Academic Editor: Graham Pawelec
Puerarin is a compound from the group of isoflavones, naturally occurring in plants of the genus Pueraria, whose representatives include, among others, Pueraria lobata and Pueraria mirifica. Relatively many scientific studies on the biological activity of puerarin have been conducted so far. It seems that most attention was paid to the effect of puerarin on bone health. However, until now, no published studies have been collected and discussed in that regard. Based on the available data obtained from in vitro studies and on the animal model, it can be clearly shown that puerarin is an effective compound in inhibiting bone resorption and improving bone structure. Consumption of puerarin may be associated with the prevention of bone mass loss and thus can reduce the risk of developing osteoporosis. However, it is necessary to conduct human intervention studies to confirm the effectiveness of such action.
Osteoporosis is a metabolic bone disease associated with reduced bone mass as well as increased degradation of bone microarchitecture. As a result, it is characterized by reduced bone strength and increased risk of fractures [1]. Osteoporosis is a major public health challenge worldwide [2]. It is estimated that in 2010 osteoporotic fractures caused 43,000 deaths in the European Union alone [3]. As for the USA, nearly 54 million Americans have too low bone mass and show an increased risk of fractures [4]. In addition, more than 8.9 million fractures per year are reported worldwide. The majority of these fractures concern the European population (34.8%) [1]. Therefore, it seems necessary to take all measures to prevent this disease. The most important, modifiable risk factors for osteoporosis include lack of physical activity, smoking and improper nutrition, mainly related to the deficiency of nutrients essential for bone structure and homeostasis, including vitamin D and calcium [5]. However, a number of other compounds present in the diet are also significant for bone health. These include vitamin C [6], potassium, magnesium, omega-3 fatty acids [7], carotenoids (e.g., lycopene, beta-carotene), polyphenols [8]. A group of phytochemicals particularly well-studied in terms of their effect on bone health includes soy isoflavones, including, above all, genistein and daidzein [9]. However, there are many other compounds among isoflavones which are derived not only from soya. One of isoflavones, puerarin (daidzein-8-C-glucoside) (Fig. 1), has become an object of interest in recent years. It is a major bioactive component found in the root of plants of the genus Pueraria (common name kudzu). Puerarin was isolated from such species as Pueraria lobata [10] and Pueraria mirifica [11]. Puerarin was extracted for the first time in 1950 [12]. Since then, its biological activity has been broadly analyzed. A number of health benefits are attributed to puerarin, i.e., antioxidative [13], anti-inflammatory [14], neuroprotective [15], hepatoprotective [16], anticancer [17], antidiabetic [18], cardioprotective [19], and anti-atherosclerotic effects [20]. This study reviews the current state of knowledge regarding the importance of puerarin for bone health. Based on the results of in vitro experiments and animal studies, it was discussed how it affects bone mineral density, bone markers and structural parameters.
