CdO), cadmium chloride (CdCl2) and cadmium sulfate (CdS) (6). Because human and animal populations are exposed to water and foods contaminated with Cd, and to industrial emissions and cigarette smoke that contain it, this metal is considered one of the principal toxic agents present in workplaces and the environment (7). According to the WHO’s 2010 report, the maximum allowed concentration of Cd in the air is ~0.04 µg/m3, while for water it is <1 µg/L, so tolerable concentrations for monthly human ingestion of Cd must be below 25 µg/kg of body weight. However, because this metal is found in foods like mollusks, fish, the viscera of grazing animals, rice and other crops, especially grasses that are consumed in high quantities in human diets, it is relatively easy for people to unknowingly exceed allowable limits. For this reason, authorities have determined that a person can ingest an average of only ~30 µg Cd/day through alimentation (8, 9).
In contrast, in uncontaminated areas where Cd exposure through water and food is low, the most important pathway that allows Cd to enter organisms is by inhaling cigarette smoke. Although environmental contamination is a minor source of exposure, levels of 2-15 ng/m3 can be found in urban areas. Some reports from Mexico City indicate values as high as 35-40 ng/m3, making it one of the metropolises with the highest levels in the world. We can state, therefore, that one of the main exposure routes affecting Mexicans is inhalation, generally in the form of cigarette smoke, which represents approximately 25% (range: 5-50%), while the oral pathway of ingesting contaminated water and foods (viscera, seafood) is estimated to contribute only around 5% (range, 1-10%). It is important to note that one cigarette may contain 1-2.8 µg of Cd, so smokers are more exposed to this contaminant, though most are unaware of its toxic effects (10, 11). We know that Cd accumulates mainly in the kidneys, liver, testicles, ovaries, placenta, lungs and brain, and that its half-life in organisms is very long: from 20 to 40 years in humans. Reports suggest a half-life of 6-38 years in the kidneys, 4-19 years in the liver, and 75-128 days in blood, but the corporal accumulation of this particular metal is dependent on the duration and dosage of exposure, because it can mimic ions that are physiologically necessary and so interfere with biological processes in the organism that allow it to prolong the time required for excretion (9). Cd, therefore, is one of the most widely-distributed environmental contaminants, plus it has a long half-life and the ability to mimic molecules involved in vital biological activities. All this means that it exerts significant effects on human reproduction (12, 13). However, numerous aspects of the effects of Cd contamination on the neuroendocrine regulation of male sexual maturation and reproduction are not fully understood, so improving our comprehension of the cellular and physiological mechanisms involved will make it possible to implement measures that can reverse Cd’s toxic effects.
Cd is a heavy metal that can generate a broad range of toxic effects at both the systemic and cellular levels. Among the former, we can mention nephrotoxicity, carcinogenesis, teratogenesis and damage to the endocrine and reproductive systems. At the cellular level, Cd can damage the structure of DNA and proteins, interfere with several mechanisms of DNA repair, cause genic instability, modify cell proliferation and differentiation, and even activate cell death by apoptosis. Indirectly, Cd induces the generation of reactive oxygen species (ROS), damages DNA, and alters gene expression and signal transduction (14, 15). Any attempt to comprehend the cytotoxicity of Cd mechanisms must begin by clarifying how this metal is transported into the interior of the cells, and then distributed to the intracellular compartments where it accumulates. Therefore, this review analyses the principle entrance mechanisms of Cd into the reproductive cells and then describes its toxic effects and their consequences for the various structures that constitute the male reproductive system.
Cd is a transition element that belongs to Group 12 of the periodic table. Its most frequent oxidation number is +2 (with two positive charges = Cd+2 or CdII). This allows it to mimic and displace divalent cations that have physiological activity, including calcium (Ca2+), iron (Fe2+), magnesium (Mg2+), manganese (Mn2+), zinc (Zn2+) and selenium (Se), in many biochemical and cellular processes. As a result, it can bind to the proteins that transport these metals and form compounds with certain biomolecules that contain negative charges, including amino acids and proteins, nucleic acids, and the sulfhydryl or thiol groups (-SH), among others (16). Thanks to its physicochemical properties, Cd can also interact with membrane transporters involved in carrying these cations to the cells through a process of ionic mimicking (17, 18). When exposure to Cd is oral, the metal can pass through the intestine by diverse physiological mechanisms common to several species, because it utilizes the same absorption mechanism as Ca, Zn or Fe, as has been widely studied among mammals (19, 20). The mechanism that transports Cd requires proteins from the family of the Ca voltage-gated channel (VGCC) or Fe transporters (DMT family; divalent metal transporters). The DMT-1 protein is the one with the greatest affinity for Cd, but this metal can also utilize Zn transporters (ZIP family of metal transporters; Zrp, Irt-related proteins), especially the ZIP-8 protein, which is present in the gastrointestinal tract (21), and the Ca 1 transporter (CaT-1; also known as TRPV, for its initials in English: transient receptor potential cation channel, subfamily V), which is expressed in highest quantities in the intestines of mammals (22).
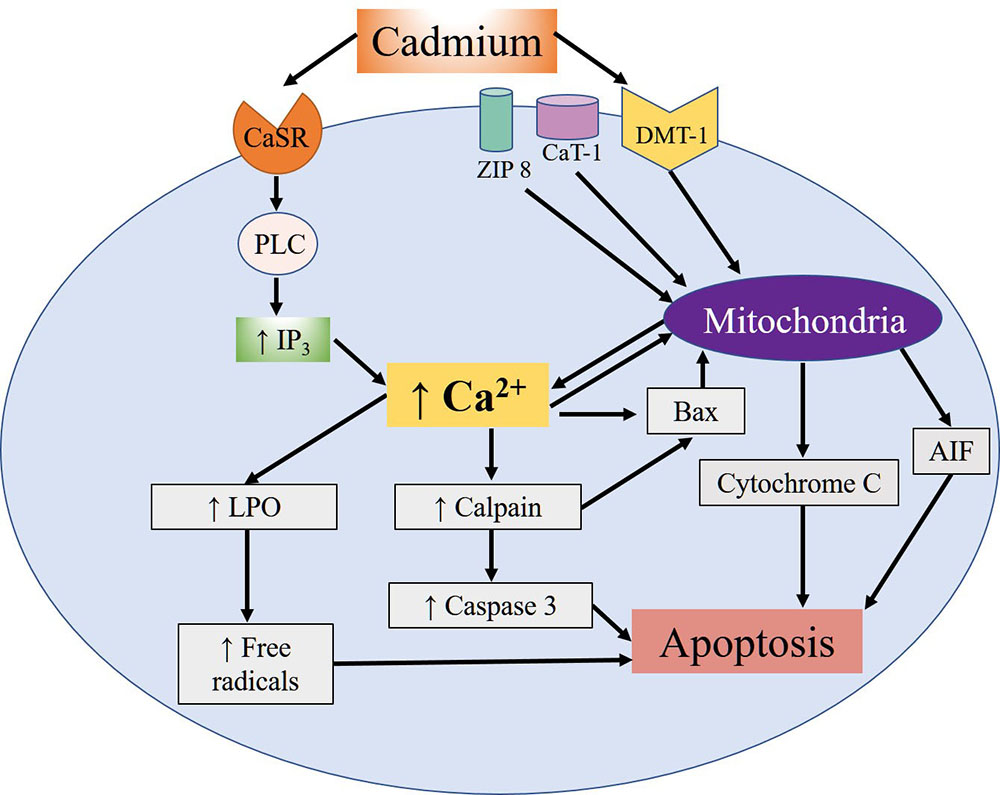
The mimicking that Cd shares with divalent metals is what allows it to interrupt certain processes, including the male reproductive function, which is one of the most severely affected since Cd exposure has often been associated with infertility (23). Specifically, Cd penetrates the spermatozoids of mammals through voltage-dependent Ca channels called CatSper. Research shows that Cd sensitivity and permeability depend on the precise composition of the channel, especially the presence of the absolute number of residues of the aspartate amino acid which, at physiological pH, are negatively-charged (24). Once absorbed, Cd binds to albumin and is carried through the bloodstream. It first reaches the liver (25), where it induces synthesis of metallothioneins (MT), a class of proteins rich in cysteines that bind to metals. The albumin and MT together protect against Cd toxicity by limiting its availability in the cells and tissues and then eliminating it in the urine. Cd’s ability to induce hepatic and renal lesions intensifies its toxic effects and allows it to accumulate for years, so that its reproductive toxicity may not appear until many years later (26). One of the most comprehensively studied effects of Cd, and the one that affects mainly reproductive tissues, is apoptosis. Studies have demonstrated that this metal can induce oxidative stress and the formation of ROS, while indirectly it can inhibit the activity of antioxidant enzymes and proteins that present Zn finger motifs (27, 28). Cd can also reach the mitochondria and damage them by interacting with the –SH groups of the cysteines present in the proteins of the internal mitochondrial membrane, causing it to lose its high impermeability and so dissipate its potential –which is necessary for adenosine triphosphate (ATP) synthesis– by uncoupling the processes of electron transport and oxidative phosphorylation. At the same time, Cd can bind to the hemoproteins that form part of the respiratory chain, causing increased ROS production, which generates mitochondrial lipid peroxidation. Studies have also shown that Cd induces activation of the intrinsic pathway of apoptosis, characterized by the release of mitochondrial cytochrome C and the apoptosis inducing factor (AIF), which can stimulate apoptosis at the nuclear level independently of the caspases. Moreover, Cd can bond to the Ca-sensitive receptor (CaSR) that induces activation of C phospholipase (PLC). This, in turn, increases concentrations of intracellular Ca mediated by the increase of the second inositol triphosphate messenger (IP3), which promotes the translocation of the calpains (Ca-dependent cysteine proteases) towards the cytoplasmic membrane, thus inducing autolysis and activation of the pro-apoptotic protein Bax. This protein allows the release of C cytochrome and the induction of initiator and effector caspases that convert mitochondrial apoptosis into an irreversible process (Figure 1). In addition to caspases, the substrates of the calpains contain membrane and cytoskeleton proteins, transcription factors and oncogenes associated with toxic effects that alter the process which allows spermatozoids to bond to ovules (29).
