1 Plant Physiology and Molecular Biology Research Unit, Department of Botany, University of Kalyani, Kalyani, West Bengal 741235, India
2 Department of Agronomy, Faculty of Agriculture, Sher-e-Bangla Agricultural University, Sher-e-Bangla Nagar, Dhaka 1207, Bangladesh
Abstract
Waterlogging or flooding in agricultural soil constructs a complex abiotic stress-web in crop plants throughout the lowland agricultural system. In rice, a staple grain crop in the world, submergence creates a long-term and recurrent problem for crops withstanding and their succeeding productivity. Therefore, to restore a satisfactory yield instead of a failure of crop in such submerged areas, the analysis of plants’ responses is important. Such analysis will facilitate research about the entity components of plants responses to anoxia or submergence. For example, the development of cellular and molecular cascade in gene expression of ROS signaling and its subsequent responses may lead to either tolerance or susceptibility in plants. Interplay of plant growth regulators [e.g., ethylene (ET), abscisic acid (ABA), gibberellic acid (GA) etc.] is the well-recognized residues in the coordination of signaling, its transmission through cellular network, and finally, modulation of gene expression are the keys to such tolerance. Nucleotide elements that are specifically sensitive to ethylene have been rescued from land-races of aus-type group of rice (Oryza sativa) and those are considered as the prime determinants for tolerance against anoxia. In this comprehensive text, we tried to accommodate and revise the fundamental and pivotal mechanisms of submergence stress at different angles of physiological and cellular responses of plants. These have also been reviewed for modern state of art techniques deciphering the molecular rejoinders like microRNA, protein-protein interaction, feedback regulation of expression, sugar sensing, amplification of elicitor’s responses and others. Finally, strategies including physiological selection, metabolic engineering, marker assisted selection, genetical manipulation, interspecific hybridization are involved in developing stress resilience and plants’ architecture to support sustainable agriculture under lowland systems.
Keywords
- Abiotic stress
- Anoxia
- Climate change
- Deepwater rice
- Ethylene
- Low oxygen stress
- sub1A
Climate change has been a pre-historic event that appears in different scenarios with facets to affect the vegetation. Time immemorial such a change of climate has been more accurately vulnerable to agriculture through a failure of crops survival, growth and productivity. As a result, yield loss occurs which leads to insecurity of food supply to the society. Thus, agriculturists and scientists worldwide face challenges to ensure proper inputs (e.g., soil, water, temperature, air and other edaphic factors) for crop cultivation [1]. These constraints of abiotic factors are technically called stressors and to overcome these stressors there are certain measures, policies and technical acts adapted so far besides the development of stress resistant plant types by the plant breeders. Adaptation of the strategies in updating and improving the agricultural land is based on nutrient cycling, decontamination, water availability, gaseous enrichment etc. [2]. So, it would be a positive thrust to select tolerant genotypes to accustom to such a climate change. Of course, most of the selected plants for stress tolerance may not necessarily be linked to adequate productivity. To combine those two traits together the research and technology rely on primarily, to dissect out the mechanism of tolerance. On the other hand, another module of research aims to find out the traditional cultivars or landraces with low yield but wider ranges of adaptability [3]. The analysis, even at the molecular level through phenological, physiological, cellular pathways, for regulation and induction of tolerance are the enriched insights. This is also to exercise for any genotype and its retrieval of traits that might have been lost in course of evolution.
Significant loss of genetic diversity including stress responses of different cultivars might possess some transferable traits to support tolerance to other related species in question. This approach essentially includes a mass selection or trial of wider ranges of genotypes either to adopt to a particular, or multi-stress factor(s). This is followed by understanding the mechanism of stress reactions as well as its underlying regulation mechanism [4]. The latter is the most finely detected to locate for specific sequences, heritable as well as linked to one or more quantitative trait locus (QTL). The later may satisfy the integrated breeding program updated with high yielding cultivars and that conceives the marker-assisted selection in a breeding program [5].
Among the different abiotic stresses, abundance of water, technically referred to flooding constitutes a complex situation before crop growth and development. Flooding causes either partial waterlogging or complete inundation. When the water level covers only roots or half of the aerial trunk, the situation is termed as waterlogging [6]. Submergence refers to when it completely sunk the shoot of the plants for significant days with varying depths of water. This essentially ensures the depletion of oxygen to the plants below its optimum level for growth. In case of submergence; this also induces other abiotic stressors like moisture deficits, an abundance of dissolved ions, limited irradiance and biotic stressors (pathogen, insect etc.) too. Even after recede of excess water, an oxidative exposure along with remobilization of reserved materials also hampered plant sustainability [7]. Stagnation of water in the different ecological niche would also dictate the compositional species diversity, dominance, specific traits to such environment and finally declaration of ecotypes. The impetus of research in the domain of submergence stress additionally allows de-folds plants reaction to depleted gases with special reference to partial oxygen pressure. An anoxic or hypoxic condition is manifested in plants with other factors of stress responses directly or indirectly for lowland submerged rice cultivation. Plants retort the submergence stress and allied events through changes of multi-level gene expression. These include changes in epigenetic manifestation to transcriptomics operation followed by translational modification. So, breeding for tolerance aspects under such oxygen deficit condition ought to communicate every method in the analysis of plants’ come back including phenomics, metabolomics, proteomics, genomics etc. [8]. Still, this chapter may encompass the updates of proceedings regarding submergence tolerance with other advanced content of signaling cascade, regulation with microRNA, the contribution of polymorphism of proteins, specific environmental elicitation etc. This would also cover the approaches for biotechnological interventions, modern state of art and strategies in developing specific crop ideotypes against anoxic reaction as well as to modulate in favor of sustenance. The insights are also expected to have the identification of heritable determinants in lieu of climate resilient crops under changing global environment.
