Frontiers in Bioscience-Landmark (FBL) is published by IMR Press from Volume 26 Issue 5 (2021). Previous articles were published by another publisher on a subscription basis, and they are hosted by IMR Press on imrpress.com as a courtesy and upon agreement with Frontiers in Bioscience.
1 Department of Zoology, School of Biological Sciences, Dr. Harisingh Gour Central University, Sagar (MP)-470003, India
2 Department of Pharmaceutical Sciences, Dr. Harisingh Gour Central University, Sagar (MP)-470003, India
3 Chitkara School of Health Sciences, Chitkara University, Punjab-140401, India
4 Department of Biotechnology, All India Institute of Medical Sciences, (AIIMS), New Delhi-110029, India
5 Divison of Endocrinology, School of medicine, Emory University Atlanta, Georgia- 30322, USA
Abstract
Osteoporosis is a progressive and chronic bone disorder characterized by low bone mass and microarchitectural deterioration of skeletal tissues. Osteoporosis leads to alteration in bone mineral content resulting in decreased bone strength with elevated fracture risks frequently associated with greater morbidity. The latest research in the area of photomedicine had sparked interest in harnessing the active components from plants in both disease control and management across the globe. We in the present review have taken a comprehensive approach to identify forty known plants and their phytoconstituents, which encompasses (i) the genetic diversity of various plants, (ii) their active components and (iii) their osteoprotective role in osteoporosis. Thus, the present review is an attempt for the first time to collectively document the therapeutic properties of valuable medicinal plants in preventing and treating bone loss in osteoporosis.
Keywords
- Phytoconstituents
- Osteoporosis
- Bone health
- Bone loss
- Osteo-protective and Review
Osteoporosis and Osteopenia are two common progressive metabolic bone disorders that occur especially in postmenopausal women and aging population. Clinical manifestation comprises damaged bone with decreased strength, thereby making bones more fragile and susceptible to fractures in areas of wrist, spine and hip. Approximately 200 million women globally are predicted to be affected by osteoporosis, with nearly one-fifth of females at the age 70 and one-tenth of females at the age 60 (1). The U.S. National Osteoporosis Foundation published that by 2020, nearly 14 million Americans above 50 years age and an additional 47 million are expected to suffer from osteoporosis and low bone mineral density (BMD) respectively, accounting for total of 55% American population which are believed to be affected with osteoporosis above the age of 50 years (1). The design of various anti-osteoporotic remedies is based mainly on methods or interventions to modulate bone remodeling. Both non-pharmacologic methods (i.e. adequate vitamin-D and calcium intake, balanced diet, fall prevention and exercise) and pharmacologic remedies are generally integrated to prevent further progression of the disease (2). Presently there are approximately ten US Food and Drug Administration (FDA) approved drugs for osteoporosis anticipation and treatment i.e. anti-resorptive drugs including bisphosphonates (Alendronate, Ibandronate, Risedronate and Zoledronic acid), denosumab (an inhibitor of receptor activator of nuclear factor-κβ ligand-RANKL), raloxifene (as selective estrogen receptor modulator-SERMs) and calcitonin. Anabolic drugs viz. parathyroid hormone in its recombinant form (Teriparatide) and fluoride enhance bone development (3,4). Associated risks of HRT (hormone replacement therapy) such as heart attack/stroke, endometrial breast and ovarian cancers are suspected as side effects of estrogen treatment (5). Administration of calcium and Vitamin D in conjugation with pharmacological agents can be used as a therapeutic substitute for treatment of osteoporosis (6). People all over the world are now switching to various plants derived products (phytoconstituents) due to their preventive, curative, healing with numerous therapeutic and immunomodulatory properties. A number of medicinal plants are found to hold valuable properties and various moieties playing essential role in the treatment of several inflammatory diseases and ailments including bone health (7).
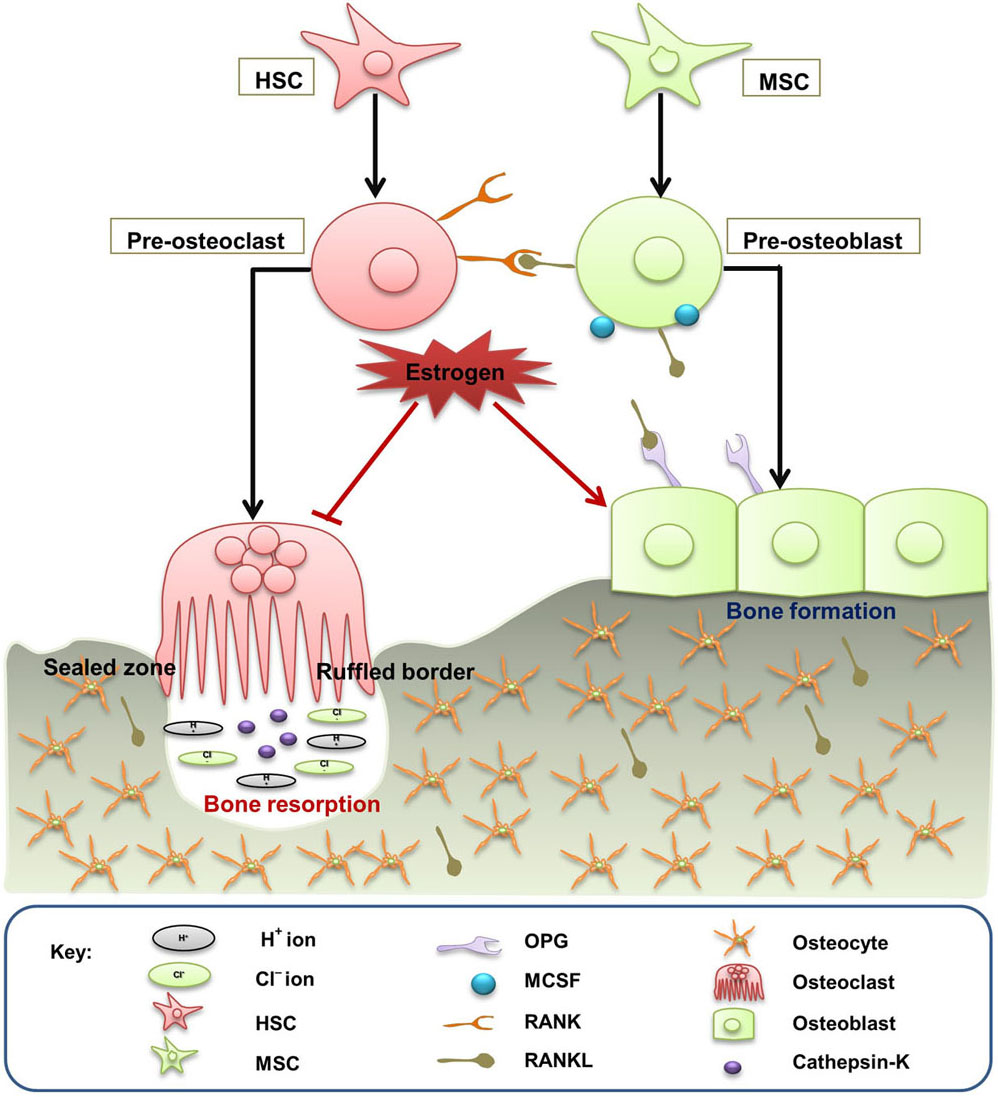
Bone remodeling is associated with restoration of micro damages in the bones thereby ensuring mechanical integrity by balancing the calcium and phosphorous release in host. Bone is remodelled by the combined actions of osteoblasts, osteoclasts and osteocytes, resulting in mineralisation of bone with the help of both collagen type-I and non-collagenous proteins (Figure 1). The remodeling process corroborates a tight relationship between osteoclastogenesis and osteoblastogenesis, which is regulated at three different levels: a) modulation of bone by immune cells b) direct interaction between osteoblasts and osteoclasts and c) neuro-endocrine system. RANKL (RANK ligand) is essentially accountable for induction, differentiation and proliferation of various immature pre-osteoclasts to multinucleated osteoclasts leading to prolonged life of mature osteoclasts (8).
