Diet and environment are two critical regulators that influence an individual’s epigenetic profile. Besides the anterograde signaling, mitochondria act as a key regulator of epigenetic alterations in cancer either by controlling the concentration of the cofactors, activity of vital enzymes or by affecting the transcription of NF-kappaB and associated signaling molecules. As epigenetic modifications are the major drivers of aberrant gene expression, designing novel nutri-epigenomic strategies to modulate reversible epigenetic modifications will be important for effective cancer protection. In this regard, nutraceuticals such as flavonoids holds significant promise to modulate the epigenome through a network of interconnected anti-redox mechanisms. However, low solubility, rapid metabolism and poor absorption of flavonoids in gastrointestinal tract hinder their use in clinical settings. Therefore, it is imperative to develop nano-engineered systems which could considerably improve the targeted delivery of these bioactive compounds with better efficacy and pharmacokinetic properties. Concerted efforts in nano-engineering of flavonoids using polymer, lipid and complexation based approaches could provide successful bench-to-bedside translation of flavonoids as broad spectrum anti-cancer agents.
Non-communicable diseases including cancer are the major cause of global deaths. According to a recent estimate, 18.1 million people have been newly diagnosed for cancer and 9.6 million deaths being recorded globally in 2018. This high rate of mortality can be prevented if detected early. As the treatment strategy varies with the pattern, type and form of cancer, management of this disease requires further development of better therapeutics and preventive strategies. Broadly, cancer shows the following seven characteristic features: uncontrolled proliferation, self-sufficient growth factors, contact-independent growth, absence of apoptosis, abnormal angiogenesis, increased inflammatory response and prevalence of metastasis and invasion. The abnormal cellular physiology of cancerous cells is due to altered molecular patterns at genomic, epigenomic, transcriptomic, proteomic and metabolomic levels. Unlike genetic control which was described as the trigger for evolutionary mechanisms, epigenomics deals with the study of a set of processes (above the DNA) involving DNA methylation, histone modifications and remodeling, and miRNA expressions. These epigenetic modifications play a vital role in chromatin remodeling for the regulation of gene expression. Altered epigenetic patterns have been strongly associated with cancer related events (1, 2). Recent studies have confirmed that mitochondria regulate epigenome either by controlling the concentration of the cofactors or by the redox-mediated alteration of the activities of epigenetic modifiers (3-5). However, finding potent epigenetic targets for therapeutic intervention intended for cancer prevention is an emerging aspect where different epigenetic inhibitors/modulators are being discovered, validated and verified (6-8). Over the centuries, many herbal and medicinal plants and their active constituents have been analyzed as possible remedies for several chronic and metabolic diseases. Secondary metabolites from plants, such as polyphenols including alkaloids, flavonoids, and stilbenes, which are present in small quantity have been tested for preventive management of several non-communicable diseases such as diabetes, cardiovascular diseases, asthma, neurological disorders and cancer (9-11). Interestingly, these molecules not only possess anti-oxidant, anti-inflammatory, cyto-protective and geno-protective properties but also modulate epigenomic re-programming through a mitochondrial mediated pathway (12-14).
Mitochondria not only control cellular oxidation via oxidative phosphorylation but also regulate programmed cell death, calcium concentration, metabolite concentrations, and diverse signaling pathways. These multifaceted roles make it dynamic controllers of cellular health and disease. Moreover, mitochondria may directly influence genome methylation, covalent histone modification, and expression of miRNA arrays to eventually alter the expression of target nuclear genes (15-17). However, the therapeutic outcome of flavonoids is limited due to reduced bioavailability as a result of limited solubility, poor permeability and pre-systematic metabolic effects. The other mechanisms involved in restricting the application of flavonoids include metabolism by gut micro flora, absorption across the intestinal wall, active efflux, and susceptibility to modification by environmental factors such as temperature, pH and light. In addition, recent studies have suggested that mitochondria are the central target of mechanistic basis of flavonoid’s mode of action, thus modulating the epigenetic machinery and complex cancer signaling, thereby suppressing the cancer progression programming. Therefore making a nanocarrier based system targeting mitochondria would offer a suitable strategy (Figure 1). Our previous studies have also explored numerous anti-cancer biological activities of the flavonoid-rich contents of an important medicinal herb Selaginella bryopteris (Sanjeevani) (11, 14). Our recent preclinical study demonstrated how nano-engineered flavonoid rich fraction isolated from S. bryopteris (NP.SB) act through mitochondria to alter the epigenetic landscape from the tumorigenesis route to normal cellular physiology (14). This contemporary approach offers a promising strategy to treat cancer with minimal side effects.
 Figure 1.
Figure 1.
An outline sketch of the proposed nano-engineered flavonoid based approach for effective mitochondrial targeting, maintenance of epigenomic machinery and induction of protective anti-cancer effects.
Epigenetic modifications play a vital role in gene regulation beyond the genetic material and exhibit stable meiotic and mitotic inheritance. Such epigenetic processes include covalent methylation of 5-cytosine of DNA, covalent modifications of N-terminal amino acid moieties of nucleosomal histone cores, and regulation through non-coding miRNA expressions. These modifications are important, intricate underlying mechanisms for maintenance of chromatin remodeling status. Epigenetic modifications occur commonly in all cell types which instruct genes either to turn off or on. Thus, cells with same genetic material respond differentially depending upon the internal and external environmental cues. Basically, epigenetic modifications are catalyzed by epigenetic modifiers known as writers (that add a specific modifying moiety); erasers (that remove a specific modifying moiety); and readers (that recognize and bind to specific modified moieties). Several distress points observed in the above mechanisms are implicated in various forms and types of cancers (18-22).
DNA methylation involves attaching a methyl group to the 5 carbon of cytosine nucleotide by DNA methyltransferases (DNMTs). On the other hand, mechanism of removal of a methyl group from cytosine either involves replication independent via TET demethylases or replication dependent through DNMT mediated demethylation (23). Methyl-CpG binding protein is the reader protein that specifically recognize and binds 5-methyl-cytosine nucleotides. Studies have confirmed that cancerous cells show aberrant promoter hypermethylation of tumor suppressor genes. In contrast, promoter hypomethylation of oncogenes increases the access of polymerase and transcription factors that mediate efficient transcription thereby causing increased expression of oncogenic proteins (24, 25).
Unlike nuclear genome, mitochondrial DNA is complexed with non-histone proteins and does not remain in a naked form. mtDNA methylation has been a subject of controversy so far, irrespective of existence of CpG and non-CpG sites (26-28). However, several studies have demonstrated that dysfunctional mtDNA methylation not only implicates the process of aging but also play an important role in onset and development of several age-associated degenerative diseases such as cancer (16, 29). It has been observed that several key enzymes such as DNMT1, DNMT3B and TET can translocate into the mitochondria and initiate the process of de novo mtDNA methylation (29).
Histone modifications are covalent modifications of N-terminal amino acid moieties of nucleosomal histone cores that include acetylation, methylation, phosphorylation, ubiquitination, and ribosylation. Several experimental investigations have documented the significant role of altered histone code and altered catalytic potentials of histone-modifying enzymes (21). Histone codes greatly influence gene expressions and significantly manipulate cellular processes by the inactivation or activation of tumor suppressor genes or oncogenes, respectively. For example, histone methylation of the tumor suppressor gene RASSF1A and histone de-acetylation of the transcription factor gene GATA4 silence the genes or more specifically reduces the binding of the transcription machinery, which increases the cancerous phenotypes (30) (Liu et al., 2016).
Histone acetyl transferase (HAT) is a writer for histone acetylation that uses acetyl-CoA as a cofactor to attach the acetyl group at the lysine or arginine amino acid residue of N-terminus of nucleosomal histone cores. There are four classes of histone deacetylases (Class I-IV) that function as erasers. Known mechanism for acetylation mediated regulation involves presence of electric charge mediated conformational change in nucleosome. For instance, highly acetylated nucleosomal histone cores create nucleosomes with loose configuration due to electric repulsion thus making euchromatin transcriptionally active. On the other hand, hypoacetylation marks the tight arrangement of several nucleosomes together, thus, producing heterochromatin or silent regions. Recent reports have shown acetyl transferase and deacetylases as the crucial players in the tumorigenesis of many cancers (31) (Su et al., 2016a). Aberrant expression and catalytic activity of different HATs cause acetylation of histone core tails, which generate distinct histone codes thus modulating normal encrypted language to the abnormal outcomes. For example, the acetylation of H4K16 is catalyzed by HATs such as males absent on the first (MOF) which belongs to MYST (Moz-Ybf2/Sas3-Sas2-Tip60) family. These modifications are associated with ovarian, breast, colorectal, gastric, lung, and renal cell cancers (31-33). Basically, silent MOF drive transcriptional gene inactivation and faulty DNA damage repair leading to genomic instability and lethality, thereby increasing the incidence of carcinogenesis.
Similarly, histone methylation has lysine and arginine methyltransferase as writers, lysine and arginine demethylase as erasers and chromodomain as readers. In most diseases, including cancer, histone methylation of the bivalent mark as tri-methylated lysine 27 of histone 3/ di-methylated lysine 4 of histone 4 (H3K27me3/H3K4me2) is found repressed and correlates with a shorter survival time (34). Lysine methyltransferase enhancer of zeste homolog 2 (EZH2) is recognized as an important contributor in the progression of proliferative tumors (35). The H3K27 tri-methylation is catalyzed by EZH2 by involving the polycomb repressive complex 2 (PRC2). Enhanced levels of H3K9me3 were also observed in the stress-induced premature senescence of ovarian epithelial cells, indicating increased heterochromatinization in response to increased histone methylation (36). Further, upregulated expression of lysine-specific demethylase 1 (LSD1) is linked to various epithelial cancers (37). It is a histone demethylase that plays a major role in modifying epigenetic pattern of epithelial-mesenchymal transition (EMT) genes (37). Dysregulated expression of EMT genes promotes metastasis and invasion. For instance, demethylation of histone H3 lysine 4 (H3K4) at the E-cadherin promoter region downregulates its expression thereby enhances metastasis.
In addition, kinases and phosphatases control phosphorylation of histone, which is recognized by 14-3-3, BRCT, and BRCA1 proteins (38). Phosphorylation of histones provides essential chromatin configuration for the binding of transcription factors and DNA repair proteins. At molecular level, DNA damage is correlated with the concentration of phosphorylated histone 2AX (H2AX), which is a sensor protein for DNA damage and its phosphorylation activates the downstream protein ATM (39). H2AX and H3.3 phosphorylation have been linked with the high-grade tumors (40). Therefore, phosphorylated H2AX can be a potential marker to detect cancer at an early stage and to recognize relapse cases (41).
