Diabetic nephropathy (DN) is one of the most serious complications of diabetes mellitus, for which no effective treatment currently exists. We tested the hypothesis that Qi-dan-di-huang (QDDH) might have therapuetic effects in an experimental rat model of DN. The levels of I kappa KinaseAlpha and Beta, p-p65, p-IκB alpha, TGF-β1 and Alpha-SMA were significantly increased in kidneys in DN. QDDH decoction only partially reversed the increased Ikappa KinaseAlpha/Beta, p-p65, p-IKappaB alpha, TGF-Beta1 and alpha-SMA in the kidneys in DN. However, treatment of diabetic rats with QDDH decoction significantly inhibited the production and release of inflammatory cytokines IL-6, IL-1 beta and TNF-alpha into the serum. QDDH decoction also significantly improved the physiologic and biochemical indicators of DN, reduced glycogen and protein deposition in DN and prevented renal fibrosis. Together, the data show that QDDH decoction exerts a protective effect on kidneys in diabetic rats and reverses the inflammatory milieu of the serum in DN.
International Diabetes Federation reported that there are over 382 million people with diabetes mellitus (DM) world-wide, and is predicted that this number will likely rise to 592 million by 2035 (1). DM is characterized by variable glucose metabolism involving complex mechanisms that results in multiple complications. Diabetic nephropathy (DN) is the most serious complication of DM, which can result in various microvascular issues and cause glomerular sclerosis as well as end-stage renal disease (ESRD) (2). It is typically characterized by nodular glomerular sclerosis. In developed countries, DN is the leading cause of chronic kidney disease. Approximately 20% - 40% patients with DM develop DN 10–20 years after DM onset, which up to 50% patients with DM develop DN after 20 years of DM onset. If the condition is not controlled well, it eventually progresses to ESRD (3). In the United States, DN progresses to ESRD in approximately 38% patients, which is the highest worldwide. In China, DN progresses to ESRD in approximately 5% patients, and this number is increasing every year. DN has become life threatening, and its symptoms can be relieved by some therapeutic methods, such as administration of angiotensin-converting enzyme inhibitors, strict restriction of blood pressure, pharmacological suppression of the renin-angiotensin system, etc; however, there is no drug available that can effectively prevent or cure DN. Hence, investigating the progression of DN and development of new target drugs are warranted (4).
Recent research has indicated that inflammation plays a role in the pathogenesis of DN via various pro-inflammatory cytokines, including interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-alpha), and interleukin-1beta (IL-1beta) both in vivo andin vitro(5, 6). Notably, the NF-kappaB (NF-κB) pathway plays a pivotal role in the inflammatory response. Under normal conditions, NF-κB exists as an inactive and latent complex bound to IkappaB (IκB) in the cytoplasm (7).Once the cytoplasm is stimulated, IκB kinase gets phosphorylated, after which degradation of IκB occurs through the proteasome, and NF-κB is allowed to translocate to the nucleus from the cytoplasm and activate the expressions of specific target genes. Therefore, inhibiting IκB phosphorylation can prevent its degradation, which may be a potential approach to suppress DN progression (8).
Traditional Chinese medicine (TCM) has traditionally used for anti-inflammation widely. One of the most important TCM treatment approaches involves formula instead of a single herb. The herbs in the formula will be compatible and act together, thus proving to be more effective against specific inflammation symptoms (9). The relationships between the herbs in the formula may include mutual enhancement, assistance, or complementation (10). A TCM theory states that the interaction between Yin and Yang generates Qi. Qi refers to the refined nutritive substances circulating in the body and functional status of tissues and organs (11). Based on this theory, our research team, via review of a large number of studies, conducted an evidence-based analysis and indicated that pathogenesis of DN is Qi and Yin deficiency and proposed YiqiYangyinHuoxue therapy for DN. After determining the pathogenesis and treatment, we optimized prescription of Chinese medicine for evidence-based treatment of DN under the support of the key project construction project of the third phase of the ‘211 Project’ in Guangdong Province. We have developed a QDDH granule, which can be used for the prevention and treatment of DN (invention patent No. ZL201310015464.5). The prescription comprisesAstragalus, Salvia miltiorrhiza, Radix Rehmanniae, Chinese yam, and licorice, which has a nourishing effect on Qi and Yin and promotes blood circulation.