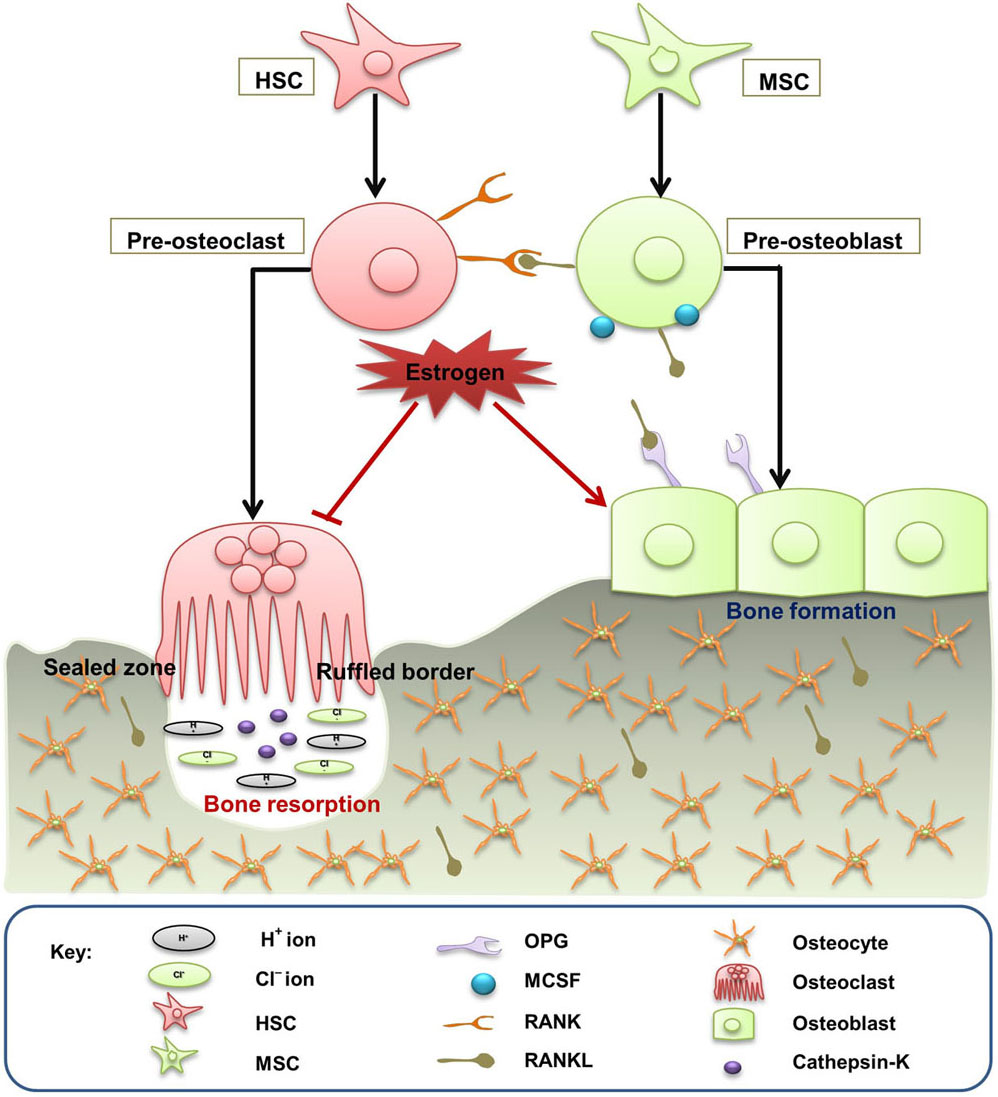 Figure 1
Figure 1Bone remodeling. Bone is remodelled by the combined actions of osteoclasts, osteoblasts and osteocytes. Activated pre-osteoblasts and pre-osteoclasts express RANKL and RANK receptor, respectively. RANK/RANKL binding induces pre-osteoclasts proliferation to form mature osteoclasts resulting in osteoclastogenesis. On the other hand, mature osteoblast expressing the decoy receptor OPG helps in the formation and prevention of excessive bone resorption. Estrogen inhibits bone destruction by maintaining the coupling between osteoclastogenesis and osteoblastogenesis.
Presently, most researchers and clinicians all around the world are focusing their research on diversities of immunomodulators, which could modulate the host immune system to combat various infections and diseases including one health (i.e. osteoimmunology) (9). Various immunomodulatory drugs viz. organic synthetics and biological agents i.e. cytokines and antibodies through their action on the pathways or single targets are used to cure or prevent immune-related ailments but only with partial success (10). In recent years, herbal/plant-based medicines are gaining momentum for use as a suitable or effective immunomodulatory agent for prevention and cure of various inflammatory and autoimmune diseases like arthritis and osteoporosis. Plant extracts are now being reported to have various immunomodulatory and anti-osteoporotic effects along with other therapeutic benefits (11). These properties are ascribed to the presence of several phytochemicals such as phytoestrogens, polysaccharides, flavonoids, lactones, diterpenoids, glycosides and alkaloids showing their remedial effects by suppressing or stimulating several components of the immune system (10). The present review thus highlights the scientific evidence on naturally present compounds extracted from potent medicinal plants, which are known to possess osteo-protective properties and thus can be effectively utilized for therapy in osteoporosis.
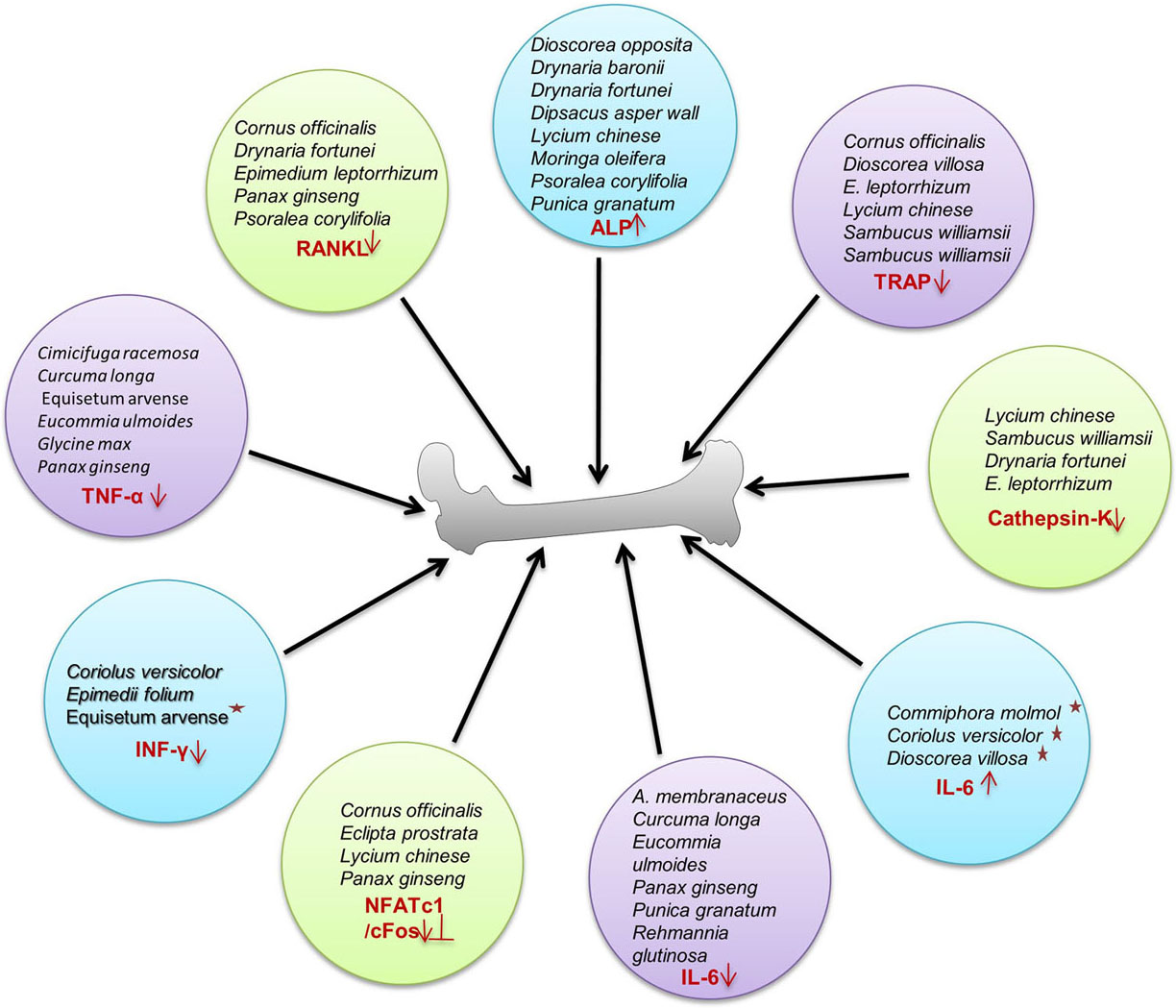
Plants are valuable sources of medicinal products with a broad spectrum of biological activity. Nearly 25 to 50% of present pharmaceuticals are derived from plant by-products (12). Phytoestrogens which are homologous to human estrogen are polyphenols present in plants owning the potential to mimic estrogen receptors and prevent osteoporosis (13). Plants have triterpenoids, flavonoids, steroids, cornusides, isoflavinoid, saponins, steroidal, non-steroidal estrogens and polyphenols capable of inducing prophylactic efficiency to reduce and prevent postmenopausal osteoporosis (14). In the present review, we have compiled an exhaustive list of 40-potential medicinal plants across the globe, whose compounds can be exploited for use as an osteo-protective agent (Figure 2). These are discussed as under and summarized in Table 1.