Along with the regulation of protein homeostasis by degradation, ubiquitination of histone plays a major role in transcription activation/inactivation, DNA repair, and condensation. Histone ubiquitination at the epigenomic level involves modification of lysine residue at the tails of nucleosomal histone core (13). Ubiquitin-protein ligase such as RING finger proteins RNF20 and RNF40 are the writers, and deubiquitinases (DUBs) such as USP7, USP22, USP44, and HAUSP are the erasers. Inverted ubiquitin interaction motif (IUIM) is a protein that binds at the ubiquitinated lysine (42, 43). Previous studies have shown that signaling pathways such as replication-dependent histone mRNA 3’-end processing, DNA repair response, transcriptional elongation and stem cell differentiation are controlled by CDK9 directed H2Bub1 modification (13). H2Bub1 has been implicated in tumor progression and prognosis of the aggressive cancers; thus, its loss can be an indicator of onset of the disease (44).
miRNAs are small non-coding RNAs transcripts of 21 to 23 nucleotides, which play a major role in gene expression regulation. Unlike regulation by DNA methylation and histone modifications at the genetic and epigenetic levels, microRNA functions at the post-transcriptional level. Molecular mechanisms of miRNAs functions involve binding to its complimentary mRNA where complete binding induces mRNA degradation and partial binding causes mRNA silencing (45). miRNAs are the important regulators of various fundamental processes such as growth, differentiation, stress response, and cellular homeostasis. miRNAs are known to interact with oncogenes and tumor suppressor genes and are thus involved in the control of tumor development and progression, invasion, metastasis, and in epithelial–mesenchymal transition of several cancers (46). Analysis of miRNA profiling suggests that some of miRNA are upregulated, and some are down-regulated in different sets of cancer (47).
Nutraceuticals are bioactive molecules with nutritional value such as dietary food components including phytochemicals. Phytochemicals are a group of secondary metabolites such as polyphenols, flavonoids, terpenoids, xanthones, organo-sulfur compounds, phytosterols, alkaloids, and carotenoids, which are known to have curative properties for diverse human ailments. Presence of hydroxyl groups and aromatic ring in polyphenols categorizes them into following subclasses: flavonoids, phenolic acids, stilbenes, and lignans. Flavonoids are widely distributed cluster of seven subgroups categorized on the basis of their chemical structures. There are several representative bioactive compounds in each subgroup of flavonoids (see Figure 2). These are synthesized via phenyl propanoid pathway, which converts phenylalanine into 4-coumaroyl-CoA to produce flavonoids. All subgroups of flavonoids consist of the same structural backbone of two phenolic rings A and B connected by the third oxygen-containing heterocyclic C ring (see the ring structures in Figure 3 and 4). These subgroups are named as flavones, flavonols, flavanones or dihydroflavones, flavanonols or dihydroflavonols, isoflavones or phytoestrogens, flavanols or catechins or anthocyanins, and proanthocyanins (Figure 3 and 4).
 Figure 2.
Figure 2.
The flow diagram showing detailed classification of polyphenols.
 Figure 3.
Figure 3.
A combined figure showing structures of different flavonoids (a) Flavones that consists of 4H-chromen-4-one with a phenyl substituent at position 2 and are found in fruits and vegetables, including onions, apples, broccoli, and berries, thyme, parsley, celery and capsicum pepper. (b) Flavanones or dihydroflavones which consists of flavan bearing an oxo substituent at position 4. It is derived from a hydride of a flavan and is generally found in grapes, orange and lemon juice. (c) Flavonols, a monohydroxyflavone that comprises a class of compounds with 3-hydroxy derivative of flavone. It is a conjugate acid of a flavonol (1-) and is found in green and black tea, yellow onions, apples, broccoli, black grapes, tomato, red wine, carrot, cherry, tomato, curly kale, leek, lettuce, nuts, walnuts, ginger, and blueberry.
 Figure 4.
Figure 4.
Figure showing structural representation of (a) flavanonols or dihydroflavonols are a class of flavonoids with 3-hydroxy-2,3-dihydro-2-phenylchromen-4-one backbone and are generally isolated from sources such as lemon, sour orange, cocoa, cocoa beverages, and chocolates. (b) Isoflavones or phytoestrogens are a class of polyphenolic compounds which consists of 4H-chromen-4-one ring in which the hydrogen at position 3 is replaced by a phenyl group and are generally found in sources such as soya bean, legumes, red clover chickpeas, peanuts, soy cheese, soy flour and tofu. (c) Flavanols or flavan-3-ol is a hydroxyflavonoid which are found in various sources such as green and black tea, apple, chocolate, beans, apricot, cherry, grapes, peach, red wine, cider, and blackberry. (d) Anthocyanidins, which are the salt derivatives of the 2-phenylchromenylium cation, also known as flavylium cation and most commonly found in blue berries, black grapes, and red wine, blackcurrant, cherry, rhubarb, plum, red cabbage, and cocoa.
These molecules have the potential to alter the health status as they have molecular targets within the cell, however, timing of food consumption, absorption, metabolism, and excretion are vital along with the presence of other factors like the use of tobacco and alcohol. Being a part of our ancient system of medicine, phytochemicals-based therapy is emerging as one of the most efficient therapies not only against cardiovascular and metabolic diseases, but also in cancer treatment (Table 1). Previous preclinical studies have demonstrated the therapeutic aspects of these bioactive molecules. Anti-oxidative, anti-inflammatory, anti-proliferative, cyto-protective and geno-protective effect are the potential therapeutic outcome of these flavonoids (11, 14, 47, 48). These therapeutic properties of flavonoids are due to their interaction with the intermediate of cell signaling proteins. Major regulatory mechanisms of phytochemicals involve modulation of redox reactions, enzyme activities, mitochondrial-retrograde responses, and cellular signaling pathways that significantly alter the metabolome and gene expression or vice-versa (Figure 5) (49, 50). Flavonoids possessing epigenetic restoration property have also been reported (51, 52).
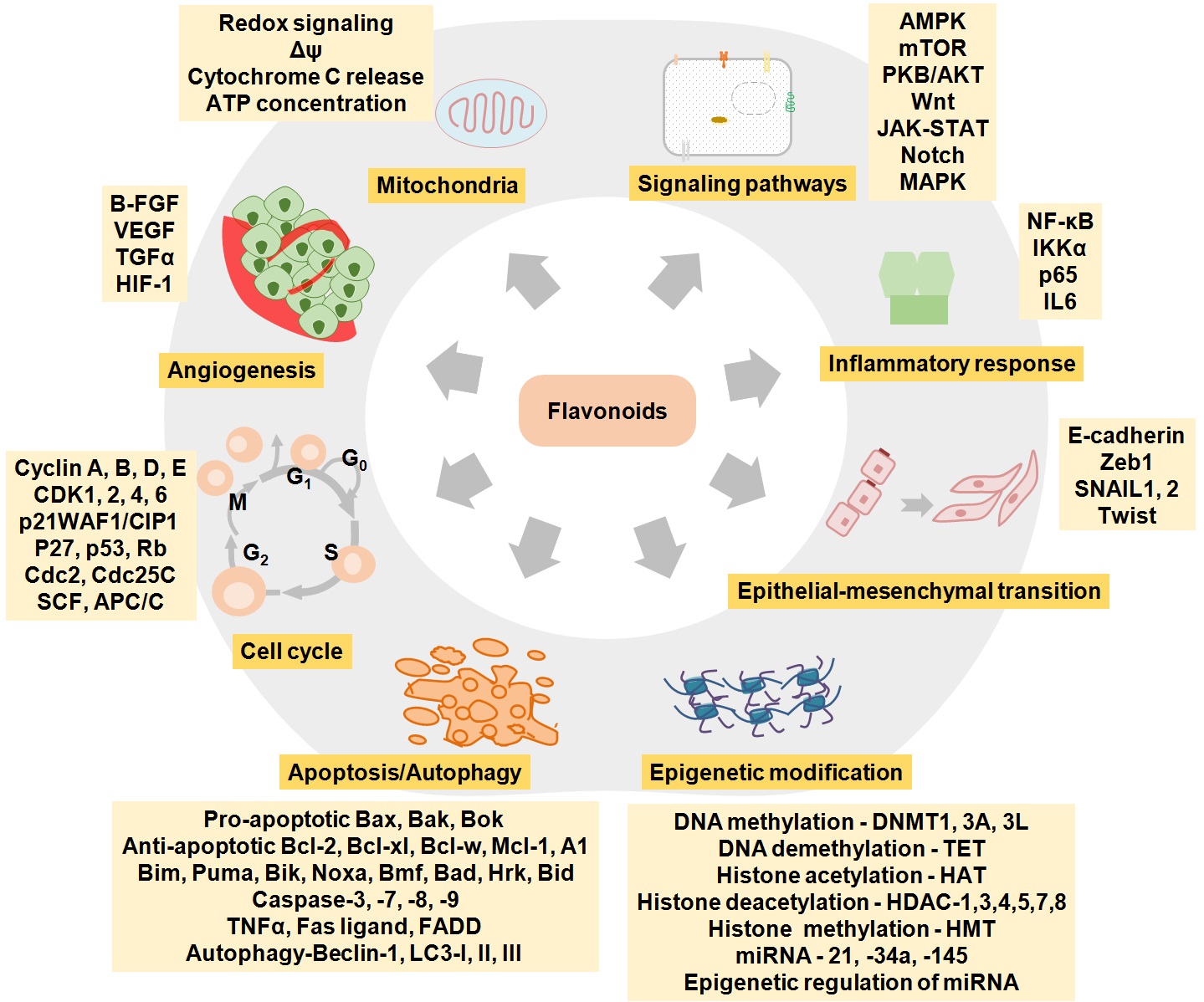 Figure 5.
Figure 5.
