Background: Mesenchymal stem cells (MSC) were shown to induce
beneficial effects in animal models of neurodegeneration and in pilot human
trials in multiple sclerosis and amyotrophic lateral sclerosis (ALS).
Aim: An open-label, clinical trial to evaluate the safety and efficacy
of repeated intrathecal administrations of autologous-MSC in ALS-patients.
Methods: The study included 20 subjects (age: 20–70) with definite
diagnosis of ALS and Amyotrophic Lateral Sclerosis Functional Rating Scale
Revised (ALSFRS-R) score of
Amyotrophic lateral sclerosis (ALS) is a degenerative disease affecting the motor neurons in brain and spinal cord with a rapidly progressive course, leading to generalized muscle paralysis and death, usually within 3–5 years from onset. The pathogenesis of ALS is complicated and still poorly understood. 10% of cases are familial and 90% sporadic. No therapy has shown sufficient efficacy in halting the progression of the disease. The two FDA approved therapies, Riluzole and Edravarone showed a mild to moderate effect in terms of reduction of the rate of progression and prolongation of survival by few months [1, 2].
Stem cells in general, were shown to induce neuroprotection and immunomodulation, by secreting growth factors and immunomodulatory cytokines, and by increasing the regulatory T cells [3, 4, 5, 6, 7, 8, 9, 10]. Mesenchymal stem cells (MSC) are non-hematopoietic stromal cells, which can be obtained from various sources, i.e., bone marrow (BM-MSC), adipose tissue (AT-MSC), embryonic tissue (E-MSC), cord blood (CB-MSC), reprogramming of mature cells (iMSC) and perinatal tissue-Wharton’s jelly (WJ-MSC) [11] and amniotic membrane [12].
Their classical role is to support hematopoiesis and produce cells of the mesodermal lineage [13, 14]. Additional properties of the MSC, including immunomodulatory and neurotrophic effects, have been described [4, 5, 6, 7, 15, 16, 17, 18, 19, 20]. In animal studies, intravenous (IV) and intrathecal (IT) administration of MSC has been shown to suppress experimental autoimmune encephalomyelitis (EAE) [7, 21, 22] and support remyelination following spinal trauma or induced demyelination [23, 24, 25]. In the mouse model of ALS, MSC administered intravenously (IV), intrathecally (IT) or intraspinally, improved motor performance and extended the survival of the animals [26, 27, 28, 29, 30, 31, 32].
Few small, mostly open-label, clinical trials have reported indications of favorable effects of MSC treatment in neurological diseases, such as stroke, multi-system atrophy, multiple sclerosis (MS), and amyotrophic lateral sclerosis (ALS) [33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50]. Specifically, in ALS, the first phase I/II trial from our group, in which 19 patients received both intrathecal and intravenous injections of MSC, showed that this combined administration was safe, in the short term follow up and induced a trend for stabilization of disease progression during the 6 months following transplantation [37]. This was followed by a phase I/II and IIa clinical trial, in collaboration with Brainstorm® company, in which escalating doses of modified, neurotrophic factors-producing MSC (MSC-NTF) administered intrathecally or intramuscularly, were shown to induce beneficial effects, ameliorating by at least 25% the progression rate of the disease, especially in the intrathecally treated group [41]. The same type of modified MSC was used in a phase II, randomized, placebo-controlled trial, in which although the rate of disease progression (ALSFRS-R slope change) did not differ between the MSC-NTF and placebo-treated patients, an improvement in the progression rate was noted in a prespecified rapid progressor subgroup, paralleled by an increase of neurotrophic factors and decrease of inflammatory biomarkers in the CSF [51]. Other groups showed in open pilot trials, similar results in terms of safety and indications of efficacy after intrathecal injections of MSC, obtained from various sources [50, 52, 53].
An additional way of MSC-administration through a direct intraspinal injection of the cells, was also tested in phase I and phase IIa small studies in ALS, and showed that the procedure was rather safe and induced short-term clinical improvements in a number of patients [54, 55, 56, 57]. Intraspinal administration of neural stem cells showed similar to MSC results, in terms of safety and short-term clinical improvements [58, 59, 60, 61, 62].
In most of the above studies the observed beneficial effects were rather short-living, possibly indicating the need of repeated injections. To this direction, a Korean group performed a phase I and a phase II trial using two IT injections of MSC, 26 days apart [48, 63]. In the phase II trial from this group, which was the first randomized controlled study, 64 patients were randomized in 2 groups and received either Riluzole only or Rizulole and two IT injections of MSC; a significant reduction in the rate of progression of the ALS-FRS scores was noted in the MSC-treated group [63]. A larger phase III trial with three bimonthly IT injections of MSC-NTF (Nurown cells, Brainstorm®) or placebo, was recently completed and the final results from it, are pending.
