Background: Atherosclerosis is the primary cause of the cardiovascular disease (CVD). Several risk factors lead to atherosclerosis, and altered nutrition is one among those. Nutrition has been ignored quite often in the process of CVD risk assessment. Altered nutrition along with carotid ultrasound imaging-driven atherosclerotic plaque features can help in understanding and banishing the problems associated with the late diagnosis of CVD. Artificial intelligence (AI) is another promisingly adopted technology for CVD risk assessment and management. Therefore, we hypothesize that the risk of atherosclerotic CVD can be accurately monitored using carotid ultrasound imaging, predicted using AI-based algorithms, and reduced with the help of proper nutrition. Layout: The review presents a pathophysiological link between nutrition and atherosclerosis by gaining a deep insight into the processes involved at each stage of plaque development. After targeting the causes and finding out results by low-cost, user-friendly, ultrasound-based arterial imaging, it is important to (i) stratify the risks and (ii) monitor them by measuring plaque burden and computing risk score as part of the preventive framework. Artificial intelligence (AI)-based strategies are used to provide efficient CVD risk assessments. Finally, the review presents the role of AI for CVD risk assessment during COVID-19. Conclusions: By studying the mechanism of low-density lipoprotein formation, saturated and trans fat, and other dietary components that lead to plaque formation, we demonstrate the use of CVD risk assessment due to nutrition and atherosclerosis disease formation during normal and COVID times. Further, nutrition if included, as a part of the associated risk factors can benefit from atherosclerotic disease progression and its management using AI-based CVD risk assessment.
Cardiovascular diseases (CVD) are a major cause of mortality and morbidity in the world. According to World Health Organization (WHO), globally, every year, 17.7 million people die due to CVD [1]. Atherosclerosis is the primary cause of CVD [2, 3, 4], and plaque rupture. It is responsible for almost 70% of fatal cardiovascular events (CVE) [5]. There are several risk factors responsible for atherosclerotic disease, but the prolonged exposure to hyperglycemia and insulin resistance, clustered with other risk factors such as obesity, arterial hypertension, and dyslipidemia constitutes an important stimulus in plaque development and progression [6, 7, 8]. It is therefore important to look into unhealthy lifestyles that lead to CVD [9, 10]. A sedentary lifestyle, a decrease in physical demands during the job workflow, and increased availability of affordable, palatable, high-calorie foods have fueled the epidemic of obesity [11, 12, 13]. Obesity is a modifiable risk factor for atherosclerosis [14, 15, 16, 17], and its prevalence in childhood is seen to have adverse effects on cardiovascular health [18].
Dietary and nutritional risks are responsible for 55% of cardiovascular events in countries with high socio-demographic index (SDI) [19]. In terms of the nutrition paradigm, increasing availability and consumption of processed and pre-packaged foods is associated with a twofold increased risk of atherosclerosis [20, 21, 22, 23]. Epidemiological evidence suggests that diets low in saturated fat, with a balanced amount of fruits and vegetables, helps against the progression of atherosclerosis [24, 25]. Nutrition is often ignored in the CVD risk assessment paradigm, mainly due to (a) its appearance in the early CVD risk management pipeline and (b) its categorization in the preventive cardiology framework compared to CVD treatment paradigms [26]. Looking at the impact of nutrition on atherosclerosis and CVD, it is important to consider the effect of nutritional factors on the CVD risk assessment. According to the American Heart Association (AHA) guidelines on the primary prevention of CVD, adults diagnosed with obesity are at an increased risk of CVD, atrial fibrillation, and heart failure [27, 28]. It is therefore recommended for these individuals to have a mindful food intake [29]. Prevention of CVE can be possible by identifying risk factors associated with atherosclerosis. This can be accomplished by screening moderate to high-risk patients who are likely to experience CVE and then recommending an appropriate treatment plan for reversing the effect of atherosclerotic plaque buildup, so-called “plaque regression”.
It is well established that conventional calculators such as Framingham risk score (FRS), Systematic Coronary Risk Evaluation (SCORE), and Pooled Cohort Risk Equation by ACC/AHA, are used for estimating the CVD risk [30]. These calculators use conventional risk factors that include office-based and laboratory-based biomarkers to produce results that are age-driven [31]. The INTERHEART [32] and INTERSTROKE [33] studies have shown nearly 90% of the CVD-related mortalities are associated with such types of conventional cardiovascular risk factors. Even though these conventional calculators are popular, they do not integrate image-based phenotypes, that directly reflect plaque vulnerability.
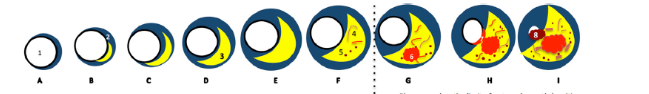
Ultrasound imaging is the most popular low-cost, preventive screening solution, which is currently adopted for CVD/stroke risk assessment [34, 35]. Compared to its other non-invasive counterparts [34], it is a portable and user-friendly imaging technique. For example, AtheroEdge™—a carotid image-based CVD risk calculator takes advantage of image phenotypes such as carotid intima-media thickness [36], plaque area, and its variability derived from the carotid scans [31, 35, 37]. Such a tool can be better used in the preventive framework provided that it can be applied to study the effect of nutrition [38, 39, 40, 41] (see Fig. 1).
 Fig. 1.
Fig. 1.Global view and CVD stages for risk assessment.
CVD risk assessment becomes even more important in the era of COVID-19 since it has a great deal of impact on underlying conditions, complications of infections, and other diseases [42]. A high incidence of thromboembolic events has been found in patients with COVID-19 [43]. It is also observed that COVID-19 leads to the worsening of conditions in the presence of CVD [44, 45, 46]. We will focus on the molecular pathways that can cause epithelial dysfunction and atherosclerotic plaque formation leading to the risk of major cardiovascular events during COVID-19.
Thus the overall objective of this study is to understand how (a) nutrition affects the formation of atherosclerosis and plaque formation, (b) the role of low-cost surrogate CVD such as carotid ultrasound-based screening which can help in effective AI-based risk stratification and monitoring the atherosclerotic disease, (c) manifestations types, and (d) study the effect of COVID-19 on the CVD cycle.
PRISMA model was adapted for the search strategy (Fig. 2). We used several repositories to retrieve the maximum amount of relevant information as required. PubMed and Google Scholar were used for searching the keywords such as “nutrition and atherosclerosis”, “nutrition, atherosclerosis, myocardial infarction”, “nutrition stroke atherosclerosis”, “atherosclerosis risk factors”, “nutrition, ethnicity, geographical effects and heart disease”, “nutrition obesity diabetes atherosclerosis”, “arterial imaging in atherosclerosis”, “atherosclerotic tissue classification and characterization”, “plaque tissue characterization”, “diet and atherosclerosis”, “cardiac injury and COVID-19”, “atherosclerosis and COVID-19”. We found about 332 articles on PubMed and about 17,400 articles on Google scholar. A total of 16,000 studies were identified, and duplicates were removed using the feature called “Find Duplicates” in EndNote software by Clarivate Analytics [47]. After applying advanced filters such as time and relevance, this narrowed down to 600 articles. The three exclusion criterias were (i) studies not related, (ii) non-relevant articles, and (iii) having insufficient data. This excluded 15,200,200, and 320 studies (marked as E1, E2, and E3), leading to the final selection of 280 studies.
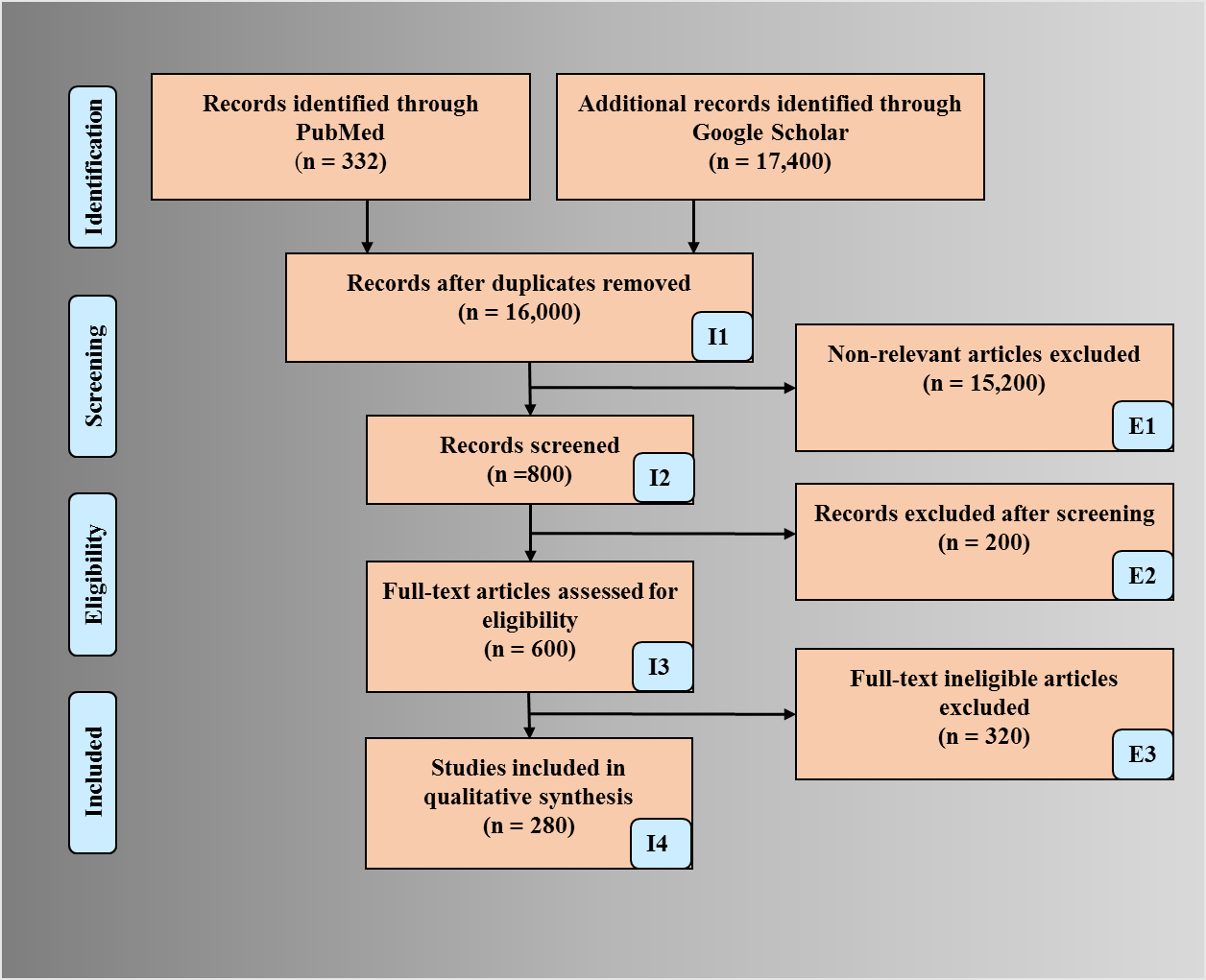 Fig. 2.