The diagrammatic representation of different mechanistic aspects of flavonoids
| Sub-classes of flavonoids | Examples | Preclinical cancer model | References |
|---|---|---|---|
| Flavones | Apigenin | B-cell lymphoma | (148) |
| Flavones | Apigenin | Bladder | (149) |
| Flavones | Apigenin | Breast | (150) |
| Flavones | Apigenin | Cervical | (151) |
| Flavones | Apigenin | Chronic lymphocytic leukemia | (152) |
| Flavones | Apigenin | Colon | (153) |
| Flavones | Apigenin | Colorectal | (154) |
| Flavones | Apigenin | Esophageal | (155) |
| Flavones | Apigenin | Gastric | (156) |
| Flavones | Apigenin | Glioblastoma | (157) |
| Flavones | Apigenin | Glioma | (158) |
| Flavones | Apigenin | Hepatocellular | (159) |
| Flavones | Apigenin | Leukemia | (160) |
| Flavones | Apigenin | Lung | (161) |
| Flavones | Apigenin | Melanoma | (76) |
| Flavones | Apigenin | Neuroblastoma | (162) |
| Flavones | Apigenin | Oral | (163) |
| Flavones | Apigenin | Osteosarcoma | (164, 165) |
| Flavones | Apigenin | Ovarian | (166) |
| Flavones | Apigenin | Pancreatic | (167) |
| Flavones | Apigenin | Prostate | (168) |
| Flavones | Apigenin | Skin | (169) |
| Flavones | Apigenin | Thyroid | (170) |
| Flavones | Baicalein | Breast | (171) |
| Flavones | Baicalein | Cervical | (172) |
| Flavones | Baicalein | Colon | (173) |
| Flavones | Baicalein | Colorectal | (174) |
| Flavones | Baicalein | Gastric | (175) |
| Flavones | Baicalein | Glioma | (176) |
| Flavones | Baicalein | Hepatocellular | (177, 178) |
| Flavones | Baicalein | Leukemia | (179) |
| Flavones | Baicalein | Lung | (180) |
| Flavones | Baicalein | Melanoma | (181) |
| Flavones | Baicalein | Osteosarcoma | (182) |
| Flavones | Baicalein | Ovarian | (183) |
| Flavones | Baicalein | Pancreatic | (184) |
| Flavones | Baicalein | Prostate | (185) |
| Flavones | Baicalein | Skin | (186) |
| Flavones | Baicalein | Sarcoma | (187) |
| Flavones | Baicalin | B-cell lymphoma | (188) |
| Flavones | Baicalin | Breast | (189) |
| Flavones | Baicalin | Cervical | (190) |
| Flavones | Baicalin | Colon | (191) |
| Flavones | Baicalin | Colorectal | (192) |
| Flavones | Baicalin | Glioma | (193) |
| Flavones | Baicalin | Hepatocellular carcinoma | (194) |
| Flavones | Baicalin | Leukemia | (195) |
| Flavones | Baicalin | Lung | (196) |
| Flavones | Baicalin | Osteosarcoma | (197) |
| Flavones | Baicalin | Ovarian | (198) |
| Flavones | Baicalin | Prostate | (199) |
| Flavones | Chrysin | Breast | (200) |
| Flavones | Baicalin | Cervical | (201) |
| Flavones | Baicalin | Chronic lymphocytic leukemia | (202) |
| Flavones | Baicalin | Colon | (203) |
| Flavones | Baicalin | Colorectal | (204) |
| Flavones | Baicalin | Gastric | (205) |
| Flavones | Baicalin | Glioma | (206) |
| Flavones | Baicalin | Hepatocellular | (207) |
| Flavones | Baicalin | Lung | (208) |
| Flavones | Baicalin | Leukemia | (78) |
| Flavones | Baicalin | Melanoma | (209) |
| Flavones | Baicalin | Osteosarcoma | (210) |
| Flavones | Baicalin | Prostate | (211) |
| Flavones | Baicalin | Skin | (31) |
| Flavones | Baicalin | Thyroid | (212) |
| Flavones | Baicalin | Ovarian | (213) |
| Flavones | Hispidulin | Colon | (214) |
| Flavones | Hispidulin | Gastric | (215) |
| Flavones | Hispidulin | Renal cell | (216) |
| Flavones | Luteolin | Breast | (217) |
| Flavones | Luteolin | Cervical | (218) |
| Flavones | Luteolin | Colon | (219) |
| Flavones | Luteolin | Colorectal | (220) |
| Flavones | Luteolin | Esophageal | (221) |
| Flavones | Luteolin | Gastric | (222) |
| Flavones | Luteolin | Glioblastoma | (223) |
| Flavones | Luteolin | Hepatocellular | (224) |
| Flavones | Luteolin | Leukemia | (202) |
| Flavones | Luteolin | Lung | (225) |
| Flavones | Luteolin | Neuroblastoma | (226) |
| Flavones | Luteolin | Pancreatic | (227) |
| Flavones | Luteolin | Prostate | (228) |
| Flavones | Luteolin | Skin | (229) |
| Flavones | Luteolin | Thyroid | (230) |
| Flavones | Luteolin | Sarcoma | (231) |
| Flavones | Luteolin | Urinary bladder | (232) |
| Flavones | Nobiletin | Acute myeloid leukemia | (233) |
| Flavones | Nobiletin | Breast | (234) |
| Flavones | Nobiletin | Colon | (235) |
| Flavones | Nobiletin | Colorectal | (236) |
| Flavones | Nobiletin | Gastric | (237) |
| Flavones | Nobiletin | Glioma | (238) |
| Flavones | Nobiletin | Hepatocellular | (239) |
| Flavones | Nobiletin | Lung | (240) |
| Flavones | Nobiletin | Melanoma | (241) |
| Flavones | Nobiletin | Neuroblastoma | (242) |
| Flavones | Nobiletin | Ovarian | (243, 244) |
| Flavones | Nobiletin | Prostate | (245) |
| Flavones | Pectolinarigenin | Nasopharyngeal | (246) |
| Flavones | Pectolinarigenin | Osteo-sarcoma | (247) |
| Flavones | Tangeretin | Breast | (248) |
| Flavones | Tangeretin | Colon | (249) |
| Flavones | Tangeretin | Colorectal | (250) |
| Flavones | Tangeretin | Gastric | (251) |
| Flavones | Tangeretin | Glioma | (252) |
| Flavones | Tangeretin | Lung | (253) |
| Flavones | Tangeretin | Melanoma | (254) |
| Flavones | Tangeretin | Ovarian | (255) |
| Flavones | Wogonin | Breast | (256) |
| Flavones | Wogonin | Melanoma | (257) |
| Flavones | Wogonin | Glioma | (258) |
| Flavones | Wogonin | Prostate | (259) |
| Flavones | Wogonin | Leukemia | (260) |
| Flavones | Wogonin | Lung adenocarcinoma cell | (261) |
| Flavones | Wogonin | Gastric | (262) |
| Flavones | Wogonin | Ovarian | (263) |
| Flavones | Wogonin | Colorectal | (264) |
| Flavonols | Fisetin | Acute myeloid leukemia | (265) |
| Flavonols | Fisetin | Breast | (266) |
| Flavonols | Fisetin | Cervical | (267) |
| Flavonols | Fisetin | Colon | (268) |
| Flavonols | Fisetin | Colorectal | (269) |
| Flavonols | Fisetin | Gastric | (270) |
| Flavonols | Fisetin | Glioma | (271) |
| Flavonols | Fisetin | Hepatocellular | (272) |
| Flavonols | Fisetin | Lung | (273) |
| Flavonols | Fisetin | Melanoma | (274) |
| Flavonols | Fisetin | Ovarian | (275) |
| Flavonols | Fisetin | Prostate | (276) |
| Flavonols | Isorhamnetin | Breast | (277) |
| Flavonols | Isorhamnetin | Colon | (278) |
| Flavonols | Isorhamnetin | Colorectal | (279) |
| Flavonols | Isorhamnetin | Lung | (280) |
| Flavonols | Isorhamnetin | Gastric | (281) |
| Flavonols | Isorhamnetin | Skin | (282) |
| Flavonols | Kaempferol | Breast | (283) |
| Flavonols | Kaempferol | Cervical | (284) |
| Flavonols | Kaempferol | Colon | (285) |
| Flavonols | Kaempferol | Colorectal | (286) |
| Flavonols | Kaempferol | Esophageal | (287) |
| Flavonols | Kaempferol | Gastric | (288) |
| Flavonols | Kaempferol | Glioma | (289) |
| Flavonols | Kaempferol | Hepatocellular | (290) |
| Flavonols | Kaempferol | Leukemia | (291) |
| Flavonols | Kaempferol | Lung | (292) |
| Flavonols | Kaempferol | Ovarian | (293) |
| Flavonols | Kaempferol | Pancreatic | (294) |
| Flavonols | Kaempferol | Prostate | (295) |
| Flavonols | Kaempferol | Skin | (296) |
| Flavonols | Kaempferol | Urinary bladder | (297) |
| Flavonols | Myricetin | Breast | (298) |
| Flavonols | Myricetin | Cervical | (299) |
| Flavonols | Myricetin | Colon | (300) |
| Flavonols | Myricetin | Colorectal | (301) |
| Flavonols | Myricetin | Esophageal | (302) |
| Flavonols | Myricetin | Gastric | (303) |
| Flavonols | Myricetin | Glioblastoma | (304) |
| Flavonols | Myricetin | Hepatocellular | (305) |
| Flavonols | Myricetin | Lung | (306) |
| Flavonols | Myricetin | Ovarian | (307) |
| Flavonols | Quercetin | Breast | (308) |
| Flavonols | Quercetin | Cervical | (309) |
| Flavonols | Quercetin | Chronic lymphocytic leukaemia | (310) |
| Flavonols | Quercetin | Colon | (311) |
| Flavonols | Quercetin | Colorectal | (312) |
| Flavonols | Quercetin | Esophageal | (313) |
| Flavonols | Quercetin | Gastric | (314) |
| Flavonols | Quercetin | Hepatocellular | (315) |
| Flavonols | Quercetin | Osteosarcoma | (316) |
| Flavonols | Quercetin | Ovarian | (317) |
| Flavonols | Quercetin | Pancreatic | (318) |
| Flavonols | Quercetin | Prostate | (319) |
| Flavonols | Quercetin | Thyroid | (320) |
| Flavonols | Quercetin | Urinary bladder | (321) |
| Flavonols | Rutin | Colon | (322) |
| Flavonols | Rutin | Lung | (323) |
| Flavonols | Rutin | Neuroblastoma | (324) |
| Flavonols | Rutin | Promyelocytic leukemia | (325) |
| Flavonols | Rutin | Prostate | (326) |
| Flavonols | Rutin | Skin | (327) |
| Flavonols | Alpinetin | Lung | (328) |
| Flavonols | Alpinetin | Hepatoma | (329) |
| Flavonols | Alpinetin | Pancreatic | (330) |
| Flavanones | Hesperidin | Acute myeloid leukemia | (331) |
| Flavanones | Hesperidin | Breast | (332) |
| Flavanones | Hesperidin | Colon | (333) |
| Flavanones | Hesperidin | Hepatocellular | (334) |
| Flavanones | Hesperidin | Lung | (335) |
| Flavanones | Hesperidin | Urinary bladder | (336) |
| Flavanones | Hesperidin | Ovarian | (337) |