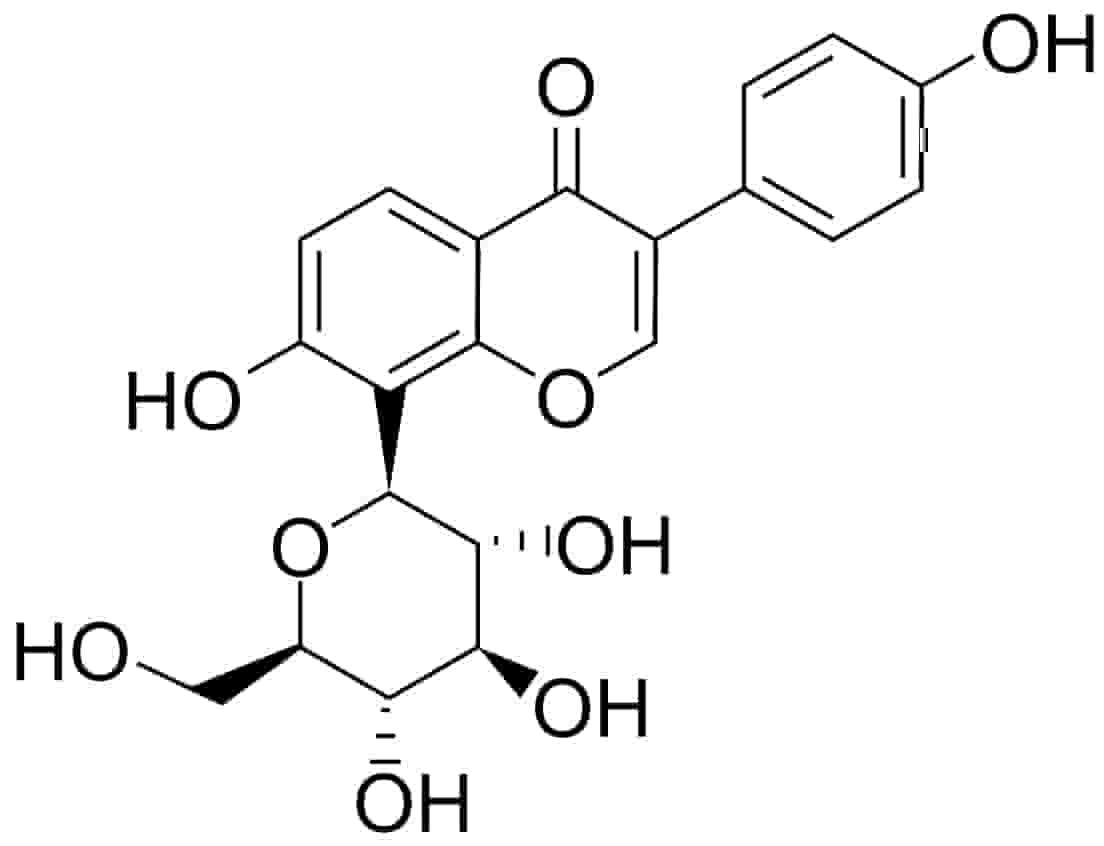 Fig. 1.
Fig. 1.Chemical structure of puerarin (daidzein-8-C-glucoside).
Many in vitro experiments in which the effect of puerarin on bone health is analyzed via various mechanisms, have been conducted so far (Fig. 2). In their research, scientists focused primarily on determining the effects of puerarin on osteoblasts and osteoclasts. The results obtained are discussed below.
 Fig. 2.
Fig. 2.The effect of puerarin on bone health.
Li et al. [21] analyzed the effect of puerarin (5–20
An experiment conducted by Yuan et al. [22] has proven that puerarin
(10
Li and Peng proved that puerarin (10–100
| Cell cultures | Study design | Source of puerarin | Effects | References |
| Osteoblasts from female, 3-months old mice | Puerarin 6’O-xyloside, 5, 10, 20 mM | Shanghai Tauto Biotech Ltd. Co., Shanghai, China | [21] | |
| RAW264.7 cells | Puerarin, 10 |
PUMC Pharmaceutical Co., Ltd., Hebei, China | [22] | |
| Mouse osteoblastic MC3T3-E1 cells | Puerarin, 1 |
PUMC Pharmaceutical Co., Ltd., Hebei, China | [22] | |
| Human osteoblastic MG-63 cells | ICI Puerarin, 0.1 µM, Puerarin + ICI | The National Institutes for Food and Drug Control, Beijing, China [purity |
[23] | |
| Osteoblastic MC3T3-E1 cells | Puerarin, 5, 10, 20, 40 |
National Institutes for Food and Drug Control, Beijing, China | [24] | |
| Osteoblastic MC3T3-E1 cells | Puerarin, 0.1, 1, 10 |
No data | [25] | |
| Osteoblasts from Sprague-Dawley rats | Puerarin, 0.01, 0.03, 0.1 mM | National Institutes for Food and Drug Control, Beijing, China | [26] | |
| Osteoblastic MC3T3-E1 cells | Puerarin, 1, 10, 20 |
Nanjing TCM institute of Chinese Materia Medica (TCM054-110528, China) | [27] | |
| Rat calvaria osteoblasts | 17-beta-estriadol group, Puerarin, 2.5, 5, 10, 25, 50, 100 µmol/L | Beijing Institutes for Food and Drug Control, Beijing China [purity 98.5%] | [28] | |
| Human osteoblasts from femur | Puerarin, 10 |
Sigma, Inc., USA | [29] | |
| Human osteoblastic MG-63 cells | ICI, 100 µM, Puerarin, 0.1 µM, Puerarin + ICI, Puerarin, 0.01, 0.1, 1 |
National Institutes for Food and Drug Control, Beijing, China [purity |
[30] | |
| Primary baboon osteoblasts | P. mirifica extract, 100 |
P. mirifica (Chiang Mai Province, Northern Thailand) | [31] | |
| Puerarin (LKT Laboratories Inc., St. Paul, MN, USA) | ||||
| Osteoblasts from calvarial bone of Wistar rats | Puerarin, 10 |
National Institutes for Food and Drug Control, Beijing, China | [32] | |
| Osteoblasts from newly born Sprague Dawley rats | Puerarin, 0.01, 0.1, 1 |
National Institutes for Food and Drug Control (NIFDC), China | [33] | |
| RAW264.7 cells | LPS + Puerarin, 10, 25, 50 |
National Institutes for Food and Drug Control, Beijing, China [purity |
[34] | |
| Bone marrow-derived macrophages from femur of male C57BL/6J mice | Puerarin, 1, 5, 25 |
Sigma-Aldrich (St. Louis, USA) [purity |
[35] | |
| RAW264.7 cells | Puerarin, 10, 25, 50, 100 |
No data | [36] | |
| Human periodontal ligament stem cells (PDLSC) | Puerarin, 10, 50, 100 |
Inchem Corp., Rock Hill, SC, USA [purity |
[37] | |
| Bone marrow cells | Puerarin, 0.01 mg/mL | - | [38] | |
| from the midshafts | ||||
| of 6- to 8-week-old | ||||
| male and female | ||||
| mouse femurs | ||||
| ALP, Alkaline phosphatase; BAP, Bone alkaline phosphatase; BMC, Bone mineral
content; BMD, Bone mineral density; BV/TV, Bone surface/total volume; BS, Bone