Fig. 2.PRISMA model depicting search strategy.
A person’s susceptibility to developing atherosclerosis may be identified by the risk factors associated with it. The risk factors responsible for atherosclerotic disease can be categorized into (a) conventional, such as hypertension, obesity, body mass index, ethnicity, gender, ethanol use, and smoking [48] and (b) blood biomarkers such as lipids, hemoglobin A1c (HbA1c) as diabetes index [35, 49], estimated glomerular filtration rate (eGFR) as renal index [50], erythrocyte sedimentation rate (ESR) as rheumatoid arthritis index [51], homocysteine, triglycerides, and hCRP/c reactive protein. Fig. 3A (Ref. [52]) shows the prevalence of these risk factors in relation to the cause of atherosclerosis and related cardiovascular diseases.
 Fig. 3.
Fig. 3.Statistical distribution on CAD. (A) Risk factors. (B) %
deaths (HbA1c
These risk factors are significantly associated with an increased risk of coronary artery disease. We discuss here several types of risk factors that assist in atherosclerotic lesion formation. Individuals with high blood pressure are more likely to develop atherosclerosis. It has been observed that hypertension causes vascular dysfunction and facilitates the development and progression of atherosclerosis.
This progression is because hypertension oxidatively injures the endothelium leading to the activation of redox-sensitive mechanisms. These mechanisms act in recruiting the mononuclear leukocytes into the wall of the arteries [53]. Another associated risk factor is obesity. It is a known fact that obesity and physical inactivity are some of the leading causes of atherosclerosis. This is due to the influence of many mechanisms such as systemic inflammation, an increase in glucose levels by causing insulin resistance and increasing blood pressures and abnormal lipid profiles [54]. Further, it can be seen that adipokines, a cytokine secreted by adipose tissue, regulate the function of endothelial, arterial smooth muscle, and macrophage cells by directly modulating the atherogenic environment of the vessel wall [55]. Adipose tissue in excess is found in obese patients, and obesity is also seen to have effects on left ventricular remodeling [56]. Further, it is also pointed out that insulin resistance and gallstone disease potentiate each other’s effects and might lead to excessive weight gain in the individuals who are prone to be obese [57]. In young adult men with higher BMI, atherosclerotic lesion formation was more frequent and advanced [58].
Several studies on ethanol have been associated with atherosclerosis progression as well as regression. A two-way effect has been observed in high, low/moderate intakes of alcohol consumption. It is argued that even though alcohol consumption may have its benefits, it equally harms if consumed in high amounts. Benefits are seen as it increases HDL, has anti-inflammatory properties, and has thrombolytic functions. However, if consumed in high amounts, it promotes hypercholesterolemia, is pro-inflammatory, and also leads to hypertension [59, 60]. Not only ethanol intoxication but also smoking is seen to have adverse effects. It has been researched that the toxins in tobacco have a lowering effect on HDL simultaneously raising the levels of LDL [61]. Nicotine and carbon monoxide present in tobacco and smoke are also seen to have adverse effects on plaque buildup as they damage the endothelium [62]. This is because nicotine produces superoxide anions, which in turn reduces the bioavailability of nitric oxide. Further, this increases the production of endothelin that results in endothelial dysfunction [63]. If patients with hypertension are chronic smokers, their risk of developing coronary artery disease is also seen to be high [64].
When discussing unmodifiable conventional risk factors, we can take into account ethnicity and gender. Both ethnicity and gender influence the likelihood of engaging in a healthy lifestyle. It was further observed in a study that black women had higher BMI and ASCVD scores, they were less likely than white women to have reported healthy dietary habits or have themselves engaged in losing weight. In the same study, it was also seen that white men were more likely than Hispanic men to have engaged in healthy eating habits [65]. In another study, progression of atherosclerosis was seen in premenopausal women [66].
It is seen that hyperglycemia acts on the vascular tissue at cellular levels and
alters it, leading to the acceleration of the formation of atherosclerosis. The
main reaction that is often involved in this formation process is referred to as
“Maillard or browning reaction” (reaction between reducing D-sugar and
amino acid) [67]. In patients with diabetes mellitus, incidental micro and
macro-calcification are considered as a biomarker for atherosclerotic burden as
depicted in Fig. 3B–D [52]. It is proved that moderate to severe renal
impairment has a greater effect on atherosclerosis and its progression [68]. CKD
is seen to promote the formation of the foam cells by down regulating cholesterol
efflux through activation of nuclear factor and repression of cholesterol
transporter. Further, in CKD patients, systemic and macrophage angiotensin 2 is
seen to promote atherosclerosis [69]. It is seen that the mechanism of
atherosclerosis is driven by rheumatoid arthritis (RA) [70]. Pro-inflammatory
cytokines such as TNF-
Type 1 interferon causes vascular damage in systemic lupus erythematosus through pathways that are crucial steps towards atherosclerosis progression [71]. Research suggests that homocysteine is an independent risk factor for atherosclerosis [72]. More than 80 cross-sectional, case-control, and cohort studies have linked hyperhomocysteinemia with CHD risk. Triglyceride levels appear to provide unique information as a biomarker of CVD risk. The association of triglyceride levels with apolipoproteins and atherogenic lipoproteins, especially apo C-III, gives unique information as a biomarker for the risk of CVD. Thus hypertriglyceridemia is also a risk factor for atherosclerosis [73]. Similarly, dyslipidemia is an abnormal level of lipid content in the blood. It causes a major risk of developing CVD [74]. Hence, it is important to manage dyslipidemia for the prevention of atherosclerosis and CVD. Research suggests that monomeric C reactive protein is a potential regulator of signaling pathways that are linked to endothelial cell inflammation, thrombosis, and angiogenesis [75]. By exerting pro-inflammatory effects, it contributes to atherosclerosis formation. It is also seen to bind with multiple ligands and is involved in multiple steps of atherosclerosis [76].
It is a well-established fact that nutrition and variations of food play an important role in the development of atherosclerosis and plaque build-up. Thus, there is a link between nutrition and atherosclerosis, and we will explore that in this section.
Trans fat and saturated fat are seen as the major contributors to plaque
formation as they act by increasing the amount of LDL in the body [9, 25].
Several epidemiological studies have been seen to promote trans-fat as a major
risk factor for atherosclerosis [77, 78]. Trans fat acts by suppressing
TGF-
Lipoproteins are complexes of amphibiatic proteins that are comprised of lipids, proteins, and phospholipids [80]. The ratio of protein to fat determines the density of the protein. Plasma lipoproteins have been grouped into five major classes based on their densities: (i) chylomicrons, which are the transporter of dietary triglyceride, (ii) very-low-density lipoproteins (VLDL), that transports endogenous triglyceride, (iii) intermediate-density lipoproteins (IDL), (iv) low-density lipoproteins (LDL), and (v) high-density lipoproteins (HDL) [81]. It is seen that atherogenic lipoproteins floating in the VLDL-IDL region promote atherosclerosis. These atherogenic lipoproteins may be classified as LDL and lipoprotein remnants. Antiatherogenic lipoproteins like HDL prevent atherosclerosis [82, 83]. Even though low-density lipoproteins are the major contributors to plaque cholesterol, it is seen that chylomicron remnants are also taken up into the subendothelial space [84]. ApoB100 is a form of LDL and is a protein that helps in moving cholesterol around our body. It has a central role in the development of atherosclerosis and plaque formation as seen in Fig. 4 [85]. Plaque may develop over time due to bad nutritional habits or it may be due to familial hypercholesterolemia [87].