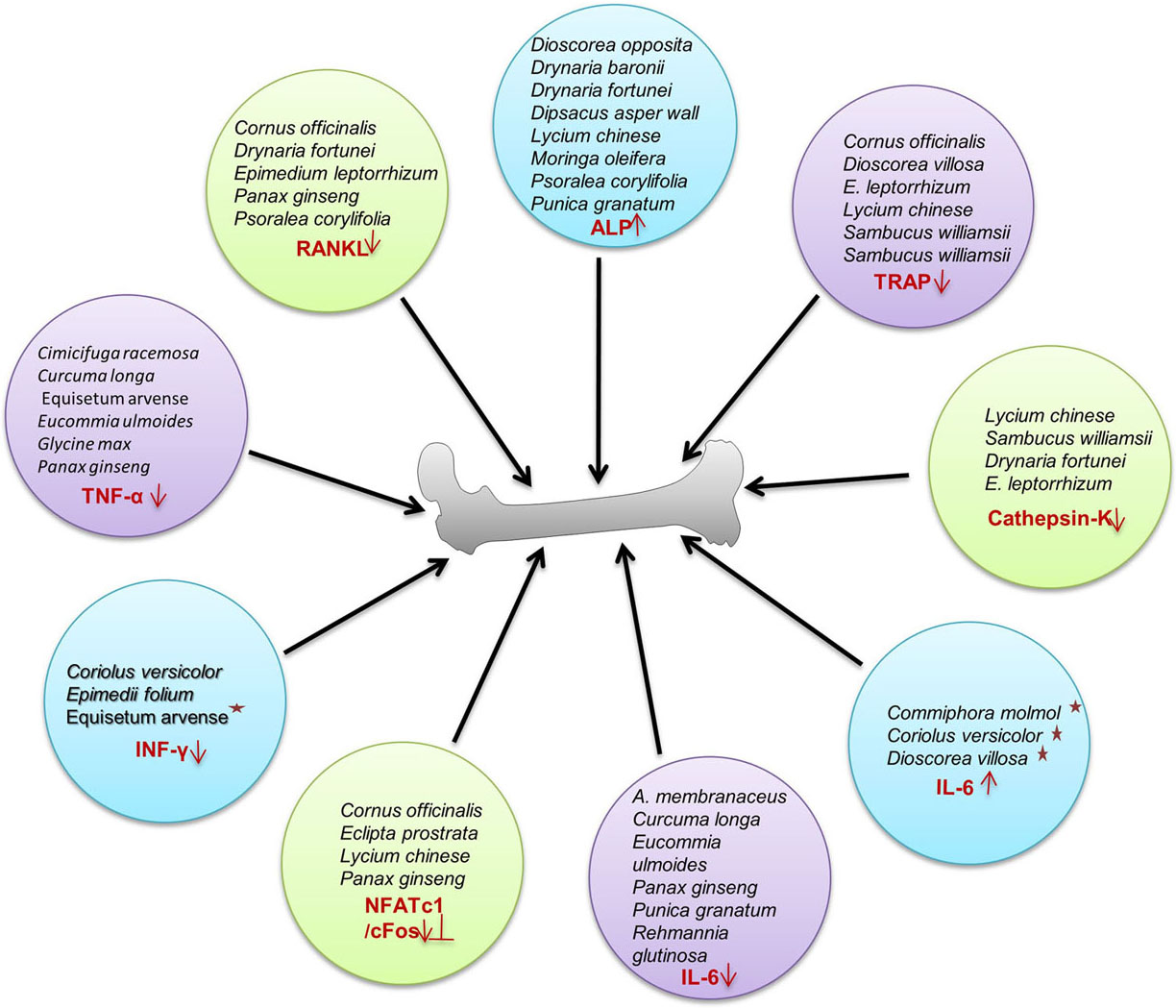 Figure 2
Figure 2Action mechanism of phytoconstituents. Phytoconstituents regulating bone health via up and down regulation of various transcription factors/cytokines for inhibition of osteoclastogenesis and inducing proliferation of osteoblasts resulting in enhanced bone health.
| S No. | Plants (Family) | Parts used | Extract | Phytoconstituents | Immunomodulating properties | References |
|---|---|---|---|---|---|---|
| 1. | Achyranthes aspera |
Roots | Butanol soluble fraction | Flavonoid | Anti-inflammation | (23) |
| 2. | Actaea heracleifolia |
Rhizome | Methanol /Aqueous extract | Triterpenoids | Immunopotentiating effects | (28) |
| 3. | Astragalus membranaceus |
Roots | MeOH extract | Flavonoids & Isoliquiritigenin | Inhibits production of IL-6 and IL-12 | (34) |
| 4. | Cibotium barometz |
Rhizome | Anti-inflammation | (35) | ||
| 5. | Cimicifuga racemosa (Ranunculaceae) | Roots & rhizomes | Iso-propanolic extract | Triterpenes | Suppresses TNF-α production | (40) |
| 6. | Cissus quadrangularis |
Stem | Ethanolic extract | Flavonoids phenolic and steroids | Increases phagocytic index and serum immunoglobulin levels | (43) |
| 7. | Coffea canephora or arabica |
Aerial parts | Aqueous extract | Caffeine | Anti-inflammatory | (53) |
| 8. | Commiphora molmol |
Resins | Ethanolic extract | Resinous exudate | Reduce production of prostaglandins &increase IL-6 | (61) |
| 9. | Coriolus versicolor |
Whole plant | Aqueous extract | Polysaccharide | Increases productions of IL-2, IL-6, IL-12, TNF-α and IFN-γ | (64) |
| 10. | Cornus officinalis |
Fruit | Aqueous extract | Cornuside | Anti-inflammatory | (68) |
| 11. | Crocus sativus |
Petals & gynoecium | Aqueous extract | Crocin | Anti-inflammatory | (73) |
| 12. | Cryptolepis buchanani (Asclepiadaceae) | Roots | Ethanolic extract | Cardenolides | Stimulates delayed type hypersensitivity reaction and humoral antibody production | (80) |
| 13. | Curcuma longa |
Rhizome | Ethanolic extract | Curcumin | Decreases levels of TNF-α, IL-1 and IL-6 | (85) |
| 14. | Cuscuta chinensis |
Seeds | MeOH extracts | Decreases IL-1β, IL-6, NF-κB, TNF-α, and COX-2 levels | (87) | |
| 15. | Dioscorea opposita |
Roots & Bark | Aqueous & MeOH extracts | DOI-protein | Prevents decline in immune functions during menopause | (25) |
| 16. | Dioscorea villosa |
Roots & rhizome | Ethanolic extract | Diosgenin | Enhances production of |
(95) |
| 17. | Dipsacus asper wall |
Roots | Dichloro |
Saponin | Not reported | (25) |
| 18. | Drynaria baronii/ |
Rhizome | Aqueous & MeOH extracts | Flavonoids (Naringin & neoeriocitrin) | Reduces expression of RANKL and NF-κβ | (107) |
| 19. | Eclipta prostrate |
Aerial parts | MeOH extracts | Triterpenoid saponins, flavonoids, | Anti-inflammatory | (108) |
| 20. | Epimedii folium |
Aerial Parts | Ethanolic extracts | Flavonoid | Decreases IL-4 and IL-5 |
(117) |
| 21. | Epimedium leptorrhizum |
Leaves | Aqueous extract | Flavonoids | Anti-inflammatory by inhibiting the production of NO, IL-1β and IL-6. | (122) |
| 22. | Equisetum arvense |
Stem | Hydromethanolic extracts | High silica content | Reduced production of IFN-γ, TNF-α and IL-2 | (131) |
| 23. | Eucommia ulmoides |
Stem & |
Ethanolic extract | Lignan | Reduces TNF-α, IL-1β, IL-6 and IL-8 | (134) |
| 24. | Fructus psoraleae |
Whole herb | Aqueous extract | Psoralea | Increase level of IL-2 and |
(139) |
| 25. | Glycyrrhiza glabra |
Root | Aqueous extract | Isoflavones | Anti-inflammatory | (146) |
| 26. | Glycine max |
Soya cotyledon | Non-fermented soy foods | Flavonoids | Anti-inflammatory | (154) |
| 27. | Herba cistanches |
Stalk | Aqueous extract | Polysaccharides | Anti-inflammatory, and immuno-enhancing effects | (157) |
| 28. | Herba epimedii |
Leaf | Aqueous extract | Flavonoids | Low level of histamine and TNF-α | (163) |
| 29. | Ligustrum lucidum |
Fruits | Aqueous extract | Flavonoids, iridoids and polysaccharide | Increasing IL-2 and IFN-γ | (165) |
| 30. | Lycium chinese |
Root bark | Aqueous & Ethanolic extract | sesquiterpene glucoside | Anti-inflammatory and immuno-enhancing effects | (169) |
| 31. | Moringa oleifera |
Leaves | Methanolic extracts | Vit. A, B, |
Increases both cell-mediated and humoral immune | (173) |
| 32. | Panax ginseng |
Roots | Chemical grade or Raw roots | Total saponins, Ginsenosides | Anti-inflammatory | (179) |
| 33. | Psoralea corylifolia |
Dried fruit & seeds | Acetone extract | Flavonoid, glycoside, Prenyl group | Anti-inflammatory | (183) |
| 34. | Punica granatum |
Seeds, Totum, juice & peel | Supercritical fluid extraction | Steroidal, Non-steroidal estrogens & isoflavones | Suppress inflammatory cytokines TNF-α & NF-κB in serum | (189) |
| 35. | Rehmannia glutinosa (Scrophulariaceae) | Roots | Aqueous extracts | Acteoside | Reduces serum IL-6, TNF-α, and type I collagen | (191) |
| 36. | Sambucus williamsii |
Root bark & Fruit | Ethanolic extract | Lignans | Immunopotentiation | (192) |
| 37. | Sambucus nigra |
Fruit | Polyphenolic extract | Polyphenols | Increases inflammatory cytokines/chemokines | (197) |
| 38. | Terminalia arjuna |
Stem bark | Ethanolic extract | Isoflavones | Releases IL-17 with other cytokines and pro-inflammatory cytokines | (199) |
| 39. | Withania sominifera |
Roots | Ethanolic extract | Withanolides | Increases IFN-γ, IL-12 and decreases IL-4, IL-10 and TGF-β | (202) |
| 40. | Zingiber officinale |
Rhizome | Hexane extract | Zerumbone, Sesquiterpene | Anti-inflammatory | (205) |
It is commonly known as Chaff-flower, Apang, Niuxi, Chirchra, Chirehitta, Latjira, Prickly Chaff flower, Ongaand Apamarga and devil's horsewhip. It is widely distributed throughout the tropical world and also can be seen in many places as introduced weed or invasive species in many areas especially the Pacific Island A. bidentata is rich in active phytochemical compounds including oleanolic acid glycosides, saponins, ecdysterone, ketosteroids and flavonoids having effect that includes liver and kidneys invigoration, bone and muscle strengthening, promoting blood flow with removal of blood stasis and increased longevity (15,16,17). A. bidentata also possess five new oleanolic acid glycosides with bone potentiating properties by inhibiting the osteoclasts formation. (18). Ecdysterone and daucosterol strongly encouraged proliferation of osteoblast like UMR106 cells, while ecdysterone enhanced osteoblast activity (19). The flavonoid quercetin in A. bidentata decreases differentiation of osteoclasts (20). A. bidentata extract (ABE) prevents steroid induced osteonecrosis of the femoral head (ONFH) (21). In connection with bone diseases, He and group (22) reported that n-butanol soluble fraction from A. bidentata roots, prevented the bone loss in ovariectomized rats and thus has potential to the substitutional remedy for osteoporosis and inflammation (23). A. bidentata polysaccharide (ABPB), a root extract taken out with alkali treatment at room temperature, considerably augmented the bone mineral content, BMD, trabecular number, thickness and biomechanical properties of OVX rats, demonstrating that ABPB had outstanding therapeutic outcome on osteoporosis in OVX rats (24).