We further explored the relationship between DN and inflammation to improve the understanding of DN pathogenesis. It has been confirmed that fenofibrate has a protective effect against type 1 DN, and TCM is of great significance for preventing and treating DN. The results of this study can provide a theoretical basis for the treatment of DN using QDDH decoction.
Eight-week-old male Sprague-Dawley rats weighing 250–320 g were purchased from the Laboratory Animal Centre of Southern Medical University (Guangzhou, China). Under a 12-h light–dark cycle and controlled temperature (23°C±2°C), all rats were bred under specific pathogen-free conditions with water and food ad libitum. The animal ethics censorship number is 44002100009793.
The animals were acclimatized to the laboratory conditions for one week prior to experimentation and randomly divided into four groups: Control (Con) group (n=8), DN model group (n=8), fenofibrate (Fen) group (n=8), and QDDH decoction group (n=8).Rats in the DN, Fen and QDDH groups were intraperitoneally injected with sodium citrate streptozotocin (STZ) buffer(65mg/kg; Sigma Aldrich, St. Louis, MO, USA), and those in the Con group were intraperitoneally injected with sodium citrate buffer; a week later, mice with type 1 DM were successfully bred when FBG was ≥16.67 mol•L−1, and feeding was continued until the urine protein appeared.
Astragalus, S. miltiorrhiza, R. Rehmanniae, Chinese yam, and licorice which were to be used for preparing the QDDH decoction were purchased from the Southern Hospital of Southern Medical University. QDDH decoction, containing Astragalus: S. miltiorrhiza: R. Rehmanniae: Chinese yam: Raw Licorice in a ratio of 6:3:3:3:1,was soaked in 500 mL double-distilled water for 8 h, in which 10 times the volume of water was added to fry dilute twice, each time for 90 minutes, the decoction was centrifuged twice at 2000×g for 2 min at 4°C, and the precipitate was discarded; the obtained solution was concentrated to a suitable volume with a rotary evaporator at 50°C. Fenofibrate in a capsule form (Lipingzhi; commercially available, purchased from Recipharm Fontaine, France; specification: 200 mg per tablet) was used as a positive control drug. Prior to the gavage, a solution was prepared in double-distilled water. The amount of QDDH decoction to be administered was calculated according to the clinical dose, which was 80 g (30 g Astragalus, 15 g R.Rehmanniae, 15 g S.miltiorrhiza, 15 g Chinese yam, and 5 g licorice), and the equivalent dose for rats was 7.2 g•kg−1. Rats in the Fen group were administered QDDH decoction at a dose of 0.02 g•kg−1, and those in the Con and DN groups were administered the same volume of double-distilled water. The drug was administered once a day for 8 weeks.
Blood glucose (by the tail cut method) and body weight were measured at 0, 2, 4, 6, and 8 weeks after drug administration. Urine was collected in a metabolic cage for 24h at the 8th week of drug intervention, the volume was recorded and 24-hour urine protein(24hU-pro) output was measured. After 8 weeks of drug intervention, the rats were sacrificed with 2 percent pentobarbital anesthesia and the abdominal aortic blood was collected and centrifuged at 2500 ×g for 4 minutes at 10°C; the serum was separated, and serum creatinine (Scr) and blood urea nitrogen (BUN) were measured. Some mount of serum was stored at −80°C until further use. The rat kidneys was weighed and recorded. All protocols and procedures conformed to the guidelines of the Laboratory Animal Ethics Committee of South Medical University.
The levels of TNF-alpha, IL-1beta, and IL-6 in serum were determined using commercial ELISA kits (Pierce, USA) according to the manufacturer’s instructions.
Under deep anesthesia that was induced with urethane (1.2g/kg, intraperitoneal injection), the kidneys were excised and same part of the left kidney was fixed in 4 percent paraformaldehyde solution for further morphological analysis using hematoxylin-eosin (HE), periodic Acid–Schiff (PAS), and Masson staining (Nikon E-100 A12.0705; Tokyo, Japan).