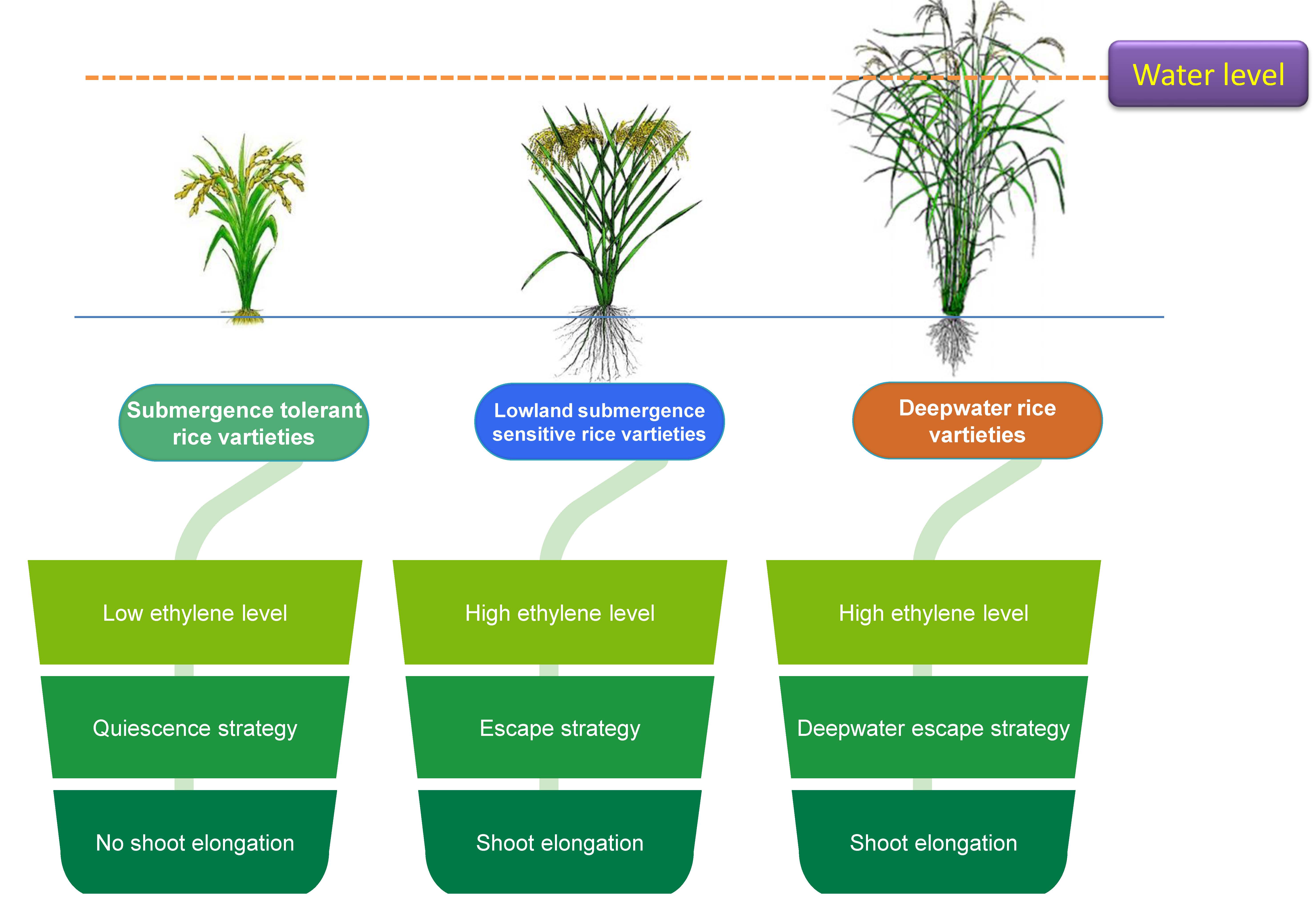
This has been well referred that submergence vis-a-vis anoxia can induce significant changes in gene expression. Collectively submerged tissues are coordinated with metabolic and physiological manifestation pre-dominantly to meet the fermentative mode of energy-yielding metabolism [9]. In the case of submergence with special reference to rice plants two general strategies of tolerance are required: escape and quiescence (Fig. 1). For both the cases, it is the interplay of growth substances to dominate any of those and complement respective alteration of plants responses. The key for these two strategies is primarily based on the management of oxygen deficiency under such ecological niche either to avoid or adapt to the anoxia. Physiologically flooded plants hardly having photosynthetic activities under water is lagged behind optimum, even minimum oxygen tension to survive through growth respiration. Only aquatic or semi-aquatic plant species can tolerate anoxia accordingly and grow and reproduce under water. In contrast, non aquatic species may also thrive under such conditions but for a very short span of exposure and thereafter exerts to be overgrow the water level by increasing their linear growth. For example, tall indica landraces of rice have been in the practice of this strategy where a significant intermodal and leaf sheath elongation occur to pursue escape strategy [10]. This later may be granted as an avoidance phenomenon with some improved morphological and anatomical features facilitating the exchange of oxygen and other gases with that niche. The development of aerenchyma at the sub-cellular level by coalesces of few cortical thin-walled tissues. Besides, at the morphological level the shoot-borne adventitious roots under water are other inducible characters adhered to this strategy, mostly found in rice as well as other species [11]. In some special adaptations, the aerenchyma bound tissues have distinct layers with suberin to check the radial depletion of oxygen or radial oxygen loss (ROL). Few plant species are good consumers of a huge amount of carbohydrates to support the available substrate for elongating cells. Specific gene expression with starch hydrolyzing enzymes may be a factor to put forth the escape strategy.
 Fig. 1.
Fig. 1.Escape and quiescence strategies in submergence tolerant and intolerant rice varieties.