Now we shall look at a few nutrients, food groups, and their effect on atherosclerosis. Observations showed that low omega-3 fatty acids intake leads to arrhythmia, which may further cause thrombosis [88]. It also promotes platelet aggregation leading to an increase in fibrous plaque and increased inflammation-causing injury to coronary arteries [89, 90]. Glancing at the foods of animal origin, eggs in specific quantities are considered to be unhealthy for patients suffering from CVD. A recent study reported that consumption of egg yolk regularly should be avoided as it is seen to have increased carotid plaque [91]. As we know that dietary cholesterol itself is harmful and potentiates the effect of saturated fats. Therefore, the egg yolk must be avoided by patients at high risk. The egg may also be associated with the increase of type 2 diabetes mellitus [92]. Other foods in animal nutrition such as processed and red meat have also been seen to promote CVD risk in patients. This is due to the presence of trimethylamine, phosphatidylcholine, choline, and l-carnitine [93]. It is therefore important to understand this link and see how these foods cause adverse effects on the body.
The pathophysiological link between nutrition and atherosclerosis
Animal dietary products increase the risk of atherosclerosis. Many recent articles show that dysbiosis (impaired microbiota) of intestinal microbes are linked with certain animal dietary products that can generate metabolites (intermediate products) like trimethylamine (TMA) and trimethylamine N-oxide levels (TMAO), which acts as key factors in developing atherosclerosis and results in cardiovascular diseases [94, 95, 96, 97] (see Fig. 5). Additionally, the recent clinical data has shown that TMA is formed from precursors like lecithin, choline, betaine, and L-carnitine. These are abundantly present in animal-based dietary components like meat, milk, and fish [96, 98, 99].
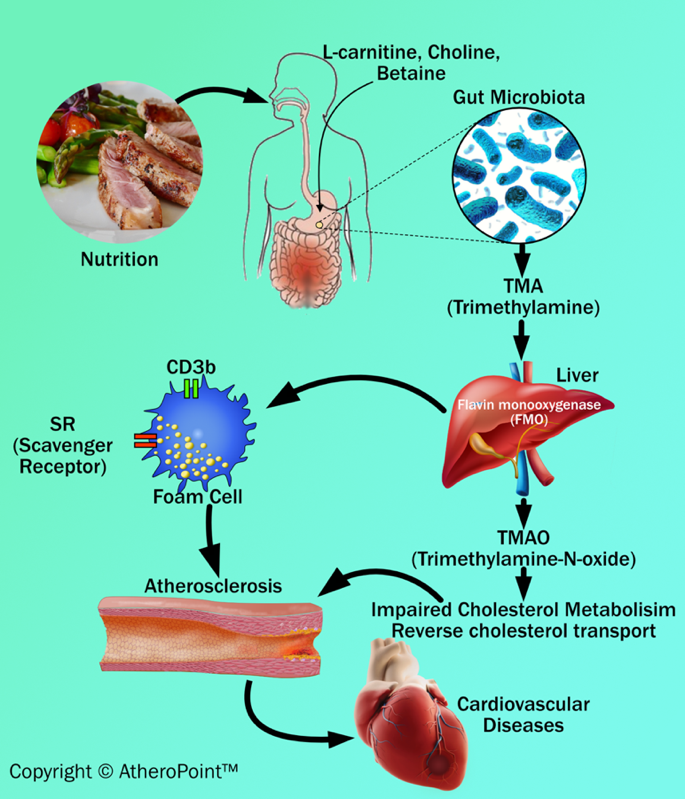 Fig. 5.
Fig. 5.Dietary adulteration and atherosclerosis development.
TMA is an organic compound produced from dietary quaternary amine components (i.e., L-carnitine and choline) by gut microbiota. Further, TMA is subsequently oxidized by hepatic flavin monooxygenase (FMO) to form TMAO in the liver [93]. TMAO can cause inflammatory reactions of the vascular wall by (i) increasing reactive oxygen species production, (ii) impairing cholesterol metabolism, and (iii) increasing sterol metabolism. These three, when combined, are involved in the formation of plaque in the blood vessel [100]. In addition to an impact on cholesterol and sterol metabolism, TMAO also increases the expression of scavenger receptors such as CD36 and scavenger receptor type 1 on the surface of macrophages. This results in a decrease in reverse cholesterol transport and promotes foam cell formation. Foam cells are also known as lipid-laden macrophages that contain cholesterol inside. In abundance, they can result in the formation of plaque that initiates the progression of atherosclerosis and triggers cardiovascular diseases [96, 98].
With prolonged exposure to risk factors, it is observed that early fatty streak development begins in childhood and adolescence. Further, early fibroatheroma occurs in the population in their teenage and young adulthood years [101, 102]. Fig. 6 shows the development of an atherosclerotic lesion in the inner lining of an artery.
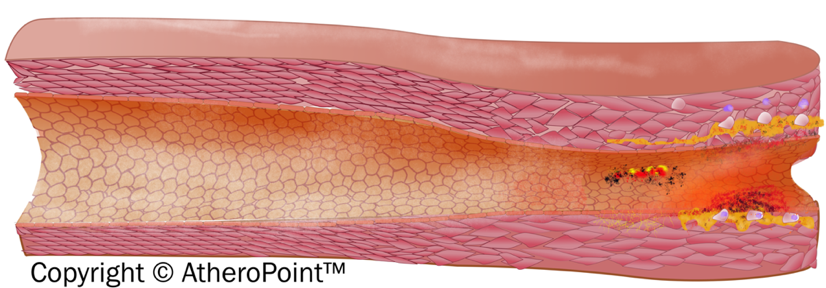 Fig. 6.
Fig. 6.Development of atherosclerotic lesion.
The initiation of the fatty streak phase of atherosclerosis begins with dysfunctional endothelial cells and the retention of apoB-containing lipoproteins in the sub endothelial space. It is seen that chronic endothelial injury is caused by various factors, including hyperlipidemia, hypertension, smoking, homocysteine, hemodynamic factors, toxins, viruses, or immune reactions. Injury in the endothelial-related region contributes to antithrombotic properties that facilitate the platelet adhesion and activation of the dysfunctional area [10]. The lipoproteins are then modified by the process called oxidation at the extracellular matrix of the subendothelial space [103]. Further, modified LDL promotes the activation of endothelial cells (EC) and smooth cells (SCM) and starts the recruitment of immune cells (i.e, monocytes) into the endothelial layer. These recruited immune cells cause a reduction in nitric oxide inside the endothelial wall resulting in endothelial dysfunction, which further triggers the atherosclerosis process [104]. Collectively, an increased monocyte interaction further differentiates into macrophages and leads to the internalization of VLDL, modified LDL, and apoE remnants. This leads to the formation of foam cells [105].
The foam cells are cholesterol-containing lipid-laden macrophages that lead to the formation of plaque. The endothelial dysfunction begins as atheroma or benign tumors of smooth muscle cells within the blood vessels. It is characterized by increased permeability, leukocyte adhesion, monocyte adhesion, and emigration. Progression of atherosclerotic plaque is seen when numerous macrophage foam cells and other inflammatory cells continue to promote the formation of other monocytes and immune cells.
The proliferation of tunica-media smooth muscle cells leads to the formation of the fibrous fatty lesion [106]. Vaso Vasorum neovascularization exists in atherosclerosis as a compensatory reaction to provide adequate nourishment and oxygen to atherosclerotic arteries [107]. The developing lesions sometimes represent the next stage beyond fatty streaks and are pearly plaque with dome shape appearance often referred to as “cap”. These lesions may further advance and can be represented as calcified fibrous areas of arteries along with visible ulceration [108]. The plaque, which is covered with a thick fibrous cap and has a well matured necrotic core, is referred to as stable plaque. This fibrous cap proves as an effective barrier in preventing plaque rupture and exposure of prothrombin factors in the vascular pathway [109]. An observation of atherosclerosis is that the macrophage cholesterol pathway becomes inefficient, leading to an imbalance in the lipid metabolisms and cholesterol, further progressing to the disruption of cellular functions [110]. Vulnerable plaque is a result of the unresolved and heightened inflammatory status of the lesions of foam cell core. Data reported in a study indicated that some nutritional and toxic trace metals may have a role in the progression or rupture of plaque [111]. Rupture or erosion of the advanced lesion initiates platelet activation and aggregation on the surface of the disrupted atherosclerotic plaque (see Appendix Fig. 13). Thrombotic vascular occlusion is associated with ischemic episodes, including acute coronary syndromes or cerebral infarction [112].
Nutritional components along with traditional cardiovascular risk factors alone cannot predict the overall risk of atherosclerotic CVE. Such traditional risk factors neither provide any information about the presence or absence of atherosclerotic plaque nor capture morphological variations in atherosclerotic plaque burden [113, 114]. Due to this limitation, many moderate to high-risk patients remain asymptomatic and undiagnosed from most atherosclerotic diseases [115]. This limitation enables the need for imaging modalities that not only can visualize the atherosclerotic plaque, but also significantly contribute to the CVD risk assessment [113, 116, 117].
Some of the popular imaging modalities are magnetic resonance imaging (MRI) [118, 119, 120], computed tomography angiography (CTA) [121], optical coherence tomography (OCT) [122], and positron emission tomography (PET). Each of these imaging modalities offers a unique capability of visualization and characterization of atherosclerotic plaque components. However, since imaging using these modalities is expensive and time-consuming, they are generally not preferred by the physician for preventive screening of patients and CVD risk assessment [28].