Additional support to possible clinical neuroprotective effects of MSC-treatment was obtained from a recently completed, controlled double-blind phase II study from our group, that examined the effects of MSC transplantation, comparing the intravenous with the intrathecal way of administration, in 48 patients with active progressive multiple sclerosis (MS). In this study [64] during the one-year follow up, 58.6% and 40.6% of patients treated with MSC-IT and MSC-IV, respectively, exhibited no evidence of disease activity (NEDA) compared with 9.7% in the sham-treated group. The intrathecal transplantation induced additional benefits, on relapse rate, MRI and fMRI measurements, walking ability and cognitive tests, possibly indicating that it is superior to the intravenous way of cell-delivery.
The observation that in most of the previously mentioned studies, both in MS and ALS [2, 41, 51, 58, 59, 65, 66], there were signs of fading-off of the beneficial effects by time, prompt us to evaluate the effect of repeated/multiple intrathecal injections of MSC in ALS.
This is a single center open, phase II clinical trial, evaluating the safety and the clinical effects of repeated IT injections of MSC in ALS patients. Patients received 1–4 intrathecal injections of autologous MSC, at intervals of 3–6 months. The scheduled treatment protocol was intended to include IT injections every 3 months for up to 2 years. However, due to limitations in the number of cultured cells or the unwillingness of the patients to undergo repeated lumbar punctures and additional bone marrow harvesting, the treatment intervals were modified/extended in several of the patients to a maximum of 6 months. The study was conducted at the Department of Neurology & Unit of Neuroimmunology and Cell therapies, at the Hadassah Hebrew University Medical Center, Jerusalem, Israel under license from the hospital’s Ethics committee and the Israel Ministry of Health, from 2016 to 2019.
The number of the patients recruited (n = 20) was calculated based on an assumption of efficacy of at least 50%, in reduction of the ALSFRS-R monthly progression rate, compared to the rate of progression during the run-in period. We based our calculations both on published cohorts (PRO-ACT, [67, 68]) that showed a mean monthly change ranging from 0.59 to 1.2 in ALSFRS-R and on our previous cohort, in which there was a higher monthly ALSFRS-R change of –1.4 [41]. Various models were evaluated with assumptions of efficacy ranging from 50% to 75%, based on our previous experience with MSC-treatments, indicating that the minimal size of the experimental group should be of at least 20 patients.
250 applications from patients were received in our centre. An independent
selective committee was set by hospital’s administration and applications were
examined anonymously, according to the predefined inclusion and exclusion
criteria (see Fig. 1: Flowchart of the trial). Following inclusion and screening
visit, patients were followed up for a mean
 Fig. 1.
Fig. 1.Flowchart of the trial. *: Patients were either lost to follow up and/or stopped treatments due to their inability to travel to our Center for further infusions (USA residences) or due to their unwillingness to undergo additional lumbar punctures.
One month later, patients underwent bone marrow aspiration (BMA) and MSC cells
were produced from the bone-marrow aspirated. On the treatment visit, the
patients were transplanted with an IT injection of MSC (1
Patients had bimonthly follow up after the treatment, which included observation for side effects, full neurological evaluation and muscle chart, ALSFRS-R score and forced vital capacity (FVC) test. Safety was assessed following treatment by the MSC, using measurements of the following variables: physical examination, vital signs (heart rate, blood pressure, body temperature), and laboratory parameters: WBC with differential and platelet count, hemoglobin (Hb), hematocrit (Ht), blood chemistry for electrolytes, creatinine and liver enzymes.
All selected patients underwent bone marrow aspiration under light general anaesthesia and an inoculum of crude bone marrow cells (150 mL) was obtained and two thirds of it was frozen. One third was cultured under GMP conditions at the human cell cultures clean room facility of Hadassah HMO. The MSCs were obtained from the bone marrow of each patient and prepared using a previously described protocol with slight modification [37].
One month later the participants were hospitalized and a lumbar puncture was
performed under standard conditions and local anaesthesia at the L4-5 lumbar
level; 3 mL of CSF were removed and the cultured purified MSCs (1
The rate of progression was calculated based on the ALSFRS-R and FVC scores per month during the run-in period and at each time point of MSC-injection and follow up visit, for up to 2-years. Two-tailed Wilcoxon signed rank and Man-Whitney tests were used to compare the changes in the rates of progression in each patient during the run-in period and after each MSC-treatment and over the whole period of the study. The rationale for such comparison, derives from the fact that progression in ALSFRS-R was shown to be linear during the course of the disease, in large cohorts [69, 70, 71, 72, 73]. Additionally, a Kaplan-Meyer progression curve was calculated defining as treatment-failure each patient who progressed/deteriorated by more than 2 degress in ALSFRS-R.