surface; Ct, Cortical bone tissue; CTx, C-terminal telopeptide; Ca, calcium; DPD,
Deoxypyridinoline; EE, 17 alpha-ethinylestradiol; FM, Femoral metaphysis; FD,
Femoral diaphysis; L4, Fourth lumbar vertebra; LPS, Lipopolysaccharides; MCF-7
cell, epithelial luminal cell line; MMP, Matrix metalloproteinase; NFATc1,
Nuclear factor of activated T-cell, cytoplasmic 1; Ob.S, Osteoblast surface;
Oc.S, Osteoclast surface; OVX, ovariectomized group; ORX, orchidectomized; OPG,
Osteoprotegerin; OPN, Osteopontin; PPAR | ||||
The results obtained from in vitro studies are widely confirmed in tests carried out on animal models. It has been proven many times that the administration of puerarin had a beneficial effect on bone markers, bone mineral density and bone structural parameters.
Li et al. [21] proved the anti-osteoporotic activity of puerarin 6-O-xyloside. They showed that ovariectomized rats (OVX) treated with puerarin (intraperitoneal injection) (20, 40, 60 mg/kg/d) had significantly higher blood calcium levels compared to the control OVX group. Additionally, higher blood phosphorus levels were observed in animals treated with puerarin. However, this effect was differed depending on the dose. The highest level of the indicated components was found with the administration of puerarin at 60 mg/kg/d. This research also assessed ALP activity. It was observed that the use of puerarin at doses of 40 or 60 mg/kg/d increased serum levels of ALP. At the same time, an increase in osteoprotegerin levels was observed [21]. Another study that proved the anti-osteoporotic effect of puerarin was conducted by Michihara et al. [39]. They showed that in ovariectomized mice during 8-week oral (per os) administration of puerarin (5 mg/kg/d) urinary DPD (doxypyridinoline) levels were lower compared to mice that did not consume this isoflavonoid. In the conducted experiment it was observed that the control group had a significantly lower number of femoral trabeculae than the OVX-puerarin group, which indicates that puerarin may protect the trabecular structure from destruction. In the group fed with puerarin a significantly lower activity of TRAP, which is a bone absorption marker, was also found. It should be noted, however, that the administration of puerarin did not affect the level of osteocalcin. Based on the observed lack of effect of puerarin on morphology and weight of oviducts and uterus, the researchers indicated that the observed properties are independent of the estrogen receptor-mediated pathway. They also proved this effect by observing that puerarin did not stimulate the growth of MCF-7 cells [39]. Similarly, Tanaka et al. [40] noted that puerarin may inhibit ovariectomy-induced bone loss. In the experiment, the researchers administered kudzu vine ethanol extract (PVEE) per os to female mice. Of isoflavones present in it, puerarin was found in the highest amounts (52%). This extract was administered at 20 mg/kg/d for 8 weeks. The authors of this research showed that animals which consumed PVEE had lower levels of bone resorption markers such as DPD and TRAP compared to OVX mice. At the same time, it was noted that the use of PVEE inhibited the reduction in femoral bone mineral density. A lower number of matured osteoclasts in the distal femur was also observed in the group treated with PVEE. On the other hand, administration of PVEE