Carotid B-mode ultrasound (BMUS) is a comparatively less expensive and user-friendly imaging modality [4, 28, 123, 124, 125, 126]. Therefore, it has a wide scope to be used for routine preventive screening of atherosclerotic plaque and CVD risk assessment [51, 70, 123, 127, 128, 129, 130]. Automated systems have been well developed for image phenotype measurements [131, 132, 133, 134] and validated using CT, MRI, and the gold standard [135, 136]. Recent studies have demonstrated the ability of carotid ultrasound image-based phenotypes such as carotid intima-media thickness (in common carotid or bulb region [137]), total carotid plaque area, carotid intima-media thickness variability (cIMTV) [138], maximum plaque height [139], and morphology of the plaque play a vital role in the prediction of risk of CVE [31, 70, 128, 140, 141]. Studies have also indicated that these carotid image-based phenotypes when combined with traditional cardiovascular risk factors have improved in their prediction ability [70, 128, 129]. Several studies have indicated an annual progression of cIMT and carotid plaque with the traditional risk factors [142, 143, 144, 145, 146].
Similar to the traditional risk predictors, these carotid atherosclerosis
biomarkers are also associated with several nutrition-based components [147, 148, 149].
Several studies have indicated a regression in cIMT by adhering to the
Mediterranean diet in patients with elevated cIMT [150, 151, 152]. A recent randomized
controlled trial by Sala-Vila et al. [153] indicated the progression of
cIMT in the internal carotid artery by 0.052 mm in the group with a control diet.
On the contrary, the regressive effect of ICA-cIMT (regression by –0.030 mm) was
observed in the group that was taking the Mediterranean diet. Petersen et
al. [154] presented a randomized controlled trial that demonstrated the
reduction in the progression rate of cIMT in diabetes patients when dietary
quality was improved for a year. Their trial indicated regression of cIMT by
–0.02
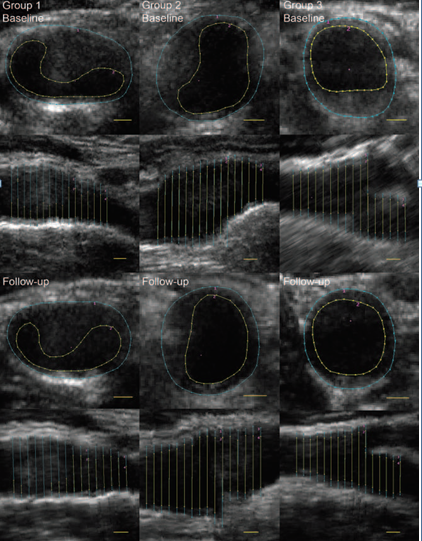
Similarly, diet-related metabolites such as L-Lysine and L-Carnitine are also associated with reduced cIMT [147]. Another randomized control trial of a two-year follow-up indicated a significant 5% reduction in the carotid vessel wall volume (VWV) and –1.1% change in the cIMT from baseline with dietary weight-loss strategies such as following low-fat, Mediterranean, or low-carbohydrate diets [156]. Fig. 7 (Ref. [156]) depicts the changes in the vessel wall volume of the common carotid artery at baseline and follow-up for the population consuming a low-fat diet (Group 1), the Mediterranean diet (Group 2), and low-carbohydrate diet (Group 3). Besides all this, the adoption of Western diet plans is associated with increased cIMT in the common carotid artery and leads to adverse vascular events [149]. A recent study indicated that patients with ischemic stroke, especially with carotid atherosclerosis, have a high intake of saturated fatty acids and low consumption of fruits, vegetables, and unsaturated fat nutrients [157].
Looking at the profound association between carotid atherosclerosis and nutrition components, there is a need to design an accurate diet quality index metric. This will assess the nutrition quality and predict the carotid plaque burden, supporting the CVD risk assessment of patients. Currently, the Diet Quality Index (DQI) questionnaire is available, but it does not show significant linkage with the carotid atherosclerotic plaque burden [158]. Further, traditional risk predictors along with carotid ultrasound image-based phenotypes and nutrition components could be useful in providing the long-term CVD risk of participants. Therefore, there is wide scope to develop such an integrated risk calculator for preventive CVD risk assessment.
Knowing the role of nutrition in the formation of carotid atherosclerosis can
determine a severe cerebrovascular or cardiovascular event. It becomes essential
to understand different ways in which cardiovascular risk can be predicted
particularly via vascular imaging or left ventricle imaging [159]. At present,
several cardiovascular risk prediction algorithms are available that consider
traditional risk factors as input and provide an estimation of the overall
cardiovascular risk of patients on the scale of 0% to 100%. Recently, the
American College of Cardiology (ACC) and American Heart Association (AHA)
recommends the use of pooled cohort risk equation (PCRE) to predict
atherosclerotic cardiovascular risk [160, 161, 162, 163, 164, 165, 166, 167, 168]. Similar to the PCRE, there are
several other popular conventional cardiovascular risk calculators such as
Framingham risk score, Reynold’s risk score, the systematic coronary risk
evaluation calculator, and QRISK3 [169, 170, 171, 172, 173, 174]. The primary idea behind using these
conventional risk prediction algorithms is to identify the patients with a high
risk of CVD and initiate statin therapy (see Table 1, Ref. [163, 166, 175, 176, 177, 178, 179])
to lower down the atherosclerotic plaque buildup [160, 161, 162, 163, 164, 165, 166, 167, 168]. ACC/AHA recommended
the initiation of statin for CVD risk prevention when PCRE
| Guideline | Risk score | Cut-off with statin initiation |
| ACC/AHA 2013 guideline [163] | Pooled Cohort | Cut off of |
| Risk Score | ||
| NICE 2014 guideline [177, 178, 179] | QRISK2 risk engine | Offers atorvastatin 20 mg daily who have a score |
| Canadian 2012 guideline [175] | FRS CVD risk score | Cut off of |
| U.S. Preventive Services Task Force [166] | Pooled Cohort | Low-to-Moderate dose of statin in Risk |
| Risk Score |
Besides the widespread use of CCVRC, there are several challenges while performing risk stratification in a diverse population. The CCVRC is mainly derived using the regression-based models that handle only a limited set of risk predictors such as patient demographics and clinical parameters. Such traditional risk predictors do not provide any information about atherosclerotic plaque-build or morphological changes in the plaque. Another challenge with such regression-based risk prediction algorithms is that such algorithms assume a linear relationship between the risk predictors and the endpoints. This oversimplification of complex non-linear relationships present in the risk predictors limits the ability of CCVRC to accurately estimate the CVD risk. Because of these challenges, there is an increasing need to look for better solutions that can provide more accurate and robust preventive CVD risk assessment.
Recently some efforts were made to integrate the traditional risk factors with the carotid ultrasound image-based phenotypes such as cIMT and plaque area [28, 48, 70, 129]. cIMT and plaque area capture the morphological plaque variations and are considered as the surrogate biomarkers of coronary artery disease (Fig. 8). Therefore, when such an integrated approach was compared against the CCVRC, it reported improved risk stratification.
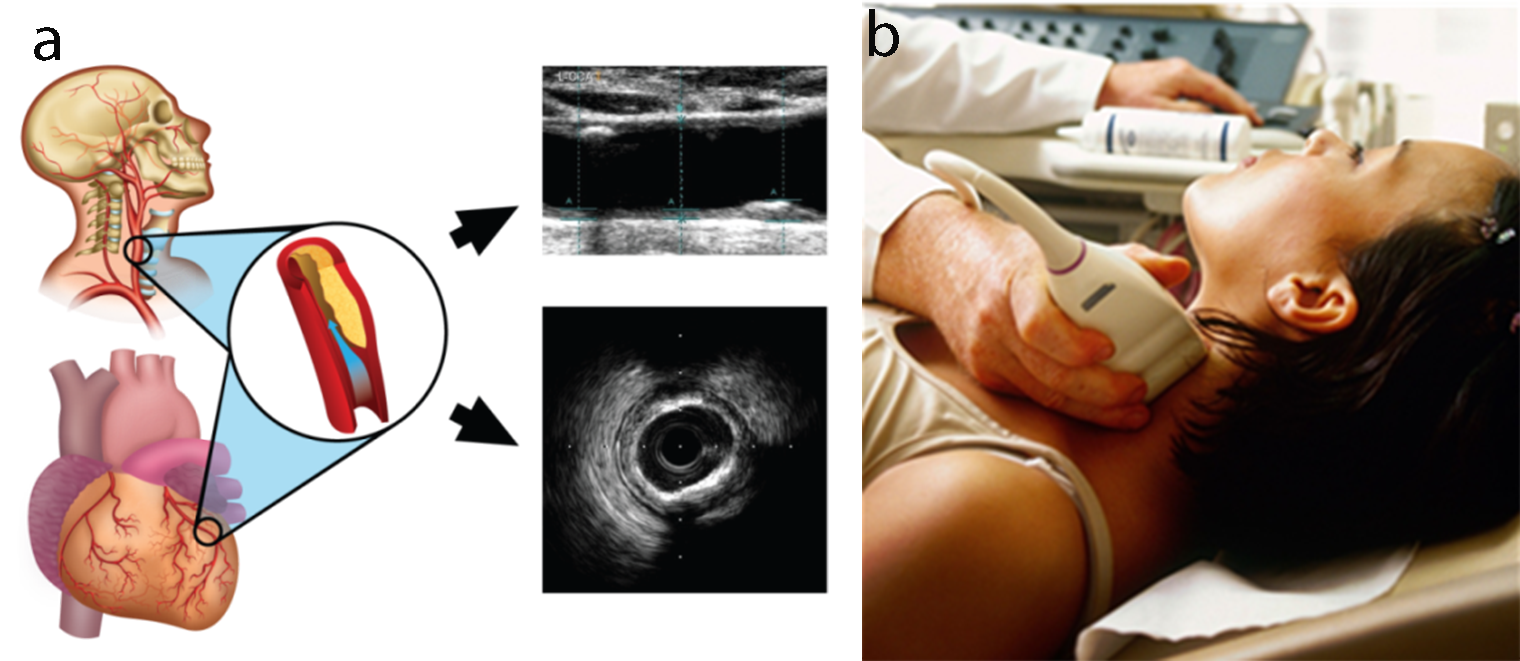 Fig. 8.