Patients demography is shown in Table 1, which summarizes the baseline parameters of ALS, i.e., disease duration, baseline ALSFRS-R score, rate of progression before enrolment, and ALS prominent symptomatology (neurological involvement at the onset of ALS). Patients were allowed to continue other ALS treatments, i.e., Rizulole or Edravarone during the study. Summary of concomitant medications is shown on Table 1.
| Patient number | Gender | Age (Years) | Disease duration (months) | ALSFRS-R at Baseline | Mean monthly progression rate from ALS onset to: | number of treatments | Concomitant medications | Main symptoms | |
| Inclusion | Baseline | ||||||||
| 001 | M | 55.0 | 19.4 | 34 | –0.617 | –0.720 | 4 | Lipitor | Gross Motor |
| 002 | M | 60.6 | 20.6 | 26 | –1.031 | –1.066 | 4 | Riluzole, Edaravone | Bulbar |
| 003 | M | 37.5 | 19.3 | 34 | –1.240 | –0.725 | 4 | Riluzole | Gross Motor |
| 004 | M | 38.1 | 24.0 | 32 | –0.684 | –0.667 | 4 | Riluzole, Edaravone | Fine Motor |
| 005 | M | 50.9 | 18.1 | 38 | –0.552 | –0.552 | 1 | Riluzole, Escitalopram | Bulbar |
| 006 | M | 32.9 | 28.2 | 24 | –0.737 | –0.851 | 3 | Riluzole, Nuedexta, Baclofen, Robinul | Bulbar |
| 007 | M | 42.1 | 28.4 | 29 | –0.459 | –0.668 | 4 | - | Fine Motor |
| 008 | M | 45.6 | 24.3 | 30 | –0.776 | –0.739 | 2 | Riluzole | Fine Motor |
| 009 | F | 49.7 | 20.6 | 41 | –0.317 | –0.339 | 4 | Riluzole, Synthroid, Rizatriptan, Tazarotene | Gross Motor |
| 010 | M | 50.8 | 16.5 | 23 | –0.790 | –1.510 | 1 | Riluzole | Bulbar |
| 011 | M | 44.5 | 18.1 | 33 | –1.425 | –0.828 | 2 | Riluzole | Gross Motor |
| 012 | F | 51.6 | 20.7 | 35 | –1.117 | –0.626 | 4 | Riluzole | Gross Motor |
| 013 | F | 59.9 | 20.0 | 39 | –0.495 | –0.449 | 4 | Riluzole, Escitalopram, Clonazepam | Gross Motor |
| 014 | M | 66.0 | 8.1 | 43 | –1.064 | –0.613 | 3 | Lipitor Aspirin | Gross Motor |
| 015 | M | 57.8 | 15.0 | 37 | –0.543 | –0.733 | 2 | Riluzole | Gross Motor |
| 016 | M | 55.2 | 9.2 | 30 | –2.299 | –1.948 | 2 | - | Gross Motor (Familial) |
| 017 | M | 50.4 | 13.3 | 32 | –1.729 | –1.201 | 1 | Mesalazine, Loperamide, Lorazepam | Fine Motor |
| 018 | M | 48.1 | 34.9 | 27 | –0.186 | –0.601 | 8 | Riluzole | Fine Motor |
| 019 | F | 47.1 | 26.0 | 6 | –1.686 | –1.613 | 0 | Clonazepam, Mirtazapine, Esomeprazole, Glycopyrrolate, Citalopram, Quetiapine | Gross Motor |
| 020 | M | 55.5 | 27.4 | 29 | –0.600 | –0.691 | 4 | Buspirone, Alprazolam | Gross Motor |
| Total | 16-M | Mean | Mean | Mean | Mean | Mean | Total | 4: Bulbar | |
| Mean | 4-F | 49.97 | 20.61 m | 31.1 | –0.937 | –0.857 | 61 | 11: Gross Motor | |
| … | … | 5: Fine Motor | |||||||
To evaluate the prognostic factors in the included patients, we calculated the rate of progression in ALSFRS-R from the onset of symptoms till the enrollment to the study or till the first MSC-treatment. As seen in Table 1, 18 out of the 20 included patients had a progression rate of more than 0.5 points in ALSFRS-R scale per month, indicating that this group consisted of patients with severe disease and bad prognosis [69, 70, 71, 73, 74].