There are few plant species including rice land-races which are efficient
survivors under depleted oxygen concentration particularly for their dwarf
nature. This adaptation is exclusively based on reduced or suppressed growth
under water to minimize the loss of energy for living tissues and thereby adopt
fermentative metabolism [12]. The limitation of respiration under low oxygen
pressure is exclusively glycolytic following fermentative ATP generation which
becomes the sole source of tissue viability. However, sensitive species despite
their ability to ferment may not survive due to the presence of toxic products
for a considerable period. Therefore, plants are forced to suppress the growth in
the internodes by the up/downregulation of few growth regulators like ethylene
(ET), gibberellic acid (GA), abscisic (ABA) etc. The tolerant genotypes can
exercise the quiescence strategy exclusively by downregulation of GA metabolism
through interference with ET. At metabolic level under oxygen deficient
condition, the precursor of ET, 1-amino 1-propane carboxylic acid (ACC) may
accumulate and impose negative feedback to the GA biosynthesis. This sets a
number of genes to distribute in the number of QTL, initially derived from
semi-tall Aus type landraces like FR-13A and the loci is recognized as
sub1[13]. Physically the locus size covers around 200 kb of which a
significant variation (about 40–65%) occurs for two subspecies of rice viz.
indicaand japonica according to their tolerant and susceptible
responses. This QTL is exclusively seated on chromosome No.19 of the FR-13A.
Basically, the sequence of the locus is responsible to encode three transcription
factors (sub1A, sub1B and sub1C) for genes sensitive to ET
(C
Rice, a common semi-aquatic plant has three distinct ecotypes in habitat in
relation to water level. In rice fallows, partial submergence or stagnation of
water is considered as normal for its growth and development. Despite drastic
fall in diffusion rate of O
It is well admitted that ET signaling on cell surface and plasma membrane is triggered on its binding to specific receptor proteins called ET receptors [22]. A number of ET receptors (ETR1) have been identified from Arabidopsis and their molecular organization, functioning and induction of different genes were properly elucidated [23]. Still, more updated information regarding different ETR1, ETR2 and their commonness or variability, interacting proteins regulation and evolutionary trends through higher plants are yet to be scrutinized and understood. The different cross-talks between ET signaling and other plant hormones particularly, ABA, GA and few elicitors like jasmonic acid (JA)are integrated for molecular insights of ET functioning under submergence or any other inducing anoxia. Identification and characterization from different mutants where triple response is lacked and could also isolate the responsible genes and their epistatic interaction in ET signaling pathways [24]. Binding with ET and ETR1 could regulate; however, negatively the developmental role of ET in plants as well as stress response. In absence of ET those receptor proteins interact to ETR1 at a constitutive triple response (CTR) that inhibits ET signaling [25]. The direct interaction between receptor proteins and ETR1 in downstream restricts the binding with ET insensitive 2 (EIN2) [26]. EIN2 is dissociated by some F-box proteins and thereby, ET signaling is inhibited. ET can change the confirmation of receptor complex and those results to inactivate CTR1. This even allows the binding of kinases domain to EIN2. On downstream, phosphorylated form of EIN2 undergoes hydrolysis to dissociate its C-terminal ends and enters into nucleus. The fragment of C-terminal ends in the nucleus functions to replace EIN3 binding mRNA to translate outside the nucleus [27]. This activates the other transcription factors EIN3-like 1 (EIL1). On the same time in the nucleus the C-terminal of EIN2 interacts with other transcription factors: EIN2 nuclear-associated protein1 (ENAP1) that regulates histone acetylation of some histone kinases (H3K14, H3K23) and as a whole promotes the ET sensitive genes [28]. ET signaling has been more updated with reference to submergence stress where anoxia may trigger the various chemical modifications like phosphorylation/dephosphorylation, acetylation and others complex networks to induce and amplify ET signaling in downstream. As for example CTR1 is able to interact many ET receptors in differential affinities. The binding of ET to those receptors brings the conformational changes that would disintegrate the receptor-CTR1 interaction to set free the CTR1 [29]. The released CTR1 is the key to activate ET responses. Still, functional mechanism of EIN2 also raises the question like what function of transmembrane domain of EIN2 and what mechanism for entry of C-terminal domain from EIN2 into nucleus is responsible. Additionally, a complex web on transcriptional, post transcriptional/translational and other epigenetic regulation are well studied for plant development with ET. Still, there are no significant reports on epigenetic control of ET signaling. The function of ETR1 signaling from loss-of-function mutant has included the intervention of ABA [30]. ETR1 has the analogy to replace the ABA-induced ETR1 transcription, and on the other hand ETR2 down regulates the ABA induction upon ETR1 and EIN4 gene expression. Other growth regulators like chemical elicitors (JA, salicylic acid) have relative effectiveness on signaling through EIN3. SA activates NPR1 (Nonexpressor of pathogenesis-related genes 1) which physically binds with EIN3 that as a whole curtails the binding to its specific promoters [31]. This undoubtedly suggests that the ET signaling for development and stress responses is not simplified at all rather may encompass other signaling residues where EIN3, EIL1 are important. All these researches invariably indicate the existence of crosstalk between receptor-based signaling paths of ET and plant growth regulators/related moieties; however, detail mechanisms are not clear till date.
According to experimental evidence, ABA may inhibit the growth of the
inter-nodal meristem and leaf sheath of submergence-sensitive rice genotypes
under water by negative effects of GA
ABA undoubtedly is a pivotal growth regulator in almost disciplines of growth and development as well as stress response. A question by default is raised that how the ABA could send the different stress signals from the environment even with commonness of dehydration under soil-moisture deficits and submergence. Numbers of proteins of the plant cell membrane, particularly on chloroplast are reported as putative ABA receptors; however, their précised roles are yet to be deciphered. The molecular signaling for ABA reveals almost 14 members of proteins in Arabidopsis which are identified to interact with ABA. These proteins classically belong to pathogen related proteins and included under type 2C protein phosphatases. On ABA counterpart these proteins upon binding to ABA induce a pyrabactin resistance like (PYL) protein to associate with type 2C protein phosphatases (PP2Cs) such as ABI1/ABI2 those inhibits the activities [35]. These proteins are collectively called PYLs. In plants, the common PP2Cs include ABI1 and ABI2 those replace the ABA signaling in downstream [36]. PYLs could inactivate PP2Cs on presence of ABA and that establishes the PYLs and assists as core of ABA. In brief or for simplicity PP2Cs including ABI1 and ABI2 are set as the principal components for ABA signaling those replace the ABA responsiveness at initial step [37]. It has also been noted that PYLs may down regulate PP2Cs to perceive ABA signaling and that indicates PYLs may act as co-receptor for ABA. The protein structure of PYLs from the START (Star-related lipid transfer) family has an ABA binding site. The binding of ABA and PYLs also increases by several fold when a phosphatase for ABI2 is present [38]. The binding site of ABA for PYLs is shared by both PYL9 and ABI2 where ABA acts as binding agent. Another intuitive explanation suggests a conformational or allosteric change of PYL9 is required to develop the binding ligand on ABI2 when ABA is present [39].