did not affect the serum bone-specific alkaline phosphatase activity. At the same time, Liang et al. [41] noted that puerarin may have a beneficial effect on bones in rats with diabetes. It was observed that daily intraperitoneal administration of puerarin (60 mg/kg/d) caused a decrease in caspase-3 expression in osteoblasts. The authors of the experiment suggest that high blood glucose levels are associated with an increase in the expression of caspase-3, which in turn may contribute to osteoblast apoptosis and lead to the development of osteoporosis. Moreover, Luo et al. [42] proved that oral administration of kudzu root extract (0.45, 0.9 and 1.8 g/kg/d) to ovariectomized rats may have a beneficial effect on bone health. In the conducted studies it was observed that the use of kudzu root extract resulted in lowering the serum levels of C-terminal telopeptide of collagen type I (CTx). This indicates that this extract may inhibit bone resorption. Additionally, it has been shown that the administration of P. lobata extract (100 mg/kg/d) results in a statistically significant reduction in the levels of osteocalcin as well as ALP and CTx, which are markers of bone resorption and remodeling [43]. It is also worth mentioning that studies conducted by Wang et al. [38] on an animal model showed that puerarin may inhibit alcohol-induced osteonecrosis. Marrow and bone necrosis were found in mice belonging to the model group treated with ethanol (intragastric). An increase in the number of empty osteocyte lacunae in the femur was also observed. Such adverse lesions were not found in mice treated with intramuscular injection of puerarin (0.5 g/kg/d). Additionally, higher expression of osteocalcin mRNA was found in animals treated with puerarin [38]. Research results published in 2020 also indicated that puerarin (15.4 and 30.8 mg/kg/d) administered i.p. inhibits titanium particle-induced osteolysis [44]. Zhang et al. [34], on the other hand, proved that calvarial injection of puerarin (1 mg/kg/d) is capable of inhibiting LPS-induced bone loss. The authors found that after the injection of LPS there was a significant increase in the number of osteoclasts and an increase in the area of osteolysis. At the same time, significant reductions in calvaria weight were observed. Mice additionally treated with puerarin had a significantly lower number of osteoclasts as well as decreased osteolysis area. Lower bone mass loss, compared to the puerarin-free group (68.5 vs. 47.9 mg of calvaria weight), was observed as well [45]. The beneficial effect of Pueraria lobata extract (PE) was also confirmed by Lee et al. [46]. They observed that the administration of PE (25–1600 mg/kg) to ovariectomized (OVX) rats resulted in a reduction in the level of bone turnover markers, such as osteocalcin, C-terminal telopeptide fragment of type I collagen, deoxypyridinoline, and pyridinoline, the concentration of which was elevated in OVX rats. The highest effect was seen in animals receiving 1600 mg PE/kg. Moreover, researchers confirmed that puerarin caused an increase in the plasma level of estradiol, which indicates that PE has estrogenic activity and its activity is similar to that of phytoestrogens. Therefore, it seems that puerarin may slow down the osteoporotic changes associated with estrogen deficiency, which is characteristic in postmenopausal women [46].