| Flavanones | Hesperitin 5,7,3’-trihydroxyl-4’-methoxylflavanone | Breast | (338) |
| Flavanones | Hesperitin | Thyroid | (339) |
| Flavanones | 5,7,3’-trihydroxyl-4’-methoxylflavanone | Prostate | (340) |
| Flavanones | Hesperitin | Carcinoid | (341) |
| Flavanones | Naringenin | Breast | (342) |
| Flavanones | Naringenin | Colon | (343) |
| Flavanones | Naringenin | Colorectal | (344, 345) |
| Flavanones | Naringenin | Gastric | (346) |
| Flavanones | Naringenin | Hepatocellular | (347) |
| Flavanones | Naringenin | Lung | (348) |
| Flavanones | Naringenin | Leukemia | (349) |
| Flavanones | Naringenin | Pancreatic | (350) |
| Flavanones | Naringenin | Prostate | (351) |
| Flavanones | Naringenin | B-cell lymphoma | (352) |
| Flavanones | Furanoflavanone | Gastric | (353) |
| Flavanonols | Taxifolin | Prostate | (354) |
| Flavanonols | Taxifolin | Skin | (355) |
| Flavanonols | Silibinin | Acute myeloid leukemia | (331) |
| Flavanonols | Silibinin | Breast | (79) |
| Flavanonols | Silibinin | Cervical | (356) |
| Flavanonols | Silibinin | Colon | (357) |
| Flavanonols | Silibinin | Colorectal | (358) |
| Flavanonols | Silibinin | Gastric | (359) |
| Flavanonols | Silibinin | Glioblastoma | (223) |
| Flavanonols | Silibinin | Lung | (360) |
| Flavanonols | Silibinin | Esophageal | (361) |
| Flavanonols | Silibinin | Ovarian | (362) |
| Flavanonols | Silibinin | Pancreatic | (363) |
| Flavanonols | Silibinin | Prostate | (364) |
| Flavanonols | Silibinin | Skin | (365) |
| Flavanonols | Silibinin | Bladder | (366) |
| Flavanonols | Silibinin | Thyroid | (367) |
| Flavanonols | Silymarin | Cervical | (368) |
| Flavanonols | Silymarin | Colon | (369) |
| Flavanonols | Silymarin | Hepatocellular | (370) |
| Flavanonols | Silymarin | Lung | (371) |
| Flavanonols | Silymarin | Neuroblastoma | (372) |
| Flavanonols | Silymarin | Ovarian | (373) |
| Flavanonols | Silymarin | Promyelocytic leukemia | (374) |
| Flavanonols | Silymarin | Prostate | (375) |
| Flavanonols | Silymarin | Bladder | (376) |
| Flavanonols | Pinobanksin | B-cell lymphoma | (377) |
| Isoflavones | Genistein | Acute myeloid leukemia | (378) |
| Isoflavones | Genistein | Breast | (379) |
| Isoflavones | Genistein | Cervical | (380) |
| Isoflavones | Genistein | Colon | (381) |
| Isoflavones | Genistein | Colorectal | (382) |
| Isoflavones | Genistein | Esophageal | (383) |
| Isoflavones | Genistein | Gastric | (384) |
| Isoflavones | Genistein | Glioblastoma | (385) |
| Isoflavones | Genistein | Hepatocellular | (386) |
| Isoflavones | Genistein | Leukemia | (387) |
| Isoflavones | Genistein | Lung | (388) |
| Isoflavones | Genistein | Melanoma | (389) |
| Isoflavones | Genistein | Oral | (390) |
| Isoflavones | Genistein | Osteosarcoma | (391) |
| Isoflavones | Genistein | Ovarian | (392) |
| Isoflavones | Genistein | Pancreatic | (393) |
| Isoflavones | Genistein | Prostate | (394) |
| Isoflavones | Genistein | Uterine | (395) |
| Isoflavones | Genistein | Bladder | (396) |
| Isoflavones | Biochanin A | Breast | (397) |
| Isoflavones | Biochanin A | Colon | (398) |
| Isoflavones | Biochanin A | Glioma | (399) |
| Isoflavones | Biochanin A | Hepatocellular | (400) |
| Isoflavones | Biochanin A | Leukemia | (401) |
| Isoflavones | Biochanin A | Lung | (402) |
| Isoflavones | Biochanin A | Pancreatic | (77) |
| Isoflavones | Biochanin A | Prostate | (403) |
| Isoflavones | Glycitein | Breast | (404) |
| Isoflavones | Formononetin | Colon | (405) |
| Isoflavones | Formononetin | Cervical | (406) |
| Isoflavones | Formononetin | Osteosarcoma | (407) |
| Isoflavones | Formononetin | Prostate | (408) |
| Isoflavones | Formononetin | NSCLC | (409) |
| Isoflavones | Formononetin | Breast | (61) |
| Isoflavones | Formononetin | Glioma | (410) |
| Isoflavones | Daidzein | Breast | (411) |
| Isoflavones | Daidzein | Colon | (381) |
| Isoflavones | Daidzein | Hepatic | (412) |
| Isoflavones | Daidzein | Melanoma | (413) |
| Isoflavones | Daidzein | Neuroblastoma | (414) |
| Isoflavones | Daidzein | Pancreatic | (415) |
| Isoflavones | Daidzein | Prostate | (416) |
| Isoflavones | Daidzein | Skin | (417) |
| Flavanols | Catechin | B-cell lymphoma | (418) |
| Flavanols | Catechin | Breast | (419) |
| Flavanols | Catechin | Bladder | (420) |
| Flavanols | Catechin | Cervical | (421) |
| Flavanols | Catechin | Glioma | (422) |
| Flavanols | Catechin | Hepatocellular | (423) |
| Flavanols | Catechin | Lung | (424) |
| Flavanols | Catechin | Melanoma | (425) |
| Flavanols | Catechin | Pancreatic | (426) |
| Flavanols | Catechin | Promyelocytic leukemia | (427) |
| Flavanols | Catechin | Prostate | (428) |
| Flavanols | Epigallocatechin-3-gallate | Acute myeloid leukemia | (429) |
| Flavanols | Epigallocatechin-3-gallate | B-cell lymphoma | (418) |
| Flavanols | Epigallocatechin-3-gallate | Urinary Bladder | (430) |
| Flavanols | Epigallocatechin-3-gallate | Breast | (431) |
| Flavanols | Epigallocatechin-3-gallate | Cervical | (432) |
| Flavanols | Epigallocatechin-3-gallate | Chronic lymphocytic leukaemia | (433) |
| Flavanols | Epigallocatechin-3-gallate | Colon | (434) |
| Flavanols | Epigallocatechin-3-gallate | Colorectal | (435) |
| Flavanols | Epigallocatechin-3-gallate | Esophageal | (436) |
| Flavanols | Epigallocatechin-3-gallate | Gastric | (437) |
| Flavanols | Epigallocatechin-3-gallate | Glioblastoma | (438) |
| Flavanols | Epigallocatechin-3-gallate | Glioma | (439) |
| Flavanols | Epigallocatechin-3-gallate | Hepatocellular | (440) |
| Flavanols | Epigallocatechin-3-gallate | Lung | (441) |
| Flavanols | Epigallocatechin-3-gallate | Melanoma | (442) |
| Flavanols | Epigallocatechin-3-gallate | Neuroblastoma | (443) |
| Flavanols | Epigallocatechin-3-gallate | Oral | (444) |
| Flavanols | Epigallocatechin-3-gallate | Osteosarcoma | (445) |
| Flavanols | Epigallocatechin-3-gallate | Ovarian | (446) |
| Flavanols | Epigallocatechin-3-gallate | Pancreatic | (447) |
| Flavanols | Epigallocatechin-3-gallate | Prostate | (448) |
| Flavanols | Epigallocatechin-3-gallate | Skin | (449) |
| Flavanols | Epigallocatechin-3-gallate | Thyroid | (450) |
| Flavanols | Epigallocatechin-3-gallate | Sarcoma | (451) |
| Anthocyanidins | Cyanidin | Breast | (452) |
| Anthocyanidins | Cyanidin | Colon | (453) |
| Anthocyanidins | Cyanidin | Lung | (454) |
| Anthocyanidins | Cyanidin | Ovarian | (455) |
| Anthocyanidins | Cyanidin | Prostate | (456) |
| Anthocyanidins | Delphinidine | Breast | (457) |
| Anthocyanidins | Delphinidine | Colon | (453) |
| Anthocyanidins | Delphinidine | Glioblastoma | (458) |
| Anthocyanidins | Delphinidine | Hepatocellular | (459) |
| Anthocyanidins | Delphinidine | Lung | (460) |
| Anthocyanidins | Delphinidine | Ovarian | (461) |
| Anthocyanidins | Delphinidine | Prostate | (462) |
| Anthocyanidins | Pelargonidin | Hepatic | (463) |
Epigenetic modifications are greatly affected with the change in the environmental conditions and act as interface between the gene and environment. Defined concentration of polyphenols in the diet can modulate epigenome-mediated genomic expressions essentially by controlling the metabolic fate of a cell hence influencing the mechanisms underlying human health and disease (9, 51, 53-55). Accumulative literature on epigenetically active polyphenols suggests that these molecules have anti-cancer effects as these can potentially manipulate the activities of epigenetic modifiers such as writers (DNMT, HAT, and HMT) and erasers (HDAC, KDM) (52). A diet containing epigenome-modifying and chemo preventive nutraceuticals influence the level of bioactive metabolites that in turn modulate cellular pools of methyl donor groups, acetyl-CoA, NAD+, and ATP, resulting in altered DNA methylation pattern and histone and non-histone protein acetylation/methylation/phosphorylation (3-5). For instance, alteration in DNA and histone methylation level is associated with the imbalance in the SAM/SAH ratio. A significant change in the SAM/SAH ratio level has been reported following ingestion of flavanol-rich diets (56-59). In vitro and in vivo tumor/cancer models treated with specific polyphenolic bioactive components have revealed efficient antitumor potential by stimulating the signaling pathway for programed cell death mediating epigenetic machinery (60). Such observations are enabling the modifiers of epigenetic mechanisms to emerge as anti-cancer therapeutics. Therefore, currently a powerful tenet is to explore how dietary molecules may influence prevention and treatment of various chronic diseases based on nutri-epigenomic intervention. Recently, the anti-cancer therapeutic potential of some bioactive compounds with the epigenetic reversal ability have been tested in clinical trials (see Table 2) (61-64).