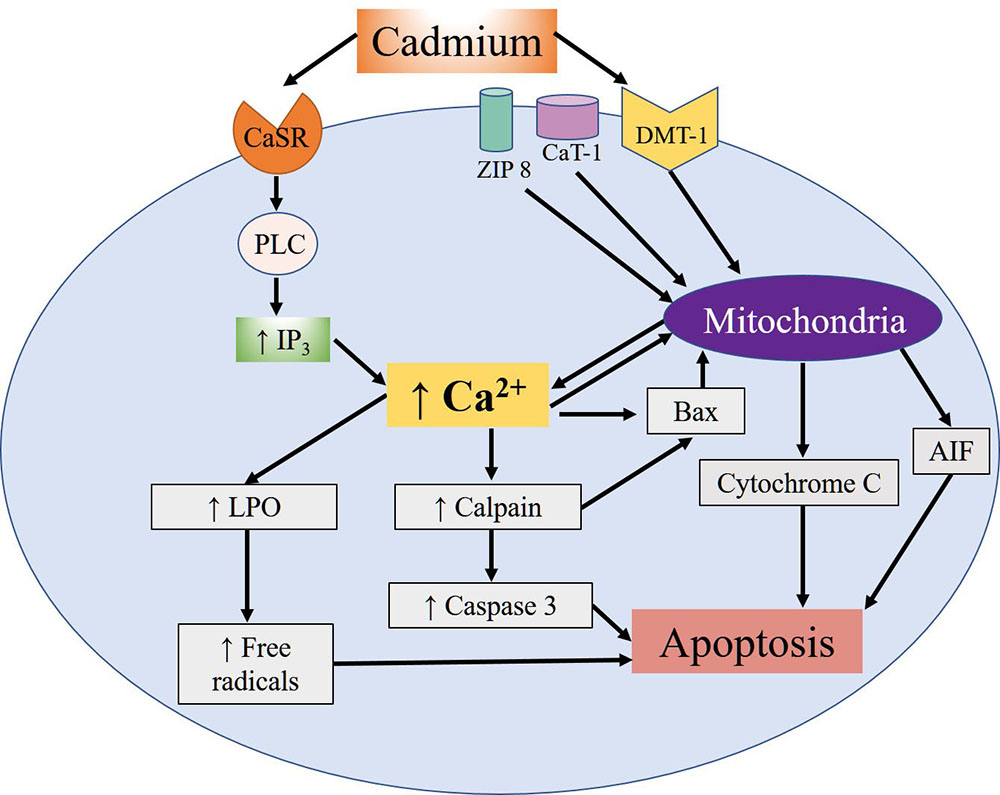 Figure 1
Figure 1Summary of the apoptotic effects of cellular Cd reported in different cell types. Cd's entry mechanisms are the DMT-1, Zip-8 and CaT-1 ion transporters. Once inside, Cd exerts diverse effects on the mitochondria, including the release of the AIF apoptosis-inducing factor and cytochrome C. In addition, Cd can bond to the Ca-sensitive receptor (CaSR), which activates the PLC-IP3 pathway and leads to an increase in intracellular Ca concentrations that, in turn, promotes activation of apoptotic calpains and caspases. This increase of Ca also fosters activation of the pro-apoptotic Bax protein and release of the mitochondrial cytochrome C and AIF proteins. Moreover, the increase of Ca induces the lipoperoxidation (LPO) of lipids and mitochondrial proteins, leading to the formation of free radicals, and promoting apoptosis.
As mentioned above, Cd is directly related to the induction of mitochondrial apoptosis, but it can also inhibit, through indirect mechanisms, anti-apoptotic systems, including such antioxidant enzymes as catalase (CAT), glutathione peroxidase (GPX), and superoxide dismutase (SOD). This results in the accumulation of ROS (30). Exposure to Cd is further associated with reductions of both glutathione (GSH) content and the activities of GPX, CAT and SOD, effects that can be analyzed as functions of the mechanisms of protein synthesis from the nucleus and in the cytoplasm because Cd can increase both the expression of transcription factors and the stability of transduction initiation factors located in eukaryotes, specifically eIF4AE (31). Although Cd’s principle target is the mitochondria, other cytotoxic effects occur because it interacts with receptors in the plasmatic membrane thanks to its ability to form multiple unions that include covalent and ionic bonding to the sulfur, oxygen and phosphorus atoms present in some amino acid residues. This explains Cd's ability to modify signal-transducing pathways. Another widely-studied effect is the extracellular entrance of Ca through ionic channels that, as mentioned previously, increases ROS, Bax and AIF concentrations and, consequently, induces apoptosis. Contradictory findings, however, have shown that at sub-lethal concentrations Cd can promote cellular adaptation and survival by activating the extracellular signal-regulated kinase ½ (ERK1/2), phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt-PKB) signaling pathway and regulating transcription factors like redox effector factor 1-nuclear factor E2-related factor 2 (Ref1-Nrf2), nuclear transcription factor-kappa B (NF-κB), int/Wingless protein (Wnt), B-cell lymphoma 2 (Bcl-2) and activator protein-1 (AP-1) (29). Other cellular processes that Cd can alter include adhesion and migration, since it can regulate the structure and function of the cytoskeleton and modify the concentrations and localization of E-cadherin, N-cadherin and β-catenin proteins, all of which are important components of tissue integrity (32). Because the cadherins are Ca-dependent proteins, Cd can mimic them, modify their structure and function, and lead to the loss of intercellular adherence, possibly activating uncontrolled processes of proliferation and cancer in both reproductive and non-reproductive tissues (33).
In summary, Cd is a highly toxic metal that affects various tissues, including those of the reproductive tract. Its principle cytotoxic effects are due to its ability to mimic divalent cations (Ca, Fe, Mn, Mg, Zn, Se) that perform physiological functions. However, this section has presented only a few of Cd’s many effects usually associated with reproductive processes. The following sections will, therefore, explain Cd’s effects on specific processes and tissues.
Male reproduction depends on the precise coordination of the HHT axis, which governs correct testis function and the subsequent production of steroid hormones and spermatogenesis. Male reproductive capacity results from the activation of the HHT axis during puberty, which begins with the pulsatile secretion of the gonadotrophin-releasing hormone (GnRH) by the hypothalamus to stimulate the gonadotrophs in the hypophysis to biosynthesize and secrete hypophysis gonadotrophins and the luteinizing (LH) and follicle-stimulating hormones (FSH). These, in turn, sustain intragonadal testosterone (T) synthesis and spermatozoid production. This function is maintained by a negative feedback mechanism controlled by the increase in the levels of T, which induces the reduction of both the hypothalamic secretion of GnRH in the hypothalamus-hypophyseal portal circulation and the release of both gonadotrophins into the bloodstream by the hypophysis. The hypophysis decreases the production and release of LH through a negative feedback mechanism that also reduces GnRH and LH and, therefore, lowers plasma levels of T (34). Multiple exogenous factors –including environmental contaminants– can alter the function of the HHT. Long-term exposure to Cd will cause toxic effects due to its accumulation over time in a variety of tissues, including the hypothalamus, hypophysis and testicles (35).
The hypothalamus is located at the base of the brain, beneath the thalamus and third ventricle, but above the optic chiasma It is an extremely complex cerebral structure that forms a functional unity with the hypophysis gland to coordinate such functions of the organism as somatic growth, maturation and gonadal function, among others (36). Its neurosecretory cells synthesize and secrete the GnRH neurohormone, which is responsible for stimulating the HHT axis (37, 38). GnRH is a decapeptide secreted by approximately 1000 neurons located in the hypothalamus (arcuate nucleus and middle eminence). It is responsible for stimulating the secretion of gonadotrophins (FSH and LH) by bonding to the membrane receptors in the gonadotrophs of the hypophysis. The neurovascular linkage between the hypothalamus and hypophysis is the hypophysis stalk, which contains the hypothalamic-hypophyseal portal system. The hypophysis gland is located immediately below the hypothalamus, resting on a depression in the base of the cranium called the sella turcica. It is divided into the anterior and posterior hypophysis (39). The FSH and LH secreted by the anterior hypophysis belong to a family of dimeric glycoprotein hormones that share certain structural characteristics (40). Each hormone consists of two sub-units: subunit-α and subunit-β, which are bonded non-covalently. Subunit-α is common to all glycoprotein hormones, but each subunit-β presents a specific sequence of amino acids –111 for FSH and 121 for LH– which gives each one its biological specificity (41). Both FSH and LH act through the classic mechanisms of the protein hormone receptor, which involves transmembrane receptors associated with the G proteins located in organs like the testicle (42).
Several studies have shown that Cd at different concentrations can alter the neuroendocrine function of the hypothalamic-hypophysis axis and so affect the release of various hormones (43-46). While few studies have analyzed Cd’s effects on the hypothalamic secretion of GnRH, some reports indicate that Cd exposure increases the expression of ARNm for GnRH 1 and GnRH 2 in salmon, and of GnRH 2 in Micropterus salmoides (47, 48), while chronic administration (4 weeks) of 5 mg/kg of CdCl2 in male rats decreased plasma GnRH levels (49).
Regarding Cd’s effect on concentrations of hypophysis hormones, studies in humans show contradictory results. Research on males exposed to Cd in the workplace has found that it reduces FSH secretion after only short exposure periods (50). Other working groups, however, report positive correlations between plasma Cd values and serum LH concentrations, but not with FSH (51). But Cd also reduces concentrations of intrahypophyseal LH (52). Studies in men who suffer from azoospermia or oligospermia associated with infertility have found positive correlations between serum and seminal Cd concentrations and concentrations of FSH (53), but other work failed to find correlations between Cd levels and concentrations of FSH or LH (54-56). Chronic exposure to 50 ppm of Cd introduced into rats’ drinking water reduced serum LH concentrations, but increased FSH (57), while chronic intraperitoneal administration of 1 or 5 mg/kg of CdCl2 decreased concentrations of both gonadotrophins (49, 58). However, the administration of high doses (4 or 6 mg/kg) in an acute or sub-chronic scheme reduced FSH and LH concentrations (59). It is well-known that Cd produces apoptosis in cells of the anterior hypophysis (60) through the mechanisms described above, and that it modifies total lipid content (44). These effects could, therefore, affect the functions performed by the testicle.