An experiment by Tanaka et al. [40] showed that ovariectomized mice fed with kudzu vine ethanol extracts (PVEE) had a lower degree of trabecular destruction than mice belonging to the control OVX-group. The beneficial effect of puerarin on bones was also noted in a research conducted by Yuan et al. [22]. Ovariectomized mice received a diet containing puerarin at different doses: 2, 4 or 8 mg/d. The authors observed that mice receiving food enriched with puerarin (2 mg/d) had a higher mineral density of various regions of the femur – proximal femur, middle femur, distal femur compared to animals fed without this additive [22]. In an experiment conducted by Liang et al. [41], femur X-rays showed that animals treated with puerarin had a lower loss of bone mass compared to those not treated with this additive. It was shown that femoral BMD (bone mineral density) in rats treated with puerarin was 9.7% higher than in the control group. Moreover, rats with diabetes had a higher number of osteoclasts clumped together as well as higher cortical bone reduction. In rats treated with puerarin, these lesions were milder [41]. Similar results were also obtained by Cho et al. [47] who administered isoflavones from Pueraria lobata per os to ovariectomized mice. Of isoflavones found in P. lobata extract, puerarin was present at the highest concentration (7.5%). Daidzein (4.2%) and genistein (1.9%) were present in smaller amounts. The researchers found that mice fed with P. lobata extract (200 and 500 mg/kg/d) had significantly higher femoral BMD than those fed without this additive [47]. Similarly, in another study, researchers showed that rats consuming the kudzu root had higher BMD of the middle femur [42]. In turn, Wang et al. [26] proved that puerarin stimulates bone formation. They showed that ovariectomized rats treated with puerarin (i.g.; 20 mg/kg/d) had a higher femur BMD and BMC (bone mineral content). The positive effect of Pueraria mirifica administration on bone health was also noted by Suthon et al. [48]. The researchers showed that rats treated with P. mirifica (50 mg/kg/d) by gavage had a higher total BMD of L4 (fourth lumbar vertebra), as well as trabecular BMD of L4 and tibial metaphysis, compared to rats not treated with isoflavones. The effect of dietary isoflavones from Puerariae Radix on bone metabolism in ovariectomized rats was also studied by Lim et al. [43]. The researchers administered orally P. lobata extract, containing high amounts of isoflavones, to rats. Puerarin constituted 57.6% of all isoflavones. The remaining isoflavones were daidzein (30.4%) and genistein (12.0%). They found that rats treated with the extract at 100 mg/kg/d had a significantly higher femoral bone mineral density compared to the control OVX-group. Such an effect was not observed in the group treated with P. lobata extract at 30 mg/kg/d. Yang et al. [49] showed that rats with ligature-induced periodontitis and treated with puerarin at 200 mg/kg/d by gavage had a lower volume of bone loss compared to those not treated with isoflavonoids. In this research, it was noted that the consumption of puerarin inhibited alveolar bone loss by inhibiting RANKL production and reducing the number of active osteoclasts [49]. The experiment performed on healthy male C57BL/6J mice showed that the intraperitoneal administration of puerarin (10 and 50 mg/kg/d) caused inhibition of osteolysis induced by titanium particles. The authors of the research noted that mice treated with puerarin had higher BMD and BV/TV (bone surface/total volume). However, this effect was dose-dependent, and a more beneficial effect was observed when a higher dose of puerarin was used. In addition, micro-CT scanning showed a lower number of pores in mice treated with puerarin. The researchers also noted a statistically significant reduction in eroded surface area, in the number of TRAP-positive cells, as well as in the osteoclast surface to bone surface ratio [49]. Interesting research results were observed by Liu et al. [50], who noted that administration of puerarin combined with zinc (50 mg/kg/d + 0.25 mg/kg/d) by gavage could prevent mandibular bone loss in OVX rats more effectively than administration of these compounds separately. The experiment showed that rats treated with puerarin + zinc had statistically significantly higher bone mineral density compared to rats fed without these additives, as well as compared to those consuming puerarin + zinc separately. In contrast, studies performed on adult ovariectomized rats showed that rats treated with puerarin (administered i.p.) (50 mg/kg/d) from Pueraria lobata had a lower BMD of the proximal tibia compared to animals not treated with this isoflavonoid. This means that puerarin was not capable of reducing loss of BMD induced by ovariectomy [47]. In 2020, the results of a meta-analysis on the effect of puerarin administration on bone mass for OVX-induced postmenopausal osteoporosis in a murine model were published. Based on eight randomized studies, the authors showed that the use of puerarin can have a beneficial effect on the increase in bone mineral density [51]. The beneficial effect of puerarin on bones was also noted by Li et al. [52]. They showed that administration of puerarin at 100 mg/kg/d resulted in a statistically significant increase in cortex and trabeculae BMD. Simultaneously, the authors of the experiment noticed that in the tested rats there was an improvement in the integrity of the intestinal mucosa. Moreover, positive changes in gut microbiota were noted. For example, Lactobacillaceae and Bifidobacteriaceae bacteria were found to grow. Additionally, puerarin increased the content of SCFAs. The authors suggested that puerarin may prevent osteoporotic changes by modulating the community of gut microbiota and repairing intestinal mucosal integrity [52]. The essence of the presented results of puerarin studies in animal models is presented in Table 2 (Ref. [21, 22, 26, 34, 35, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 52, 53, 54, 55, 56]).