The protein levels of phospho-p65(1:1000, AF2006, rabbit; Affinity Biosciences, USA), p65(1:1000, AF5006, rabbit; Affinity Biosciences, USA), phospho-IkappaB alpha (p-IκBα) (1:1000, AF2002, rabbit; Affinity Biosciences, USA), IkappaB alpha (IκBα) (1:1000, AF7776, rabbit; Affinity, USA), NF-kappaB (NF-κB) p105/p50 antibody (1:1000, AF6217, rabbit; Affinity, USA), IkappaB beta (IκBβ) (1:1000, #94101, mouse; Cell Signaling technology, USA), IKK alpha/beta(1:1000, AF6014, rabbit; Affinity Biosciences, USA), beta-actin(1:1000, BF0198, rabbit; Affinity Biosciences, USA), TGF-beta1(1:1000, AF1027, rabbit; Affinity Biosciences, USA), and alpha-SMA (1:1000, AF1032, rabbit; Affinity Biosciences, USA) from the kidney tissue of rats from the four groups were measured using Western blot analysis. The proteins were extracted and quantified by BCA Protein assay kit (Thermo Fisher Scientific, Inc.).
Data are expressed as mean ± SEM. Variation between groups was analyzed by one-way analysis of variance and the least significant difference was measured using post hoc multiple comparison tests. The Statistical Software for Social Sciences20 provided by IBM (USA) was used. A Pvalue of 0.05 was considered to be statistically significant.
There was no difference in the starting body weight before random feeding among the groups. The back hair of the rats administered with STZ injection was towering, yellowish and dull; they showed signs of weight loss (P<0.05), reduced mobility, lack of energy, and developmental delay. There was no significant change in the body weight of rats at two weeks after drug intervention. At 8 weeks, rats in the QDDH decoction group had higher mobility and reaction rate than those in the DN and Fen groups. The body weight was significantly increased (P< 0.05) in the QDDH group, as shown in (Figure 1A)
 Figure 1.
Figure 1.
Effect of QDDH decoction on body weight (A) and fasting blood glucose (B) in four groups at 0, 2, 4, 6, 8 weeks after administration. Compared with Con group,*P<0.05; compared with DN group, #P<0.05.
The FBG levels of rats from all the four groups at 0, 2, 4, 6, and 8 weeks have been shown in Figure 1B. Compared with the Con group, STZ injection significantly increased the blood glucose level in the QDDH decoction group. After administration, the blood glucose level of each group was still significantly higher than that of the Con group at 0, 2, 4, 6, and 8 weeks (P<0.05). Compared with the Con group, there was no significant difference in the FBG level between the Fen and QDDH decoction groups within 2 weeks after administration. During the4th to 8th week after administration, the FBG level of the Fen and QDDH decoction groups decreased significantly (P<0.05). There was no significant difference in the FBG level between the Fen and QDDH decoction groups (P>0.05).
The Scr level in the QDDH decoction, DN and Fen groups was markedly increased compared with that in the Con group. Compared with the DN group, the level of Scr in the Fen and QDDH decoction groups was significantly decreased and compared with the Fen group was observably decreased in the Fen group (Figure 2A).
 Figure 2.
Figure 2.
Effect of QDDH decoction on Scr (A), BUN (B), 24h-pro (C), KW/BW (D), IL-6 (E), IL-1beta (F), and TNF-α (G) in the four groups after administration. Comparison with the Con group,*P<0.05; Comparison with the DN group, #P<0.05.
After administration of QDDH decoction administration, the BUN level in the DN, Fen and QDDH decoction groups were significantly increased compared with that in the Con group. Compared with the DN group, the BUN level was observably decreased in the Fen and QDDH decoction groups. There was no significant difference between the BUN levels in the Fen and QDDH groups (Figure 2B).
The 24hU-Pro level in the Con group was significantly lower than that in the DN, Fen and QDDH decoction groups. After 8 weeks of treatment, the 24hU-Pro level in the Fen and QDDH decoction groups was dramatically decreased than that in the DN group. The 24hU-Pro levels in the Fen and QDDH decoction groups showed no difference (Figure 2C).
The KW/BW value is an index of kidney function. Compared with the Con group, the KW/BW values in the DN, Fen, and QDDH decoction groups were significantly increased. After administration of Fen or QDDH decoction, the KW/BW values in the Fen and QDDH decoction groups increased compared with that in the DN group. The KW/BW values in the Fen and QDDH decoction groups showed no difference (Figure 2D).