Neurosecretion of GnRH in the hypothalamus is regulated by multiple factors, including neurotransmitters, since it receives information from neurons that produce noradrenaline, dopamine, serotonin (5HT), γ-aminobutyric acid (GABA) and glutamate. There are reports that glutamate and noradrenaline stimulate the HHT axis, while GABA, dopamine and serotonin seem to inhibit it (38, 61-63). Glutamate is an important neurotransmitter that stimulates the GnRH neurons, which are innervated directly by glutamatergic neurons. We also know that the GnRH neurons express receptors for α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) and kainate and, though to a lesser extent, N-methyl-D-aspartate receptors (NMDA) (64). Hence, it is considered an activator in the pulsatile release of GnRH, since some reports suggest that stimulation of the NMDA receptors leads to early-onset puberty in rats and monkeys. The increases in glutamate synthesis in the hypothalamus initiate puberty in those species (37, 65, 66). In adult rats, stimulating the NMDA receptors triggers the release of LH via GnRH (67).
GABA, meanwhile, is the neurotransmitter that inhibits mature neurons, though it can also perform inhibiting or stimulating functions on GnRH secretion, depending on changes in its receptors during the development of different individuals (68, 69). GnRH neurons express the GABAA (70, 71) and GABAB (72) receptors. In rodents, administering bicuculline (a GABAA antagonist) and phaclofen (a GABAB antagonist) reduces the release of GnRH in young animals, but increases it in adults (73).
Regarding noradrenaline, we know that this neurotransmitter contributes to facilitating an increase in the release of GnRH/LH. Also, activation of the α1-adrenergic receptor facilitates the release of noradrenaline, but not α2 or β, while infusions of the α1-adrenergic blocker prazosin into the middle eminence suppress GnRH release (74).
With respect to dopamine, the precise role that it plays in initiating puberty has not yet been established completely, though research has demonstrated that hypothalamic concentrations of dopamine increase before and around puberty onset (75).
Turning to 5HT, we find that it has an inhibitory effect on GnRH synthesis, and there are reports that treatment with 5HT or agonists of the serotonin 1A receptor (5HT1A) and serotonin 2 receptor (5HT2) receptors in male rats reduces the neurons that contain ARNm and codify for GnRH (61).
Exposure to Cd modifies the amounts of neurotransmitters, as has been reported in adult male rats under chronic exposure to high doses of CdCl2 (25 mg/kg) in drinking water, as they suffered a reduction in 5HT, dopamine and noradrenaline concentrations in the hypothalamus (43, 76). We also know that acute treatment with this same dose of CdCl2 decreases GABA concentrations in the striate of rats (77), and that chronic treatment in drinking water reduces glutamate concentrations, though without affecting GABA in the hypothalamus (78). These modifications of neurotransmitters could affect GnRH secretion.
Testicle development is controlled by a hormonal balance in a microenvironment of somatic and germinal cells that involves cell-to-cell interactions and hormonal signaling by the FSH and LH secreted by the hypophysis (79). It is well-known that while FSH is important for controlling the proliferation of Sertoli cells and inducing spermatogenesis (39, 80, 81), LH is required for steroidogenesis because it stimulates the membrane receptor in the Leydig cells which, in turn, stimulates the enzymatic conversion of cholesterol into T (39). T synthesis and spermatozoid production are the testicle’s principle functions, and both are affected by exposure to Cd. The decrease of androgens can alter not only the morphology and functioning of the testicle but also the morphophysiology of the epididymis and accessory glands and reproductive parameters, including fertility.
The testicle is the organ that generates gametes; that is, the male sexual cells called spermatozoids. But it is also capable of synthesizing T; hence, it is considered the primary male sex gland. Testicles are found in pairs, are oval-shaped and covered by a layer of connective tissue called the tunica albuginea that invaginates inside the testicular body to form the testicular mediastinum and septa. The septa separate the testicle into lobes that contain the seminiferous tubules, the site where spermatogenesis –or spermatozoid synthesis– takes place. Spermatogenesis refers to the process of spermatozoid production and their subsequent differentiation from round diploid cells to haploid cells with the characteristic form of spermatozoids. This process is divided into three main phases: 1) mitosis; 2) meiosis; and 3) differentiation. The first phase is also called spermatocytogenesis. During this process, type A spermatogonia divide repeatedly by mitosis to produce other spermatogonia of the same type, which serves to maintain a cellular reserve. These type A spermatogonia then give rise to the type B spermatogonia, which are also divided by mitosis to produce more type B spermatogonia. Studies speak of the “maturation” of the type B spermatogonia because they increase in size and will be transformed into primary spermatocytes. Upon reaching this state, they migrate to the adluminal compartment of the seminiferous tubule, where they will undergo the first meiotic division. Secondary spermatocytes are obtained as a result of this first meiotic division, while spermatids result from the second meiotic division. The phase of differentiation, or spermiogenesis, occurs when the round spermatids are differentiated into spermatozoids. Sertoli cells are of great importance in this, and all, stages of spermatogenesis. They are located in the seminiferous tubules where they provide physical support for germinal cells and maintain the tubular environment to allow the differentiation of spermatocytes into spermatozoids (82).
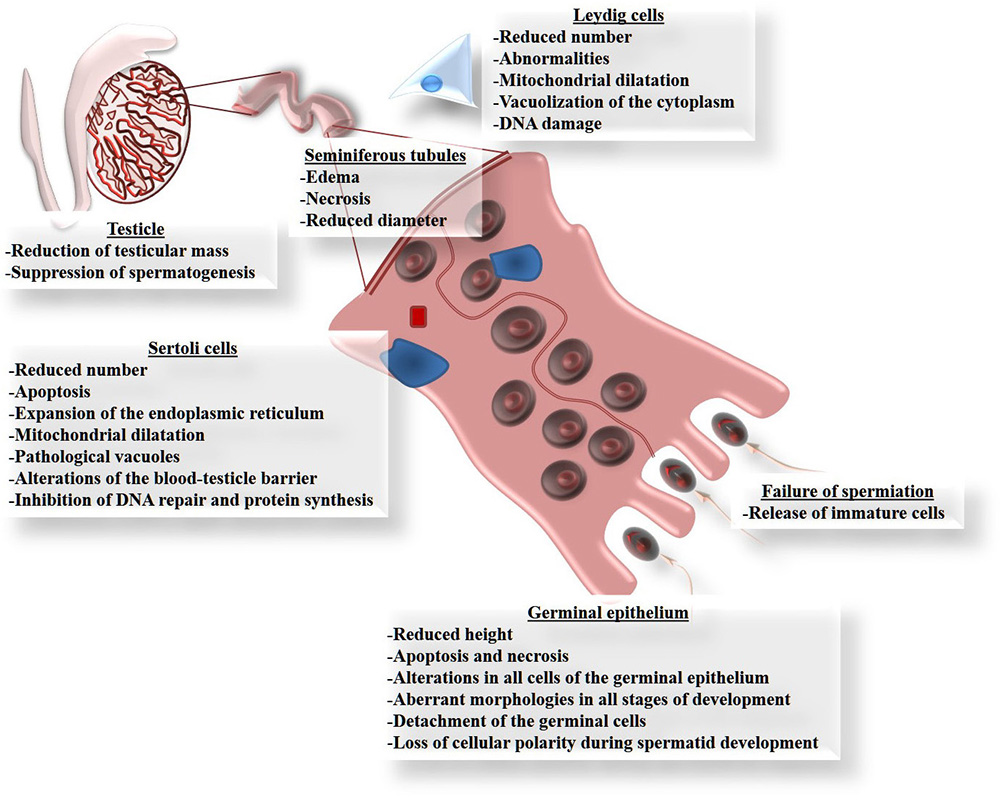
Although Cd can damage several organs, the testicles are the main targets of Cd toxicity (83, 84), since this metal can migrate to, and cause alterations in, these organs (85) because they are particularly susceptible to the effects of oxidative stress due to their high content of polyunsaturated fatty acids (86). Figure 2 summarizes the principle affectations of the testicle caused by Cd exposure, especially the reduction of testicular size and mass (87-89). Cd exposure reduces the diameter and length of the seminiferous tubules (88, 90, 91). This is explained by their high content of germinal cells that die by apoptosis (89, 92-102), which in some cases is observed as vacuolization of the epithelium, or even the complete loss of cell layers from the germinal epithelium (88, 99). This causes interstitial edema and hemorrhaging, together with a marked reduction in the height of the epithelium and its calcification (90, 91, 103). In this regard, alterations have been seen in all cells of the germinal epithelium, including unusual morphologies in all cell types and decreases in the number of spermatogonia and spermatocytes (90, 91, 104-106). But Cd also induces poor orientation and the loss of cellular polarity in the development of spermatids in adult rats (107); a finding that may be associated with failures in spermiation (107) caused by the release of immature cells (104-106).