Fig. 8.Carotid ultrasound imaging for preventive CVD risk screening. (a) Carotid artery being used as a surrogate marker for coronary artery. (b) Imaging device.
The AECRS2.0 is an integrated risk prediction algorithm that is comprised of 13 predictors, including 5 carotid ultrasound image-based phenotypes [128]. The independent contribution of each risk predictor in the 10-year CVD risk is shown in Fig. 9 and is preferred as a preventive screening tool [35]. This tool has been applied to the bulb region and correlated to renal scores [50, 180, 181, 182] or rheumatology patients [183].
 Fig. 9.
Fig. 9.Independent contribution of risk predictors in the 10-year CVD risk profile of a patient estimated for the left common carotid artery, right common carotid artery, and mean of left and right common carotid artery [AtheroEdge™ 2.0]. (a,b) Original and reproduced ultrasound scans for moderate-risk patient. (c,d) Original and reproduced ultrasound scans for high-risk patient. (e) Independent contribution of risk predictors in the 10-year CVD risk. The figure was reproduced with permission [46] (courtesy of AtheroPoint, Roseville, CA, USA).
Another way of improved risk assessment is to use artificial intelligence (AI)-based algorithms for cardiovascular risk stratification. AI-based algorithms can handle a large number of risk predictors in their model. Therefore, diverse types of risk predictors could be included in the risk prediction algorithm. Further, since AI-based algorithms are data-specific algorithms, they capture and learn from the complex non-linear interrelationship among the risk predictors [123]. This characteristic makes AI-based algorithms unique compared to CCVRC. Recently, several studies have demonstrated the superior performance of AI-based algorithms over the CCVRC. A recent study by Kakadiaris et al. [184] reported the outperformance of ML-based atherosclerotic CVD event prediction over the conventional PCRE (0.92 vs. 0.71). The authors demonstrated that the PCRE over-recommended the statin to nearly 46.0% of the selected population. However, the AI-based algorithm recommended statin to 11.4% population who required the initiation of statin therapy.
Another study by Weng et al. [185] also supported the outperformance of AI-based algorithms over the PCRE for CVD risk assessment. Knowing that the integration of carotid ultrasound image-based phenotypes can provide better CVD risk assessment, Jamthikar et al. [130, 186] presented a study to compare the AI-based algorithm against the 13 different types of CCVRC for CVD risk assessment (Fig. 10, Ref. [186]). In their study, AI-based algorithms showed an overall risk-stratification accuracy of 92.52%, which was higher compared to all the 13 types of CCVRC. The authors also showed the role of machine learning for better risk prediction that used carotid ultrasound plaque characteristics to improve the risk stratification accuracy [187, 188]. The same authors also supported their claim of superiority of AI-based algorithm over CCVRC in a recent study that predicted multiclass risk profile in Canadian patients [140]. A comparison between AI-based vs. statistical-based calculators can be shown in Fig. 10 [186]. Such ML-based strategies for office-based settings are now available [31].
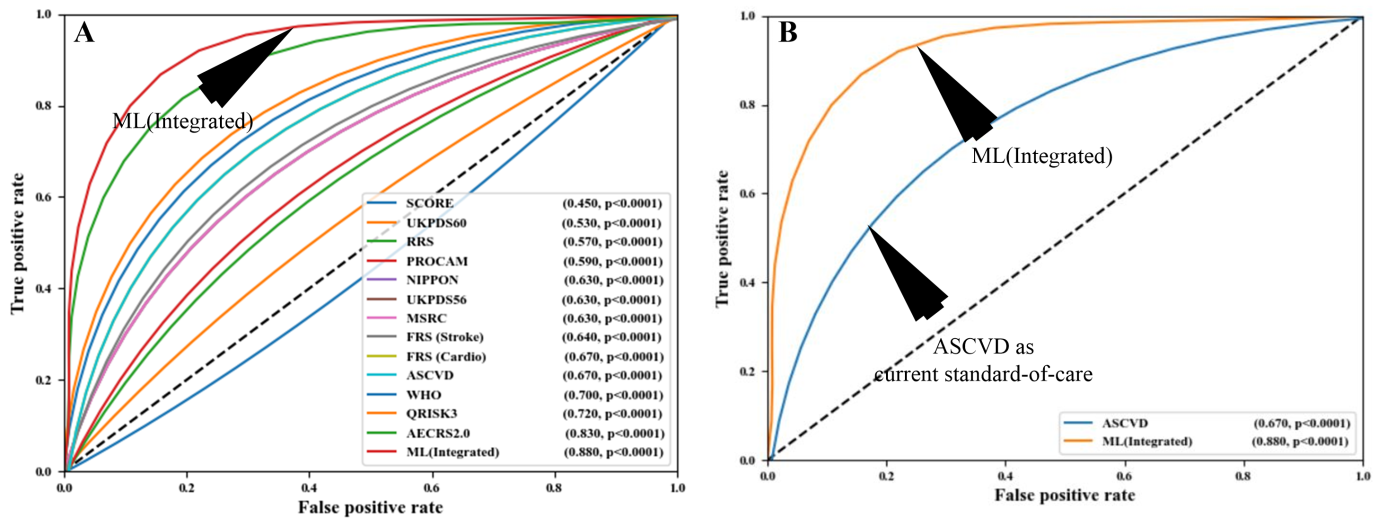 Fig. 10.
Fig. 10.Comparing the ML-based CVD risk assessment with conventional calculators. (A) Comparing ML-based system with 13 types of CCVRC and (B) comparing ML with the standard-of-care ASCVD calculator (Reproduced with permission [186]).
Ultrasound-based Tissue characterization (UTC) is one of the upcoming methods to morphologically evaluate the tissue that is at risk. The team has developed several applications of UTC in several organs such as the thyroid [189, 190], arrhythmia [191], liver [192], prostate, cardiovascular, etc. The potential of AI-based algorithms has also been explored in the domain of atherosclerotic plaque tissue characterization. Studies have been conducted to risk stratify the carotid ultrasound images into symptomatic and asymptomatic plaques [193, 194]. This study indicated an overall tissue characterization accuracy of 90.66% using the AI-based algorithm. Another study by Lekadir et al. [195] used the deep learning model, called the convolutional neural network, to identify different carotid plaque components such as the lipid core, fibrous cap, and calcified tissues with a classification accuracy of 78.5%.
Looking at the broader view of the discussion, it is clear that an AI-based algorithm should be used for accurate cardiovascular disease risk assessment. Further, a combination of traditional risk factors along with carotid ultrasound plaque phenotypes should be used for preventive screening of patients for atherosclerosis and risk estimation. Another point to note was that although there were attempts made to predict the CVD risk in machine learning using nutrition components [196, 197], there is still a wide scope to investigate the efficacy of ML-based CVD risk assessment and event prediction using a combination of nutrition, traditional risk factors, and the image-based biomarkers of atherosclerosis.
Although there are several pharmacotherapies available to treat atherosclerosis and reduce the overall mortality rate, CVD is still the top challenge global healthcare providers are facing. Therefore, there is a need to look for an additional treatment strategy that can go hand-in-hand with existing therapies. A nutritious diet and lifestyle modification, even at an early age, has proven to be an effective solution for the prevention of CVD [198, 199, 200, 201, 202, 203]. Nutraceuticals are natural nutrition-based compounds that provide health benefits and are used in preventing atherosclerotic CVD [204, 205]. They are generally categorized into either dietary supplements or functional foods [204, 205].
Polyunsaturated fatty acids (PUFA) are the essential type of dietary supplements that are generally used in the (i) regulation of blood pressure and blood clotting and (ii) in the modulation of the inflammatory response by forming the eicosanoids mediators [205]. PUFA are generally divided into two types such as omega-3 PUFA and omega-6 PUFA. Rich sources of omega-3 PUFA are fish oil, nuts, and flax seeds. The benefits of Omega-3 PUFA are well-known over the past decades [206]. In addition, American Heart Association (AHA) has recommended eating 200 grams of oily fish every week [207]. Fish oil or fish oil supplements have shown a reduced risk of atherosclerotic markers. Omega-3 PUFA contains eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), both helpful in CVD prevention [205, 208]. Low levels of DHA and EPA both are associated with reduced endothelial function, which may further lead to arterial stiffness [209]. This stage can be improved by daily consumption of omega-3 PUFA for a minimum of 12 weeks [210]. A Japan EPA Lipid Intervention Study (JELIS) trial with 18645 Japanese patients showed a 19% reduction in the risk of major cardiovascular events in a group taking statin and 1.8g of EPA than the group that statin alone [211]. A very recent REDUCE-IT trial also investigated the effectiveness of using a pure form of EPA in patients with elevated triglyceride levels [212]. This study also showed a reduction in overall ischemic events, including cardiovascular deaths, among patients who received 2g icosapent ethyl twice daily [212]. Thus, omega-3 PUFA can reduce the risk of arrhythmia and thrombosis by slowing down the rate of atherosclerotic plaque build up [207, 213]. Therefore, it must be part of the treatment plan for CVD prevention.