Therefore, the exact molecular mechanism of ABA recognition and ABA-mediated inhibition of PP2Cs by PYLs, the isolation of PYLs is required. The cloning is also done from cDNA library of Arabidopsis for PYLs and PP2Cs. The over expression of those proteins is also done which is analyzed through homogenecity and crystallization procedure. The precise structure of PYL2 in both ABA dependent [ABA (+)] and ABA independent [ABA (–)] is revealed to have a catalytic core domain of ABI1 [40]. As a whole, the regulation and signaling of ABA at molecular level may summarize: first, presence of PYLs for ABA receptor but less chances to be the co-receptor; second, ABA binding is responsible for allosterism phenomenon on PYLs that in downstream induces binding with PP2Cs and third, ABA in conjugation with PYLs could inhibit PP2Cs that may hinder the substrate protein entry.
sub1A has already been established as submergence tolerant gene
discovered in FR13A. Later on, few IR64 and IR64-AUB1 have been
identified with this factor and that might partly explain the level of
differences between these two under submergence. The promoter of sub1A
with its comparative sequence analysis in two distinct alleles (sub1A
and sub1B) reveals the occurrence of a few single nucleotide
polymorphisms (SNPs), variable in number. Few of those may represent
allele-specific regulatory sequences or cis elements those are specific for
upstream targeted TFs [41]. Few of those in sub1A allele may constitute
putative motifs. SNP4 may comprise a SITE 1 motif that is alike to G–box which
was first found in a proliferating cell nuclear antigen (PCNA) promoter and
cloned from rice. Site 1element in fact is transcriptional activators which is
responsible for specific meristematic tissue expression. Likewise, SNP 5 in
sub1A alleles constitutes two distinct cis elements for CAREOSREP1
motif. In rice coleoptiles, this CAREOSREP motif renders the GA-induced responses
for the proteinase gene [42]. In context to sub1A-2 allele, there
recoded three SNPs like SNP3, SNP5 and SNP9 responsible for allele-specific
putative cis-regulatory elements. Of those, SNP3 covers an ABA-responsive motif
(ABRE). The latter is proved with almost ABA-responsive genes in plants having a
core sequence GAATCC and thereby suggesting the ABA dependence of sub1
QTL. Submergence often induces an in-built dehydration stress in rice plants and
there in contiguous to ABRE other cis elements are also found in
sub1A as dehydration response elements or C repeats (DRE/CRT) in SNP5.
These are found in many drought-responsive genes in Arabidopsis and rice
[43]. SNP9 constitutes a site II motif which represents the response elements
which is seated on more upstream in transcription start sites of sub1A
allele. Last but not the least, a third site (site III) basically corresponds to
5
sub1A has been well-recognized in its concurrent occurrence with other differentially expressed genes (DEG) allied to confer submergence tolerance. This was mostly detected in IR64 and IR64 sub1A at their intermodal tissues duly quantified by q PCR platforms for expression profiling of genes transcription factors. A total of 2508, of which 2487 were novel transcription factors were derived from annotation with existing databases [44]. This analysis predicts around a sequence of 2 kb stretch as upstream of almost the Deutsche Forschungsgemeinschaft (DFGs) promoter with 32 motifs. According to the activation by different allied sub-stressors (anoxia/ hypoxia, carbohydrate metabolism, irradiance, ABA, GA and other hormonal elicitations etc.) these are divided into four groups. Similarly, under submergence, the unavailability of irradiance and hypoxia appears to involve the responsive motifs in their cis-elements as ANERO1-4 and GT1CONC4:C36SENSUS, respectively in almost the DFGs [45]. For the anaerobic energy metabolism, there are about 16 recognized motifs, common of those PYRIMIDINEBOXOSRAMY1A, TATCCAOSAMY etc. For the carbohydrate metabolism with amylase promoter RMY motifs is the most important in both up and downregulated DFG in IR64, IR64 sub1A. For the amylase gene AMY-3 motif with the core sequence (CGACGO), essentially GC loaded was found to act as a coupling element in the association of G-box elements. In rice, MYB protein is an interconnector of hormone-sugar metabolism and distinct AMY motifs are also found, the most conserve sequence is TATCCA offering the cis-elements for TFs of OsMYBS1, OsMYBS2 and OsMYBS[46]. For the GA metabolism, the response element as GARE and RAMY1A is important for their involvement in the inhibition of solubilization of carbohydrate in culm and leaf sheath. In rice grain protein, gluten also is characterized with gene OsGluB-1 having four GLUB1 motifs with exclusively endosperm bound expression. In recent literature, gluten has been identified as another foremost energy-yielding protein in rice grains subjected to induce dormancy. There are also some motifs amenable for TFs like TATA box, EF1, EF2, expression of meristem genes like LEAY-TAG and also with some genes in homeotic development. In respect to IR 64, 80% upregulated genes contain EF2 motifs but few in downregulated genes [47]. Dehydration/Cold response elements as DRE/CRE and ABA response elements (ABRE) are contiguous with four of the DFG motifs more frequent. Though submergence often induces some sort of nutrient deficiency like Fe, still motifs for those like IRO2 are less recurrent [48]. At the molecular level also that submergence is integrally coupled with dehydration and ABA mediation response to recovery has been advocated with the frequency of DRE/CRT and ABRE motifs present in almost DFGs.