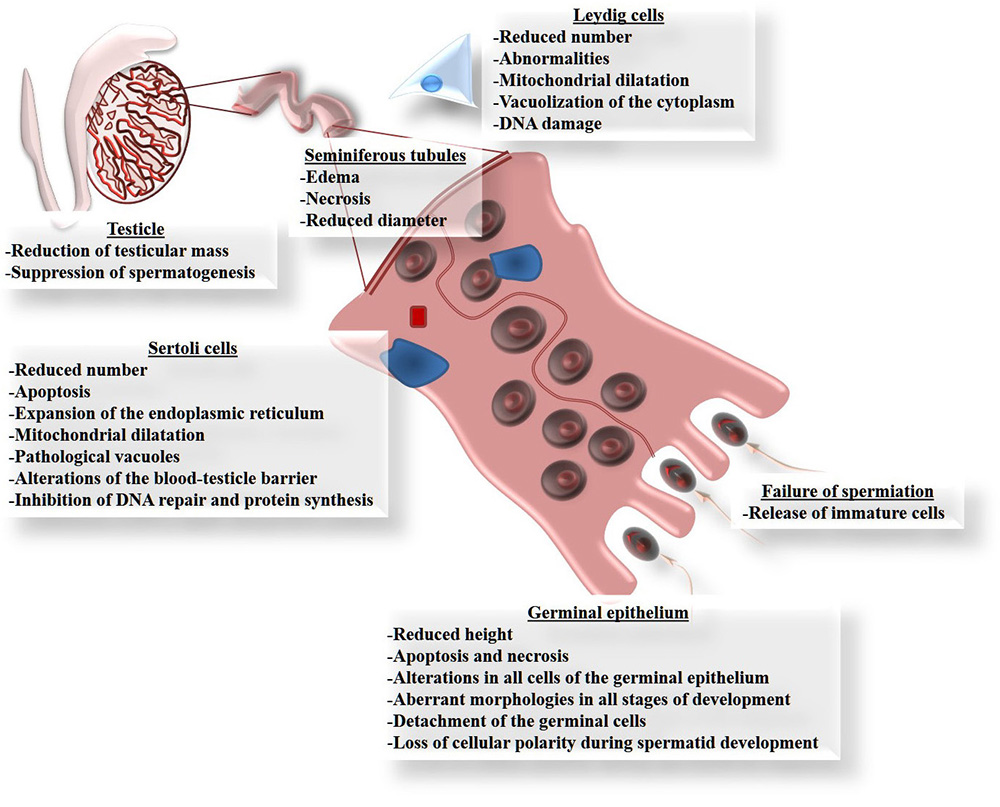 Figure 2
Figure 2Principle damage that cadmium causes in the testicles. All these forms of damage can be explained by the involvement of reactive oxygen species (ROS), because exposure to Cd increases oxidative stress in the testicle, determined as lipoperoxidation.
Sertoli cells play a crucial role in the process of spermatogenesis by participating from the differentiation stage of the germinal cells through to their maturation as spermatozoids. Thus, any changes that these cells suffer due to Cd exposure will affect this process directly. There are reports that, like Leydig cells, Sertoli cells are highly vulnerable to damage by Cd (105, 108), which can even inhibit their proliferation (109) and decrease the number of cells available (90). This can occur because Cd has been identified as an apoptosis-inducer (110). Other reports on Sertoli cells indicate that Cd causes an expansion of the endoplasmic reticulum, mitochondrial dilatation, vacuolization and DNA damage in birds and mammals like rodents and hogs (100,106,109,111). Another well-known effect of Cd is that it interacts with extracellular Ca binding domains present in E-cadherins (103), which are the principle molecules of cellular adhesion like those found between Sertoli cells as part of the blood-testicle barrier (BTB), and between Sertoli cells and germinal cells. The union of E-cadherin with Cd interrupts this cell-cell binding (100). Also, Sertoli cells from human cells, after forming an epithelium, were used as a model to determine the effects of Cd on BHT. In this study, Sertoli cells were sensitive to Cd toxicity, as well as changes in localization in cell adhesion proteins caused by alterations in the cytoskeleton, especially the depolarization of the F-actin filaments caused by the delocalization of the proteins: Eps8 (substrate of the epidermal growth factor 8 receptor pathway) and the branched actin polymerization protein. Furthermore, Cd prevented endocytic vesicle trafficking and the delocalization of c-Src and annexin II proteins (actin regulatory proteins) (112). In addition, administering Cd can reduce spermatogenesis by affecting the permeability of the BTB (107, 113), which is regulated by certain tight bonds: occludin, N-cadherin and vimentin (100). Leydig cells can also be affected in males exposed to Cd, for studies show abnormalities and reductions in the number of cells in those individuals (87, 90). These affectations include dilatation of the mitochondria, vacuolization in the cytoplasm and DNA damage (114, 115).
Additionally, to these effects Cd reduces the effectiveness of the antioxidant defense system by decreasing the enzymatic activity of SOD, CAT and GPX, as well as that of GSH. This means that levels of oxidative stress in rats’ testicles will rise due to alterations of the antioxidant system (116). For this reason, when organisms have been exposed to Cd, Zn can be utilized to reduce this metal’s effects by competing with Cd. However, many enzymes require Zn to be activated (117), so it has been associated with numerous enzymes in the body that can prevent damage by activating the antioxidant system (118-121).
When the spermatozoids leave the testicle, they cannot yet fertilize the oocyte. They must pass, first, through the epididymis to acquire this ability. This stage of the process is called epididymal sperm maturation. The epididymis is an elongated, highly-contoured tube, whose length varies by species. It may measure 1 m, as in the mouse, 3 m in rats, between 5 and 7 m in humans, and up to 70 or 80 m in horses (122). It is attached to the testicles by the efferent conducts and contains three main anatomical regions –caput, corpus and cauda– though additional regions can be recognized histologically, so the number of regions depends on both species and the form of the initial segment (123). Observations of most species show that epididymal sperm maturation is achieved before the spermatozoids reach the cauda, where they are stored until ejaculation (82). However, studies of males exposed to Cd reveal that the weight of the epididymis decreases (124) and that the highest accumulations of Cd occur in the corpus. This suggests that Cd can affect epididymal sperm maturation, the functionality of the spermatozoids and, therefore, the reproductive success of males exposed to this metal (125).
The epididymal tubule consists of a pseudostratified epithelium where we find principle cells, basal cells, clear cells, narrow cells, halo cells and apical cells. However, the principle and basal cells are present throughout the epididymal tubule, while the clear, narrow and apical cells appear only in certain segments of the epididymis (124). The disposition and localization of the cells in the epithelium along the epididymal tubule constitute microenvironments by secreting and absorbing ions, sugars and proteins, etc., that, upon coming into contact with the spermatozoids, undergo biochemical, morphological and physiological modifications that gradually lead them to acquire –during their transit through this organ– their ability to move and become capacitated to interact with the oocyte; that is, the capacity to achieve fertilization (126).
There are reports that the epididymis requires the participation of androgens to maintain its epithelium in good condition (127) and to perform and control its functions. We know that the androgen receptor (AR) is found in great quantities in the caput and corpus, though in lower amounts in the cauda (123, 128). While the epithelium of the epididymis is androgen-dependent, estrogens also participate in maintaining its morphophysiology (129) by secreting T, which is necessary for the correct expression and secretion of the proteins, glycoproteins, glycolipids and phospholipids required for the spermatozoids to mature and survive (123, 130, 131). This becomes especially important when we consider that animals exposed to Cd suffer reductions in their T content (132, 133), which decreases the size and weight of the epididymis (122, 133) as a consequence of histological damage (133). This damage may increase the height of the epithelium in the caput (134). This could be interpreted as indicating a problem with fluid absorption, which depends on a contribution of androgens (135) that, as mentioned above, decreases when Cd is administered. In addition to these problems with morphology and histology, the epididymis also suffers a reduction in the number of spermatozoids in the lumen of the tubule located in the region of the initial segment; that is, the caput and cauda. This parameter is inversely proportional to the concentration of Cd administered (134, 136).
One of the most important changes that spermatozoids undergo as they pass through the initial segment and caput of the epididymis is hyper-compaction of the DNA, which is achieved through the formation of disulfide bridges between cysteine residues that bond to protamines (137). This change is essential for conserving the spermatozoid’s genetic material while it transits to the fertilization site (138). However, Cd causes decompaction of the chromatin by eliminating the disulfide bridges, an action that increases the fragmentation of spermatic DNA (139).
Changes in the carbohydrates in the membrane of the spermatozoid constitute other modifications that spermatozoids may undergo while passing through the epididymis, because the adequate distribution of carbohydrates is what gives them the capacity to cross the cervical mucosa, approach the oocyte, and fertilize it (126). This process is dependent on the following enzymes: glycoside hydrolases (hydrolytic enzymes that break down carbohydrate residues) and glycosyltransferases (enzymes that add carbohydrate residues from another donor sugar). Both kinds of enzymes function by modifying the glycoproteins on the spermatozoids’ surface during epididymal maturation (140). We have found that administering Cd lowers the concentration of N-acetyl-glucosamine, sialic acid and fucose in the membrane of spermatozoids obtained from the three main regions of the epididymis (122). These changes can be explained by the reduction of androgens caused by exposure to Cd, which may impede the synthesis of such proteins as the glycoside hydrolases and glycosyltransferases, though this remains to be demonstrated.
Other intracellular changes that occur in spermatozoids during their maturation are the modification of Ca and cyclic adenosine monophosphate (cAMP) concentrations, and distinct patterns of tyrosine phosphorylation, all of which are essential for the adequate development of spermatozoid motility (141-144). For spermatozoids to develop this ability, they must pass through a process of protein activation in the epididymis called tyrosine phosphorylation, which involves a cAMP-dependent tyrosine kinase (145-147). It has been suggested that both non-human animals and humans exposed to Cd present alterations in sperm motility, possibly caused by changes in the process of tyrosine phosphorylation, energy loss, and decreased concentrations of ATP/AMP (148). One in vitro study of spermatozoids obtained from the caudal region of the mouse’s epididymis (149) found that the percentage of phosphorylated proteins increased under Cd exposure, compared to a control group. This does not necessarily mean that the increase in phosphorylated proteins in tyrosine residues favors the development of sperm motility because this parameter was not analyzed in the study. However, a study by our working group found that Cd also increases the number of phosphorylated proteins, and that phosphorylation increased as the spermatozoids advanced from the caput to the cauda in subjects treated with Cd. This result was distinct from observations of the control subjects, where the pattern of protein phosphorylation was similar between caput and corpus. In contrast, the spermatozoids obtained from the cauda showed a marked reduction (122).
According to these reports on the causes of damage to the morphophysiology of the epididymis in organisms exposed to Cd, it is not a question of oxidative stress, even though numerous studies argue that this is the case for the testicle (150-152). This is affirmed because the activity of the SOD, CAT and GPX enzymes does, indeed, decrease, but does not generate lipoperoxidation (133), as occurs in the testicle. Therefore, the principle factor in the damage that Cd causes in the epididymis is the reduction of androgen concentrations.