As discussed in section 6, the Mediterranean diet (MedDiet) is considered one of the most popular functional food diets that offer several preventive benefits for cardiovascular health [214, 215, 216, 217]. A well balanced Mediterranean diet includes (i) high intake of minimal processed plant-based food such as vegetables, fruits, whole-grains, and nuts, (ii) high consumption of monosaturated rich fat from fish, olive oil, and (iii) lower intake of saturated fats, meat, and dairy products [218]. Recent studies have indicated a reduced risk of myocardial infarction, heart failure, and stroke by strictly adhering to the MedDiet [219, 220]. Even diabetic patients who have a high chance of dying due to CVD have shown a reduction by 30% in the mortality rate by each 2 point increase in the MedDiet score [221, 222]. Another major PREDIMED study also indicated a reduction in the composite CVD risk by 30% when following the MedDiet supplemented with olive oil and nuts [223]. Paterson et al. [220] presented a 17-year follow-up study which demonstrated a population strictly adhering to the MedDiet has a reduced risk of stroke events. Due to the widespread benefits of MedDiet, it can be considered as a primary choice for food intake. As a result, the physicians have recommended this diet for preventing atherosclerotic-driven CVD-related events.
Besides the MedDiet, several studies have shown the reduction in overall CVD risk with an appropriate intake of foods containing soluble fiber [224, 225, 226, 227], plant sterols/stanols [228], B3 vitamin-like Niacin [229], vitamin E, vitamin C [230], vitamin D [231, 232, 233, 234], and amino acids like Taurine [235]. Along with this, time-restricted eating also proves beneficial in managing obesity and thereby preventing CVD [236]. Most of these nutrition components generally target the reduction of LDL cholesterol and increase in HDL cholesterol level, [10] thereby reducing the atherosclerotic plaque build-up and CVD events.
COVID19 is a highly transmittable and pathogenic viral infection that is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [237]). It has led to a dramatic loss (nearly infecting ~155 Million and perishing ~3.3 Million people as of 5th May 2021) of life worldwide [238].
Several studies have suggested that COVID-19 severely affects the lungs of an individual and has adverse effects on other organs of the body [239]. It is seen that the increased mortality due to SARS-CoV-2 is mainly due to viral pneumonia induced Acute Respiratory Distress Syndrome (ARDS) [240]. ARDS leads to lung damage (refer to Appendix Fig. 14). SARS-CoV-2 enters the lungs via aerosol transmission, which further attaches to the host cells. This leads to respiratory symptoms that can be categorized as the earliest clinical presentation of COVID-19 [44].
Imaging has shown to be very useful in understanding COVID-19 severity [241, 242]. Comorbidity has been shown to affect the lungs and heart more aggressively [45, 46, 49]. SARS-CoV-2 causes brain and heart injuries via different pathways (as seen in Fig. 11).
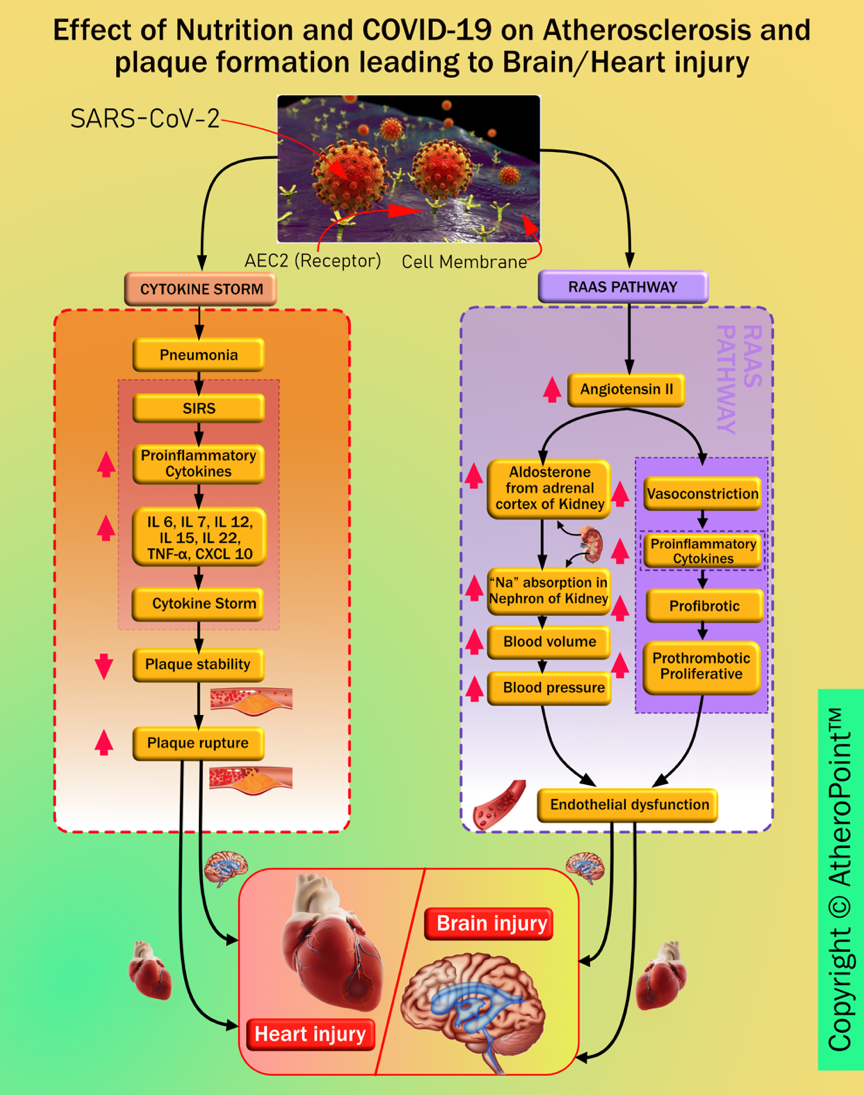 Fig. 11.
Fig. 11.Pathways linking COVID-19 to heart and brain injury.
During the SARS-CoV-2 outbreak, governments have imposed a quarantine to reduce the spread of the virus. This quarantine is an unpleasant experience that is causing changes in lifestyles such as unhealthy nutritional habits [243]. In this section, we illustrate how nutrition and lifestyle changes during COVID-19 affect atherosclerosis [244]. Alteration of nutritional habits is occurring because of the reduced availability of fresh food and its replacement with unhealthy food due to restrictions in the opening of stores.
A recent clinical journal on the psychological impact of quarantine also reported negative effects, including post-traumatic stress symptoms like depression, confusion, and irritability [245, 246]. An increase in consumption of packaged foods, frozen foods, and reduced consumption of fresh foods like fruits and vegetables are major consequences of weight gain. Increased weight gain with reduced consumption of fresh food results in a decrease in antioxidants in the body. Additionally, physical inactivity with oxidative stress increases the risk of atherosclerotic plaque formation [247, 248, 249]. COVID-19 has direct effects on basic, underlying, and immediate drivers of acute and chronic malnutrition. Several sectors like (a) food insecurity due to poor-quality diets, (b) reduced income because of limited financial resources, (c) limited care and restricted health services, (d) interrupted education for children and adults, and (e) an unhealthy household environment are critical to reducing childhood undernutrition at particular risk of collapse or reduced efficiency due to widespread impact of COVID-19 [250]. It is very important to focus on the importance of nutrition to boost immunity and to keep professional and authentic dietary guidelines about nutrition and food safety to withstand COVID-19 [251, 252]. Therefore, utmost nutritional practices need to be figured out to provide critically ill patients with utmost enteral and parenteral nutrition as required [253, 254].
The immune system protects the host from pathogenic organisms. Virally infected cells directly activate natural killer cells which act to kill the infected cell. The processes involved in antiviral immunity are therefore linked to various nutritional consequences [255]. Patients with critical COVID-19 conditions should be included in life-saving therapies in which nutrition plays a great role. The supplementation of amino acids and lactoferrin must be accompanied by drug therapies followed by an ICU hospitalization. It is seen that lung damage caused by coronavirus is from the overexpression of proinflammatory molecules (cytokines) which has shown to be lowered by an adequate integration of amino acids.
Nutritional therapy, together with pharmacological therapy, undoubtedly helps the COVID-19 patient to overcome the acute phase of the disease first and to shorten recovery time [256]. Inflammatory molecules are involved in infection, and certain dietary components, including cytokines, growth factors, and other components, may interact with them [257]. Nutrition modulates disease severity and progression. Nutritional status has the potential to influence susceptibility to the risk of COVID-19 through its integral role in immune function. For example, micronutrients support mucosal immune function (vitamin A), epithelial tissue integrity (vitamins A, C, and D), enhancing the function of certain adaptive and innate immune cells (vitamins A, C, D, E, iron, zinc, and PUFAs) and potential pro-oxidant effects (vitamin C) [258]. Obesity and related comorbidities like endothelial risk function, altered Interferon production, oxidative stress, sarcopenia, and insulin resistance, increased ACE2 inhibition in adipose tissues can lead to cytokine storm, impaired immune response, thrombotic events, which further make an individual more prone to infection and transmission of COVID-19 [259, 260, 261, 262, 263, 264, 265].