The rhizome of A. heracleifolia, also named as Cimicifugae rhizome is commonly known as “Sheng-Ma” in Chinese medicine (25). Triterpenoids constituents from C. rhizoma were demonstrated to show inhibitory effects on osteoclastic cells, bone resorption and bone deterioration in OVX mice (26). Ethyl acetate soluble part of methanol extracted from plant carries triterpenoids and is used for the treatment of osteoporotic condition and strengthening of bone by elevating bone mineral density (BMD) (26). The administration of triterpenoids at a dose of 50 mg/kg body weight every other day has positive effect on the bone health by reducing serum alkaline phosphatase (ALP) levels and conserving trabecular bone mass, number and thickness leading to enhanced BMD in female mice (27). The aqueous extract of C. rhizoma possesses immunomodulating property in Raw 264.7 macrophage cell lines (28).
It is commonly famous as Mongolian milkvetch in English and in Chinese as bei-qi or Huang-hua, Huangqi and exists as a flowering herb. It is native to Mongolia, Korea and China. It has been reported with anti-osteoporotic, cardiotonic, antitumor, neuroprotective, antiaging and anti-inflammatory properties (29,30). In recent studies, it has been proved that formononetin; a bioactive phytoestrogen constituent of this plant is a favorable agent for the inhibition of osteoporosis (31). Formononetin encourages angiogenesis and osteogenesis through the inhibition of osteogenic markers expression and inflammatory cytokines, thereby also preventing osteoarthritis by promoting growth factor activation, endothelial repair and wound healing (32). The flavonoids content inhibits the secretion of IL-6 and IL-12 proinflammatory cytokines. This Asian traditional herb shows estrogenic effect, appreciably enhancing biomechanical strength, BMD and ash contents of the tibia and femur in OVX rats (33). It is also a potential source of various natural inhibitors of inflammation (34).
It is commonly famous as Gou-ji, Mythical lamb plant. Strict recognition of Cibotium species is not easy as all are having shiny and waxy fronds, with difference of powdery-pale blush from underneath. Its natural habitat is amid the dripping trees and stream gullies of the rainforests on Hawaii’s windward volcanic slopes, South East Asia, cloud forests of Central America and Mexico. C. barometz is best known for its role as an anti-inflammatory agent in ancient traditional Chinese medicine. It also inhibits osteoclast formation without any effect on viability of macrophages derived from bone marrow (35). The plant extracts have been reported to significantly contribute to either prevent or treat postmenopausal osteoporosis (36).
Cimicifuga racemosa also known as Actaea racemosa, commonly known as Black cohosh, Bugbane, Bugroot, Snakeroot, Fairy candle, Rattle root, Black root and Black snake root, is a native to North America. It is used in the treatment of osteoporosis and additional bone related issues such as osteomyelitis (37). Isopropanolic extract from roots and rhizomes of C. racemosa, are found to have similar effects as that of raloxifene on bone strength in OVX rat model of osteoporosis (38,39). Its anti-inflammatory properties are also known to provide relief from pain associated with osteo-arthritis (OA) and rheumatoid arthritis (RA) (37). The ethanolic extract of C. racemosa has also been reported to suppress lipopolysaccharide-induced TNF-α production in blood monocytes (40).
It is commonly known as Veldt grape and Devil's backbone in English, Vajravalli in Sanskrit, Adamant creeper, Asthi-samharaka, Hadjod or Hadjoda in Hindi and Pirandai in Bangladesh, India and Sri Lanka. It is reported to be one of the most important herbal medicine for osteoporosis by enhancing osteogenesis (traditionally used to repair broken bones), thereby improving bone strength (41). An unidentified, phytogenic isolated anabolic steroid from the plant may be one of the main constituents, which affects osteopontin and CD4+ T cells (42). Flavonoids and phenolic compounds from ethanolic extract of C. Quadrangularis have been reported with immunomodulatory potential due to its activity on neutrophils stimulation leading to increase in phagocytic index and serum immunoglobulin levels in human blood (43). Recently, C. Quadrangularis is also reported with many properties such as reduction of proinflammatory cytokines (44), anti-inflammatory (45), antioxidant (46), antiglucocorticoid properties (47), and reduction in OVX induced bone loss in long bones with a site-specific manner. In femur it affects cancellous bone followed by tibia. C. Quadrangularis undoubtedly reduces bone resorption mainly by decreasing proinflammatory cytokines which are frequently elevated after ovariectomy (48).
It is a shrub of the genus Coffea, which produces berries for the extraction of coffee. The commercially cultivated species of coffee are C. canephora (robusta) and C. Arabica. C. canephora are native to western and central sub-Saharan Africa, from Guinea to Uganda and southern Sudan, while C. arabica, the most highly regarded species, is native to the southwestern highlands of Ethiopia and the Boma plateau in south eastern Sudan and possibly mount Marsabit in northern Kenya. Caffeine, a purine alkaloid (1, 3, 7-trimethylxanthine), is not merely a chief component of caffeinated roasted coffee but also present in tea and caffeinated soft drinks. The role of caffeinated drinks especially coffee in bone loss always remained controversial. Consumption of coffee has been reported to exert some negative effects on the skeletal system and is thus considered as a threat for osteoporosis (49). Caffeine reportedly decreases the BMD (50) and in turn increases the risk of hip fractures (51), by negatively influencing calcium retention (52). Various cellular and pharmacological responses are exhibited by caffeine by producing several biological effects such as anti-mutation, anti-oxidation, antibiotic action, angiogenic, anti-hypercholesterolemia, anti-hypertension and anti-inflammatory (53). Regular intake of caffeine have been linked to enhanced calcium excretion thereby negatively reducing the effectiveness of calcium absorption causing effective loss of nearly 4 to 6 mg of calcium with single cup of coffee. Thus, its regular consumption may result in decreased BMD leading to osteoporosis. The evidences of its detrimental effect on bone health are little and conflicting (54) and need further validation.
This plant grows in tropical regions especially in semi-arid and arid regions producing oleo gum resinous exudate also called myrrh. This resin reportedly has multiple beneficial therapeutic effects such as anti-bacterial (55), anti-inflammatory (56), antioxidant (57), hypoglycemic (10) and cardioprotective (58). In the serum of rat, IL-6 levels were prominently elevated after the administration of DEN/PB (Diethyl nitrosamine/Phenobarbital) when compared with their respective controls. C. molmol helps in decreasing both circulating tumor markers and inflammatory cytokines in DEN/PB-induced rats (59). The ethanolic extract of C. molmol resin have been reported with marked increase in anti-inflammatory effect in rat model of formalin-induced hind paw edema (60). In this model, Su et al also ascribed the anti-inflammatory effect of C. molmol in reducing the production of prostaglandins (61).
It is a variety of mushroom commonly known as Turkey tail, Kawaritake or Cloud mushroom (Japan), Yunzhi (China). The polysaccharide content (62) in water extract of C. versicolor inhibits osteoclast activity thereby improving bone formation and and is thus used as a preventive treatment for various bone diseases (63). A study performed by Luo (64) reported that the root aqueous extract of C. versicolor possess anti-tumor, anti-metastasis and immunomodulatory properties in metastatic breast cancer mouse model. Immunomodulatory properties of C. versicolor are due to its properties of stimulating lymphocytes viz. T-cells, B-cells and natural killer (NK) cells (65,66). It has also been reported that C. versicolor increases production of TNF-α, IFN-γ, IL-2, IL-6 and IL-12 in splenic lymphocytes of tumor bearing mice and thus could help in preventing bone deterioration induced by onset of breast cancer (64). The induction of pro-inflammatory cytokine profile by the polysaccharopeptide (PSP) isolated from the C. versicolor further establishes its profound effects on other immune cells (67).
It is a dogwood species, which is also familiar as Japanese cornel, Japanese cornelian cherry and Cornelian cherries. The precise word for C. officinalis is Korean cornel dogwood or Chinese cornel dogwood because the flowers are originated from China and Korea. C. officinalis is known to inhibit RANKL-mediated osteoclast differentiation in a dose dependent manner without any cytotoxicity against BMMs (Bone marrow-derived macrophages). It inhibits the expression of tartrate resistance acid phosphatase (TRAP), c-Fos, osteoclast associated receptor (OSCAR) and nuclear factor of activated T-cells cytoplasmic 1 (NFATc1) in BMMs treated with M-CSF (macrophage colony-stimulating factor) and RANKL. The compounds obtained from C. officinalis are recognized to inhibit inflammation by regulating nuclear factor kappa-β (NF-κB) (68).