| Clinical trial phase and type | Flavonoids used | Cancer type | Participant number | Dose amount, duration & mode | Finding | References |
|---|---|---|---|---|---|---|
| Pre-surgical trial | Silybin-phosphatidylcholine | Breast cancer | 12 | 2.8 g daily for 4 weeks prior to surgery; oral | High blood concentration of silybin-phosphatidylcholine ensures selective accumulation | (464) |
| Phase Ib; Single site; Clinical study | AXP107-11, a multi-component crystalline form of genistein | Unresectable pancreatic cancer | 16 | 400 mg-1600 mg daily in combination with 1000 mg/m(2) gemcitabine per week orally for 6 months | Favorable PK-profile was reported | (465) |
| Phase II; Randomized; Double-blind; Placebo-controlled | Genistein | Prostate cancer | 47 | 30 mg genistein daily for 3-6 weeks | Modulate genes related to cell cycle and androgen expression | (466) |
| Phase II; Placebo-controlled; Block-randomized; Double-blind | Genistein | Localized prostate cancer | 44 | 30 mg daily for 3 to 6 week prior to prostatectomy | Well tolerated and reduced the level of serum PSA | (467) |
| Phase II; Randomized | Soy isoflavone | Prostate cancer | 32 | 2 slices of soy bread containing 34 mg isoflavones/slice or soy bread containing almond powder prescribed daily as a source of β-glucosidase for oral consumption for 56 days | Suppressed pro-inflammatory cytokines expression and reduced immunosuppressive cells | (64) |
| Phase II; Randomized crossover trial | Isoflavone | Asymptomatic prostate cancer | 32 | Crossover of two formulations as soy bread and soy almond bread to deliver approximately 60 mg aglycone equivalents of isoflavones per day for 20-weeks | Metabolizing phenotypes of isoflavones to distinguish cancer patients resistance to preventive strategies were reported | (62) |
| Randomized; Placebo-controlled study | Isoflavones | Breast Cancer | 140 | 2 packets of 25.8g soy protein powder or 25.8g milk protein per day with water or juice until surgery | High genistein level modulates gene expression prolife; and over-express cell proliferating genes | (468) |
| Randomized; Double-blind; Placebo-controlled | Isoflavone | Prostate cancer | 86 | 80 mg/day of total isoflavones & 51 mg/day aglucon units | Serum hormone levels, total cholesterol, or PSA remain unchanged upon short-term intake of soy isoflavones | (469) |
| Phase II; Randomized; Placebo-controlled | Isoflavone | Urothelial bladder cancer | 59 | 300 or 600 mg/day as purified soy extract G-2535 for 14 to 21 days before surgery | More effective responses at the lower dose in bladder cancer tissues were recorded | (398) |
| Phase II; Randomized; Double-blind; Placebo-controlled | Isoflavone | Prostate cancer | 158 | 60 mg/day isoflavones prescribe orally for 12 months | Isoflavone reduces prostate cancer risk | (470) |
| Phase I; Randomized; Dose-escalating | Isoflavones | Prostate cancer | 45 | 40 -60-80 mg purified isoflavones from biopsy to prostatectomy | Safe dose of purified isoflavones for future examinations were established | (471) |
| Phase II | Isoflavones | Advanced pancreatic cancer | 20 | 531 mg isoflavones twice daily from day 7 until the end of study; 1,000 mg/m² gemcitabine on days 1, 8, and 15; 150 mg erlotinib once daily on day 1 to day 28 | Non-significant survival of patients | (472) |
| Double-blind; Randomized; Placebo-controlled | Isoflavones | Breast cancer | 205 | Isoflavone tablet - 1 mg genistein, 26 mg biochanin A, 16 mg formononetin, and 0.5 mg daidzein | Ineffective to increase mammographic breast density and has no effect on lymphocyte tyrosine kinase activity, oestradiol, gonadotrophins, or menopausal symptoms. | (473) |
| Randomized; Placebo-controlled; Crossover | Phytoestrogens | Breast cancer | 62 | 114 mg isoflavonoids as Phytoestrogen tablets for 3 months | Non-significant to eliminatemenopausal symptoms in breast cancer patients | (474) |
| Phase I | Epigallocatechin-3-gallate (EGCG) | Breast cancer | 24 | 40 to 660 μmol l(-1) in 7 levels for 2 weeks; topical | Well tolerated and but no maximum tolerated dose was detected. Effective treatment of radiation dermatitis was reported | (475) |
| Phase II | EGCG | Stage III lung cancer. | 37 | 440 μmol/L given orally three times a day during the radiation. | Effective and safe method against acute radiation-induced esophagitis | (476) |
| Phase II; Two-stage; Single-arm | EGCG | Advanced stage ovarian cancer | 46 | 500 mL double-brewed green tea daily until recurrence or during a follow-up of 18 months | Doesn’t show promising maintenance intervention in advanced stage ovarian cancer after standard treatment. | (477) |
| Randomized controlled trial | The Minnesota green tea trial; green tea catechin | Breast cancer | 1075 | 800 mg daily for one year | Study verified breast cancer patients for the differences in tea catechins metabolism based on COMT genotype to propose biomarkers of breast cancer risk | (478) |
| Placebo-controlled; Randomized clinical trial | Green tea catechins | Prostate Cancer | 97 | Polyphenon E® 400 mg/day for 1 year | Well tolerated but not enough to overcome prostate cancer | (428) |
| Phase II; Randomized clinical trial; Open label | Brewed green and black tea | Prostate cancer | 113 | 1300 mg green tea polyphenol including 800 mg of EGCG | Green tea modulates systemic oxidation and inflammatory arm through NFkB in prostate tissue | (479) |
| Phase IB; Randomized; Placebo-controlled; Dose escalation | Green tea extract (Polyphenon E) | Breast cancer | 34 | 400 mg; 600 mg; and 800 mg twice daily given orally for 6 months | Promising role of tea polyphenols in signaling pathways of growth factors such as HGF and VEGF, angiogenesis and lipid metabolism was observed | (63) |
| Phase II; Randomized; Double-blind; Placebo controlled | Polyphenon E | Low grade cervical intraepithelial neoplasia | 98 | 800 mg epigallocatechin gallate daily for 4 months | Well tolerated and safe but unable to resist high-risk HPV infections | (480) |
| Phase II | Polyphenon E | Rai stage 0-II chronic lymphocytic leukemia | 42 | 2000 mg twice daily for 6 months | Well tolerated and its daily consumption reduces ALC and/or lymphadenopathy | (481) |
| Randomized; Double-blind; Placebo controlled | Polyphenon E | Prostate cancer | 50 | 800 mg epigallocatechin gallate in Polyphenon E daily for 3–6 weeks before surgery | Low bioavailability and/or bioaccumulation reported in prostate tissue | (482) |
| Phase I | Polyphenon E | Asymptomatic Rai stage 0 to II chronic lymphocytic leukemia | 33 | 400 to 2,000 mg twice a day for up to 6 months; Orally | Well tolerated and observed to decreased lymphadenopathy observed in the majority of patients | (483) |
| Phase II; Multicenter study; Single-arm; Open-label | P276-00, a flavone-derived molecule | Refractory mantle cell lymphoma | 13 | Intravenous infusion of 185 mg/m2/day from days 1-5 of a 21-day cycle | A novel well-tolerated CDK isoforms inhibitor evaluated in patients with relapsed or refractory MCL | (484) |
Flavonoids or mitochondria-targeted drugs act through redox-dependent and redox-independent regulation of nuclear gene expression. Although many studies have emphasized the anti-oxidative properties of flavonoids, their effect on mitochondria in a redox-independent manner has not been extensively studied (65). However, individual cancer type has different cellular and molecular disorientation due to abnormal expression of susceptible genes. Therefore, a tumor cannot be treated effectively by targeting a distinct gene or protein or a single signal transduction pathway (11, 14, 47, 66). Since, several intra- and extracellular signaling pathways are governed by mitochondria and therefore, it is the primary site of action for flavonoids and also promotes apoptosis and inhibition of cell growth (Figure 6). Various mitochondria-targeted flavonoid compositions have been analyzed for their anti-cancer effects. Numerous mitochondria-targeted drugs have also been identified and validated for their anti-cancer effects (67-69) and based on their mode of action, these mitochondria-targeted anti-cancer drugs as classified as mitocans. However, (68) Gorlach et al., described the molecular mechanisms of each mitocan underlying anti-cancer activity.
 Figure 6.
Figure 6.
Scheme illustrating the vital role of mitochondria in regulation of major cellular processes involved in signaling, cell cycle, autophagy, apoptosis, angiogenesis, and epigenetic modifications.
Further, harboring healthy mitochondria greatly impact the redox status and related processes. Most observation favors the flavonoid induced upregulation of mitochondrial biogenesis through increase in the PGC-1α level. Recent reports have shown that (-)-epicatechin increase biogenesis by G-protein coupled estrogen receptor, fisetin enhance biogenesis by downregulation of glycogen synthase kinase 3β activity, and digitoflavone upregulate biogenesis by enhancing AMPK phosphorylation (70-72). However, results are conflicting for quercetin, as it can both increase and attenuate this process (73).