| Study size | Study design | Source of puerarin | Effects | References |
| 50 female rats, 3 months old | Puerarin 6’O-xyloside, 40, 60 mg/kg/d (i.p.) | Shanghai Tauto Biotech Ltd. Co., Shanghai, China | [21] | |
| Duration of treatment: 12 weeks | ||||
| 48 Kunming female mice 8 weeks old | OVX + Puerarin, 2, 4, 8 mg | PUMC Pharmaceutical Co., Ltd., Hebei, China | [22] | |
| Duration of treatment: 4 weeks | ||||
| 12 Sprague-Dawley rats | OVX group treated with puerarin (20 mg/kg/d, intragastric administration) | National Institutes for Food and Drug Control, Beijing, China | [26] | |
| Duration of treatment: 12 weeks | ||||
| 36 ICR male mice, 6–8 weeks old | Puerarin, 1 mg/kg/d (calvarial injection) | National Institutes for Food and Drug Control, Beijing, China [purity |
[34] | |
| Duration of treatment: 2 weeks | ||||
| Pathogen-free and healthy male C57BL/6J mice, 6–8 weeks old | Puerarin, 10, 50 mg/kg/d (i.p.) | Sigma-Aldrich (St. Louis, USA) [purity |
[35] | |
| Duration of treatment: 14 days | ||||
| 216 female Kunming mice, 4 weeks old | experimental group received spirits (20 mL/kg) [46% ethanol; intragastrically] + puerarin (0.5 g/kg) [intramuscular injection] | - | [38] | |
| Duration of treatment: 4–10 months | ||||
| Slc: ddY female mice, 9 weeks old | Sham-puerarin group (5 mg/kg/d) | - | [39] | |
| OVX-puerarin group (5 mg/kg/d) | ||||
| Duration of treatment: 8 weeks | ||||
| Slc: ddY female mice, 10 weeks old | OVX-PVEE group (20 mg/kg/d) | Kudzu vine ethanol extract (PVEE) | [40] | |
| Duration of treatment: 8 weeks | ||||
| 30 Sprague-Dawley male rats | Diabetes treated with puerarin (intraperitoneally injected, 60 mg/kg/d) control group | Limin Pharmaceutical Corporation, Jinan, Shandong, China | [41] | |
| Duration of treatment: 6 weeks | ||||
| 60 female Sprague-Dawley rats, 6 months old | OVX + kudzu, 0.45, 0.9, 1.8 g/kg/d | Kudu root extract | [42] | |
| Duration of treatment: 6 weeks | ||||
| Female Sprague-Dawley rats, 8 weeks old | OVX + 17 |
P. lobata extract (Kapdang Co., Seoul, Korea) | [43] | |
| OVX + PTIF (30 or 100 mg/kg/d) | ||||
| Duration of treatment: 8 weeks | ||||
| 20 Male Sprague-Dawley rats | Puerarin, 15.4, 30.8 mg/kg/d (i.p.) | Sigma-Aldrich, Saint Louis, MO, USA | [44] | |
| 60 adult Sprague-Dawley rats | Estradiol, 10 µg/kg (i.p.) | Beijing Four Rings Biopharmaceutical Co., Ltd. | [45] | |
| Puerarin, 50 mg/kg (i.p.) | ||||
| Duration of treatment: 12 weeks | ||||
| 48 nine-week-old female Sprague Dawley rats | Puerarin, 25, 100, 400, 1600 mg/kg/d | Sejun F & B Co., Ltd., Gangwon, Korea | [46] | |
| Duration of treatment: 8 weeks | ||||
| 20 female mice, 30 weeks old | OVX treated with 200 mg or 500 IPL/kg/d | Pueraria lobata (Willd.) extract | [47] | |
| Duration of treatment: 4 weeks | ||||
| Sprague-Dawley female rats, 6 months old | OVX + P. mirifica, 5, 25, 50 mg/g/d (by gavage) OVX + Puerarin, 7 mg/kg/d (subcutaneously injected) | P. mirifica powder (Smith Natural Co., Ltd) | [48] | |
| Duration of treatment: 12 weeks | ||||
| 40 male Sprague-Dawley rats, 7 weeks old | Puerarin, 200 mg/kg/d (by gavage) | Shanghai Winherb Medical S&T Development Co. Ltd., Shanghai, China [98% purity] | [49] | |