It is a flowering plant of Crocus genus, which is generally known as Autumn or Saffron crocus. C. sativus is presently well-known and grow in India, East Asia, Mediterranean and Irano-Turanian region. C. sativus is also much popular in folk medicine for various diseases such as antispasmodic, expectorant, stomachic, aphrodisiac and emmenagogue (69). The main component responsible for saffron’s color is a water soluble and unique carotenoid deciphered as crocin with various known health benefits (70). Previous findings suggested the pharmacological effects of crocin and saffron extracts which includes antitumor (71), antianxiety (72) and anti-inflammatory roles (73). Recent findings have also suggested the protective effects of crocin against bone diseases i.e. osteosarcoma and articular cartilage degeneration (74). Crocin has also been revealed to revitalize cartilage damage with decreasing bone weakening from oxidative damage and inflammation; due to its antioxidative nature (74). In-vivo studies carried out in a rabbit osteoarthritic model showed amelioration effect of crocin on cartilage degeneration by inhibiting the expression of the MMP-1, -3 and -13 (matrix metalloproteinase) genes in cartilage (75). Oral administration of crocin daily for 16 weeks prevented the worsening of trabecular microarchitecture, inhibited estrogen deficiency induced bone loss by maintaining biomechanical proficiency of bone and helps decrease bone turnover rate (76).
In India, it is commonly known as Harjoralata and Krishna-Anantamul (Assamese), Karanta (Hindi), Kalasariba (Bengali), Jambasariba Khasi-Kombat-ugiang and Garo-Darikhal (Sanskrit). In Thailand, C. buchanani is known as “Thao-En-On” (77,78). The plant is known as ‘Ganglong’ (by Mishing tribes of Arunachal Pradesh in India) and its aqueous extract (79) is used traditionally for the treatment of bone fracture in east Siang district of Arunachal Pradesh. The ethanolic extract (95%) of plant root possess potent immune-stimulant activity. When administered orally, it leads to reduction in TNF-α release, delayed type hypersensitivity reaction and humoral antibody production (80).
It is commonly known as Turmeric (English), Kurkum (Arabic), Halodhi (Assamese), Halud (Bengali), Halad (Gujarati), Haldi (Hindi, Urdu) Gurkemeje (Danish), Aneshtasharvari (Ayurvedic), Haridra (Sanskrit), Zardchob (Unani). It is a routine spice and a common ingredient in curries and other ethnic meals in Indian dishes. Turmeric is also known as 'Indian saffron’. Curcumin, the main active constituent present in rhizomes of C. longa improves both the bone microarchitecture by activation of microRNA-365 via regulating matrix metalloproteinase (MMP)-9 (81) and mechanical strength of bones by reducing serum estradiol levels in non-ovariectomized rats with normal estrogen levels, thereby increasing cancellous bone formation, but in case of ovariectomized rats curcumin slightly increase some bone histo-morphometric parameters impaired by estrogen deficiency (82). Chen et al., performed in vivo study on rat bones with dexamethasone (DXM) induced osteoporosis and reported improvement in bone health due to upregulation in the levels of osteocalcin in serum and downregulation in the levels of collagen type-I fragments, thereby protecting osteoblasts from apoptosis (83). Curcumin administration has been reported to decrease the levels of various proinflammatory cytokines viz. IL-1, IL-2, IL-6, TNF-α and MIP1α (84) and reducing NF-κB activity, thereby decreasing inflammatory responses (85).
Cuscuta is a parasitic plant, commonly known as Dodder, a genus with 100-170 species having orange, yellow or red colour. Earlier it was considered as a single genus and included in the family Cuscutaceae, but now it has been moved to another family called Convolvulaceae (morning glory family) depending on the work of the Angiosperm Phylogeny Group. They are found in temperate and tropical regions with higher diversity in subtropical and tropical regions. The common name of the genus includes angel hair, beggar weed, scald weed, devil's hair, devil's ringlet, devil's guts, fire weed, gold thread, hail weed, hellbine, lady's laces, love vine, pull-down, strangle tare, strangle weed, wizard's net and witch's hair. To-Sa-Za (TSZ) are the mature dry seeds of C. chinensis. TSZ-Aqueous extract upregulates ALP activity, BMP-2 (bone morphogenic protein) expression, collagen synthesis and mineralization in MG-63 cells suggesting the important role of C. chinensis in osteoblastogenesis thereby enhancing bone strength and formation (86). C. chinensis extract is also known to exhibit wide range of biological applications such as antioxidant, anti-inflammatory antiosteoporotic, antiestrogenic and antiprogestogenic in various ailments (87,88).
Chinese Yam (Shan Yao) earned its name as “fairy food” because it is administered mainly as congenital and acquired tonic and produces a novel protein DOI. This protein of D. opposita has constructive effects both on bone microarchitecture and BMD in vivo. Using high resolution micro computed tomography (μCT), it was observed that treatment of mice with D. opposita reportedly have increased BMD, trabecular number, bone volume fraction, trabecular thickness along with simultaneously decreasing structure model index and trabecular separation of the vertebra L2. These changes in BMD and microarchitecture support its property of augmenting bone strength (89). In other species, D. nipponica synergistically regulated bone health after ovariectomy due to the presence of natural products from Dioscorea members that could possibly retain the balance of mineral deposition and absorption (90). Complete extracts from roots of D. batatas stimulate the maturation of osteoblasts by increasing collagen production, ALP activity, extracellular matrix synthesis and calcium deposition (91). In some other studies, yam bark and root (H2O and hexane) extracts help upregulate collagen synthesis and its accretion in osteoblast. The ALP activity was also elevated by yam root and bark extracts signifying that both extracts have stimulatory effect on osteoblast differentiation and matrix maturation via stimulating collagen synthesis and ALP activity (91). DOI has also been reported to be advantageous in the keeping a check on immune cells functioning during menopause (25).
Dioscorea villosa known by common names Wild yam, Aluka, Colic root, Mexican wild yam, China root devil’s bones, Rheumatism root, Yuma, Shanyao (Chinese), Igname (French), Silvestre (Spanish), Vildjams (Swedish) and is also native to North and Central America. There are many species of Dioscorea, whose dried roots and rhizome are useful for therapeutic purposes and have been studied for treatment of various bone diseases. Diosgenin: a steroidal sapogenin present in D. villosa is believed to perform an important role in bone formation by stimulating osteogenic activity of osteoblasts in vitro, along with inhibiting osteoclastogenesis, it has also been reported with anti-osteoporotic effects in rats in vivo (92) and improves bone microarchitecture in aging (93). Recent in vivo study shows reduction in the contents of TRAP, alanine aminotransferase (ALT), serum bone gla protein (BGP), osteocalcin, hydroxyproline/creatinine ratio and calcium/creatinine ratio (HOP/Cr and Ca/Cr) on administration of ethanolic extract of rhizomes of D. spongiosa on glucocorticoid induced osteoporosis, It has also been found to up-regulate bone mass by boosting its calcium content (94). The different species of yam shows immuno-modulating properties by activation of lymphocytic immune cells and enhancing the secretion of cytokines such as TNF-α and IL-6 in the cell culture of RAW 264.7 macrophage cells (95).
It is found in China with common name Xu-Duan. Dipsaci radix is the dehydrated root of D. asper wall and is used by Koreans in herbal medicine treating bone related fractures. The saponin in D. radix is the major constituent showing osteoprotective and anti-inflammatory activity (25), which downregulates BMP-2 expression leading to reduction in osteoblastic differentiation (96); thereby modulating the events of bone remodeling to enhance bone strength (97). The dichloromethane part of D. radix causes elevated ALP activity, expression of sialoprotein and osteocalcin, thus enhancing bone health (98). The saponin component of the plant, AsperosaponinVI, causes osteoblast differentiation by increasing the activity of BMP-2/p38 and extracellular signal-regulated kinases 1/2 (99). Thus, this traditional Chinese medicine is effectively used for the treatment of osteoporosis and bone fracture.
D. baronii commonly known as basket fern is a genus of ferns containing 16 species and a single natural hybrid. Basket ferns are mostly epipetric or epiphytic and are native to tropical East Asia, South East Asia, Australia, Africa, South Asia and Oceania. Drynaria is mostly used for its bone healing ability in stress fractures, knee pains, tooth related maladies and week loins. Drynaria extract has been reported to improve trabecular thickness that is steady with the complete increase in the bone volume/tissue volume ratio, which is indeed an indicator of bone density (100). Other species of the Drynaria like, the rhizome of D. fortune (Kunze), family Polypodiaceae is a variety of the commonly used Chinese herb Gusuibu, which is useful in prevention and treatment of various bone-related diseases. Wang et.al reported that the chemical constituents of Gusuibu can reveal its role in proliferative action of osteoblast-like UMR106 cells (101). D. fortunei have also been described as beneficial for treating arteriosclerosis, inflammation, hyperlipidemia and bone related diseases such as osteoporosis. D. fortune has been found with therapeutic effects on bone fracture and osteoporosis in the OVX rat model (102). It enhances bone formation by enhancing bone matrix proteins i.e. type I collagen, up-regulation of Runx2 and by upregulating osteocalcin expression via induction of BMP-2 and ALP (102). The flavonoids present in Drynaria rhizome i.e. Naringin, neoeriocitrin, kaempferol-3-O-β-D-glucopyranoside-7-O-α-Larabinofuranoside are anti-osteoporotic chemical constituents used to activate the estrogenic receptor (ERs) and replace estrogen for clinical use (101). The chief dynamic constituent of Drynariae flavonoids namely Naringin prevents the retinoic acid encouraged osteoporosis in rats and BMP-2 expression leading to enhanced bone formation. It can also enhance the proliferation and differentiation of osteogenic human bone mesenchymal stem cells (BMSCs) in osteoporosis (103). Naringenin the main metabolite of Naringin reveals a dual regulating function of estrogenic and anti-estrogenic actions through its selective binding with estrogen receptors and thus helps in treatment of osteoporosis (104). The dried rhizome extracts of Rhizoma Drynariae (Gusuibu) containing flavonoid, exhibits bone anabolic ability through angiogenesis and/or osteogenesis (105) and is found to have opposing effects on both inflammatory responses and bone destruction. Through in vitro and in vivo study its anabolic effects are revealed to be responsible for the treatment of bone related defects by increasing osteoblast proliferation and increasing the expression of BMP-2 and ALP (106). It has also been reported to inhibit osteoclast activity by downregulating the expression of RANKL (107) and cathepsin K (106). The extract containing flavonoids exert oestrogen-like shielding effects and increases osteoblastic activities in OVX-induced osteoporotic mice (105). D. fortunei has also been reported to suppress the inflammatory responses and exert anabolic property on bone by decreasing the induction of inflammatory cytokines and NF-κB (107).