We did not observe any serious side effects related to the treatment. 1 patient had aspiration pneumonia after the BMA, 1 suffered a fracture of the ankle, 1 had renal colic/urolithiasis, all of them not related to the treatment with MSC. 3 patients suffered from headache and back pain after the stem cells injection, related to the lumbar puncture and 1 patient suffered from general weakness and fatigue for two weeks post MSC injection, possibly related to the treatment. All side effects were mild and resolved. One patient deceased 4 weeks after the first treatment, due to rapid deterioration of his disease and respiratory failure. The summary of all adverse events is shown on Table 2.
| MedDRA | MedDRA | Number of patients | Patient number |
| Body System class | Preferred term | ||
| General disorders & administration site conditions | Injection site pain | 1 | #010 |
| Fatigue | 3 | #006, #011, #014 | |
| Weakness | 3 | #006, #011, #014 | |
| Skin and subcutaneous disorders | Swelling of face |
1 | #009 |
| Nervous system disorders | Headache | 2 | #005, #011 |
| Lower respiratory tract inflammatory and immunologic conditions | Aspiration pneumonia |
1 | #004 |
| Pneumonitis |
1 | #019 | |
| Renal and urinary disorders | Urolithiasis | 1 | #004 |
| Accidents and injuries | Fracture of ankle | 1 | #004 |
| Death | Death NOS | 1 | #016 |
Clinical analysis was performed in 19 out of 20 patients. Out of the 19 patients who underwent the first transplantation, 16 had a second injection, 12 a third injection and 10 patients had four cycles of MSC-transplantation. In total, 9 patients were lost to follow-up and stopped additional treatments between the four cycles of MSC-transplantation till the end of the trial, due to their difficulty to travel from abroad to our center or their unwillingness to undergo additional lumbar punctures and bone marrow aspiration, but they were included in the statistical analysis at each treatment point.
All the 20 recruited patients were followed before the transplantation for a
mean period of 7.7 months (range: 3.5–18) and 19 out of 20 showed deterioration
(reduction in the ALSFRS-R score during this period (mean monthly change: –1.036
The monthly rate of progression in ALSFRS-R was ameliorated (improved) by more than 25% (which arguably represents a clinically meaningful change) [75] in:
Overall, during the whole period from the 1st to last transplantation:
The overall beneficial effect was statistically significant (p = 0.0038, Mann and Whitney and Wilcoxon signed ranked test) (Fig. 2A,B and Tables 3A,3B).
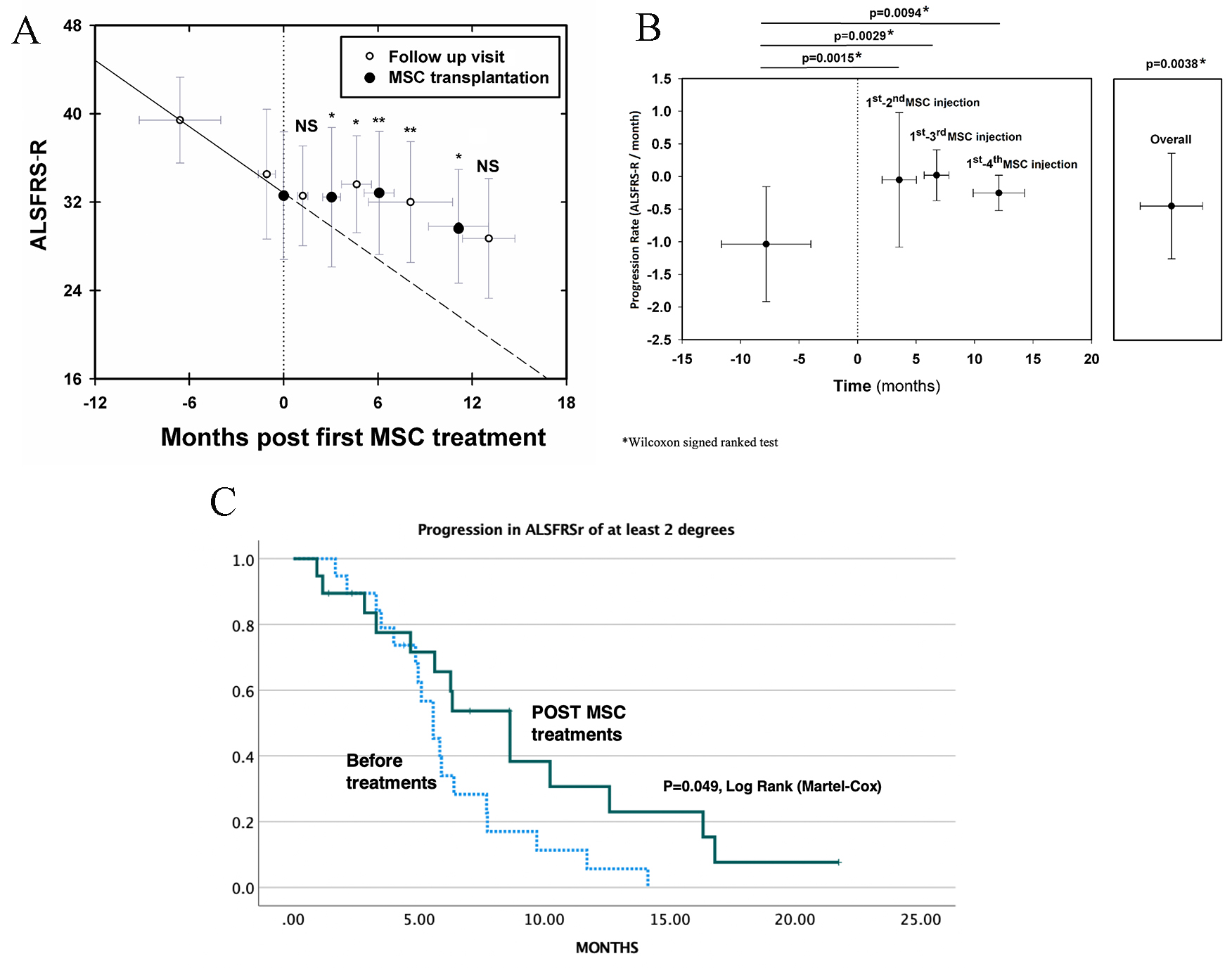 Fig. 2.
Fig. 2.Clinical effects of repeated intrathecal MSC transplantations on the progression of ALS. (A) Follow up of ALSFRS-R before and after MSC treatments. *: p