The accessory sex glands include the seminal vesicles, prostate, and bulbourethral (or Cowper) glands. The seminal vesicles are joined to the epididymis by efferent ductules and contribute over 60 % of the seminal fluid. They are sack-like structures located on one side of the bladder. The prostate surrounds the ejaculatory ducts at the base of the urethra, just below the bladder. It is nut-shaped and its secretions also form part of the seminal fluid. The bulbourethral (Cowper) glands are located at the base of the penis, where the urethra begins. They secrete an alkaline liquid that lubricates and neutralizes the acidity of the urethra and provide the first fraction of the seminal fluid. The sum of the secretions of these accessory glands constitutes the seminal fluid that, together with the spermatozoids, form the semen (153). High Cd levels in the seminal fluid have been associated with alterations of the sperm parameters in infertile males (154) and a decrease in seminal volume (133). Thus, in addition to the morphophysiological damage that Cd inflicts on the testicles and epididymis, it also reduces the weight of the prostate and seminal vesicles (124, 155), which could be associated with the decreased seminal volume.
Cd also plays a critical role in male infertility by increasing incidences of prostate cancer (156). Cd concentrations in the prostate increase under chronic exposure and, as mentioned above, T synthesis decreases in animals exposed to Cd. Here, it is important to consider that the production of testicular androgens is essential for maintaining prostate tumors since the development of this type of cancer can be delayed –for a time– by castration, estrogen administration, or both (98). The fact that Cd exposure decreases T in organisms might explain the development of prostate cancer in men exposed to this metal because this condition is an aberrant genic expression that stimulates cellular proliferation or blocks apoptosis (157). Activation of such transcription factors as the metallothionein gene and some proto-oncogenes can increase the proliferation of cells that contain damaged DNA (158) and, perhaps, suppress DNA repair, thus augmenting the population of cells with DNA damage (157). This notion is sustained by the fact that chemically-induced apoptosis can be blocked by Cd and so foster the accumulation of aberrant cells (98).
Most reports on Cd’s effects on sperm quality are based on studies of humans exposed to Cd, or experiments that exposed rats and mice to this metal (Table 1). Observations show that individuals exposed to Cd have low sperm concentrations, motility and vitality, accompanied by a higher number of sperm abnormalities (159-163). In addition, infertile patients have Cd in the bloodstream, and studies have determined that as Cd concentrations rise, sperm concentrations and motility decline (162,164). In this regard, when Cd is administered to rats and mice, they also present low sperm concentrations and motility, accompanied by an increase in the incidence of sperm abnormalities (124, 136, 152, 159, 162). Research on another sperm parameter studied in mice exposed to Cd –apart from concentration, motility and vitality, all of which decrease under exposure to Cd– has found marked increases in the number of abnormalities in
| Parameter | Description | Species | Reference |
|---|---|---|---|
| Motility | Reduction | Rats | (135, 151, 158, 161) |
| Mice | (164) | ||
| Asthenozoospermia | Humans | (158-163) | |
| Concentration | Reduction | Rats | (123, 154, 161) |
| Mice | (164) | ||
| Oligozoospermia |
Humans | (158-163) | |
| Morphology | Reduction | Rats | (123) |
| Teratozoospermia | Humans | (163, 158, 160, 162) | |
| Live | Reduction | Mice | (164) |
| Humans | (158, 160) |
the heads of the spermatozoids, associated with a premature acrosomal reaction and an increase in DNA fragmentation (165). Certain studies help us understand the mechanisms through which Cd causes alterations in sperm parameters. Thus, we know that while sperm motility can be affected by various mechanisms, one of the most important ones is Cd, because it is the principle competitor of Ca, an especially important regulator of sperm motility (166, 167). Here, studies with mice have demonstrated that the activity of the CatSper channel is modified by Cd exposure and that this affects sperm motility (136, 168). In addition to this, however, Cd can also reduce levels of sialic acid (the terminal carbohydrate of the glycoproteins) in spermatozoids (169). Sialic acid is important because it enhances sperm motility by preventing “friction”. But Cd also increases ROS concentrations (149) that, according to some reports, can cause oxidative stress that damages the spermatozoids’ membrane and alters tyrosine phosphorylation in the sperm. This further diminishes sperm motility and their capacity to fuse with the oocyte, thus interfering with the fertilization process (170). Cd, however, is not an active metal in the redox system; that is, it does not generate ROS through a pathway similar to the Fenton reaction. Rather, Cd can bond to the sulfhydryl groups of ROS regulators, like GSH, to prevent them from functioning as antioxidants. This allows the ROS to increase –indirectly– and generate greater oxidative stress (171, 172).
Pulsatile secretion of GnRH by the hypothalamus is necessary for maintaining male reproduction. However, GnRH and gonadotrophin secretion varies constantly during sexual development. Neuroendocrine stimulation of the HHT reproductive axis begins during fetal development (173) when GnRH neurons are observable, though the connections between these neurons and the portal system of the hypothalamus and hypophysis do not become functional until around week 16 of gestation in humans, thanks to the secretion of GnRH (174). GnRH neurons are functional at birth but remain tonically-repressed during childhood after the cascade of perinatal androgens. Hypothalamic GnRH secretion increases at the onset of postnatal life and leads to the temporal activation of gonadal steroidogenesis, especially in males, though this remains quiescent because the release of pulsatile GnRH is suppressed until the onset of puberty, when GnRH release is reactivated (175). As puberty progresses, the gonadotropins begin to secrete continuously to stimulate T synthesis, which is what fosters sexual development (176). At that moment, the increase in T levels, together with its conversion into the active metabolite dihydrotestosterone (DHT), triggers the development of the secondary sex characteristics and growth of the reproductive organs, while also increasing the libido by converting T into estradiol (177). In adulthood, pulsatile GnRH continues to stimulate the biosynthesis of LH and FSH that, in turn, maintain the production of intragonadal T and spermatogenesis, as well as the systemic secretion of T (178).
Puberty is a period that comprises a complex series of changes that lead to sexual maturation and the acquisition of reproductive capacity (179). Its onset is controlled by a sophisticated regulatory system in which the brain centers –hypothalamus and hypophysis– govern the peripheral sex glands: the testicles in males. GnRH secretion is of two distinct types: pulsatile and cascade (180), but the latter occurs only in females. The pulsatile mode refers to the episodic release of GnRH through distinct pulsations of GnRH secretion into the circulation portal, while GnRH levels are undetectable during the inter-pulse intervals (181). The precise neuroendocrine detonator of puberty is GnRH which, in turn, is stimulated by kisspeptin (KISS1) (39). During this process, the HHT axis is characterized by a marked increase in the secretion of pulsations of LH that stimulate the testicles to secrete T (39). As puberty advances, the gonadotropins begin to secrete continuously, bringing sexual development to completion (39). KISS1 was identified in 2003 as a 54-amino acid codified peptide and, with its receptor (KISS1R, or GPR54), is now recognized as the key actor in the HHT axis (182). KISS1 is synthesized and secreted by hypothalamic nuclei in the arcuate nucleus and anteroventral periventricular nucleus (183). There are reports that stimulation of the GnRH neurons by KISS1 activates the hypothalamic-hypophysis-gonad (HHG) axis and secretion of gonadotrophins in the hypophysis (184). We also know that the expression of KISS1 and its receptor increases during puberty in rodents and primates (185, 186). Therefore, administering KISS1 in animals –both males and females– can stimulate the secretion of GnRH and gonadotropins (187).
The rat is a useful animal for studying sexual development, and one widely used to analyze the mechanisms that underlie the process of sexual maturation, which are quite similar across numerous species and so can be extrapolated to other animals, including humans. The mechanisms involved in sexual development include the onset of gonadotropin secretion, the action mechanisms of the sex steroids, and positive and negative feedback circuits (188). In male rats, puberty is considered the stage in which fertility becomes evident, which occurs between days 42 and 49 of postnatal life (188, 189). This phase is associated with behavioral events like genital grooming (GG), which consists of licking the testicles, penis and anogenital region. This behavior intensifies between 46 and 48 days of age (190-192), and its frequency is regulated by LH and T levels (193).
Turning now to the context of male sexual behavior, studies have described that for intromissions to occur, there must be penile reflexes that include erections and dorsiflexion of the penis, described as fast whipping (190, 194). This also requires the formation of a cup in the penis (i.e., the broadening of the glans penis associated with the moment of ejaculation). It is well-known that in prepubescent male rats there is a close relation between circulating T concentrations and the development of penile reflexes (195, 196). Another marker of puberty in males is preputial separation (PS), which occurs in the peripubertal stage, and becomes evident when the prepuce begins to gradually retract from day 41 to day 47 of life (192, 197).
There are reports that peripubertal exposure to Cd affects the functioning of the HHT axis by reducing the weight of the male sex organs –testicles, epididymis, seminal vesicle, prostate– and decreasing blood T levels in adulthood (132). We also know that prenatal administration of Cd affects some puberty markers in male rats, and studies report that administering high doses of CdCl2 (10 and 20 mg/Kg) to gestating mothers delays the descent of the testicles in their offspring (198), although other reports have found that maternal exposure to Cd advances this parameter (199). Several other puberty markers, however, remain to be evaluated, including PS and GG, so that exposure to Cd in the perinatal stage could still be found to affect the onset of puberty. Studies in young humans living in contaminated zones of Italy found a delay in the onset of puberty accompanied by a decrease in testicular volume and low T levels (200). However, no one has yet analyzed whether these findings affect the first pulse of GnRH or KISS1 in this stage. In this regard, some studies have found that exposure to Cd and other contaminants increases the expression of KISS1 in the human placenta (201).
Recently, evidence has accumulated to indicate that certain heavy metals –including Cd– can be considered endocrine disruptors (ED) (202). ED are compounds –natural or synthetic– that interfere with biosynthesis, metabolism, or the action of endogenous hormones. Numerous chemical substances belong to this category, including Cd. Most studies show that ED can imitate the activity of endogenous hormones and so reproduce equivalent effects. The biological effects of these compounds occur through a genomic action mechanism; that is, they act as hormonal agonists for a specific receptor (203). However, there are also clear indications that non-genomic action mechanisms are present and fully capable of altering, or at least affecting, the synthesis, transport and availability of endogenous hormones (203). Also, ED blocks the activity of hormones by competing for receptors or affecting the physiological concentration of a specific one (203). There is disagreement in the scientific community regarding evaluations of the possible risks of exposure to ED. Several researchers believe that this is harmful to health because ED can contribute significantly to the development of breast, ovarian, or testicle and prostate cancer (204-208). Others, however, sustain that additional studies are needed to adequately analyze whether exposure to ED can produce adverse effects on human health (209-211). The effects of both ED and Cd, however, do include inhibition of the steroidogenic enzymes and activation of estrogen receptors (ER) or AR, as will be described in greater detail below. Cd is included among the ED because it affects the synthesis and/or regulation of various hormones, including LH and FSH (212, 213). Cd also affects the synthesis of progesterone in JC-410 porcine granulose cells and activates estrogen receptor alpha (ERα) and/or mimics its estrogenic effect in various tissues, such as the uterus and mammary gland, as well as in breast cancer cell lines (214-216).