Research has found that inflammation of the kidney plays a key role in promoting the development and progression of DN. Using ELISA, we detected the levels of IL-6, IL-1beta, and TNF-alpha in the sera of rats in each group. Compared with the Con group, the expression levels of IL-6, IL-1beta, and TNF-alpha in the DN, Fen and QDDH decoction groups were significantly increased (P<0.05). The expression levels of IL-6, IL-1beta, and TNF-alpha in the Fen and QDDH decoction groups were significantly lower than those in the DN group (P<0.05). The expression level of IL-6 in the QDDH decoction group was significantly lower than that in the Fen group (P<0.05); however, there was no significant difference between the expression levels of IL-1beta and TNF-alpha in the Fen and QDDH groups (P>0.05) (Figure 2E, Figure 2F, and Figure 2G).
HE, PAS, and Masson staining were carried out to evaluate the renal injury in glomerular and tubular structures, as shown in Figure 3
 Figure 3.
Figure 3.
Effect of QDDH decoction on renal injury in DN rats, as assessed by HE staining (A), PAS staining (B), and Masson staining(C). Representative photomicrographs (400×) of staining of kidney tissues obtained from rats in the Con group (a), DN group(b), Fen group (c), and QDDH decoction group (d).
In each pathological group, the Con group showed normal glomeruli and tubule structures, and the structures and culture were clear; there was no obvious pathological manifestation.
In the DN group, pathological sections of HE staining (Figure 3) showed severe fibrosis in the glomeruli, significantly reduced, significantly reduced cell number around the fibrosis, widened and diffuse glomerular mesangial area, and thickened capillary wall. Glomerular atrophy could not be assessed owing to the unclear structure. The balloon wall was thickened and the balloon was connected, and tubular expansion was accompanied by vacuolar-like changes in the epithelial cells along with renal interstitial fibrosis accompanied by obvious inflammatory cell infiltration. The Fen group exhibited moderate glomerular fibrosis, glomerular mesangial thickening, thickening of the capillary wall, and balloon adhesion as well as mild thickening of the balloon wall. In addition, moderate renal tubular epithelial cells with vacuolar changes and mild to moderate interstitial fibrosis with mild inflammatory cell infiltration were observed. In the QDDH decoction group, the rats showed mildfibrosis and slightly thickened mesangial mesangium and capillary wall.
PAS staining (Figure 3) revealed a large amount of glycogen protein deposited in the glomerular and tubular cells of rats in the DN group. In the Fen group, tight globules and medium-weight glycogen protein deposition in the renal tubular cells were observed. In the QDDH decoction group, a small amount of glycogen protein was deposited in the glomerulus and renal tubular cells.
The pathological structure seen on Masson staining (Figure 3) performed for rats in the DN group was characterized by severe fibrosis in the glomerulus, severe hyperplasia of the mesangial matrix, thickening of the basement membrane and capillary wall, glomerular atrophy, unclear structure, increased balloon wallthickness, and interstitial fibrosis in the DN group. In the Fen group, moderate glomerular fibrosis, mesangial matrix, thickening of the basement membrane, and interstitial fibrosis as well as a thickened balloon wall were observed whereas in the QDDH decoction group, mild glomerular fibrosis, mildly hyperplasic mesangial matrix, slightly thickened basement membrane, and mild to moderate interstitial fibrosis were observed with no obvious balloon wall thickening.
Protein expression was detected by Western blotting (Figure 4). The results showed that there were no significant differences in the expressions of p65, p50, IκBα, and IκB in the kidneys of rats in the Con, DN, Fen, and QDDH decoction groups. Compared with the Con group, the expressions of Ikappakappa alpha/beta (Iκκα/β), phospho-p65, and phospho-IkappaB alpha (p-IκBα) were significantly increased in the kidneys in the DN, Fen and QDDH decoction groups (P<0.05). After 8 weeks of drug administration, the expressions of Iκκα/β, phospho-p65, and p-IκBα in the kidneys of rats in the Fen and QDDH decoction groups were significantly lower than those in the DN group (P<0.05). There was no significant difference in the expressions of Iκκα/β, phospho-p65, and p-IκBα in the kidneys of rats in the Fen and QDDH decoction groups (P>0.05).
 Figure 4.
Figure 4.