Two basics are applied to consider ET as a key to forward rice plants in way of
submergence: submergence escape/avoidance and submergence quiescence. In
conferring to quiescence, ET must be regulated by sub1A ERFs in
different motifs along with other differentially expressed genes that could
tender a feedback inhibition of ethylene. Plants under deepwater situation can
withstand the flood so long that elevating water regime outruns plant standing.
Occasionally this ensures the waterlogging sensitivity of the plants,
particularly effective to tall indica genotypes. Still, submergence
tolerant lowland genotypes were also exposed to inundation but did not continue
beyond two weeks. Tolerant genotypes are well competent to acclimatize within
these days by their subdued growth and regulated anaerobic mode of respiration.
For the escape strategy, there are fair accumulation of ET and its command on GA
over-synthesis resulting in increased plant height. Whatever the case is, the
adaptation under flooding between two types of strategies may be due to gene
duplication of sub1 locus with distinct recurring submergence regimes.
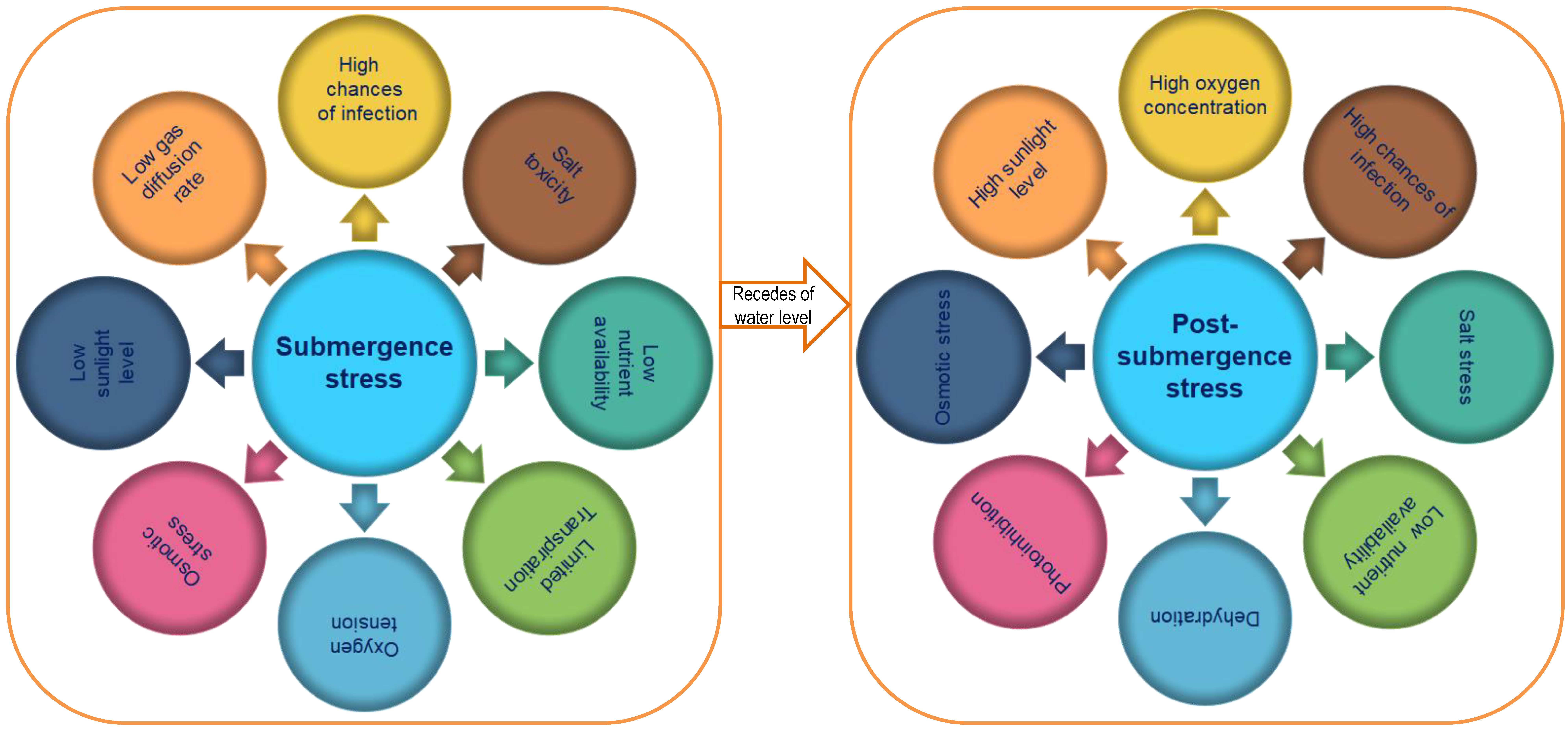
Submergence not alone exerts its ill effects but also with other multiple factors
like timid diffusion of gases, improper membrane activity, fall in root hydraulic
conductivity, inefficient light transmission, variable temperature, low nutrient
acquisition etc. [49] (Fig. 2). In rice, submergence tolerance is mediated by a
cluster of putatively expressed genes: sub1A, sub1B and
sub1C. sub1B and sub1C cover for all rice genotypes
invariably in all accessions but sub1A is exclusively present in
submergence tolerance-specific rice genotypes [50]. sub1A in fact is an
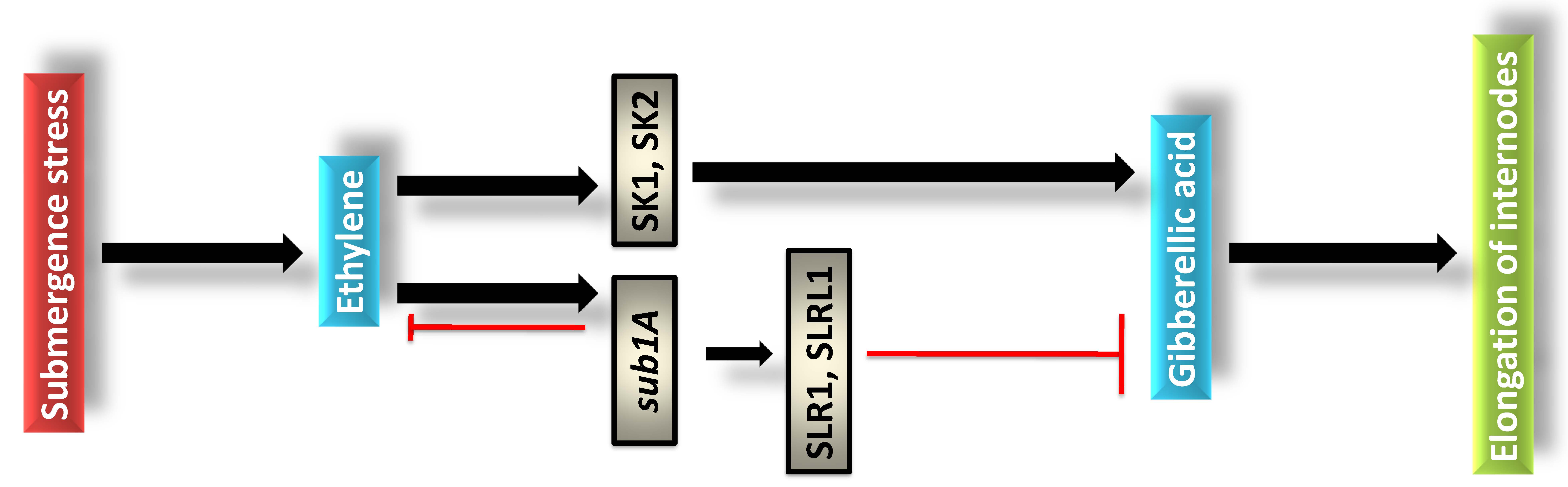
allele that encodes an ERF. The molecular mechanism of sub1A mediated
tolerance has been attributed in quiescence strategy by specific gene cascade
(Fig. 3). Likewise, in escape strategy rice plants are expressed with SNORKEL1
(SK1) and SNORKEL2 (SK2) through ET mediated GA accumulation in stem and leaf
sheath under deep water condition [51]. Contrarily, in quiescence SLENDER RICE 1
(SLR1) and SLENDER RICE LIKE1 (SLRL1) are involved with ABA mediated
down-regulation of GA influence on carbohydrate metabolism. The latter otherwise
controls the mobilization of carbohydrate to the developing tissues under water
and restricts the growth of plants under water. Now, submergence also reinforces
the status of water deficits; however, partially in tissues even under water in
excess. Physiologically, rice plants are trailed with inadequate hydraulic
conductivity on roots membrane due to insufficient rhizospheric dissolved O
 Fig. 2.
Fig. 2.Various effects of submergence and post-submergence stress in rice varieties.
 Fig. 3.
Fig. 3.Physiological and molecular function of ethylene and sub1A in rice plants under submergence stress.
Genes expression under submergence-induced anoxic condition are not independent,
rather those are directly or indirectly correlated with other expression webs
under dehydration, metal toxicity, pH variations, light intensities differences