| Patient number | ALSFRS-R change | During run-in period | between 1st & 2nd injection | between 1st & 3rd injection | between 1st & 4th injection | Overall |
| 1 | Monthly change | –0.928 | 0 | –0.632 | –0.322 | –0.260 |
| Absolute change | 40 |
34 |
34 |
34 |
34 | |
| Time (in months) (% improvement in the rate of progression in ALSFRS-R) * | 6.47 | 3.07 (100%) | 6.33 (31.9%) | 12.43 (65.3%) | 15.37 (72.0%) | |
| 2 | Monthly change | –1.103 | 1.224 | 0.656 | –0.070 | –0.119 |
| Absolute change | 37 |
26 |
26 |
26 |
26 | |
| Time (in months) (% improvement in the rate of progression in ALSFRS-R) * | 9.97 | 3.27 (211.0%) | 6.10 (159.5%) | 14.23 (93.7%) | 16.77 (89.2%) | |
| 3 | Monthly change | –0.466 | 0.705 | 0.171 | –0.238 | –0.238 |
| Absolute change | 40 |
34 |
34 |
34 |
34 | |
| Time (in months) (% improvement in the rate of progression in ALSFRS-R) * | 12.87 | 2.83 (251.3%) | 5.83 (136.7%) | 12.60 (48.9%) | 12.60 (48.9%) | |
| 4 | Monthly change | –0.566 | 0.909 | 0 | –0.684 | –0.689 |
| Absolute change | 34 |
32 |
32 |
32 |
32 | |
| Time (in months) (% improvement in the rate of progression in ALSFRS-R) * | 3.53 | 3.30 (260.6%) | 7.23 (100%) | 10.23 (–20.8%) | 16.97 (–21.7%) | |
| 5 | Monthly change | –0.496 | 0 |
0 | ||
| Absolute change | 47 |
38 |
38 | |||
| Time (in months) (% improvement in the rate of progression in ALSFRS-R) * | 18.13 | 2.33 (100%) | 2.33 (100%) | |||
| 6 | Monthly change | –1.231 | 0 | 0 | 0 | |
| Absolute change | 32 |
24 |
24 |
24 | ||
| Time (in months) (% improvement in the rate of progression in ALSFRS-R) * | 6.5 | 3.27 (100%) | 6.53 (100%) | 8.60 (100%) | ||
| 7 | Monthly change | –3.088 | 1.2 | 0.556 | 0.262 | 0.046 |
| Absolute change | 36 |
29 |
29 |
29 |
29 | |
| Time (in months) (% improvement in the rate of progression in ALSFRS-R) * | 2.27 | 3.33 (138.9%) | 7.20 (118.0%) | 11.47 (108.5%) | 21.73 (101.5%) | |
| 8 | Monthly change | –0.674 | –2.449 | –2.449 | ||
| Absolute change | 36 |
30 |
30 | |||
| Time (in months) (% improvement in the rate of progression in ALSFRS-R) * | 8.9 | 3.27 (–263.4%) | 3.27 (–263.4%) | |||
| 9 | Monthly change | –0.411 | 0 | –0.158 | –0.335 | –0.260 |
| Absolute change | 43 |
41 |
41 |
41 |
41 | |
| Time (in months) (% improvement in the rate of progression in ALSFRS-R) * | 4.87 | 3.07 (100%) | 6.33 (61.6%) | 11.93 (18.5%) | 17.67 (36.7%) | |
| 10 | Monthly change | –3.097 | –1.622 | –1.622 | ||
| Absolute change | 39 |
23 |
23 | |||
| Time (in months) (% improvement in the rate of progression in ALSFRS-R) * | 5.17 | 8.63 (47.6%) | 8.63 (47.6%) | |||
| 11 | Monthly change | –0.309 | –0.214 | –0.142 | ||
| Absolute change | 36 |
33 |
33 | |||