Recent experiments have uncovered the intricate role of mitochondria in the epigenetic regulation of several processes involved in human health and disease (13, 16, 74). There are two important routes through which mitochondria regulate the epigenetic machinery. Firstly, as the hub for the metabolic processes, the mitochondrion holds several tiny co-factors and substrates that are utilized in the epigenetic modification processes, such as the methyl group from the SAM (indirect source of methionine) for DNA and histone methylation, the acetyl group from acetyl-CoA for histone acetylation, and ATP for histone phosphorylation (15). Second, being the center for the oxidative mechanisms, mitochondria can oxidatively modify the catalytic properties of the mitochondrial and cellular enzymes and proteins thereby affecting the epigenetic process and redox signaling. For instance, mitochondria alter the activity of sirtuins, which are a class of NAD-dependent deacetylases. Sirtuin 6 has been implicated in ageing, many metabolic diseases and explored for therapeutic interventions (75).
Flavones such as apigenin, chrysin, and baicalein are potential anti-neoplastic and anti-carcinogenic agents. The anti-cancer activity of these flavonoids is mediated through activation of mitochondria-dependent apoptotic pathway, which has been validated through in vitro and in vivo studies (76-81). Induction of mitochondria-dependent apoptotic pathway in peripheral blood lymphocytes from B-chronic lymphocytic leukemia patients and leukemia cell lines have been observed following chrysin treatment (78). Chrysin disturbs the mitochondrial membrane potential that causes release of cytochrome C, up-regulate the pro-apoptotic Bax and caspase-3 and down-regulate the anti-apoptotic Bcl-2 protein that in turn result in apoptosis/cell death (78). Additionally, silibinin, in breast cancer, triggers mitochondria-mediated cell death. Basically, silibinin induces ROS-dependent mitochondrial dysfunction leading to ATP depletion by involving BNIP3 to initiate autophagy (79). Similarly, apigenin also causes mitochondrial dysfunction leading to mitochondria-mediated apoptosis in hepatocytes of HCC rats (80). Apigenin-loaded PLGA nanoparticles targeted at the nucleus damage DNA and subsequently induce mitochondria-mediated apoptosis in tumor cells (76). Moreover, baicalein follows a similar path in the gastric cancer cell line where it induces S phase arrest, down-regulate Bcl-2, up-regulate Bax and disrupt mitochondrial membrane potential in dose dependent manner (81). Additionally, mitochondriotropic nanoemulsified genistein-loaded carriers have shown pro-apoptotic anti-cancer effects due to mitochondrial damage through depolarization-mediated cytochrome C release (82). Moreover, a pro-oxidant action upon treatment of colorectal cancer with 5-Hydroxy-7-methoxyflavone was observed due to ROS triggered mitochondria-mediated apoptosis (77).
Several preclinical evidences have suggested that flavonoids possess anti-cancer properties; however, proper exploitation of the associated health benefits is limited. Primarily, limitations are due to poor bioavailability as a result of low solubility, insignificant permeability, first-pass metabolic effects, metabolism by gut micro flora, and absorption across the intestinal wall, active efflux mechanism, and easy modification by environmental factors such as temperature, pH, and light. Moreover, differences in isolation, purification, treatment strategy, interpretation, and analysis create an additional level of challenge in using flavonoids as anti-cancer agents.
Many challenges are associated with the extraction and isolation of flavonoids, that may include the presence of low levels of active compounds (ranging from few micrograms to milligrams per kg of plant), complexity of the compounds, difficulty in isolation, identification and purification of individual constituents, complicated analytical procedures, time-consuming processes, high costs, and low product yield. The variation in flavonoid composition decreases the predictive yield and their labile nature make them susceptible to a high degree of degradation or chemical modification during the purification (83).
Flavonoids possess characteristic impaired solubility, compromised oral absorption, and vulnerability to high hepatic metabolism subsequently leading to unsuitable PK profile (i.e., ADME and toxicity). Moreover, complexation or precipitation of flavonoids when ingested with other food components as well as their degradation by intestinal micro flora contributes to reduced stability and poor bioavailability (83, 84).
To allow clinical applicability of dietary flavonoids, exploration of various approaches has been reviewed.
Over the past many years, several attempts have been made to improve the yield of flavonoids by the means of modifications in extraction, purification, and isolation approaches. On large scale, numerous techniques that have shown distinctive advantages such as high caliber extraction yield of antioxidants, and good percentage of marker compounds have been employed. These techniques may include pressure based procedures, microwave assisted methods, resin based adsorption systems and many others like pre-fermentation treatment, semi-preparative high-speed counter-current chromatography, etc (85-93).
In order to surmount the limited bioavailability issues of flavonoids employment of numerous approaches have been reported. For example, flavonoids can be formulated as certain types of glycosides complex, which serve as substrates for certain intestinal epithelial transporters, leading to their increased absorption and enhanced bioavailability (94, 95). The administration of quercetin-4-O-glucoside resulted in a 5-fold increase in the plasma level of flavonoids as compared to quercetin-3-Orutinoside; thus, offering a potential substitute for augmentation of bioavailability (95). Moreover, addition of natural bio-enhancers such as piperine can significantly inhibit phase II enzymes like UDP-glucuronosyltransferase preventing the metabolic degradation of flavonoids (84, 96, 97). In addition, use of specific ABC transporter blockers such as lapatinib and nilotinib may improve the flavonoid bioavailability (83).
The numerous factors limiting the efficacy of the flavonoids can be overcome by application of nanocarrier based approaches that offers several advantages such as prevention against metabolic degradation in the gastrointestinal tract, improvement of solubilization potential, and alteration of absorption pathways (Table 3) (98).
| Nanocarrier | Flavonoid | Model system | Inference | References |
|---|---|---|---|---|
| Lipid nanoparticle system | Quercetin, Naringenin, and Hesperetin | In vitro digestion | Enhanced relative bio-accessibility as LNP preserved well during oral digestion | (113) |
| Multifunctional solid lipid nanoparticles | Baicalein and fisetin | Colon adenocarcinoma cells | Electroporation of SLN lead to increased anti-cancer effects | (485) |
| anti-cancer | ||||
| DQAsomes | Curcumin | A549 cell and Caco-2 cells | Enhanced antioxidant activity and mitochondrial targeting ability due to improved stability of curcumin-loaded DQAsomes | (126) |
| Cationic lipid-based nanocarriers | Genistein | CT26 and HepG2 cell lines | Mitochondrial depolarization, cytosolic cytochrme c release and activated caspase-9 augmented anti-cancer activity | (486) |
| TPP conjugates | Cyanostilbene derivatives | HeLa, MCF-7, Hec-1A, KGN, HCT116, A549, 293T, and CCD-18Co cellsImmunodeficientBALB/c nu/nu mice | Selective mitochondrial accumulation generates intracellular reactive oxygen species, decreases mitochondrial membrane potential and upregulates anti-cancer activity | (487) |
| Chemically modified gallic acid | Mammary adenocarcinomaTA3/Ha cell line | Increased uncoupling effect and decreased ATP levels lead to hiked anti-proliferative effect in vivo due to selective mitochondrial uptake | (488) | |
| Polymeric nanoparticles | Apigenin | Swiss Albino mice | Stable and efficient encapsulation of nanoparticles result in ROS mediated mitochondrial apoptosis | (169) |
| Hesperetin | C6 glioma cells | Delivery resulted in enhanced uptake, sustained drug release and therapeutic efficacy | (489) | |
| Carbon based nanostructures | Quercetin | Human lung epithelial carcinoma A549 line | Increased stability and no cytotoxicity of nano graphene oxide for oral delivery is reported | (490) |
| Gold nanoparticles | Quercetin | Hormone-dependent (MCF-7) and hormone-independent (MDA-MB-231) breast cancer cell lines | Induced apoptosis and suppressed EGFR signaling for -anti-neoplastic effects is reported with increased therapeutic efficacy | (491) |
| Baicalin | MCF-7 cells | Induction of apoptosis showed anti-cancer effectiveness of conjugated nanoparticles | (492) | |
| Morin | MCF-7 breast cancer cells | Reducing and stabilizing nanoparticles endocytosed by cells and stimulate cell death via promoted DNA damage, cell cycle arrest, apoptosis | (493) | |
| Hesperetin | Hepatocellular carcinoma - Hep3B cells | Polymer functionalized nanoparticles inhibit proliferation and induce apoptosis | (494) | |
| TiO2 nanoparticles | Quercetin | 3T3 mouse fibroblast cells | High bioavailability and stability with maximum antioxidant capacity | (495) |
| Iron oxide nanoparticles | Baicalein | Triple negative breast cancer MDA-MB-231 cells | Controlled release profile, cell cycle arrest, significant down-regulation of anti-apoptotic protein Bcl-2 and up-regulation of pro-apoptotic proteins | (496) |
| Superparamagnetic iron oxide nanoparticles | Luteolin | U87, MCF-7, HeLa, L929 and A549 cell lines | Reduced cell viability and higher apoptosis of cancer cells is reported | (497) |
| Mesoporous silica nanoparticles | Quercetin | MDA MB 231 and MCF-7 breast cancer cell lines | Enhanced cellular uptake and bioavailability lead to cell cycle arrest and apoptosis via Akt and Bax signaling pathway | (137) |
Extensive research work has been conducted in the direction towards enhancement of various biopharmaceutical and pharmacokinetic attributes of flavonoids. Sandhu et al., demonstrated a 3.5 and 3.3-fold enhancement in the drug release rates and 7.32 and 11.45-fold escalation in Cmax and AUC signifying the improvement in oral bioavailability of both tamoxifen and naringenin from self nano-emulsifying drug delivery system to achieve a synergistic anti-proliferative effect (99). Ragelle et al., demonstrated an improvement in the pharmacokinetic profile, a 24-fold increase in the relative bioavailability and increase in the anti-tumor efficacy of fisetin nanoemulsion in comparison to the free fisetin (100). Aminoflavone (AF) exhibits significant growth inhibitory effects at low doses in human TNBC in vitro models and complex anti-cancer effects. However, the in vivo studies and clinical trials have revealed AF mediated dose-limiting pulmonary toxicity, halting its progress in the clinic. In order to curb this menace, Brinkman et al., developed a unimolecular micellar nanoformulation of AF that displayed an increased in vivo therapeutic efficacy and significant tumor growth inhibitory effect in the xenograft model for EGFR-overexpressing TNBC (101). An increase in the water solubility, bioavailability and permeability across intestinal epithelial cells and anti-tumoral efficacy of Silibinin via encapsulation in niosomal formulation was demonstrated (102). Similarly, the improvement in the anti-cancer efficacy and oral bioavailability by encapsulation in PLGA nanoparticles- hydroxyl propyl beta cyclodextrin complex has also been reported (103). Encapsulation within a liposomal formulation significantly enhanced the inhibitory effect of luteolin on the growth on the CT26 colorectal carcinoma cell line compared with free luteolin (104). Likewise, encapsulation of naringenin in soluthin-maltodextrin-based nanocarrier resulted into a 116-fold increase in oral bioavailability, a 21-fold reduction in the in vitro cytotoxicity along with significant in vivo tumor suppression (105). The in vitro studies of naringenin loaded eudragit nanopartricles exhibited bioavailability (∼96-fold; p <0.05) as well as cytotoxicity (∼16-fold; p <0.001) and in vivo studies demonstrated significant tumor suppression (p <0.01) with subsequent improvement in survival rate compared to free naringenin (106). An increase in the in vitro cytotoxic efficacy of (−)-epicatechin against breast cancer cell lines (MCF-7, MDA-MB-231, MDA-MB-436 and SK-Br3) was reported by Perez-Ruiz et al., due to encapsulation in lecithin–chitosan nanoparticles. The developed formulation displayed a four-fold lower IC50 (85 μM) compared to free (−)-epicatechin (350 μM) and high selectivity to cancerous cells (107).