Trends have shown a worsening of cardiac events in COVID-19 patients with underlying conditions such as coronary artery disease, hypertension, and diabetes mellitus [266]. Many reports from admitted patients have also suggested cardiac injury in about 12% to 26% of them. This exists due to the presence of pericytes in the heart, which have a high expression of ACE2 that are the same receptors through which the virus gains entry into the cells of the body. The cytokine released during the infection could affect the intramural coronary vessels of the patients. It is also seen that cardiovascular diseases have a major effect on ARDS in patients with COVID-19 affected lungs [239].
Some studies also suggest that abnormal immune system response is likely to be the underlying cause of myocardial injury during coronavirus infection. Thus, it is suggested that potential cardiac involvement should be identified early to provide a prompt diagnosis for improving the outcome [45]. It is noted that the consequences of dysregulation of the renin-angiotensin system caused by SARS-CoV-2 inside atherosclerotic plaques lead to endothelial dysfunction. This progresses into thrombosis, which in turn favours the invasion of plaque by inflammatory cells. These events may transform vulnerable plaque into a complicated and ruptured plaque [267]. Some studies also suggest that if positive for SARS-CoV-2 and asymptomatic, patients could be at increased risk of developing cerebral ischemic strokes, and myocardial infarction due to increased instability of coronary and carotid plaques [49, 268].
As mentioned above COVID-19 patients develop several complications, among these is the elevated level of IL-6 (interleukin-6). It is responsible for signalling the liver to increase the synthesis and secretion of C-reactive protein (CRP) levels [269]. Higher CRP levels in the blood are diagnostic of extensive tissue damage and pathological inflammatory response. It was observed that at the early stage of COVID-19, CRP levels were positively correlated with the diameter of lung lesions and the severity of COVID-19 [270]. Moreover, CRP levels can be measured and used as a lab-based blood biomarker, which is a simple, affordable, and rapid way to assist the therapeutic conditions and to evaluate the severity of the disease [269]. Thus, CRP levels are also seen to be an effective diagnostic tool in measuring the severity of COVID-19. Anti-inflammatory agents helped in suppressing the elevated levels of IL-6, thus keeping CRP levels in check. In patients with CVD risk, a high burden of subclinical inflammation is associated with COVID-19 development of subclinical disorders or cause cardiovascular damage [271]. However, nonsteroidal anti-inflammatory drugs (NSAID) should be avoided in patients with a high risk of cardiovascular disease as they are seen to increase the risk of heart attack, stroke and high blood pressure [272, 273]. Generally, ACE2 plays an important role in the cellular entry of SARS-CoV2. Additionally, it also affects the renin-angiotensin-aldosterone system (RAAS), which acts as a central mechanism of many drugs including NSAIDs [B7]. An important study also shows the increase of expression of ACE2 levels on heart tissue of diabetic rats after treating with NSAIDs [274].
There are two major components for COVID19 screening: (i) primary and (ii) secondary. Fig. 12 shows the pictorial representation of the workflow during COVID-19 screening and diagnosis along with the role of AI for CVD screening.
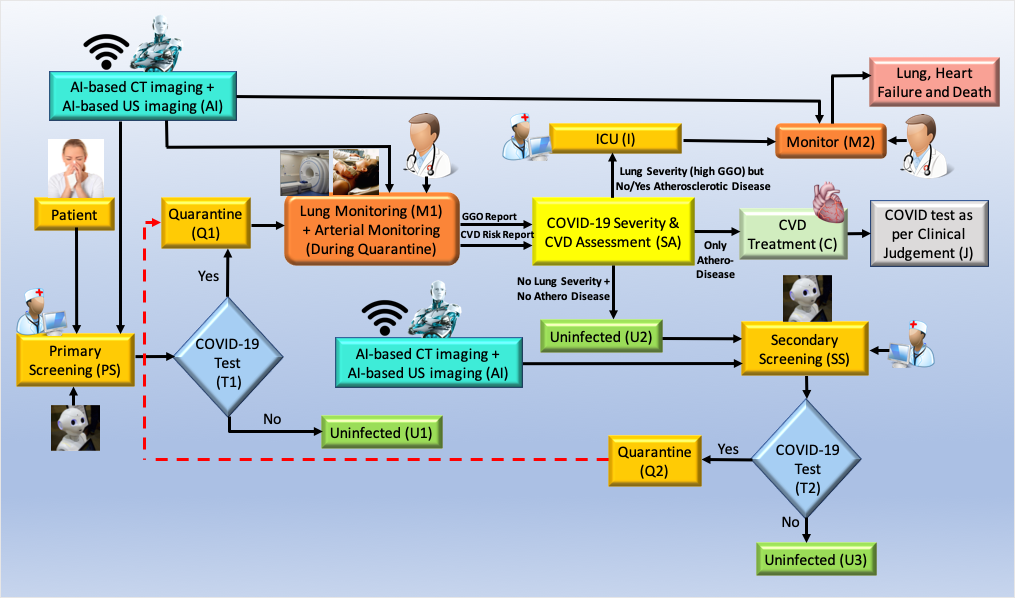 Fig. 12.
Fig. 12.Role of AI-based CVD risk assessment during pandemic (Courtesy of AtheroPoint™, CA, USA).
Primary screening: The patient undergoes screening with the help of a robot and AI in the COVID-19 framework. Questions are asked considering basic symptoms and based on the answers received, the robot analyses and to move forward if there is a need for screening tests like the RT-PCR test (shown as T1, diamond box). Note that the cross-questioning is done when the patient first appears at the clinic or hospital doors. The analysis is done with the help of the AI-based machine intelligence system and telemedicine (TM) right at the outset (shown in the yellow ellipse, where the nurse and robot sign is depicted). Based on the results of the RT-PCR test at the T1 junction (shown in diamond box), the patient needs to be quarantined (Q1, marked yellow) if the test is positive, or the patient is uninfected by COVID-19, if the test is negative (marked green, U1).
Outcome of risk assessment box (SA):While the patient is in quarantine, risk assessment is performed using AI-based system during monitoring (shown using M1). During the monitoring process, the doctor interacts with the patient to understand (a) his lung condition and (b) atherosclerotic arterial condition. The lung condition is evaluated using X-rays/CT imaging embedded with AI. The atherosclerotic arterial condition is evaluated non-invasively using carotid ultrasound for CVD risk. The output of the monitoring function is fed to the risk assessment box (SA), to take appropriate decisions on three fronts: (i) worsening lung condition and need for ICU, or (ii) worsening of CVD condition and need for CVD treatment, and (iii) uninfected.
There are three outcomes from the risk assessment box (SA). (i) The patient’s Ground Glass Opacities (GGO) conditions if worsened (from the output of the monitor), however, no atherosclerotic lesions in the carotid arteries, the patient is categorized to be extremely symptomatic, evaluated further in Intensive Care Unit (ICU) under ventilation conditions. This situation is a dire necessity because the lung conditions have started to fill up with fluid (see Appendix Fig. 14). Note that, the patient is continuously monitored in the ICU (shown by monitoring function M2). (ii) If the lung conditions are not worse (very low GGO), while the carotid arteries show plaque burden, the patient undergoes CVD treatment (C). In the last outcome (iii) the patient neither has lung worsening nor arterial deterioration, therefore he is considered uninfected (U2). Note that, since the patient was quarantined (Q1) and the risk assessment (marked as A) was performed using AI based imaging followed by CVD treatment (marked as C), the patient is assessed by the doctor based on clinical judgement (marked as J). Finally, once the patient is uninfected (U2), the long COVID process triggers where the patient can be retested for COVID (secondary screening, marked SS). If found positive, the feedback loop triggers again for quarantine (Q1) or if negative, free from COVID-19 (U3, marked green).
The role of AI for CVD risk is evaluated using a machine learning system where the imaging data can be taken to predict the CVD risk at the vascular level [31, 140, 187]. The idea is to acquire the carotid scans of the patient, collect the plaque image-based phenotypes and predict the risk using a machine learning system. The AI component requires only the trained model shown by green colour labelled as Imaging-based AI with the AI logo of a robot on it. The output of the machine learning system is to tell the clinician monitoring the patient (M1) if the risk of CVD is low, moderate or high. Based on this colour-coded scheme, the patient undergoes CVD risk assessment (marked as SA). This can involve ground-glass opacity evaluation [241] of the CT lungs or if there is any pulmonary embolism [275]. Thus, the concept shows the role of AI-based CVD screening for diagnosis during pandemic times.
This review provided an understanding of a complete cycle for tracking the effect of nutrition on heart disease. We studied the link between nutrition and the effect it has on various risk factors to have a clear understanding and isolate direct causes of the disease. A deep insight into the effects of LDL in plaque formation and the development of atherosclerotic lesions is shown in the study. We also provided quantitative wall measurement methods, which can be used for studying the effect of nutrition on atherosclerotic wall formation. With the ongoing pandemic, it is important to make sure that one provides information on how the disease might take its course in an infectious environment. Thus, we explored the link between nutrition and atherosclerosis during the non-COVID-19 and COVID-19 periods. The review presented a pathophysiological link between nutrition and atherosclerosis by gaining a deep insight into the processes involved at every stage of plaque development. Various studies provide evidence on how various foods or other factors might affect atherosclerosis, and thus, we combine and form strategies to diagnose and treat the disease. After targeting the causes and finding out results using low-cost, user-friendly, ultrasound-based arterial imaging, the study showed the role of how to risk stratify and monitor the plaque burden. The study shows the role of AI in efficient CVD risk assessments. Finally, the review presented the role of nutrition and vascular damage due to SAR-CoV-2, leading to brain and heart injury.