| Time (in months) (% improvement in the rate of progression in ALSFRS-R) * | 9.7 | 4.67 (30.7%) | 7.03 (54.0%) | |||
| 12 | Monthly change | –0.368 | 0.677 | 0.347 | –0.122 | –0.122 |
| Absolute change | 40 |
35 |
35 |
35 |
35 | |
| Time (in months) (% improvement in the rate of progression in ALSFRS-R) * | 13.6 | 4.43 (284.0%) | 8.63 (194.3%) | 16.33 (66.8%) | 16.33 (66.8%) | |
| 13 | Monthly change | –0.339 | –0.288 | –0.148 | –0.191 | –0.336 |
| Absolute change | 41 |
39 |
39 |
39 |
39 | |
| Time (in months) (% improvement in the rate of progression in ALSFRS-R) * | 5.9 | 3.47 (15.0%) | 6.73 (56.3%) | 10.47 (43.7%) | 11.90 (0.9%) | |
| 14 | Monthly change | –0.227 | –0.217 | –0.366 | –0.366 | |
| Absolute change | 44 |
43 |
43 |
43 | ||
| Time (in months) (% improvement in the rate of progression in ALSFRS-R) * | 4.4 | 4.60 (4.4%) | 8.20 (–61.2%) | 8.20 (–61.2%) | ||
| 15 | Monthly change | –0.917 | –0.337 | –0.837 | ||
| Absolute change | 44 |
37 |
37 | |||
| Time (in months) (% improvement in the rate of progression in ALSFRS-R) * | 7.63 | 2.97 (63.2%) | 8.37 (8.7%) | |||
| 16 | Monthly change | –1.912 | –1.667 | –1.667 | ||
| Absolute change | 46 |
30 |
30 | |||
| Time (in months) (% improvement in the rate of progression in ALSFRS-R) * | 8.37 | 4.20 (12.8%) | 4.20 (12.8%) | |||
| 17 | Monthly change | –0.517 | +1.428 |
1.428 | ||
| Absolute change | 35 |
32 |
32 | |||
| Time (in months) (% improvement in the rate of progression in ALSFRS-R) * | 5.8 | 1.40 (376.2%) | 1.40 (376.2%) | |||
| 18 | Monthly change | –2.008 | –0.706 | –0.415 | –0.238 | –0.221 |
| Absolute change | 43 |
27 |
27 |
27 |
27 | |
| Time (in months) (% improvement in the rate of progression in ALSFRS-R) * | 7.97 | 2.83 (64.8%) | 7.23 (79.3%) | 12.60 (88.1%) | 13.57 (89.0%) | |
| 19 | Monthly change | –1.392 | Withdrew | |||
| Absolute change | 15 |
|||||
| Time (in months) (% improvement in the rate of progression in ALSFRS-R) * | 6.47 | |||||
| 20 | Monthly change | –1.029 | 0.39 | 0.214 | –0.595 | –0.595 |
| Absolute change | 35 |
29 |
29 |
29 |
29 | |
| Time (in months) (% improvement in the rate of progression in ALSFRS-R) * | 5.83 | 2.57 (137.9%) | 4.67 (120.8%) | 8.40 (42.2%) | 8.40 (42.2%) | |
| Average | Monthly change | –1.054 |
–0.051 |
+0.019 |
–0.253 |
–0.445 |
| Time (in months) (% improvement in the rate of progression in ALSFRS-R) * | 7.82 m | 3.55 m (107.1%) | 6.75 m (91.4%) | 12.07 m (55.5%) | 10.70 m (47.4%) | |
| Confidence Interval ( |
(–1.46)–(–0.65) | (–0.51)–(+0.41) | (–0.16)–(+0.20) | (–0.38)–(–0.13) | (–0.82)–(–0.07) | |
| * % improvement in the rate of progression in ALSFRS-R during each post-treatment period, vs the run-in period. | ||||||