and also biotic infestations. Still, it is the ET that may corroborate all of
these signaling by connecting in a more complicated cascade which has been
exercised by sub1A encoded group VII encoded ERF belongs to AP2
sub-group are quite compatible to other differentially expressed genes under
anoxia as well as oxidative redox. Even terrestrial species has been reported
with five ERF-VII gene(s) expression under anoxia and ROS induction and have been
cloned from Arabidopsis[58]. It is well admitted that submergence
creates an oxidative stress condition that itself happens to be a stressful
factor. ET plays a major/key role through ABA and GA metabolic web; however,
indirectly to influence plants’ survival [59]. The tissue specific accumulation
of ET under submergence creates the cellular mechanism for cell growth and
elongation for specially escape strategy in few deep water rice plants. This is
coordinated with a simultaneous phenomenon of programmed cell death (PCD) where
ET may trigger the development of ROS. In maize and rice roots, a significant
amount of ROS generation induces ET accumulation that plays a major role in
aerenchymatous tissue formation, particularly in those involving emerged
adventitious roots [60]. Regarding the mechanism of ROS generation, its signaling
and downstream impacts on ET to promote aerenchyma formation is much contextual.
Likewise, H
In rice, this ERFs-VII are known from FR-13 A, a semi-tall indica genotype as the governing elements to prolonged inundation and has sequence homogeneity with HRE1, HRE2, RAP2.2, RAP2.3, and RAP2.12 induced under low oxygen partial pressure. In relation to submergence other tandem repeats of ERF (not sub1) could sense the flood in some deepwater genotypes commonly termed as SNORKE-l that induces culm elongation, however, directly or indirectly with GA coordination. sub1 has got its unique sensitivity to low oxygen tension even with its post-translational modification through a sequential process. A cysteine oxidase mediated proteolysis of N-terminal end of ERF encoded protein may be involved in this alteration. This is more established with findings of various polymorphisms in cysteine oxidase, however, in Arabidopsis that may draw a parallel relationship in sensitivity to ET mediation [68]. So, to let ET to reduce activity the proteolysis ERF coded protein must be checked by depleted oxygen tension under submergence. Undeniably, ET is the unique growth regulator which controls its own concentration within the tissues by auto-inhibition and this phenomenon is quite causative in signaling of submergence tolerance. This cascade of signaling is involved with different transcription factors and their activation which find its binding site in many ethylene-responsive elements (ERE). Out of those AP2/ERF, a super family happens to be the largest one in crop species and interaction with ERE in different regulation is the key to submergence tolerance in rice [69]. In most of the cases dicot species added more information in modalities of bindings with ERF and thereby the elucidation of the role of ET in diverse conditions of stressful environment is possible.