Effect of QDDH decoction on the NF-κB signaling pathway in DN rats. Western blot was carried out to evaluate the levels of phospho-p65, p65, p-IκBα, IκBα, NF-κB p105/p50, IκBβ, and Iκκα/β in the Con group, DN group, Fen group, and QDDH decoction group. Comparison with the Con group,*P<0.05; comparison with the DN group, #P<0.05.
We found that compared with the Con group, the expressions level of TGF-beta1 and alpha-SMA in the kidney were significantly increased (P<0.05) in DN, Fen and QDDH decoction groups (Fig5). After 8 weeks of drug intervention, the expressions level of TGF-beta1 and alpha-SMA in the kidneys of rats in the Fen and QDDH decoction groups were significantly lower than those in the DN group (P<0.05). There was no significant difference in the expression of TGF-beta1 and alpha-SMA between the Fen and QDDH decoction groups.
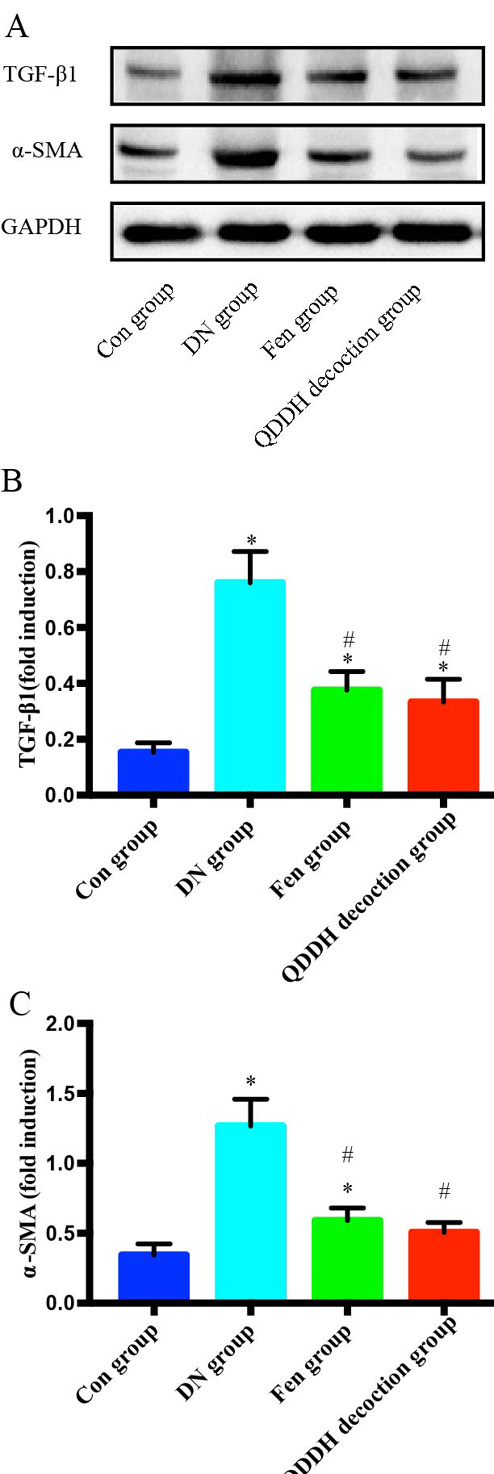 Figure 5.
Figure 5.
Effect of QDDH decoction on the TGF-beta1 and α-SMA in DN rats. Western blot was carried out to evaluate the levels of TGF-beta1 and α-SMA in the Con, DN, Fen and QDDH decoction groups. Comparison with the Con group,*P<0.05; Comparison with the DN group, #P<0.05.
DN is a major microvascular complication of DM and a progressive disease characterized by varying degrees of albuminuria and chronic kidney disease. Currently, DN can be divided into five phases according to the staging criteria proposed by Mogensen in 1989(12): stage I is characterized by renal hypertrophy and hyperfiltration; stage II by a ‘silent period;’ stage III by persistent microalbuminuria, and stage IV by presence of persistent urinary protein accompanied by increased protein output (20–200μg/min), highly selective proteinuria, and microalbuminuria, which is usually caused by early DN. In this period, the glomerular filtration rate gradually recovers to a roughly normal level, and blood pressure slightly increases but does not reach the level corresponding to hypertension. Stage IV is characterized by clinical proteinuria, that is, clinical nephropathy, and stage V by renal failure, that is, uremia. Early DN includes Mogensen stages I–III but usually refers to stage III. Appropriate treatment in the first 3 stages can control disease progression, and the stages IV and V are the mid-term and the late-stage DN, respectively, during which treatment cannot prevent further progression of the disease. The animal model we established is based on the persistent urine protein present in rat urine, suggestive of stage III. The pathogenesis of DN is very complex and involves a variety of cellular signaling pathways and regulation of molecules in each cell pathway; therefore, the entire pathogenesis of DN is still not fully understood.