| Patient | Pre-treatment run-in period: | Between 1st and 2nd injection: | Between 2nd and 3rd injection: | Pre-treatment run-in period: | Between 1st and 2nd injection: | Between 1st and 3rd injection: |
| Delta FVC | Delta FVC | Delta FVC | Monthly FVC change | Monthly FVC change | Monthly FVC change | |
| 001 | –12 | 0 | 0 | –1.85 | 0.00 | 0.00 |
| 002 | 0 | 1 | –1 | –1.40 | 0.30 | 0.00 |
| 003 | –11 | 1 | –1 | –0.70 | 0.35 | 0.00 |
| 004 | –8 | –10 | –5 | –2.26 | –3.03 | –2.07 |
| 006 | –15 | –5 | 3 | –2.31 | –1.53 | 0.45 |
| 007 | –3 | –3 | –5 | –1.32 | –0.90 | –1.11 |
| 009 | 0 | 0 | 0 | –0.82 | 0.32 | 0.00 |
| 012 | –9 | –1 | –3 | –0.15 | –0.22 | –0.46 |
| 013 | –3 | –6 | –3 | –0.50 | 0.86 | 0.00 |
| 014 | –6 | –6 | –4 | –0.45 | –2.60 | –1.95 |
| 018 | –24 | –8 | –23 | –3.01 | –2.82 | –4.28 |
| Mean ± SD | –8.27 ± 7.16 | –3.36 ± 3.85 | –3.82 ± 6.81 | –1.34 ± 0.92 | –0.84 ± 1.43 | –0.86 ± 1.41 |
| median | (–8) | (–3) | (–3) | (–1.32) | (–0.22) | (0.0) |
| Confidence Interval ( |
(–13.08)–(–3.46) | (–5.95)–(–0.77) | (–8.39)–(+0.76) | (–1.96)–(–0.73) | (–1.80)–(0.11) | (–1.81)–(–0.09) |
| p value | 0.09 | 0.08 | NS | NS |
As seen in Fig. 2A,B, there was a statistically significant reduction in
the slope of progression after each one of all 4 cycles of transplantation, as
compared to the deterioration during the pre-treatment period (–1.054
The Kaplan-Mayer survival curve that demonstrates the incidence of ALSFRS-R-progression of more than two degrees, is shown in Fig. 2C. A statistically significant difference in the curve, was observed comparing the post- and pre-treatment periods (p = 0.049, Log Rank, Martel-Cox).
Moreover, 7 out of 19 patients actually improved clinically (mean increase in
ALSFRS-R: 2.86
The changes in respiratory function (FVC) are seen in Table 3B. Data are shown in 11 patients for which there were measurements at all time points up to the 3rd transplantation. There was a trend of beneficial effect (median change in FVC: –8 during the run-in period, vs. –3, after the 1st and after the 2nd transplantation, median monthly change in FVC: –1.32 during the run-in period vs. –0.22 between the 1st and 2nd MSC-injection and 0.0 between the 2nd and 3rd injection). These changes did not reach statistical significance. Between the 1st and 2nd injection, 7 patients showed an improvement of more than 25% in the slope of progression and 5 a 100% improvement; between the 2nd and the third transplantation, six patients showed an improvement of more than 25% in the slope of progression of FVC, and 4 a 50% improvement; 2 patients improved by more than 100%. Between the 3rd and the 4th transplantation, 3 patients showed an improvement of the slope of progression of FVC, of more than 50%, and 2 an 100% improvement (data not shown).