PNPs are nano-colloidal cargos made up of a wide variety of biodegradable natural or synthetic high molecular weight polymers with size ranging from 10-1000 nm (84). A synthetic polymer usually includes poly-α-cyanoacrylate alkyl esters, polyvinyl alcohol, polylactic acid, polyglycolic acid, and polylactic- glycolic acid. Natural polymers may be further categorized into two groups: proteins and polysaccharides of plant origin and microbial or animal origin. Polymeric nanoparticles have been evaluated as potential vectors for flavonoid delivery in the recent times because their colloidal nature that may help in overcoming diverse physiological barriers, including the gastrointestinal mucosa and blood brain barrier (76, 108-110).
Polymeric micelles demonstrate promising delivery of hydrophilic drugs via oral route. They offer various advantages such as (i) ability to manipulate the hydrophobicity and hydrophilicity within the polymeric system to accommodate a wide variety of drug molecules (Table 3); (ii) guarantee a high degree of sustained release (iii) provides hydrophilicity, biodegradable and non-toxic delivery; (iv) high stability in the GI tract ; (v) preferential uptake by specialized Peyer’s patches (M cells) and isolated follicles of GALT; and (vi) protection from degradation along with economical processing (98).
The past decades have witnessed several advancements in lipids-based nanocarriers. These nanocarriers exhibit better biocompatibility, commercial scalability and adaptability, as well as distinctive mechanisms of absorption contributing to fine-tuning of the oral bioavailability, and avoidance of various physiological barriers such as gastric and colonic pH, intestinal micro flora, hepatic environment, first pass metabolism etc. Monoglycerides, diglycerides, triglycerides, and oils constitute the lipid excipients along with various combinations of phospholipids, sphingolipids, and even high fat meal. Lipid based nanosystems include micelles, solid lipid nanoparticles, nanostructured lipid carriers, nanoemulsions, microemulsions and liposomes that vary from 10 nm to hundreds of nanometers. Stabilization (electrostatic, steric or elecrosteric) acts as a critical factor for the fabrication of the colloidal systems (98).
Micelles are self-assembled colloidal nanostructures (10 to 100 nm in diameter) that are made up of amphiphilic molecules, arranged in core-shell architecture with hydrophobic chains directed inwards making up the inner core and hydrophilic portion making up the surrounding corona. Micelles may take up several forms depending on the conditions and composition of the system. Bioactive moieties can be confined or uniformly distributed either in the core or in the palisade of micelles. Moreover, micellar systems have several other features to be considered as an effective delivery system, such as small size (typically <20 nm), thermodynamic and colloidal stability (98).
The recent couple of decades have witnessed advancements in the emulsion based systems for the conveyance of hydrophobic drugs of which microemulsions and nanoemulsions has garnered significant attention owing to their potential attributes of super solvency, thermodynamic or kinetic stability, miniature droplet size and high industrial scalability (84). Microemulsion, also known as swollen micelle existing in the submicron range, are optically isotropic and thermodynamically stable systems (111) comprising of an oil phase, an aqueous phase, a surfactant, and a co-surfactant. In contrast, nano-emulsions (often called mini-emulsions) are 10 to 100 nm sized oil droplets (smaller than the droplets present in ordinary emulsions) dispersed in aqueous media. Their potential advantageous characteristics such as optical transparency, low viscosity, enhanced functionality, and physical stability has gathered considerable interest in the pharmaceutical field. Stabilization of the systems is achieved by a mixture of emulsifier and co-emulsifying agent. In addition to these, self-microemulsifying and self-nanoemulsifying drug delivery systems, have emerged as successful alternatives to current emulsion based systems for oral delivery of flavonoid. These are composed of homogeneous blend of oil, surfactant, co-surfactant, and bioactive molecule that result in the formation of an oil-in water microemulsion and nanoemulsion upon exposure to aqueous media (112).
SLNs are defined as nano size particles (50-1000 nm) comprising of a solid hydrophobic core, made up of lipids and lipid-like molecules such as triacylglycerols or waxes, surrounded by a phospholipid monolayer and stabilized by means of surfactants as stabilizers. The therapeutically active agents can be either homogeneously embedded in the core of SLNs or localized in shell. SLNs provide a wide array of benefits such as good stability, bio-compatibility, application versatility, target ability by appropriate chemical modification, effective protection of encapsulated bioactive molecules, sustained drug release, and economical scale-up strategies (98).
NLC are modified versions of SLNs with the core containing a combination of both solid (fat) and liquid (oil) lipids at room temperature. These were developed to overcome the problems associated with SLNs, such as improper drug loading, poor long-term stability, and subsequent leakage of drug during storage. NLC have therefore gathered much attention in recent times as potential alternative of SLN due to their attractive traits such as increased encapsulation efficiency, controlled release of therapeutic agents and henceforth improved bioavailability along with maintenance of physical and chemical stability (113-115).
Apart from the aforementioned approaches, better solubility and improved physical and chemical stability of therapeutically active moieties can also be achieved by physical complexation with other molecules. A molecular inclusion complex is characterized by a physical conjugation between the host and the guest (active ingredient) molecule (116, 117).
Cyclodextrins are natural oligosaccharides belonging to a family of molecules that consist of several glucopyranoses bound together to form a ring with a distinctive lipophilic cavity and hydrophilic outer surface that ensure improved aqueous solubility of the complex (enclosing hydrophobic moieties). The CD-complexation has brought about a drastic change in the delivery of therapeutically active lipophilic molecules by safeguarding against degradation and microbial contamination, reducing the incompatibility issues, and improving the availability of bioactive compound at the site of action (116, 118).
Phytosome®, a patented technology, is a vesicular drug delivery system developed to tackle various bioavailability issues and involves complexation between the phyto-constituents with naturally occurring phospholipid. Phytosome® is developed by forming a molecular complex between phosphatidylcholine and plant components (1:1 or a 2:1) through chemical bonds making them highly compatible and highly bioavailable (119). They can minimize the dose due to sustained drug release pattern (120-122).
Extensive work in the recent times have been done for the development of a delivery system with ability to surpass biological barriers; avoidance of premature deactivation of bioactive molecules; and elevation of the intracellular availability of the drugs at their target site (123). Multifaceted cellular regulatory mechanisms mark mitochondria as a critical target for the cancer-prevention strategy. Specific characteristics of mitochondria include mitochondrial trans-membrane electrochemical gradient, protein import machinery, pH difference, and fusion process. These advantages are also being exploited for developing targeted strategies, which are known as mitochondria medicine (124). For mito-targeting of therapeutic agents, approaches involve either loading of drugs into surface-modified nanoparticles or conjugation of the targeting moiety with model drugs. In this context, specialized nano-delivery systems are being utilized such as delocalized lipophilic cations, polymeric NPs, mitochondrial targeting peptide, carbon nanostructures, blended nanoparticles, metal nanoparticles, and mesoporous silica nanoparticles (Figure 7). Uptake of mitochondria targeted nano-carrier is shown in the Figure 8.
 Figure 7.
Figure 7.
Detailed classification of different nanocarriers based strategies for effective flavonoid delivery and mitochondrial targeting.
 Figure 8.
Figure 8.
Schematic representation of the delivery mechanism of different mitochondria-targeted nanocarriers
Liposomes are effective antigen carriers that possess great translational potential for mitochondrial delivery owing to various favorable features such as biodegradability, non-toxicity and non-immunogenicity, and antigen loading properties. Further, these are one of the oldest drug delivery systems and most successful FDA approved therapeutics. Over a couple of decades, liposomes have emerged as the most investigated class of nano delivery systems for the mitochondrial targeted therapeutics (125).
DQAsomes represent as the most standout systems amongst the most broadly reviewed nanocarriers. These are composed of dequalinium chloride molecules that are self-assembled into vesicle-like fashion in an aqueous suspension. These possess the ability to enter cells via endocytosis along with evading endosomal uptake (126).
MITO-Porter is a novel liposome-based approach possessing selective mitochondrial target ability that can encapsulate and deliver various types of cargo molecules irrespective of their size and physicochemical properties via membrane fusion (127). However, the delivery of bioactive natural moieties such as flavonoids by means of MITO-Porter is unexplored which require more focussed research (128).
DLCs are small lipophilic, delocalised positive charge carrying molecules; whose mitochondrial targeting ability in the current scenario is under exploration. Given the lipophilic nature and resonance stabilized delocalization of the charge, they can easily pass through the phospholipid bilayers leading to increased accumulation in mitochondria and can be conjugated to various cargos (including antioxidants, nucleic acids, and spin traps) for mito-targeting purposes. Some of the commercialized DLCs based systems include MitoTracker™ and TPP (129).
Among the available DLCs, triphenylphosphonium (TPP) conjugates stands out as the most extensively studied system for mitochondrial target ability. The TPP cation consists of three hydrophobic phenyl rings surrounding a cationic charged phosphorus atom thereby possessing intrinsic hydrophobicity and mitochondrial selective accumulating properties. Due to this particular attribute, the specificity and therapeutic efficacy can be drastically increased with a concomitant reduction of harmful effects, which is very important to overcome the resistance of some cancer cells (123).