With reference to rice, the activity of ACS has been well characterized for its regulation and imposition of submergence recovery of quiescence strategies. ACS belongs to the E.C. family depending on pyrodoxal phosphate (PLP) with a co-factor vitamin B5 (Pyridoxal pyrophosphate). On the amino acid residues in C-terminus there cloned three possible forms of ACS from rice and all of those perform multiple functions of carboxylation, deamination and transamination of amino acids. In rice, a number of genes have also been reported to complement responses of ACSs in meeting with a wider array of abiotic stresses. Under submergence, particularly, with anoxia activation of ACSs are mostly offered by auto-phosphorylation at different amino acid residues at their C-terminal end [70]. This is more illustrated in ACS II with a conserved serine residue that undergoes phosphorylated by calcium dependent protein kinase along with other same residues but phosphorylated with mitogen-activated protein kinase (MPK). In contrast, ACS III has no phosphorylation domain and thereby appears to be the least contributory. It has already been reported that ET has been self-regulated within its cellular concentration by the feedback inhibition of its intermediate or even byproducts also. In fact, when methionine, the precursor of ET is trailed by its concentration the former has to be recycled to maintain a steady homeostasis or to prevent end product inhibition. Methyl thioadenosine (MTA), a byproduct of ET can be hydrolyzed to methyl thioribose (MTR). The latter is again phosphorylated by a kinase into phosphorylated MTR. In downstream complex reactions with few other intermediates as well as different cofactors like Ni and Fe, the successive enzyme activities may forward a crucial regulation for ethylene biosynthesis. Other rate-limiting genes for ET biosynthesis pathway commonly concern S-adenosyl methionine decarboxylase (SAMDC) with its different isoforms: Os SAM-DC 1/2/3/4/5, Os MT-kinase etc. [71].
Anaerobic stress in the form of anoxia or hypoxia undoubtedly creates a
non-sustainable situation where plants modify their metabolic pathways,
particularly with carbohydrate metabolism [72]. Considering rice as a model crop
under submergence for anoxia there have been two specific growth forms those
compromise with energy yielding metabolism. An illustration with rice in both
escape and quiescence strategies are well familiar in this concern where
glycolytic flux is turned out in an anaerobic mode of metabolism, particularly
with alcoholic fermentation [73]. For the escape strategy where rice plants
execute more metabolic energy for growth of vegetative tissues like internodes
and leaf sheaths in one way represent the constraints of lodging sensitivity. To
overcome the prolonged period of submerged condition, escape strategy offers the
internodal stem elongation, changes in plant architecture and metabolism. This
uncontrolled growth of tissues of the concerned vegetative parts adds the plants
in long height over the water regime and that causes maximum lodging sensitivity
when the water subsides. Though escape strategy is a primarily adopted mechanism
for flooding resistance still, from agronomic purposes it is not full proof for
plant growth and yield potential [74]. From the corner of dry matter allocation,
the tall indica cultivars are more partitioned with vegetative growth than to
mobilize photosynthates in reproductive parts like developing grains [75].
However, this strategy would be granted as one of the pathways in avoidance for
short time submergence stress. Genetic variability in existing rice genotypes
thus, are based on pre and post submergence period dry matter accumulation in
calm and leaf sheath to offer better insurance for escape strategy. Moreover,
from allocation of photosynthetic reduced carbon plants are more prone to convert
those into starch rather than sucrose in those vegetative parts [76]. So, plants
have to execute more energy in turnover of starch-sucrose interconversion to make
readily access for transportable sugar (sucrose) and its utilization into
glycolytic pathways. Escape strategy is also set back for oxidative exposure and
waterlogging, particularly under intense illumination of natural sunshine. The
changes in photosynthetic pigment more into light energy harnessing (PSII:PSI,
chlorophyll a:b and chlorophyll:RuBisCO) than energy quenching mechanism lead the
plants to photo oxidation. Rice being a C
Anaerobic germination is the key feature in submergence during seed germination
and set of seedlings condition. In low land ecosystem, the few rice cultivars are
led to direct seeding practices by the farmer where anaerobic germination
evaluates the potential of germplasm under submerged anoxic or hypoxic soil in
low germination rate ensuring seedling death. Still, rice germplasms with their
ability to grow on that hypoxia to anoxia following complete submergence during
vegetative growth, particularly at seedling stage for a prolonged period offer
another strategy before the flood recedes [79]. The existing rice germplasms with
particular reference to FR13A like land race possessing a gene/QTL offers the
quiescence strategy for submergence tolerance at vegetative growth [80]. The
quiescence strategy technically refers the economization of carbohydrate
metabolism in calms and leaf sheaths that checks the overall plant growth under
water. The conservation of carbohydrate in the vegetative tissues and its less
utilization in major glycolytic path leads the suppression of plant growth;
however, for a particular period of submergence [81]. This essentially turns out
major glycolytic path into alternative mode like anaerobic catabolism and that is
commonly referred with alcohol fermentation. Therefore, quiescence strategy is
principally based on utilization of carbohydrate metabolism into fermentative
catabolism of sugars with less production of energy equivalent (ATP).