Therefore, clinical treatment does not completely terminate or reverse the gradual progression of DN to ESRD. In Western medicine fenofibrate is preferred for treating diabetic kidney. It is a representative of fibrates, is an agonist of the peroxisome proliferator-activated receptor alpha, and acts by lowering serum glycerol triestersand total cholesterol levels, thus playing a lipid-lowering role. It has been widely used in the clinical treatment of different types of hyperlipidemia for approximately 30 years(13).Animal experiments have found that fenofibrate can improve kidney damage caused by type 2 DM by reducing renal lipid toxicity(14). Current research shows that fenofibrate protects against DN independent of its lipid-lowering effect (15-17) and provides protection to kidneys. It controls inflammation and fibrosis by inhibiting the NF-κB signaling pathway(18). However, there are few studies on whether fenofibrate can improve kidney damage caused by type 1 DM(18).
In combination with modern pharmacological research methods, TCM intervention in DN improves the role of kidney fibers, as has been recognized by scientists.TCM has been used for treatment for thousands of years and involves a unique understanding and treatment of DM and its related complications. Astragalus polysaccharide significantly increased the activity of superoxide dismutase, catalase and glutathione peroxidaseand has antioxidant effects(19,20).Modern pharmacological studies have shown that S.miltiorrhiza can reduce free radicals and inhibit lipid peroxidation. Furthermore, numerous studies have shown that by inhibiting the activity of S. miltiorrhiza NF-κB, TNFalpha and IL-6 were released, which play an anti-inflammatory role(21). Rehmanniaglutinous contains polysaccharides, polysaccharides, sterols, and polysaccharides, which can inhibit the accumulation of extracellular matrix by inhibiting the expressions of connective tissue growth factor, TGF-1, and Ang II(22). Consequently, it can definitely prove to be beneficial in treating DN. Modern pharmacological studies suggest that yam can reduce blood sugar, and the mechanism may be owing to the fact that yam polysaccharide directly or indirectly increases the activity of sugar metabolism or key enzymes involved(23). Yam polysaccharide is effective in the prevention and treatment of DM rats(24).Licorice gamping which Spleen Qi, harmonic function of various drugs, can effectively improve the symptoms or assist the whole side DN. Modern pharmacological research believes that licorice can protect the liver and exert anti-inflammatory and anti-oxidative effects (25). In addition, licorice has certain hypoglycemic effects (26), and research has found that glycyrrhizic acid acts as a hypoglycemic agent by increasing the secretion of glucagon-like peptide-1 by TGR5 activation(27).
Our research indicates that QDDH decoction can protect the kidney by inhibiting the activation of the NF-κB signaling pathway and production and release of inflammatory cytokines and by improving the physiological and biochemical characteristics of DN in rats and histological effect on rat kidney. It has laid the foundation for further mechanism and clinical research. In the course of the research, the active ingredients for kidney protection were explored in our next step to further comprehensively test the mechanism of treatment of DN with QDDH decoction.
The authors would like to thank the Taian City Central Hospital for assistance in research planning and subject enrollment. This work was supported by funding from the National Natural Science Foundation of China (NSFC81573812 and NSFC81673840). The authors declare no potential conflicts of interests with respect to the authorship and/or publication of this article.
Abbreviations: Diabetic nephropathy (DN), Qi-dan-di-huang(QDDH), Control group (Con group), Fenofibrategroup (Fen group), Diabetes mellitus (DM), end stage renal disease(ESRD), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), tumor necrosis factor-α (TNF-α), traditional Chinese medicine (TCM), 24-hour urine protein (24hU-pro), serum creatinine (Scr),blood urea nitrogen(BUN), hematoxylin-eosin (HE), periodic Acid–Schiff (PAS)