In recent years, we have learned that many environmental compounds mimic the physiological activity of estrogens. These include xenoestrogens, phytoestrogens and metalloestrogens (217). Cd is a predominant environmental contaminant that is reported to have effects as a potent metalloestrogen (218) which mimics the activity of the estrogenic hormones (219-222) by bonding to the nuclear-type ERs denominated ERα and estrogen receptor beta (ERβ) (216, 220, 223-229), and to the transmembrane estrogen receptor GPR30 (228, 230). ERα and ERβ are codified by distinct genes and their expression varies with the type of tissue involved. ERα is expressed predominantly in reproductive organs like the uterus, breast and ovaries, as well as in the liver and central nervous system, while the β form is expressed mainly in other tissues, including bone, the endothelium, the lung and the urogenital tract, but also the ovaries, the central nervous system and the prostate (231, 232). In the absence of the hormone, ERα is sequestered in an inactive compound and repressed by such molecular chaperones as thermal shock proteins. But the union with the hormone induces a change in the conformation of the ligand-binding domain that releases the receptor from the inactive compound and, as a result, eliminates the masking effect of the ligand-binding domain. The receptor is dimerized and later bonds to the response elements of the DNA in a complex process that requires recruiting coactivators of steroid receptors (SRC-1, SRC-2 and SRC-3) (233). Once bonded, the coactivators recruit the co-integrator p300/CBP (CREB-binding protein). The co-regulator compound then stimulates transcription by remodeling the chromatin through its ability to acetylate histones and interact with the machinery of basal transcription (234).
Using transitory transfection assays to block the union of 17βestradiol to ERα in a non-competitive manner, studies have demonstrated that Cd activates ERα (219, 220, 225, 227). This suggests that it interacts with the receptor’s ligand-binding domain. The amino acids cys381, cys447, glu523, his524 and asp538 have been identified as possible interaction sites of Cd with the ERα's ligand-binding domain (235). These amino acids, which play a role in the interaction of Cd with ERα, are located in the H4, H8, H11 helixes and the interface of the loop with the H12 helix. In this way, Cd’s interaction with different amino acids can promote localized folding, as in the case of the zinc finger domain, or the assemblage of different regions of the protein in a domain (235). Finally, the effects of Cd can be blocked by an anti-estrogen, suggesting that this metal’s effects are mediated by the genomic ERα pathway and activate the non-genomic ERα pathway through ERK1/2 and Akt (216, 228).
Like E2, Cd induces cellular proliferation (216, 219, 225) and increases the transcription and expression of estrogen-regulated genes, such as the progesterone receptor (PR) (216, 219, 225). In fact, in studies with rodents Cd begins to mimic the effects of E2 in target organs (236) after just one intraperitoneal injection of 5 μg/kg of CdCl2. In ovariectomized female Sprague-Dawley rats, an increase in the net uterine weight was observed, accompanied by a proliferative response by the endometrium, which promotes the growth and development of the mammary glands and induces hormone-regulated genes. Also, the female descendants of those rats experienced early onset puberty (236).
Various reports indicate that Cd has a potential role in the development of hormone-dependent cancers (218, 219, 221, 222, 230). For example, in utero exposure to Cd increases the risk of developing breast cancer. Parodi et al. (237) observed that treating pregnant female rats with Cd altered the development of the mammary gland before the onset of puberty in female offspring by increasing the number of mother/progenitor cells, cell density and REα expression, all of which increase the risk of developing breast cancer. Finally, in vitro tests have demonstrated that Cd has proliferative action in human prostate cells through an estrogen-dependent mechanism that increases ERα and ERβ expression independently of the androgens (238).
Other research has shown that prostate tumors can be induced experimentally by oral exposure to Cd (157). While some important epidemiological reports indicate a relation between Cd exposure and rates of prostate cancer, their findings have been refuted (239). Over the past decade, research has shown that Cd has potent androgenic-type activities both in vivo and in vitro by bonding directly to AR. In transfection assays, Cd activates a chimera that contains the ligand-binding domain of AR (240), allowing it to bond to the ligand-binding domain with a high degree of affinity (240, 241) and to block androgen’s union to the receptor. Moreover, it can compete with DHT, the natural ligand of AR (240). The union of Cd with AR modifies the conformation of the receptor (242), and so could change its transcription potential. Scatchard analysis demonstrated that Cd binds to AR with a dissociation constant in an equilibrium of 1.19 × 10-10 M. Work with cell lines has shown changes in AR activity or AR expression when cells are exposed to Cd (240, 243-245). Epidemiological studies evaluating the relation between Cd and AR have analyzed blood, toenails and urine, exposure to Cd and indirect measurements of the function of AR, such as the level of the specific prostate antigen in serum (246-249). To date, however, these approaches have produced only mixed results, while failing to confirm any clear interaction between Cd exposure and AR signaling in the human prostate. Evidence from basic scientific studies suggests that Cd may play a role in prostate cancer by interrupting AR, a hormone-activated transcription factor that is the key driver of the progression of cancer in that organ (250) although, ironically, AR is necessary for normal prostate growth and development. Finally, research has demonstrated that prostate tumors can be induced experimentally by oral exposure to Cd (251).
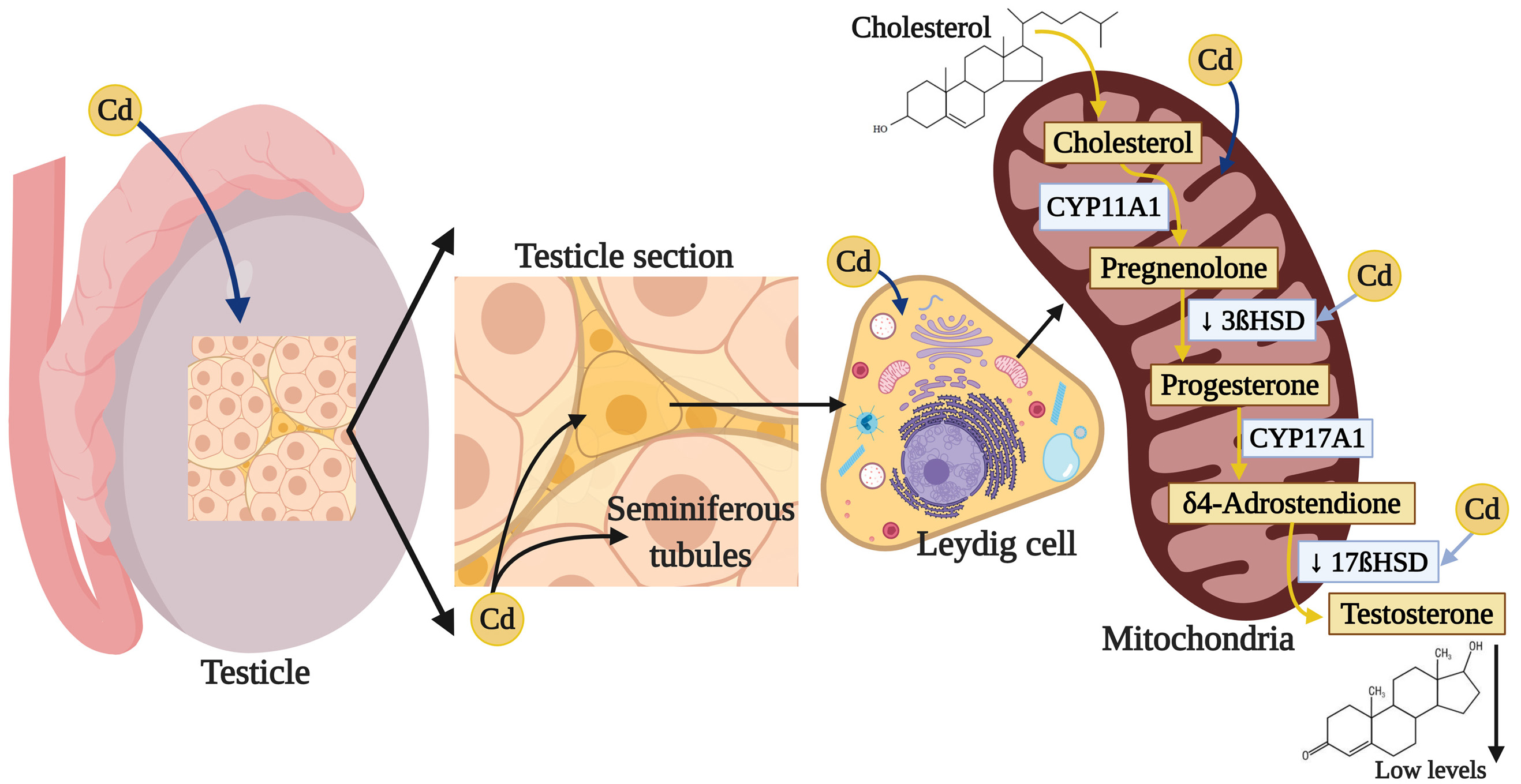
Testosterone (4-androstenol, 14-ol, 3-one) is a steroid hormone belonging to the family of the androgens, with a structure derived from cyclopentanoperhydrophenanthrene. It is composed of 19 carbon atoms, with methyl groups at carbons 10 and 13, and a hydroxyl group at carbon 17 (252). This hormone is synthesized from cholesterol in the Leydig cells in the testicles (253). The biosynthesis of T is subject to short-term regulation controlled by LH, which binds to specific receptors on the surface of the Leydig cells and stimulates cAMP production. cAMP performs two principle activities in controlling steroidogenesis in the Leydig cells. The first is to acutely stimulate the biosynthesis of T by translocating cholesterol from the external mitochondrial membrane towards the internal one, which generates the synthesis of pregnenolone through the action of the cytochrome enzyme P450scc/CYP11A1 (cholesterol side cleavage enzyme), which is found in the internal mitochondrial membrane. cAMP’s second action is to activate A kinase (PKA) that, through the phosphorylation of transcription factors related to the activation of genes that codify for steroidogenic enzymes, phosphorylates proteins involved in transporting cholesterol towards the mitochondria, as is the case of the StAR protein (steroidogenic acute regulatory protein) (254). Pregnenolone is biotransformed into progesterone by the 3βhydroxysteroid dehydrogenase Δ4-5 isomerase enzyme (3β-HSD) (252). Then the progesterone obtained through the Δ4 pathway is transformed into androstenedione by the enzymatic compound 17α hydroxylase C17-20 lyase, which is dependent on cytochrome P-450 (P-450c17). The final step in T synthesis is regulated by the microsomal enzyme 17β-hydroxysteroid dehydrogenase (17βHSD), which catalyzes the conversion of androstenedione into T. The endogenous production of steroids in the Leydig cells regulates the expression of the enzymes involved in T biosynthesis (253).