Physiologically this strategy is facilitated with one of the major plant growth
substance ET and its domination over others growth hormone like GA, auxin, ABA
etc. So, ET-dominated growth substation is the principal characteristic feature
for quiescence strategy where plants could able to sustain their life under water
mostly by suppression of growth that requires less energy. Therefore, plants
under such flash flood condition reduce the exhaustion of carbohydrate/nutrient
within few days and thereby, plant growth is prohibited that may reveal after
occurrence of flooding situation. Therefore, with less cellular energy
utilization and shortage of carbohydrate in downstream growth period, the plants
may hinder to growth and development [82]. With quiescence strategy plants are
strengthened with respiration just for shake of viability of tissues under
constrain of anoxia and hypoxia rather than growth respiration which ensure the
overall realization of adequate growth [83]. Plants are characterized with
delayed flowering, down regulation of cell wall loosening, down regulation of ET
production etc. in quiescence phenomenon. Moreover, the plants’ differential
responses to insufficient O
In rice stimulus-response mechanism under submergence particularly, with ET often is dependent on a prokaryotic two-component system of a bacterial histidine kinase [84]. This is accompanied by another histidine-containing phosphor transfers enzyme pertaining to the signaling across the cellular membrane. Rice, plants are aided with other secondary messengers in forwarding the stimulus, particularly, the anoxia and elevated redox at post submergence stress. The proteins of those systems correspond to ethylene insensitive homologues: EIN2, 5, 1. Those are also accompanied by others like orthologues of constitutive triple response (CTR1). In rice several receptor proteins have been cloned with overexpression imparting sensitivity to ET under submergence includes OsERS1, OsERS2[85]. The other sub-families are common with their variable responses and important of those are OsERT1, OsERT2 and OsETR3. The other common responses of ET includes triple responses which is also facilitated in signaling pathways for any kind of stress impulses. CTR1 is the most important of such those expressed proteins which is dominant in absence of ET. CTR1 is a recessive mutation which may be active in constitutive activation of ET functioning. In case of wild type, the gene produces a negative regulator for responses done by the paths ET, whereas its mutation can activate the responses of the ET. CTR1 is an analogue of the Raf family gene whose characteristics include a serine/threonine protein kinase [86]. In a number of cases starting from yeast to higher angiosperm the kinase is involved in many developmental as well as signal transduction pathways. In response to submergence tolerance encountered by ET the order of action with ETR1, EIN2, ENI3 and CTR1 has been ascertained by the expression of epistatic relationships of mutants. In rice there recorded three distinct CTR1 genes have been cloned: OsCTRI-1 and OsCTR1-2 have the closer proximity to CTR. For other genes like EIN2 rice acquires many polymorphisms that commonly represent OsEIN2/MHZ7, OsEIN2.2, OsEIN2.3 and OsEIN2.4 homologs. CTR1 is a protein kinase that phosphorylates EIN2 at a specific domain in absence of ET. In absence of ET the EIN2 is activated and forward the downstream signaling of triple responses. On such activation, the hydrolyzed part of EIN2 is transduced into the nucleus and activates the other downstream moieties like EIN3. In rice other EIN-like residue has also been reported and appears to be the key regulators in binding to ERE sequences [87]. Therefore, EIN3 is proved to be a novel transcription factor that finally regulates the signaling of ET function. In Arabidopsis ET signaling pathways is predicted to be the model taking ETR1 as a primary receptor. ETR1 in presence of ethylene suppress the negative regulator CTR1. On such condition, other signaling protein EIN2, which opens the ion channel on the membrane, can lead to the transducer of the signal into the nucleus. Thereby gene activation is achieved. This is the model pathways that are also appreciated in rice with significant variations in different mutants. Likewise, OsERF1 is another member of the ethylene responsive sub-family which is expressed in rice with a significant variation to ethylene sensitivity. The sensitivity is also adhered to the other stressors inducing an anoxic or hypoxic condition of plants.
Now, submergence is also related to a number of facets where the abundance of
water brings upon high salts and tilts, temperature variation, fluctuation of pH,
excess heavy and toxic ions as well as other toxic substances. Despite other
plant growth regulators, it is ET that takes command overall of the physiological
processes under submergence to be modulated. On the gross level of phenology, ET
plays major role in the development of roots and shoots, which is adventitious in
nature, along with intermodal growth, formation of the abscission zones,
development of air vacuoles etc. [88]. Therefore, the signaling essentially
covers an extended network from nuclear origin to physiological responses through
metabolic cascade. Likewise, water in abundance, or the hypoxic condition, would
be another target of photosynthetic research in cereal, the major impeded crop
under submergence. Starting from gas exchange through stomata ending to
carboxylation in mesophyll tissues would be more interesting for the rice
genotypic plasticity to adopt the stringencies of the anaerobic condition of
submergence. Attenuation of chlorophyll fluorescence with its non-photochemical
quenching of the dispersed solar intensity under water would clarify energy
dissipation paths and alteration of the targeted leaf tissues. Following
photosynthetic events, it is the allocation of sugars that faces more
vulnerability to submergence induced anoxia. Signaling to this case is initiated
from starch mobilization for glycolytic operation in rice coleoptiles under
complete to partial submergence. With reference to rice, O
To maintain an altered glycolytic path and reduction of ATP consumption, there
are the two basic domains on which submerged plants could adopt depleted redox of
submergence. There are two key genes in the starch mobilization of shifted
glycolytic paths into anaerobic mode of metabolism. Initially a sucrose phosphate
synthase (SPS) is reversibly activated with uridine diphosphate (UDP) as a
glucose carrier [91]. The second one is sucrase or invertase in -non-reversible
reaction hydrolyzing into glucose, however, in phosphorylated form. In fact, at
the cellular level these two gene products are opposing in function to sustain
the consumption of ATP in a more economical way. In presence of UTPG and fructose
the enzyme SPS can act with one mole of PPi residue. In another round of
reaction, UTP is used for fructose phosphate with the synchronized generation of
ATP. The enzyme is nucleotide diphosphate kinase. Conversely, invertase requires
extra ATPs in the hydrolysis of sucrose than SPS mediated reaction. Under the
condition of O
The calamity for sugar metabolism management under anoxia is predominantly
managed by lactate and pyruvate reactions, however, in anaplerotic paths. Two
genes in order of regulation like PDC and lactate dehydrogenase are the keys to
carry forward plants switching over aerobic to anaerobic mode of metabolism.
Still, the energetic plants are trailed for ATP generation in a more economic
mode. In the oxidation of NADP(H) + H
Scientific perceptive and its analysis to submergence mediated hypoxic or anoxic
stress in wetland species is appeared to be the most complicated with different
facets of cellular, metabolic reactions. From the most pioneer approaches, it
covers predominantly fermentative metabolism vis-a-vis a shifting of
normal glycolytic paths extending the gene-web governed cellular signaling. This
is more impulsive with other facets of the responses when plants are transferred
from complete anaerobic condition (submergence) to the high O
MKA and MH conceived the idea and made the outline of the manuscript. MKA, IS and DD collected the literature and wrote the manuscript draft. MH edited the manuscript and prepared the figures.
Not applicable.
We acknowledge Taufika Islam Anee, Department of Agronomy, Sher-e-Bangla Agricultural University, Bangladesh for her critical reading and proofreading of the manuscript.
This research received no external funding.
The authors declare no conflict of interest.