| Female Sprague-Dawley rats, 8 weeks old | OVX + 17 beta-estradiol group (10 ug/kg/d) | Sigma-Aldrich, Saint Louis, MO, USA [Analytically pure] | [50] | |
| OVX + puerarin group (50 mg/kg/d) | ||||
| OVX + zinc group (0.25 mg/kg/d) | ||||
| OVX + puerarin + zinc group | ||||
| Duration of treatment: 12 weeks | vs. OVX+puearin or OVX+zinc group | |||
| 40 10-week-old Sprague-Dawley female rats | Puerarin, 50, 100 mg/kg/d | J&K Scientific Ltd, Beijing, China [purity |
[52] | |
| Duration of treatment: 14 weeks | ||||
| Male Sprague-Dawley rats, 7 months old | ORX + P. mirifica, 0, 10, 100, 1000 mg/kg/d | Pueraria mirifica (Chiang Mai Province, Thailand) | [53] | |
| Duration of treatment: 3 months | ||||
| 18 eleven-week-old C57BL/6J mice | Puerarin, 100 mg/kg intraperitoneal injections every two days | Sigma-Aldrich, Sydney, Australia | [54] | |
| Duration of treatment: 6 weeks | ||||
| 30 7–8 weeks old Male Sprague-Dawley rats | Puerarin, 50 mg/kg/d | Sigma-Aldrich, USA [99% purity] | [55] | |
| Duration of treatment: 14 weeks | ||||
| 30 female 6-week-old outbred ICR mice | Fermented puerarin, 100 mg/kg/d | Daegu University, Daegu, Korea | [56] | |
| Duration of treatment: 12 weeks | ||||
| ALP, Alkaline phosphatase; BAP, Bone alkaline phosphatase; BMC, Bone mineral
content; BMD, Bone mineral density; BV/TV, Bone surface/total volume; BS, Bone
surface; Ct, Cortical bone tissue; CTx, C-terminal telopeptide; Ca, calcium; DPD,
Deoxypyridinoline; EE, 17 alpha-ethinylestradiol; FM, Femoral metaphysis; FD,
Femoral diaphysis; L4, Fourth lumbar vertebra; LPS, Lipopolysaccharides; MCF-7
cell, epithelial luminal cell line; MMP, Matrix metalloproteinase; NFATc1,
Nuclear factor of activated T-cell, cytoplasmic 1; Ob.S, Osteoblast surface;
Oc.S, Osteoclast surface; OVX, ovariectomized group; ORX, orchidectomized; OPG,
Osteoprotegerin; OPN, Osteopontin; PPAR | ||||
Li et al. [21] observed that the administration of puerarin
6-O-xyloside to rats had a beneficial effect on bone microarchitecture. Compared
to the control OVX group, in animals treated with puerarin, the cortical and
trabecular bone were thickened. It should be noted that the level of pathological
lesions in femoral bone tissue was dependent on the dose of puerarin
administered. The least adverse lesions occurred after administration of 60 mg
puerarin/kg/d [21]. Urasopon et al. [53] conducted research in which
they administered Pueraria mirifica to rats after orchidectomy. They
found that the enrichment of food with P. mirifica inhibited bone loss
in trabecular and cortical bones. It should be stressed that this effect was
dependent on the dose of P. mirifica. The most positive results were
achieved in the group of rats treated with the highest amounts of P.
mirifica (1000 mg/kg/d) [53]. Wang et al. [26] showed that the
administration of puerarin to rats resulted in the improvement of trabecular bone
structure. They noted that both trabecular number and trabecular thickness were
significantly higher in the OVX + puerarin group compared to the OVX group [26].