Thus our key findings of the study are:
Several articles have addressed only a few out of all these aspects, but after a perusal of various studies, we did not find any study that would address all these links together. Some of these previous studies have been tabulated in Table 2 (Ref. [10, 38, 276, 277, 278, 279]).
| Citations | Year | Nutri |
LDL & Atherogenesis | PL |
PF |
AI |
RS |
COV-19 |
| Badimon et al. [276] | 2010 | |||||||
| Anand et al. [38] | 2015 | |||||||
| Torres et al. [10] | 2015 | |||||||
| Bolla et al. [277] | 2016 | |||||||
| Tarkin et al. [278] | 2016 | |||||||
| Casas et al. [279] | 2018 | |||||||
| Proposed | 2021 | |||||||
| Nutri | ||||||||
In a review written by Badimon et al. [276],weobserved that it does link an aspect of nutrition to atherosclerosis and provides nutraceutical interventions in the treatment of atherosclerosis by discussing various effects that nutraceuticals might have on the body. However, they do not talk about various other attributes that are necessary to provide a holistic approach towards the disease.
In another study conducted by Anand et al. [38], a link between various foods, macronutrients, and CVD was established; therefore, it did not target atherosclerosis but instead gave a broader outlook towards nutrition and its link to CVD. This is also seen in the review by Casas et al. [279] who talks about nutrition and cardiovascular health as a whole. Further, Torres et al. [10], in his review, gave a very vast review on nutrition and atherosclerosis, but to target, the disease as a whole, the imaging techniques, its link with AI, and many other aspects were not mentioned in their review. A similar missing component was also observed in the article by Bolla et al. [277], who also discusses, in brief, the pathophysiological link between nutrition, but does not address other factors related to the disease. Tarkin et al. [278], in their review, talks about the imaging techniques, but apart from that, the whole nutritional component is missing. Hence, to our understanding, no other paper has provided the same approach towards the disease which is necessary to study the disease and provide interventions in its treatment.
It is well established that nutrition plays a major role in the formation of plaque as well as in the risk factors, individually contributing to the progression of the disease. But to understand and study this change and progression, it is necessary to have imaging modalities, without these, it is impossible to provide the quantifications of the risk factors associated. By studying imaging modalities, we have also established that ultrasound is the most convenient modality out of the other existing. Further, for risk assessment, the artificial intelligence-based algorithm is recommended to be the best. It is therefore recommended that all these factors be followed to identify and treat the disease at its earliest.
We believe that ineffective knowledge about the disease, progression of the atherosclerotic lesion due to bad nutritional habits and ineffective monitoring does not help in targeting the disease as a whole. Our findings and conclusions help the healthcare sector to get a one-stop solution to their problem.
By addressing all these problems, we feel that more and more people can be educated on their condition. The disease initially diagnosed at very late stages can henceforth be identified and treated accordingly. Preventing the disease will save a lot of people and thus would contribute to decreasing its overall global burden by adopting these practices and understanding the link, one has a greater chance to diagnose and treat it before it worsens or becomes life-threatening. We observed that there is very less knowledge about the effects and behavior this disease would show in the presence of COVID-19, therefore our study helps address this link and provides solutions in all environments.
This review provided a brief insight into atherosclerosis by providing a multidisciplinary approach to it. To our understanding, no other paper includes or addresses all these aspects altogether, as it requires and involves different fields. It addresses and forms a unique link between atherosclerosis and its causal risk factors that may affect it, leading to the nutritional aspects associated with its progression. The review gives a deep insight into how plaque is formed. It further reviews methods for arterial imaging and the best-suited methods to minimize risks associated. Stratifying risks and talking about treatment, prevention, and discussing these changes might allow it to prevail in the ongoing pandemic. A limitation was that though we have discussed every aspect in much detail, targeting various minute details posed a challenge for us. We feel that as a further extension of this study, we would like to throw more light on the COVID-19 section and provide more recent findings and challenges that one might face at this time. Since the imaging component has shown to have a benefit in CVD risk assessment, strong AI tools such as deep learning can be integrated as well [280].
The role of optimal nutrition is imperative in the management of atherosclerosis. A link between nutrition and lifestyle choices can trace the outcomes of the disease. An emphasis is made on the role of several dietary compounds in plaque progression. We observed that not only disease progression, but also disease regression, medical nutritional therapy, and optimal nutritional strategies proved to be beneficial. When referring to optimal treatment, it is of immense importance that prompt and effective diagnostic methods be included to prevent the disease from worsening. Therefore, for early detection and a cure, ultrasound and AI-based methods can be used to benefit the detection of plaque formation, progression, tissue characterization, and classification. For treating the disease, several compounds can actively reduce the inflammatory response and prevent the oxidation of LDL. Hence, it is concluded that a multidisciplinary approach towards this disease can help in the optimal treatment and a reduction in associated risk factors as well.
SmM, ADJ, PA, NNK, LS, JSS contributed in conception and design, SM, ADJ, PA, and JSS contributed manuscript writing, GF, IMS, PSC, MT, KV, SoM, GDK contributed in administrative support and critical evaluation, and all other authors provided vascular inputs, and proofread and approved the manuscript.
Not applicable.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest.
 Fig. 13.
Fig. 13.Atheroma development and plaque rupture (courtesy of AtheroPoint, Roseville, CA, USA). (A) Healthy blood vessel with no change in the geometry of the endothelial layer. (B) Penetration of monocytes in the intimal layer. (C) Formation of plaque with foam cells and migration of smooth muscle cells. (D) Atherosclerotic plaque rupture and formation of thrombus.
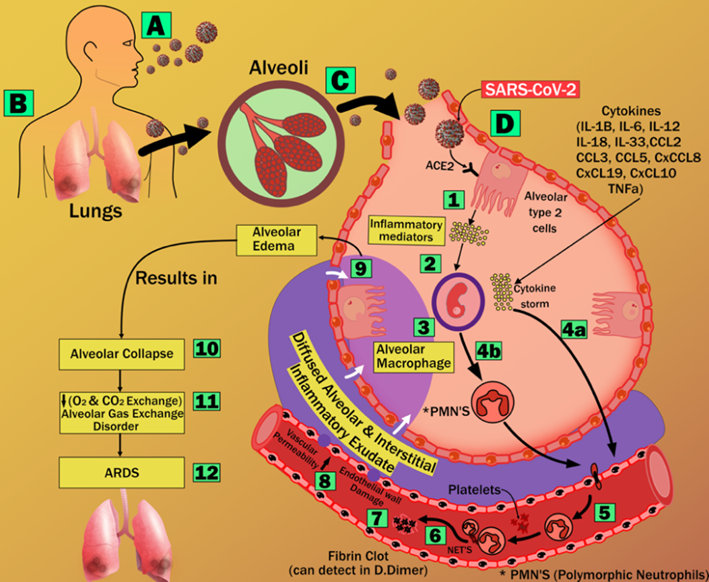 Fig. 14.
Fig. 14.Acute Respiratory Distress Syndrome (ARDS) due to COVID-19 (courtesy of AtheroPoint, Roseville, CA, USA).
SN, Acronym, Description; ACC, American College of Cardiology; AE,
AtheroEdge™, Roseville, CA, USA; AHA, American Heart Association;
ApoB100, Apolipoprotein B; ApoC-III, Apolipoprotein C-III; ASCVD, Atherosclerotic
Cardiovascular Disease; BMI, Body Mass Index; BMUS, B-mode Ultrasound; CCVRC,
Conventional Cardiovascular Risk Calculator; CHD, Coronary Heart Disease; CIMT,
Carotid Intima-Media Thickness; CKD, Chronic Kidney Disease; CTA, Computed
Tomography Angiography; CVD, Cardiovascular Diseases; CVE, Cardiovascular Events;
DHA, Docosahexanoic acid; DQI, Diet Quality Index; EC, Endothelial cells; eGFR,
Estimated Glomerular Filtration Rate; EPA, Eicosapentaenoic acid; ESR,
Erythrocyte Sedimentation Rate; FMO, Flavin Monoxygenase; FRS, Framingham Risk
Score; HbA1c, Haemoglobin A1c; hCRP, C-reactive protein; HDL, High-Density
Lipoprotein; ICA, Internal Carotid Artery; IDL, Intermediate Density Lipoprotein;
IL-17, Interleukin 17; IL-6, Interleukin 6; JELIS, Japan EPA Lipid Intervention
Study; LDL, Low-Density Lipoprotein; mCRP, Monomeric C-reactive protein; MRI,
Magnetic Resonance Imaging; OCT, Optical Coherence Tomography; PCRE, Pooled
Cohort Risk Equation; PET, Positron Emission Tomography; PUFA, Poly Unsaturated
Fatty Acid; RA, Rheumatoid Arthritis; SC, Smooth Cells; TG, Triglycerides;
TGF-