Multiple studies show that exposure to Cd can affect various enzymes that participate in T synthesis; for example, chronic exposure to CdCl2 in adult male rats increased testicular cholesterol (255) but, in contrast, decreased expression of the ARNm of class B type I Scavenger receptor (SR-BI), which facilitates the capture of cholesterol esters (256) from the StAR protein (257, 258), since Cd increases the activation of the cAMP-responsive element-binding protein (CREB) transcription factor (258). In addition, Cd reduces the expression and activity of the 3β-HSD and 17β-HSD enzymes (255, 259), while decreasing the expression ARNm by the CYP11A1, 17α-hydroxylase and 11β-hydroxysteroid dehydrogenase enzymes (11β-HSD), which intervene in steroidogenesis in the Leydig cells (256) (Figure 3). The result of this complex process is a decrease in T synthesis that has been amply reported after exposure to Cd (132).
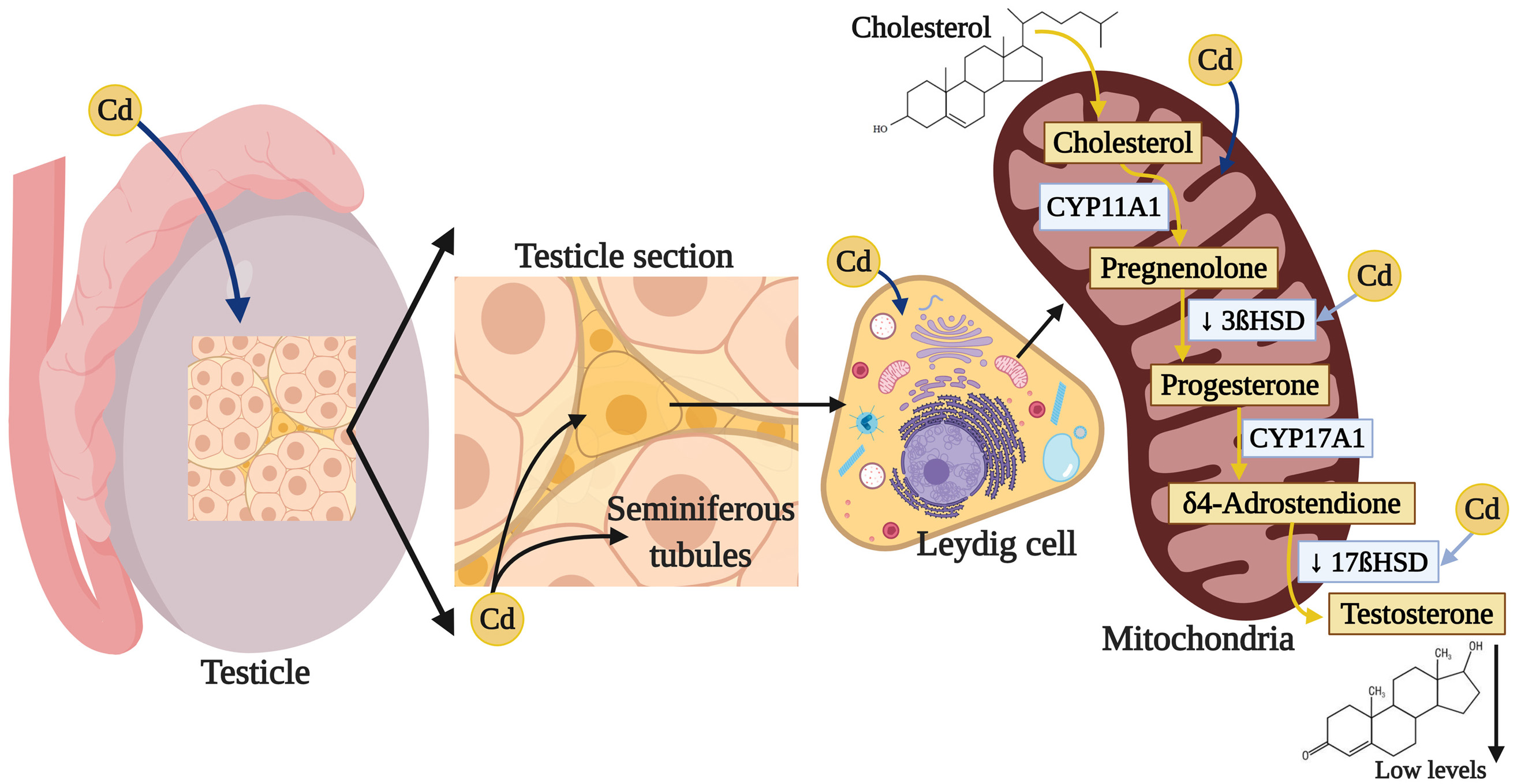 Figure 3
Figure 3Damage of cadmium in testicular steroidogenesis. Cadmium can affect various enzymes that participate in T synthesis by reducing the expression and activity of 3β-HSD and 17β-HSD, which interfere in steroidogenesis, specially in the Leydig cells, the result of this process is a decrease in T.
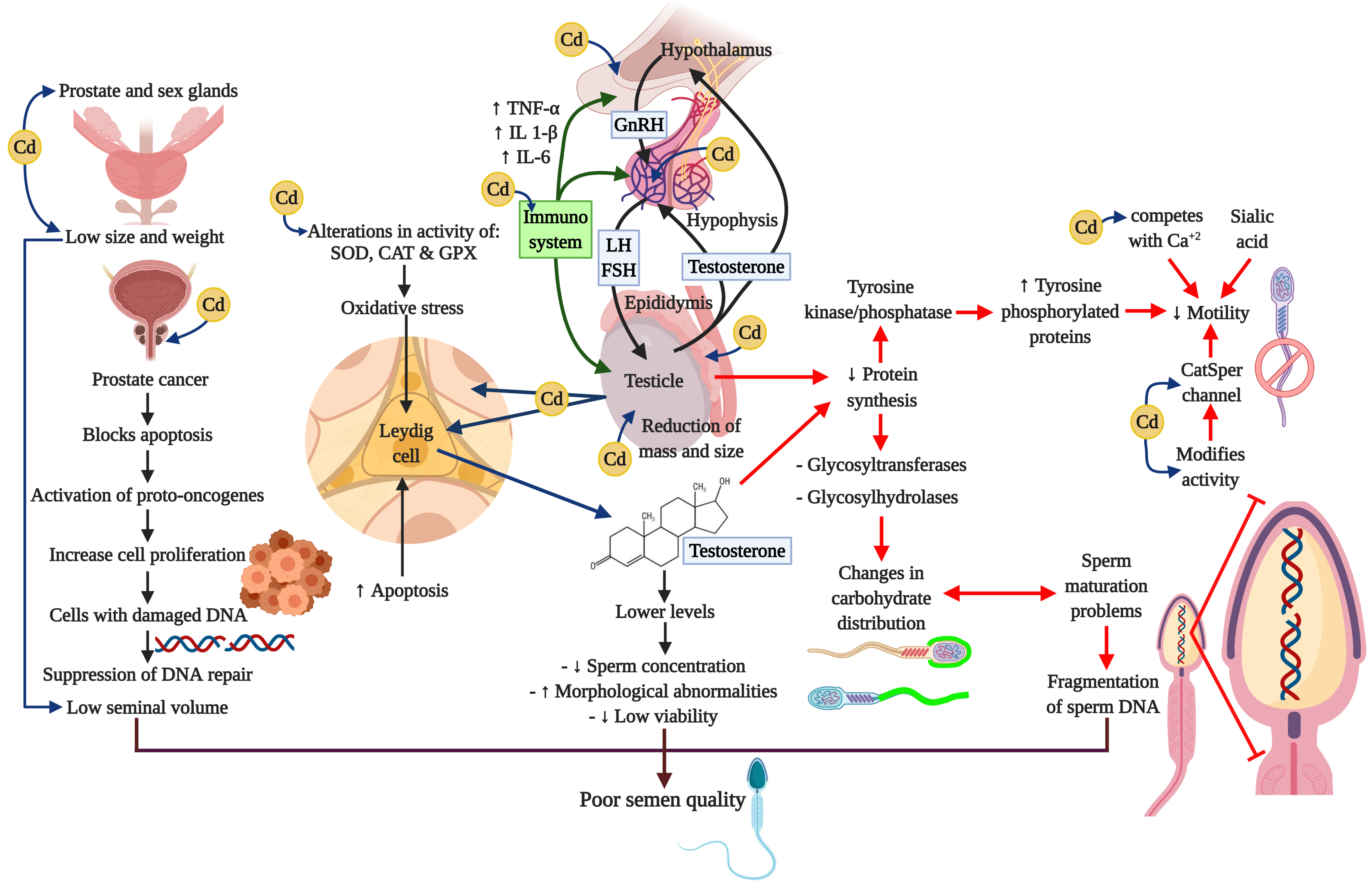
A considerable amount of information has been amassed indicating that the immune system can have profound effects on the hypothalamic-hypophysis-testis axis. Figure 4 summarizes the principle affectations of the Cd on the HHT axis, and the effects of it related to the release of cytokines from a variety of activated immune cells that can regulate the secretion of GnRH and LH or have direct actions on Leydig and Sertoli cells (260-263). Cytokines that play important regulatory roles in the normal functioning of the HHT axis include the proinflammatory cytokines tumor necrosis factor-alpha (TNF-α), interleukin-1beta (IL-1 β), and interleukin-6 (IL-6). TNF-α is released from a variety of cell types, including macrophages, and is a marker of systemic and local inflammation in smokers (264). Moreover, a recent study reports that these three cytokines cause a dose-dependent decline in steroidogenesis in the TM3 mouse Leydig cell line (265); whereas TNF-α infusion into hypothalamic fetal cells in culture decreases GnRH secretion (266). Research has also shown that chronic low-dose cadmium exposure in rats can significantly increase the interferon gamma (IFNγ) and interleukin-10 (IL-10) levels, suggesting that Cd may enhance inflammatory responses (267). It is well-known that inflammation is also associated with oxidative stress and can impair male reproductive functions (268-270).