The mito-targeted engineered polymeric nanoparticles demonstrate a flexible application in therapeutics against diseases such as cancer, diabetes, and Alzheimer’s disease. Notably, FDA-approved poly (lacticco-glycolic acid) (PLGA) and polyethylene glycol (PEG)-based materials are used for synthesis of nanoparticle, providing a distinctive edge in terms of biocompatibility. A mitochondrial-targeted polymer based systems with TPP moiety at the terminus using polymer blending technology has been reported that ensure fine tuning of the size as well as surface charge (103).
A variety of amino acid- and peptides-based approaches provide several advantages over DLCs, such as avoiding endosomal and/or lysosomal sequestration leading to their accumulation in the mitochondria, better biocompatibility, as well as straightforward synthesis and amide coupling. These sequences are normally derived from cytosolic synthesized proteins destined for trafficking to mitochondria specified by their Arg, Ala, or Ser rich N-terminal sequence having alternating arrangement of hydrophobic and positively charged amino acids forming an amphiphilic α-helix. Even though MTPs shows noteworthy mitochondrial target ability but their applicability is limited due to retracted generation of soluble and cell permeable conjugates. Numerous synthetic peptides demonstrating mitochondrial targeting activity along with intrinsic pharmacological properties have been reported (123, 130).
In the current scenario, CNTs have emerged as highly versatile and effective nanocarriers for the conveyance of various drugs; but, their role in mitochondrial medicine is still under investigation. Intracellular components target ability of CNTs is currently at its infancy stage. Several carbon based nanostructures for the targeted delivery of bioactive molecules in mitochondria have been evidenced. In an exploratory study, fluorescein isothiocyanate (FITC)-labelled PL-PEG (PL-PEG-FITC)-functionalized SWCNTs (SWCNT-PL-PEG-FITC) was found to be localized at mitochondria in tumor and normal cells in a mitochondrial transmembrane potential-dependent manner (123, 131).
Blended biodegradable polymers is a promising polymer blending technique that provide effective delivery of bioactive molecules to the mitochondria (132).
These include gold, silver, platinum, iron oxide and titanium dioxide based nanosystems ranging within 10 nm size and provide an array of anti-oxidant capabilities along with ease of attachment of mitochondrial targeting ligands. However, their safety and efficacy is one of the major concerns among scientists. Several studies elucidating the toxicity profile of metal based nanocarriers have been reported (133-135). Lately, studies have reported the delivery of plant-based anti-cancer agents by means of metal nanoparticles with enhanced target ability (125).
Mesoporous silica’s are solid porous structure with numerous empty capillaries (mesopores) arranged in a honeycomb-like arrangement that can accommodate bioactive molecules in massive manifolds. These nano-frameworks offer flexibility in tuning the pore size, chemical and mechanical stability, and subsequent biocompatibility; thereby gathering a great attention for the delivery of flavonoids (136, 137).
Several groups have illustrated the combinatorial effects of flavonoids with anti-cancer agents in reducing drug resistance, tumor recurrence and metastasis through nano-engineered systems. The mitochondrial targeting strategy through flavonoids may serve as an effective therapeutic approach to combat cancer stem cells (CSCs). For instance, one such combinatorial synergistic approach using doxorubicin and quercetin was demonstrated by Fang et al., using hyaluronic acid tethered mesoporous silica nanoparticles for gastric carcinoma chemotherapy (138). Signaling pathways such as Hh, Wnt/β-catenin and Notch pathways playing an important role in differentiation of normal stem cells offer an attractive approach to target CSCs. Wu et al., in their earlier study demonstrated anti-proliferative and apoptosis inducing activity of 2’ Hydroxyflavanone (2HF) via blockage of the Akt/STAT3 signaling pathway in prostate cancer. The subsequent studies further investigated the effect of 2HF on EMT, and cell migration and invasion using Wnt/β catenin signaling pathway by suppressing GSK 3 β phosphorylation, β catenin expression and transactivation (104). There have been several reports where the anti-tumor and anti-metastatic effects of flavonoids have been demonstrated. Dai et al., (139) reported the anti-carcinogenic effect of bacailin using tumor xenograft model reported antitumor activity of silybin in human HCC HepG2 using both in vitro and in vivo conditions (140). The results suggested a decrease in the expression of the Notch1 intracellular domain (NICD), RBP-Jκ, and Hes1 proteins, upregulation of apoptosis pathway-related protein Bax, and downregulation in Bcl2, survivin, and cyclin D1. Lim et al., illustrated caused a dose-dependent growth inhibition of various brain tumor cell cultures and neurospheres through curcumin encapsulated in polymeric nanoparticles, effectively blocking the CSC-associated Hh pathway and reducing IGF and STAT3 levels (141).
Several evidences suggesting the use of plant-derived phytoconstitutents in the treatment of chemo-resistant cells have reported. Mohammadian et al., reported significant inhibitory effect on AGS human gastric cell line than free chrysin (142). The study also has elucidated successful restoration of tumor suppressor miRNA expression levels. Minimization or reversion of drug resistance using MCF-7/ADR tumor was well demonstrated by Lv et al., using biotin conjugated doxorubicin and quercetin co-loaded polymeric nanoparticles. The resultant nanoparticle system reported a significant inhibition of the activity and expression of P-glycoprotein and in MCF-7/ADR cells both in vitro and in vivo conditions (143). A synergy in the combinantion therapy of paclitaxel and difluorinated curcumin was observed by Gawde et al., against gynaecological tumors (144).
Mitochondrial stress associated oxidative radical injury may alter cellular epigenome which in turn may initiate the process of carcinogenesis, therefore, an effective protective agent should be capable of maintaining epigenetic stability. Analysis of post-translational histone modifications showed that NP.SB potentially protects B/CMBA cells from epigenetic injury following exposure to pro-oxidants (14). In recent study, we observed that in addition to protecting mitochondria from pro-oxidants induced alterations, encapsulated SB fraction (NP.SB) significantly protects cellular epigenome. Exposure of cells to pro-oxidants resulted in significant epigenomic alterations, as evident from increased methylation of H3K9me1 and H4K20me3 and phosphorylation of H3 and H2A histones. While hyperubiquitination of uH2A/uH2B and hypoacetylation of AcH3 further highlighted the seriousness of these alterations following pro-oxidants exposure. In contrast, no such alterations were observed among cells pre-treated with NP.SB. Altered histone modification pattern modulates and affect DNA methylation machinery, which thus alters the expression of different genes important for cellular integrity and polarity. In addition, studies have shown that dysregulated mitochondrial functioning results in the activation of retrograde signalling cascade which consequently affects nuclear gene arrangements (145, 146). In a recent study, NP.SB pre-treatment regulated DNA methylation at p16 promoter regions even after pro-oxidants treatment. Furthermore, the un-altered levels of let-7a and let-7b in NP.SB pre-treated cells showed the ability of this encapsulated fraction to protect cells from transcriptional dysregulations due to altered histone profile, which were significantly reported among pro-oxidants alone treated cells.
Nutri-epigenomics is an emerging field in terms of dosage, targeting, tissue distribution, bioavailability, molecular and/or cellular interactions that limits the clinical utility of several plant secondary metabolites including flavonoids. Since, cancer is characterized by both genetic and epigenetic instability; it requires multifaceted protective strategies for effective tumour cell targeting while conferring better protection of normal cells from adverse drug-induced toxicity. In this regard, nano-engineered systems encapsulating bioactive flavonoids holds great promise for improved delivery and selective tumour cell targeting with better efficacy and pharmacokinetic properties. Recently, several nanocarrier based approaches, including polymeric nanoparticles, SLNs, liquid crystals, liposomes, and microemulsions have been employed to overcome the limitations associated with clinical use of flavonoids. Overall, nano-engineered systems offer attractive opportunities for the safe, effective, sustained and targeted delivery of flavonoids to reshape the mitochondrial mediated epigenetic regulation for cancer therapeutics. However, challenges such as inquesting their targeting efficiency, meeting up the regulatory international standards and feasibility of scale-up are few concerns which warrants attention for effective bench-to bedside translation of flavonoids as broad spectrum cancer protective agents.
The authors are thankful to Council of Scientific and Industrial Research (CSIR) and University Grant Commission (UGC), Government of India, New Delhi for providing necessary assistance. The award of Junior and Senior Research Fellowships to NB by Department of Biotechnology (DBT), Government of India, New Delhi is gratefully acknowledged.
Abbreviations: ADMET, absorption, distribution, metabolism, excretion, and toxicity; ATM, ataxia telangiectasia-mutated; sATP, adenosine triphosphate; Bcl-2, B-cell lymphoma 2; BNIP3, BCL2 Interacting Protein 3; BRCT, BRCA1 C Terminus; CMC, critical micelle concentration; CSCs, cancer stem cells; DLCs, Delocalized lipophilic cations; DNMTs, DNA methyltransferases; DUBs, deubiquitinases; EGCG, epigallocatechin gallate; EMT, epithelial-mesenchymal transition; EZH2, enhancer of zeste homolog 2; GALT, Gut-associated lymphoid tissue; GATA4, GATA Binding Protein 4; H2Bub, 1monoubiquitination of histone H2B at lysine 120; H3K4, histone H3 lysine 4; HAT, histone acetyl transferase; HDAC1, histone deacetylase 1; HMT, histone methyl transferase; IUIM, inverted ubiquitin interaction motif; KDM, histone methyl transferase; LSD1, lysine demethylates; M cells, specialized microfold cells; MeCP, methyl CpG binding protein; miRNA microRNA; MOF, males absent on the first; MYST, Moz-Ybf2/Sas3-Sas2-Tip60; NAD+, nicotinamide adenine dinucleotide; NICD, Notch1 intracellular domain; NLC, nanostructured lipid carriers; NP.SB, nano-engineered flavonoid rich fraction from Selaginella bryopteris; PARP-1, poly (ADP-ribose) polymerase 1; PD, Pharmacodynamics; PK, Pharmacokinetics; PLGA, poly(lactic-co-glycolic acid; PNP, polymeric nanoparticles; RASSF1A, Ras association domain family 1 isoform A; RNF20, RING finger proteins; RNF40, RING finger proteins; ROS, reactive oxygen species; SAH, S-adenosylhomocystein; SAM, S-adenosylmethionine; SAM/SAH ratio, SAM to SAH ratios; SB.Fr., flavonoid rich fraction from Selaginella bryopteris; SLN, solid lipid nanoparticles; TET, ten eleven translocation; γH2AX, phosphorylated histone 2AX