The aerial parts of Eclipta prostrata commonly known as Bhringraj and False daisy (Hindi), Mohanlian and Han Lian Cao (Chinese), Karisilanganni (Tamil), Eclipta humilis Kunth and Verbesina conyzoides Trew. This herb grows widely and is spread across all the provinces of China to Australia. It mostly grows near riverside, field side and roadside. Over thousands of years, traditional Chinese medicine has used Eclipta for the treatment of renal diseases. Methanolic extract have been reported to possess hepato-protective, anti-inflammatory, immunomodulatory and anti-oxidative properties (108). Predominant components isolated from the plants are flavonoids, triterpenoid saponins and coumestans. Wedelolactone from E. prostrata, a classied coumestan is known to inhibit osteoclast differentiation of RAW264.7 cells (109). Ethyl acetate extract from wedelolactone at low concentration has anti-osteoclastogenic effect, probably through RANKL/RANK induced NF-κB pathway (109), with no side effects on osteoblastogenesis. Wedelolactone never showed any cytotoxic consequence on BMSC. It is known to facilitate osteoblastogenesis by regulating Wnt/β-catenin signaling pathway and suppresses osteoclastogenesis by downregulating NF-κB/c-fos /NFATc1 pathway (110).
It is known with different common names like Barrenwort, Horny goat weed, Fairy wings and Bishop’s hat. It is also famously called as “Yin-Yang-Huo” in Chinese medicine and is one of the most important osteogenic herbs spanning throughout China, with a smaller number somewhere else in Asia and least within Mediterranean region. The flavanoids present in the ethanolic extract (111) of plant have an inhibitory action against bone resorption by stimulating osteoblastogenic factors in OVX models (112). E. folium has some notable effects on bone system such as increased bone mass and bone strength (113) attributed to osteoblast proliferation (114) and a remarkable decrease in adipogenesis (115). The combined effect of drugs from E. folium and Ligustri lucidi fructus were studied via procuring male Wistar rats as sham, OVX and OVX + treated groups. All the treated groups showed better anti-osteoporosis effects with significant increase in the concentration of the serum calcium and phosphorus along with downregulation of ALP and TRAP (116). A study was carried out in glucocorticoid-induced osteoporotic rats to elucidate the combinatorial effects of E. folium and L. fructus on BMD and hormone levels. It was reported that rise in BMD can be directly linked with high serum estradiol levels in glucororticoid-induced osteoporotic mice. The plants are found to exert anti-inflammatory effects upon oral administration in combination with E. folium, L. fructus along with dexamethasone on asthmatic model rats. Simultaneously, it also decreases the secretion of IL-5 and IL-4 in broncho-alveolar lavage fluid (BALF), adrenocorticotrophic hormone (ACTH) and corticotropic releasing hormone (CRH) levels in plasma. Along with that it further elevates the concentration of IFN-γ and glucocorticoid receptors (GCR) in BALF and restores the balance of Th1/Th2 cells (117).
It is known as Horny Goat Weed, Bishop's hat, Barrenwort, Fairy wings or Yin Yang Huoin (Chinese medicine). They occur are endemic to China with the smaller numbers also found in mediterranean region. The leaves of Epimedium species like E. wushanense, E. brevicornum, E. koreanum and E. pubescens are mainly used to check and treat osteoporosis and other menopausal related diseases in China and other countries. Phytoconstituents from these species are frequently used as herbal drugs for anti-osteoporotic remedies (118). Icariin and epimedin (B and C) are the flavonoids acting as chief anti-osteoporotic constituents, having ability to suppress urinary calcium excretion, restrain bone resorption, encourage bone formation and check osteoporosis. Its flavonoids have estrogen-like activity, thereby causing modulation of bone metabolism through estrogen receptor pathway, which further leads to enhanced osteoblastogenesis via regulating ALP activity and expression of IL-6, OPG (osteoprotegerin), M-CSF, RANKL, BMP-2, Cbfα1 (core binding factor alpha subunit 1) and SMAD4 (protein) involved in the bone remodeling (119,115). The flavonoids regulate the osteogenic differentiation and function via the BMP and Wnt/β-catenin signaling pathways. It leads to higher mRNA expression of Runt-related transcription factor (Runx)-2, cyclin D1, BMP-2 and BMP-4, all of which are involved in regulation of BMP and Wnt signaling pathways. (120). Active constituent of the E. leptorrhizum is Icariin, a flavonoid glucoside, suppressing bone loss and upregulating osteoblastogenesis in distal femur and tibia in OVX models and postmenopausal women via increasing expression ratio of OPG/RANKL, ALP activity and accelerating ERα phosphorylation (121,122). This reflects that the anabolic effects are produced by Icariin in bone probably by activating estrogen receptors (123). It also decreases TRAP positive osteoclasts, inhibits the size of lipopolysaccharide (LPS) induced osteoclasts formation and bone desorption by expression of TNF-α and IL-6. Inhibitory effect of Icariin was studied in vitro where a reduction in activation of Iκ-Bα and ERK1/2 genes were observed when stimulated with LPS. Icariin suppresses osteoclasts differentiation by inhibiting p38 activation and JNK pathway (122). When macrophage precursor cells from bone marrow and RAW264.7 cell line were stimulated with LPS and RANKL in the presence of Ikarisoside A, Osteoclastogenesis was found to be repressed. The decreased osteoclastogenesis can be attributed to the anti-inflammatory nature of Ikarisoside A, a natural flavonoid of E. koreanum. (124). Ikarisoside-A blocks the resorbing capacity of differentiated RAW264.7 cells on calcium phosphate coated plates. It has also been found to reduce the osteoclast specific genes such as TRAP, MMP-9, RANK and cathepsin K via inhibiting the RANKL mediated activation of Akt, NF-κB, and JNK, indicating that Ikarisoside-A may be beneficial for treatment of bone diseases as RA, periodontal bone erosion and osteoporosis (125).
It is nearly present in all parts of the world such as Asia, Europe, Africa, North America and South America and commonly known as Horsetail, Scouring rush and Pewterwort. Shoot (fresh or dried all plant parts from the rhizome, stem, leaf and spores) of horsetail are used for medicinal purposes including treatment for osteoporosis due to its rich source of silicon (126), a mineral necessary for bone health and responsible for absorption and utilization of calcium. An Italian study conducted on 122 Italian women with osteoporosis improved their bone density after taking horsetail extract for one year. It is known to increase the bone strength by synthesizing and stabilizing collagen by propyl hydroxylase enzyme (127). In vivo and in vitro studies elucidated the role of E. arvense in the proliferation of osteoblasts along with inhibiting osteoclastogenesis (128,129). Another study carried on hydro-methanolic extracts demonstrated its role in bone tissue regeneration (130).The extract of E. arvense is found to decrease the population of T lymphocytes, the chief cells of inflammatory immune system related diseases as their proliferation was inhibited, causing decrease in CD-69 and IL-2 surface receptor expression and IL-2 production. Its immunopotent role can be proven by decreased production of IFN-γ and TNF-α (131), thereby leading to better bone protection on administration of horsetail (21).
Its common name in Chinese medicine is Du Zhong. It is small tree native to Central China. In Chinese, the stem barks of E. ulmoides are called as Eucommiae Cortex. These barks are used for management of bone related disorders i.e. lower back pain and weak joints (25). The lignan constituent of E. ulmoides is found to have osteoprotective effects in both in vitro and in vivo studies (132) by suppressing the growth of osteoclasts and enhancing osteoblast activity (133). It is reported to have anti-inflammatory activity by reducing the secretion of proinflammatory cytokines such as IL-1β, TNF-α, IL-6 and IL-8 (134) along with inhibiting enzymatic activity of caspase-1 (135).
It is a native of China and commonly known as Buguzhi. Its aqueous extract is used to treat bone diseases by increasing bone density and altering bone histomorphology by inhibiting osteoclast formation (136) and enhancing osteoblastic proliferation (137). Its effect was explored in both calcium deficiency-induced and OVX osteoporotic rats, which mimics the estrogen like effect on the treated group of rats. The herbal mixture of H. epimedii, F. Ligustri lucidi and F. psoraleae has have been reported to activate prostaglandin EP3 receptor and OPG which are responsible for bone remodeling (138). F. psoraleae causes enhanced secretion of IL-2, along with augmented activity of NK cells, responsible for immune potentiating effects in Pneumocystis carinii infected rats (139).
It is native to parts of Asia, such as India and southern Europe. It is an herbaceous perennial legume known with different names in different languages such as in English it is known as Licorice/Liquorice, Sweet-wood in Sanskrit and various regional Indian names as Yashtimadhuka, Yashtimadhu, Jethi-madh, Mulhathi and Meethilakdi. Formononetin, chemically Formononetin-7-hydroxy-3 (4-methoxyphenyl) chromone, is a naturally found isoflavone in the roots of G. glabra and G. uralensis. The structural similarity of formononetin with 17 beta-estradiol, mimics estradiol effects thus it is regarded as a “phytoestrogen”. This isoflavinoid also has beneficial effect on osteoarthritis (140). The constituents of formononetin are natural SERM (141,142). SERMs like raloxifene, revealed estrogenic activity in the bone cells and help in the treatment of osteoporosis in postmenopausal women without mammotropic and uterotrophic effects (143). The scientific concept about the effect of formononetin on bone tissues were firstly validated through in vitro studies on bone cells (144) and later the histomorphometric parameters of laboratory animals (142). Intraperitoneal administration of formononetin has shown to increase the trabeculae area in tibia and lumbar vertebra (145). Formononetin administration to OVX rats showed positive effect on various bone biomechanical parameters leading to the prevention of osteoporosis (31). Glycyrrhizin, a triterpene saponin, is considered as a main and potent constituent of licorice G. glabra root. Licorice possesses antiulcer, demulcent, antacid, diuretic, expectorant, laxative, sedative, anti-inflammatory and tonic properties (146).