The current single center open clinical trial, shows that repeated intrathecal injections of MSC in ALS patients
was safe and well-tolerated, at least in the short/medium term. Adverse events
that were considered related to the treatment were mostly mild and transient and
occurred close to the time of cell administration. These findings match previous
observations on treatment with MSC in a variety of diseases, including ALS
[33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 48, 63]. Although our study was primarily targeted to assess safety and
not efficacy, our data provide indications of clinically meaningful beneficial
effects induced by the repeated intrathecal injections of MSC. These are
reflected by the slower rate of disease progression during the months following
each transplantation vs the run-in pre-treatment period and the clinical
improvements observed in several patients. On the basis of an individualized per
patient analysis, the vast majority (65%) of the MSC-transplanted subjects were
defined as responders, having at least 25% slower progression rate in ALSFRS-R
after MSC-treatments, compared with the pre-treatment run-in period. Overall,
during the whole period from the 1st to last transplantation 13 patients showed a
The rationale behind the approach of stem cell therapies in neurodegenerative diseases such as ALS, lies on the fact that CNS loses its capacity for efficient regeneration over time. This is especially pronounced in chronic neurodegenerative diseases, possibly due to an insufficiency of growth factors or defective mobilization of the intrinsic CNS stem cells/ progenitors [76, 77, 78, 79]. Based on their well described properties [76, 79, 80, 81, 82], stem cells seem to represent a “logical” treatment approach to achieve those unmet needs and induce neuroprotection or neurotrophism. Moreover, stem cells are strong immunomodulators and may potentially downregulate inflammatory elements upon their migration to the CNS [83, 84, 85]. Inflammation was advocated to play a role in the progression of neurodegeneration even in diseases which were previously considered as “purely” degenerative, like ALS [86, 87, 88]. Several studies have shown that embryonic, neuronal, and other adult stem cells can induce beneficial clinicopathological effects in animal models of neurological diseases [7, 8, 21, 22, 89, 90, 91, 92].
During the last decade, MSC treatments have been tested in various neurological diseases in small or pilot open-label trials [33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44], with promising indications.
The putative mechanism of action of MSC in neurological diseases is controversial. Some investigators claim that the most prominent effects are mediated through peripheral immunomodulation [4, 5, 6, 8, 78, 93, 94]. We have long advocated that neuroprotective and neurotrophic mechanisms play the most crucial role, as supported by our findings in animal models [7] and our pilot trials of MS and ALS [37, 41]. We speculate that IT injection, which brings a higher proportion of the injected cells into close proximity with damaged areas of the CNS, may induce more robust effects than intravenous injection as shown in our recent controlled trial in MS [64]. However, we cannot rule out possible immunomodulatory effects, that may also play a role in halting the progression of ALS. In this trial we haven’t performed an immunological analysis because we used only intrathecal administration and therefore peripheral effects are less likely, but based on our previous findings [37], such MSC-induced immunomodulatory mechanisms (probably mostly local in the CNS) may indeed contribute to the overall effect of MSC-treatment in ALS.
The main strength of our study is that this is a pivotal trial to evaluate the safety and clinical effect of repeated (up to four) injections of autologous MSC (every 3–6 months) and a follow-up period that exceeds that of previous studies. A controlled trial from a Korean group [48, 63] used two injections, very close one to another (26 days apart). In preliminary results from a recently completed phase III controlled study using 3 bimonthly injections of MSC-NTF (Nurown cells, Brainstorm®) the investigators reported that the study did not meet its main goal. While 34.7% of patients who received the stem cell therapy showed a numerical improvement according to the ALSFRS-R scale, that change did not differ significantly from the 27.7% in the placebo group, which also achieved the study goal. A subgroup of patients with baseline ALSFRS-R score of 35 or higher, showed improvement that was “clinically meaningful” compared to those given a placebo. The analysis of cerebrospinal fluid confirmed a statistically significant increase in the nerve growth factors, as well as a reduction in biological indicators of neurodegeneration and inflammation, only in the stem cell-treated patients and no in the placebo group.
An additional strength of our study is related to the inclusion of patients with bad prognosis, as evidenced by the high rate of progression from onset of symptoms to inclusion to the study. The obvious limitations of our trial are the small number of patients and its open-label design. Being an open trial without a controlled group, an additional possible limitation of our findings could be theoretically related to a “regression to the mean” phenomenon. However, such regression, although may have affected the clinical changes at some degree, especially after the first transplantation and the first months of the study, cannot —to our view— explain the benefits observed during the subsequent cycles of treatment and the actual clinical improvements of several patients.
In summary, our results provide signals of, at least short/intermediate-term, clinical efficacy and possible indications of neuroprotection, induced by the repeated administrations of autologous MSC in patients with ALS. These data may contribute to the design of future trials with cell therapies. Larger studies are warranted to confirm our observations and further evaluate the therapeutic potential of cellular therapy in neurodegenerative diseases such as ALS.
PP—design and performance of the study, participation in writing of the manuscript; IK—design and performance of the study, participation in writing of the manuscript, analysis of the data. AG—design and performance of the study, analysis of the data. NEY—performance of the study. DK—design and performance of the study, participation in writing of the manuscript, analysis of the data.
The study was approved by our Hospital Ethics committee (Approval number: 0208-16) and all participants signed an informed consent for the trial. Clinical Trials.gov Identifier: NCT04821479.
Not applicable.
There was no external funding from a commercial company. The trial was supported by the Hospital and the Investigator’s funds.
The authors declare no conflict of interest.