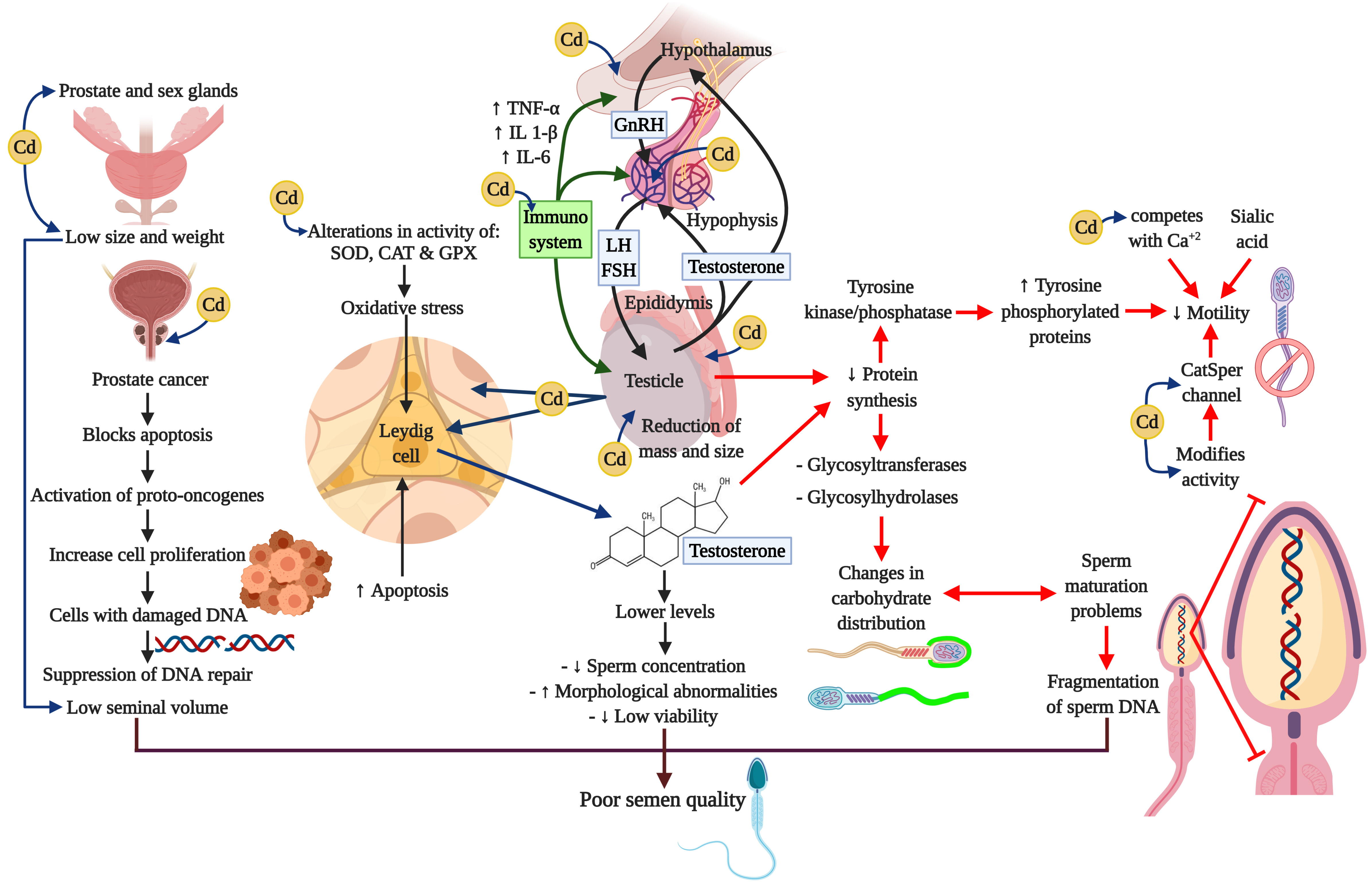 Figure 4
Figure 4Cadmium and negative impacts on neuroendocrine axis. Cadmium alters the oxidative stress, the signaling pathways, and interrupts neuroendocrine processes. These actions cause serious damages in male reproductive organs such as the prostate, the testis and the epididymis, and prevents processes like steroidogenesis, spermatogenesis, and epididymal maturation. Besides, Cd can induce adverse effects in immune system through the cytokines. All these alterations may compromise the reproductive capacity.
Adverse effects of Cd exposure induce testicular dysfunction, inhibition of spermatogenesis, and male infertility. This phenomenon is attributed to activation of the “inflammasome” and augments the inflammatory response, an intracellular multiprotein complex, which is activated by tissue damage induced by Cd (271). Several studies have reported that Cd exposure increased TNF-α, IL-6 and IL1β generation in testicular tissue. When adult male mice were injected intraperitoneally with CdCl2 at a dose of 2 mg/kg body weight per day for seven consecutive days, Cd-treatment increased IL-1β and TNF-α concentrations in a statistically-significant manner, though this effect was precluded by incubation with melatonin, thus decreasing oxidative damage to the sperm (272). Similar results were reported recently in rat and chicken testes. In the first experiment, male rats received only one injection of CdCl2 (2 mg/kg, i.p.) on day 9 and TNF-α increased 2-fold; while the administration of a potent anti-inflammatory compound (Diacerein) inhibited the Cd-induced effect (271). In the latter report, chickens were fed with normal full-fodder with 140 mg/kg of CdCl2, and their TNF-α, IL-1β, and IL-6 mRNA levels were significantly higher in the Cd group. In those experiments, levels of an inflammatory factor were precluded with Ganoderma, a medicinal mushroom that has enhanced anti-inflammatory effects (273).
Taken together, these studies suggest that exposure to Cd induces the inflammatory response by activating the “inflammasome” in different cells of the male reproductive system.
Cd is a toxic agent present in the environment that can have negative impacts on the HHT axis through diverse toxicity mechanisms, including oxidative stress, inhibition of the Ca channels, alterations of the signaling pathways, and immuno-endocrine interruption. These actions cause the primary male reproductive organ –the testicle– to lose such key functions as steroidogenesis, spermatogenesis, and epididymal maturation. Moreover, Cd can exert adverse effects on the HHT axis through the activation of the “inflammasome” in different cells of the male reproductive system, modifying the onset of puberty, the development of sexual maturity, adult sexual behavior and even fertility; thus compromising the reproductive capacity of individuals so affected.
This work was supported by the Division of Biological and Health Sciences at the Autonomous Metropolitan University, Campus Iztapalapa. CONACyT funding was provided to JHR (CVU: 622536).
HHT
hypothalamic-hypophysis-testis
cadmium
particles measuring 2.5 micrometers
sulfur dioxide
carbon monoxide
nitrogen dioxide
ozone
mercury
arsenic
lead
chrome
nickel-cadmium
cadmium oxide
cadmium chloride
cadmium sulfate
World Health Organization
oxygen species
positive charges
positive charges
calcium
iron
magnesium
manganese
zinc
selenium
sulfhydryl or thiol group
Ca voltage-gated channel
family divalent metal transporters
family of metal transporters
transporter CaT-1
transient receptor potential cation channel, subfamily V
voltage-dependent Ca channels
metallothioneins
adenosine triphosphate
apoptosis-inducing factor
Ca-sensitive receptor
C phospholipase
inositol triphosphate
pro-apoptotic protein
catalase
glutathione peroxidase
superoxide dismutase
glutathione
transduction initiation factors located in eukaryotes
extracellular signal-regulated kinase ½
phosphatidylinositol 3-kinase
protein kinase B
redox effector factor 1
nuclear factor E2-related factor 2
nuclear transcription factor-kappa B
int/Wingless protein
B-cell lymphoma 2
activator protein-1
gonadotrophin-releasing hormone
luteinizing hormone
follicle-stimulating hormone
testosterone
serotonin
γ-aminobutyric acid
α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid
N-methyl-D-aspartate
serotonin 1A receptor
serotonin 2 receptor
blood-testicle barrier
substrate of the epidermal growth factor 8 receptor pathway
androgen receptor
cyclic adenosine monophosphate
dihydrotestosterone
kisspeptin
kisspeptin receptor
kisspeptin receptor
hypothalamic-hypophysis-gonad
genital grooming
preputial separation
endocrine disruptors
estrogen receptor
estrogen receptor alpha
estrogen receptor beta
transmembrane estrogen receptor
steroid receptor coactivator-1
CREB-binding protein
cytochrome enzyme
cholesterol side cleavage enzyme
kinase A
steroidogenic acute regulatory protein
3βhydroxysteroid dehydrogenase Δ4-5 isomerase enzyme
17α hydroxylase C17-20 lyase
17β-hydroxysteroid dehydrogenase enzyme
B type I Scavenger receptor
cAMP Response Element-Binding Protein
tumor necrosis factor-alpha
interleukin-1beta
interleukin-6
mouse Leydig cell line
interferon gamma
interleukin-10