It is commonly found in North America with common name as soya bean or soybean, and is also indigenous to East Asia. It is grown as edible bean as it contains proteins and flavonoids that includes biochanin-A, daidzein and genistein and may also be used as a common dietary supplement. The UN Food and Agriculture Organization classified G. max as an oilseed than a pulse. The soy flavonoids show strong effects on bone metabolism and are structurally and functionally correlated to 17-β-estradiol. It has its own importance in the prevention and management of postmenopausal osteoporosis (147). Clinical trials suggested that isoflavones from soy have positive effects on bone turnover markers, bone mechanical strength and BMD in postmenopausal osteoporotic women. Around 22% of soybean protein in diet is equivalent to daily estrogen administration needed to improve bone loss induced by OVX, but soybean protein unlike estrogen, do not show uterotrophic side effects and can’t cause reduction in bone turnover markers (148). The modulation of flavonoids and soybean protein on nuclear receptors is related mainly for receptors expression on progesterone, androgens, estrogens, retinoic acid, vitamin D and thyroid hormones (149). Soy flavonoids slow down bone loss in osteoporotic animals and postmenopausal osteoporotic females by modulating bone metabolism related gene expression, cytokines, growth factors, calciotropic receptor, osteocalcin, ALP and collagen type I (COL I) and thus regulate trabecular microstructural properties. Soy flavonoids is anti-estrogenic on both estrogen receptor alpha and estrogen receptor beta dependent gene expression in estrogen dependent behavior in brain (150). Genistein helps in the regulation of the B-lymphopoiesis and shows estrogenic properties in bone and bone marrow (151). It does not show estrogenic action in the uterus and thus helps in preventing bone loss. Other hypothetical mechanisms derived from other biochemical actions of flavonoids include activation of an “orphan” receptor (different from estrogen type I receptor) or inhibition of enzymatic activity (protein kinases) (152). In recent studies, it has been rectified that clinical effectiveness of flavonoids, which may to a certain extent depend on equol (a gut bacterial metabolite of daidzein), shows stronger estrogenic activity (153). Genistein reportedly possess anti-proliferative, antioxidant, estrogenic and anti-inflammatory effects (154).
It is commonly known as Cistanche and Rou Cong Rong. Different species of Cistanche are distributed in Mediterranean region, Africa and Asia and is one of the best Chinese herbs. C. herba aqueous extract administration exerts preventive effects for bone loss and osteoporosis in OVX rats (155). The polysaccharides contents of C. herba has been reported to uphold the bone marrow cell cycle transition, stimulate hematopoietic function in bone marrow and enhances BMSCs differentiation into osteoblasts, which are necessary for the synthesis of new bone (156). It has also been found to have both anti-inflammatory and immunopotentiating effects (157).
It is commonly known as ‘Yin-Yang-Huo’ in China. H. epimedii is an ancient Chinese herbal medicine (158), whose aqueous extract from leaves are found to uplift estrogen levels in postmenopausal women (159) and possesses both osteoblast forming and osteoclast inhibiting properties, when tested in OVX rats (160). The combination of Herba species have been found to have osteoprotective effects by masking mast cell activity (161), thereby significantly reducing levels of histamine and TNF-α release from The Laboratory of Allergic Disease 2 (LAD2) human mast cell. These effects are due to total flavonoid contents of H. epimedii (162). H. epimedii extracts augment the non-specific immune responses and decrease level of TNF-α (163).
It is commonly known as Nu-Zhen-Zi in Chinese medicine and is found in both coastal and lowland habitats including roadsides, shrub lands and forest margins. In addition to that it is also found in countries including Australia, Argentina, Italy, Spain, South Africa, Midwest and Southeast U.S. (164). Its fruits are commonly found to be benefical for improving bone health and acts as potential candidate for management of postmenopausal osteoporosis by increasing bone calcium content and prevention of increased bone turnover. This can be attributed to decreased levels of both urinary deoxypyridinoline levels and serum osteocalcin (165). Aqueous extract of L. lucidum possesses osteo-protective effects on OVX female rats by stimulating osteogenesis along with inhibiting adipogenesis and osteoclastogenesis (166). In vitro comparative study between aqueous and ethanolic extract demonstrated that augmented circulating 1, 25-dihydroxyvitamin D3 (1,25(OH)2D3) levels do not help in improvement of calcium levels in mature OVX rats (167). Studies performed on extracts of iridoids and flavonoids of Fructus ligustri lucidi (162) reported improvement in calcium levels, bone turnover (165), upregulate Vitamin D dependent gene expression and increased calciotropic hormone levels suggesting improved calcium balance in post-menopausal women (168).
It is found in China as wolfberry or Gojiberry and another reported species is L. barbarum. The varieties recognized are L. chinense var. chinense or potaninii. L. chinense is popular as Chinese tea plant, Chinese Matrimony Vine, Chinese Boxthorn and Chinese Desert-Thorn. This plant is found to be very valuable in traditional Chinese medicine in treatment of hematemesis, pneumonia, night sweats, diabetes mellitus, cough and other inflammatory conditions (169). The known sesquiterpene glucoside (10R, 30S, 50R, 80S, 2Z, 4E)-dihydrophaseic acid 3-O-β-D-glucopyranoside (DPA3G) is recognized as the active component of the L. chinense. Treatment of Mesenchymal Stem cells and Pre-Osteoblast cells with the fraction containing DPA3G showed significant upregulation in cell proliferation and ALP levels when compared with corresponding controls. DPA3G enhanced both osteoclast differentiation of monocytes and osteoblast differentiation of MC3T3-El cells indicating its important role in maintaining normal bone remodeling process (170,171). In Korea, the aqueous extract made from root bark of L. chinense is termed as ‘Jigolpi’. LRC decreases osteoclastogenesis related markers like c-Fos, NFATc1, TRAP, cathepsin-k, RANK, MMP-9, CAII and as such reduces bone density along with trabecular microarchitectural deterioration in the OVX rat model (172). LRC also possesses various biological roles along with its anti-inflammatory properties (169).
It is prevalently known as Horse Radish Tree, Ben Tree, Drumstick tree or Miracle tree and is found in India, Pakistan, Bangladesh, Nepal, Himalayan region, Africa, Central America, and Arabia. Methanolic leaf extracts of M. oleifera possesses immunomodulatory activity and are used to boost the immune system by increasing both the cell-mediated and humoral immune responses in rats (173). Osteoblastogenic effects of methanolic extracts from M. oleifera were reported on osteoblast cell line SaOS2. The anti-osteoporotic effect can be thus due to increased bone formation, increased bone mineral and hydroxyproline content, thereby making it an important therapeutic agent for various bone related disorders (174).
It is also known as Korean Ginseng or Chinese Ginseng. Its name is derived from the Chinese word “renshen” that means “man root”. Roots are the main part of Panax (Burk), Panax notoginseng saponins (PNS), a well-known conventional Korean and Chinese herb, in presence of LPS, downregulates TNF-α production and mRNA expression of COX-2 and IL-1β in bone marrow macrophages i.e. RAW264 cells (175,176). Total saponins from P. notoginseng can amplify bone formation by encouraging the proliferation of cells, collagen, osteocalcin synthesis, ALP activity and mineralization in osteoblastic MC3T3-E1 cells in vitro (177). Another genus P. ginseng meyer commonly known as Korean ginseng is a native to Asia and North America producing ginsenosides. By-products of these ginsengs (Rb1, Rg1, Rh2 and Rh2(R)) can restrain the secretion and action of RANKL, JNK, NF-kB, c-Fos, TNF-α, NFATc1 and IL-6 (178). Saponins content of P. notoginseng possesses a multiple number of pharmacological effects along with anti-inflammatory and anti-oxidative properties (179).
It is an important plant in the Tamil (India) siddha systems of medicine, Chinese medicine and Indian Ayurveda. It is also popular as Babchi, Bavanchi, Karpokarishi, Karboga-Ari, Bhavanchi-Vittulu, Vakuchu in India. Psoralen (PSO) is an active compound of P. corylifolia extract, which promotes bone formation. The dried fruit of P. corylifolia is a renowned traditional Chinese medicine, described for its bone strengthening effects. Isopsoralenone type of P. corylifolia extract, also promote MSC differentiation to osteoblasts by promotion of OC amount, ALP activity along with enhancing mRNA level of Runx-2 in vitro (180). PSO stimulate MSCs to differentiate and develop into osteoblasts leading to enhanced bone mass formation in OVX induced osteoporotic rats (181). In China, P. corylifolia is also used as tonic and food additives for the remedy of joint diseases, bone fractures, leucoderma and psoriasis. The prenyl group present in the extract might be the one with anti-osteoporotic properties (182). The seeds of P. corylifolia exert anti-oxidative, anti-inflammatory and antimicrobial properties (183). The extract is found to possess osteoprotective effect in OVX rats and thus increases BMD by decreasing the excretion of calcium in urine and declined serum osteocalcin (184). The chemical constituent i.e. psoralin shows osteoprotective effect both under in vitro and in vivo studies in postmenopausal conditions by inhibiting RANKL induced osteoclast formation (185). Another constituent found in ethanolic extract is bakuchiol which helps in reducing bone loss in OVX rats by increasing serum ALP, Ca and Estrogen conc. and BMD along with reducing the inorganic phosphorus level (186).