The beneficial effect on bone histomorphometry was also confirmed by Suthon
et al. [48]. They observed that rats treated with P. mirificashowed an improvement in such parameters as bone volume (BV), trabecular
separation (Tb.Sp), trabecular number (Tb.N), and osteoblast surface (Ob.S) [48].
Similarly, Lim et al. [43] observed that puerarin can effectively
improve the histomorphometry parameters. It was noted that the proximal tibia had
significantly higher trabecular number (Tb.N) as well as BV/TV. However, no
difference was found for such parameters as bone surface/bone volume (BS/BV), trabecular thickness (Tb.Th) and Tb.Sp. This research also showed an increase in
plasma osteocalcin level in the group treated with puerarin, with a simultaneous
slight decrease in alkaline phosphatase activity [43]. Additionally, the bone
morphometry of the trabeculae of mandibles proved that animals treated with
puerarin + zinc showed higher values for such parameters as BV/TV and Tb/Tb.N. At
the same time, a significant reduction in Tb.Sp compared to OVX + puerarin and
OVX + zinc group was noted. The authors also compared the levels of serum bone
biochemical markers. They showed significantly higher calcium levels and
simultaneously lower levels of TRAP and RANKL. The results of this study showed
that the combined use of puerarin and zinc may be an effective way to inhibit
osteoclastogenesis [50]. Xiao et al. [54] confirmed that puerarin
protects against bone mass loss in ovariectomy-induced osteoporosis model mice.
They reported that the animals that received puerarin had improved the bone
structural features. The following parameters were improved: BV/TV, BS/BV, BS/TV,
Tb.N. The authors of the experiment proved that puerarin has a bone protective
effect by suppressing osteoclastogenesis via inhibition of the
TRAF6/ROS-dependent MAPK/NF-
The current state of knowledge clearly shows that puerarin has a positive effect on bone health. It has been repeatedly proven that this compound stimulates osteoblast differentiation and simultaneously inhibits osteoclastogenesis. The studies carried out on an animal model proved that puerarin has anti-osteoporotic properties and can inhibit bone loss in ovariectomized and orchidectomized animals. Many experiments showed that animals treated with puerarin had higher bone mineral density and bone mineral content. It should be noted, however, that despite promising indications concerning the beneficial effect of puerarin on bones, there is a lack of research that would prove such an effect among people. For this reason, the initiation of intervention studies with the participation of people, especially because there are good grounds for this, is worth considering.
Conceptualization and methodology—BK, AGM; investigation and data curation—BK, AS; writing—original draft preparation—BK; writing—review and editing—BK, JS, AGM; supervision—AGM.
Not applicable.
Thanks to all the peer reviewers for their opinions and suggestions.
This research received no external funding.
The authors declare no conflict of interest.
ALP, Alkaline phosphatase; BAP, Bone alkaline phosphatase; BMC, Bone mineral
content; BMD, Bone mineral density; BV/TV, Bone surface/bone volume; BS, Bone
surface; Ct, Cortical bone tissue; CTx, C-terminal telopeptide; Ca, calcium; DPD,
Deoxypyridinoline; EE, 17 alpha-ethinylestradiol; FM, Femoral metaphysis; FD,
Femoral diaphysis; L4, Fourth lumbar vertebra; LPS, Lipopolysaccharides; MCF-7
cell, epithelial luminal cell line; MMP, Matrix metalloproteinase; NFATc1,
Nuclear factor of activated T-cell, cytoplasmic 1; Ob.S, Osteoblast surface;
Oc.S, Osteoclast surface; OVX, ovariectomized group; ORX, orchidectomized; OPG,
Osteoprotegerin; OPN, Osteopontin; PPAR