P. granatum is deciduous fruit-bearing plant, found in the southern hemisphere, Central Asia and also in drier regions of South-East Asia, commonly known all over the world as Pomegranates (PG). PG is used in cooking, baking, juice blends, smoothies, meal garnishes and alcoholic beverages (cocktails and wine). PG seeds hold ellagic acid, punicic acid, non-steroidal phytoestrogens and steroidal estrogen including coumoestrol, comesten and isoflavones genistein, ascorbic acids and daidzein. PG also contains some other estrogenic compounds such as quercetin, estrone, luteolin, kaempferol and estradiol that account for both bone formation and prevention of bone resorption. It is logical in considering the estrogenic compounds i.e. β-sitosterol presence in PG may have acted in a way to encourage mRNA expression of collagen, ALP and the protein levels therefore stimulating the Runx2 gene expression in osteoblastic cells (187). The PG extract has been pharmacologically effective for the treatment of menopausal symptoms produced in OVX mice (188). PG seed oil (PSO) leads to inhibition in the expression of pro-inflammatory factors like CCL2, IL-1, IL-6 whereas elevating anti-inflammatory factors such as IL-6 receptor antagonists (IL-6ra, IL-6st). It is also reported that punicic acid successfully restrict the expression and secretion of inflammatory factors like TNF-α and NF-κB in serum, intestine and erythrocytes (189).
It is widely known as Rehmannia root, Sheng Di Huang, Rehmannia glutinosa Libosch. This plant is used in Chinese medicine and is known for its potential anti-osteoporotic and bone-strengthening components. Both in vitro and in vivo studies explain its prime role in the treatment and protective effect for osteoporosis. The plant extract enhances the proliferation and ALP activity of osteoblasts (190), mRNA expressions of bone-related genes, increased BMD, cortical bone thickness and OPG secretion, while decreasing resorption area and formation of TRAP multinucleated cells (25). Its immune-potentiating properties are shown by reduced serum TNF-α, IL-6 and collagen (type I) levels in postmenopausal women (191).
The common names include North China Red Elder, Jiegumu, William's elder. It is a folk medicine from China, traditionally used to handle bone fractures and joint related diseases (192). The extract of S. williamsii helps to amplify bone health in OVX rats by modulation of OPG expression and RANKL (193). The plant by-product improves the bone mass, strength and micro-structure in both OVX mice and rats (194). S. williamsii by-products reduces the number of TRAP positive cells in RANKL-induced RAW 264.7 cells and causes inhibition of bone resorption. It also helps to promote bone formation explained in various in vitro studies (195). The fruit oil of S. Williamsii has also been reported to possess immunopotentiating effects (192).
It is a flowering plant native to Europe and North America, commonly known as Elder, Black elder, Elderberry, European Elder and European Black Elderberry. It grows mainly in sunny regions and other conditions as well together with both dry, fertile and wet soils. In animal experimental models, its extract was found to possess anti-inflammatory, antioxidant, anti-osteoporosis and anti-glycosylation activities (196). There was significant improvement in the serum of rats in diabetic conditions when treated with natural polyphenols from S. nigra, leading to usual concentration of reduced glutathione and less serum concentration of malondialdehyde with improved osteoporotic conditions. The fruit extract containing the natural polyphenols appreciably improves the osteoporosis regression and extremely low BMD in diabetic male rats (196). Black Elderberry extract feeding attenuates the HFD-dependent rise in numerous serum inflammatory chemokines/cytokines (197).
Terminalia is known by several names, which include Arjuna or Arjun, Mathimara, Thellamaddi, Kumbuk, Maruthamaram, Neer maruthu and Kohda in various regions of India. It is mainly originated from river banks or near dry river beds in Bangladesh, West Bengal, South and Central India. Ethanolic extract from T. arjuna has anti-osteoporotic activity (198). Ethanolic extract of the stem bark of some other species of Terminalia like T. catappa possess immunomodulatory and anti-inflammatory activity by inhibiting the effects of proinflammatory mediators in RAW 264.7 cells (199).
It is popularly and commonly known as Ashwagandha in Ayurvedic medicine. It is widely used in the treatment of diverse diseases and ailments. W. sominifera (WS) has been reported to have anti-tumour, anti-inflammatory, cardioprotective, antioxidant, adaptogenic, anti-osteoarthritic, anti-rheumatic, anticoagulant and immunomodulatory activity etc. It has positive effect on both central nervous and endocrine systems (200). Presence of low toxicity profile and the phytosterols of Withania are of great importance to be used in chronic maladies such as osteoporosis. Active constituents of WS are the heterogeneous alkaloids which include inganaferine, tropine, pseudo-tropineana hygrine and cuscohygrine. Withania also contains withanolides, withaferin and steroidal lactones, which are estrogenic in nature and possibly have similar affects like other beneficial phytosterols helping to produce anti-osteoporotic activity (201). Ashwagandha preparations, mainly labeled as WS2, possess immunomodulatory activity with regard to IgE and cell-mediated hyper-reactivity (202).
Zingiber zerumbet is a sesquiterpene derivative of tropical ginger Z. zerumbet sp. Zerumbone, the important constituent of the essential volatile oil from tropical ginger, can suppress RANKL-induced NF-κB activation by preventing IκBα kinase (IKK) and RANKL induced osteoclastogenesis (203). Zerumbone also has the potential to completely suppress NF-κB activation significantly (203). In a RANKL-induced osteoclast model, hexane extract of ginger is found to decline the upregulation of TRAP-positive and multinucleated RAW 264.7 cells. Actin staining shows that the ginger hexane extract (GHE) treatment can inhibit actin-ring formation, thereby suppressing osteoclast differentiation and bone resorption via inhibiting NFATc1 and osteoclastogenesis related genes in RANKL-induced osteoclast model (204). Z. zerumbet has also been reported to possess anti-inflammatory responses (205).
Osteoporosis is the most common chronic condition of skeleton effecting more than 200 million individual worldwide (206). It remained one of the most imperative but often ignored bone disease related with aging and postmenopausal conditions that always leads to fragility and bone loss. In addition, it has also been reported as a heavy toll on the economy with an estimated burden of 131.5 billion USD worldwide by 2050 (207). Till date, various drugs have been proposed for the treatment of osteoporosis and other bone related implications with variable results. Extensive safety concerns are of utmost importance with treatment, as presently accessible drugs (strontium, bisphosphonates and estrogen-replacement-therapy) in treating osteoporosis are associated with several undesirable effects (208). Both in vitro and in vivo studies have suggested that herbal medicines and their respective phytoconstituents have huge potential for future medicinal use in modulating bone health. These phytoconstituents possess many advantages over the modern conventional pharmaceutical’s preparations including their low cost and least adverse side effects. These properties of phytoconstituents make them suitable to be considered for herbal medicines as substitutes to various available synthetic drugs in the market, which are costly and have adverse side effects. With the advent of isolated bioactive compounds, these plants can be better exploited for advancement of innovative pharmaceuticals compounds with quick response, leading to development and formulations of novel phytomedicines. Also, there is enormous scope for many countries with rich biodiversity, to contribute towards the development of pharmaceutical trade in this field. Although phytoconstituents are present with vast medicinal applications, but for their full-fledged clinical applications, extensive study of their mechanism of action, bioactivity and toxicology are still warranted. With the discovery and advancement in pharmacological studies of novel phytoconstituents possessing potent osteoprotective and immunomodulatory properties, the field of phytomedicine is bound to grow with immense potential to fight against various emerging inflammatory diseases including bone health.
Zaffar Azam, Vikas Pandey equally contributed to this paper. This work was financially supported by projects: DST-SERB (EMR/2016/007158), Govt. of India and intramural project from All India Institute of Medical Sciences (AIIMS), New Delhi- India sanctioned to RKS. ZA acknowledge, Department of Zoology, Dr. Harisingh Gour Central University, Sagar (MP)-India for providing infrastructural facilities. VS and VP thanks, Department of Pharmacy, Dr. Harisingh Gour Central University, Sagar (MP)-India. RKS also thanks, the Department of Biotechnology, AIIMS, New Delhi-India for providing necessary facilities. NG acknowledges Chitkara University Jhansla Punjab-India for providing infrastructural facilities. HYD thanks ICMR for research fellowship. ZA, LS and AB thanks UGC-NFST, CSIR and DST for their respective research fellowships. AA and NS thank DBT for fellowship and Department of Biotechnology, AIIMS, New Delhi-India for providing necessary facilities. We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
BMMs
bone marrow-derived macrophages
bone morphogenic protein
bone marrow stromal cells
CD40 ligand
hematopoietic stem cell(s)
interferon
glucocorticoid receptors
matrix metalloproteinase
nuclear factor κB
osteoprotegerin
osteoclast-associated immunoglobin like receptor
ovariectomy
phosphatidylinositide-3-kinase
Nuclear factor kappa beta
receptor activator of NF-κB
RANK ligand
T-cell receptor
T-helper
TNF receptor-associated factor
Tumor necrosis factor
dendritic cell
tartrate-resistant acid phosphatase
rheumatoid arthritis
Osteoarthritis
malondialdehyde
3-O-β-D-glucopyranoside
Panax notoginseng saponins
Psoralen
collagen type I pathway
mechanistic target of rapamycin
Transforming growth factor-β
monocyte chemoattractant protein
granulocyte-macrophage colony-stimulating factor
colony-stimulating factor
macrophage colony-stimulating factor
Food and drug administration
hormone replacement therapy
prostaglandins
selective estrogen receptor modulator