Academic Editor: Alexandros G. Georgakilas
This review gathers recent findings in biophysics that shed light on the biological principle of self-organization, spanning from molecules to more complicated systems with higher information processing capacity. The focus is on “feedback loops” from information and matter to an exchange component with a more fundamental meaning than “cybernetic regulation” and “maintenance of homeostasis”. This article proposes that electric and electromagnetic forces are the most important mediators over large distances. Field-like mediation is distinguished from cell-to-cell communication by special electric- or ion-guiding mechanisms that create additional pathways to the “classical” mediators such as nerve conduction or blood flow. Resonance phenomena from phonons and photons in the visible range will be discussed in relation to organelles, cytoskeletal elements and molecules. In this context, the aqueous surrounding of molecules and cells is an important aspect. Many of these phenomena are caused by quantum physics, such as the tunneling of electrons in enzymes or in other coherent working systems. This suggests that quantum information processing is also spread over large-scale areas of an organism.
The present article is a sequel to our previous publication “Biophysical mechanisms complementing “classical” cell biology” [1]. In that paper, we suggested the inherent tendency of nature was to build more and more order out of the surrounding entropy. This general tendency of biomolecules and molecules was described as “active matter” [2, 3].
In this review, we focus on the feedback mechanism behind “pure” information, wherever it originates. We also discuss “pure” matter that can be found in atoms through to the macroscopic world. This represents a third agent besides information and matter and is a driving force behind the negentropic tendency of active matter. The dichotomy of pure matter/energy and information provides the classical distinction between living and non-living systems [4, 5]. At first glance, matter and information appear to be polar entities that are not inter-convertible. However, we later show there is a close relationship between information and matter that upgrades continuously in a coherent and hierarchical order [6, 7].
What could be the third component between information and matter and what makes it living matter, or at least active matter? Here, we examine different inter-related processes between matter and information, as well as the mediators or actors involved in these feedback loops [8, 9, 10].
In the following chapters we discuss how these feedback loops build a ladder of hierarchies that originate at the base of matter and forces per se, and go up to the formation of biomolecules. Hence, the notion of “feedback loops” is more fundamental than “cybernetic regulation” and “maintenance of homeostasis”.
This review will highlight the importance of electric and electromagnetic forces and their interactions within and between cells [11, 12, 13, 14, 15, 16, 17]. Such bioelectric phenomena can be spread over large distances and should be distinguished from cell-to-cell communications that occur via special electric, ionic or other charged molecule guiding mechanisms that form additional ion gradients and electric fields [18, 19]. We will then discuss the cell interior at the molecular level. In addition to the “classical” electric interactions, resonance phenomena from phonons and photons up to the visible range will be discussed with respect to organelles, cytoskeletal elements and molecules. The aqueous surrounding of the cell is an important factor in this regard. In the final chapter we will discuss the communication principles involved, including molecular quantum phenomena. This may explain information processing within living systems not only at the fundamental molecular level, but perhaps also over large-scale areas [20, 21, 22, 23, 24, 25, 26, 27, 28, 29].
In summary, this third component allows steady feedback and feedforward communication in all of the aforementioned scales. Over time, these circular processes represent an up-lifting spiral and an apparent tendency towards higher differentiation. The forces underlying these processes can be basic physical principles, thermodynamics, chemical and “bio”-chemical in nature. Mediators are represented by charges, electromagnetic fields (EMF, photons and quantum processes), and topography such as clustering and compartmentalization.
To start this chapter, it should be emphasized that the terms “entropic” and “negentropic” are difficult to use because living systems and active matter are normally recognized as being open and dissipative systems. In other words, cells are in flow equilibrium where energy can dissipate and where this is governed by the existence of special topography and compartments [4, 30].
We begin with so-called active matter because it provides the best example of how the quality of mediators can move the third component.
The so-called Benard reaction is an example at a more basic scale, with the direct drivers of this reaction being thermodynamic forces [31, 32]. It represents a spontaneous ordering of uniform neutral molecules or particles that were moved passively inside a confined container by an external driving force. The best example of this is the movement of rice grains in a cooking pot: after a while these show a regular pattern with small outlets of steam. Hence, with the right mediator and with outside energy, even absolutely uniform and inert particles can be arranged into a geometric pattern. A more complex example is that of chemical agents that have a reciprocal action on themselves, as in the so-called Belousov-Zhabotinski (BZ) reaction [33, 34]. This reaction is characterized by distinct fields of color-switching molecules, where the front of the color switching reaction moves around. The energy for the reaction is contained within the molecules and in the medium where the molecules react. In the case of a round reaction vessel, spiral-shaped or circular patterns are formed. Moving patterns evolve over time depending on which molecules have been captured by the reaction and how the molecules are arranged in their local neighborhood.
In molecular arrangements involving activators and inhibitors, patterns can arise according to the so-called “Gierig–Meinhardt-Turing model” for self-organizing structures. These include for example the color patterns on seashells, or on the skin or fur of many different species [35, 36, 37].
The group of Vlisek et al. [2] first coined the expression “active matter” for “non-living objects” under specially designed conditions. These include fluidized granular materials, “Janus particles” driven by differences in surface tension, light-pushed colloids, and micron-sized edges or spheres that rotate due to an applied electric field and thus behave as self-propelled particles. All of these active fluid systems exhibit unusual behavior such as the emergence of long-range correlations, super-diffusive behavior, anomalous density fluctuations, and explosive aggregation dynamics. With regard to self-moving active matter, this can explain all of the observed processes at different scales, including the segregation of cells in tissues [2].
A higher level is the coordinated enzymatic interactions of biomolecules. These often lead to reaction circles, which are then linked to further circles (hypercycles) [38]. Because such reactions have specific functions, the order of the reactants also has a specific topography in order to fulfill the tasks in a stepwise fashion. The geometric order and functional neighborhood are crucial for achieving short reaction times.
Along the same lines is the recent finding in eukaryotic cells that so-called biomolecular condensates form according to functional needs in micron-scale compartments. These condensates lack surrounding membranes and concentrate proteins and nucleic acids. They are involved in various processes, including RNA metabolism, ribosome biogenesis, DNA damage response and signal transduction. Interestingly, liquid–liquid phase separation is an important organizing principle in these condensates and is driven by multivalent macromolecular interactions [39] (Figs. 1,2).
 Fig. 1.
Fig. 1.Feedback loops between information and matter during evolution. (a) Relation between information (1) and matter (2) and feedback loops (3) as mediating process. This principle can be favored by environmental cues (like in Benard reaction) or by intrinsic molecular properties (BZ reaction) or in combination. U: upgraded information consolidating the state of matter as well of the related information signature. (b) Relation between information (1) and matter (2) and feedback loops (3) in protocells. Left: information within matter (1, e.g., nucleic acid) and matter as tool (2 translating protein) is incorporated (bow arrow) into a sheltering phospholipid vesicle. 3: process of information/matter feedback, e.g., for replication.
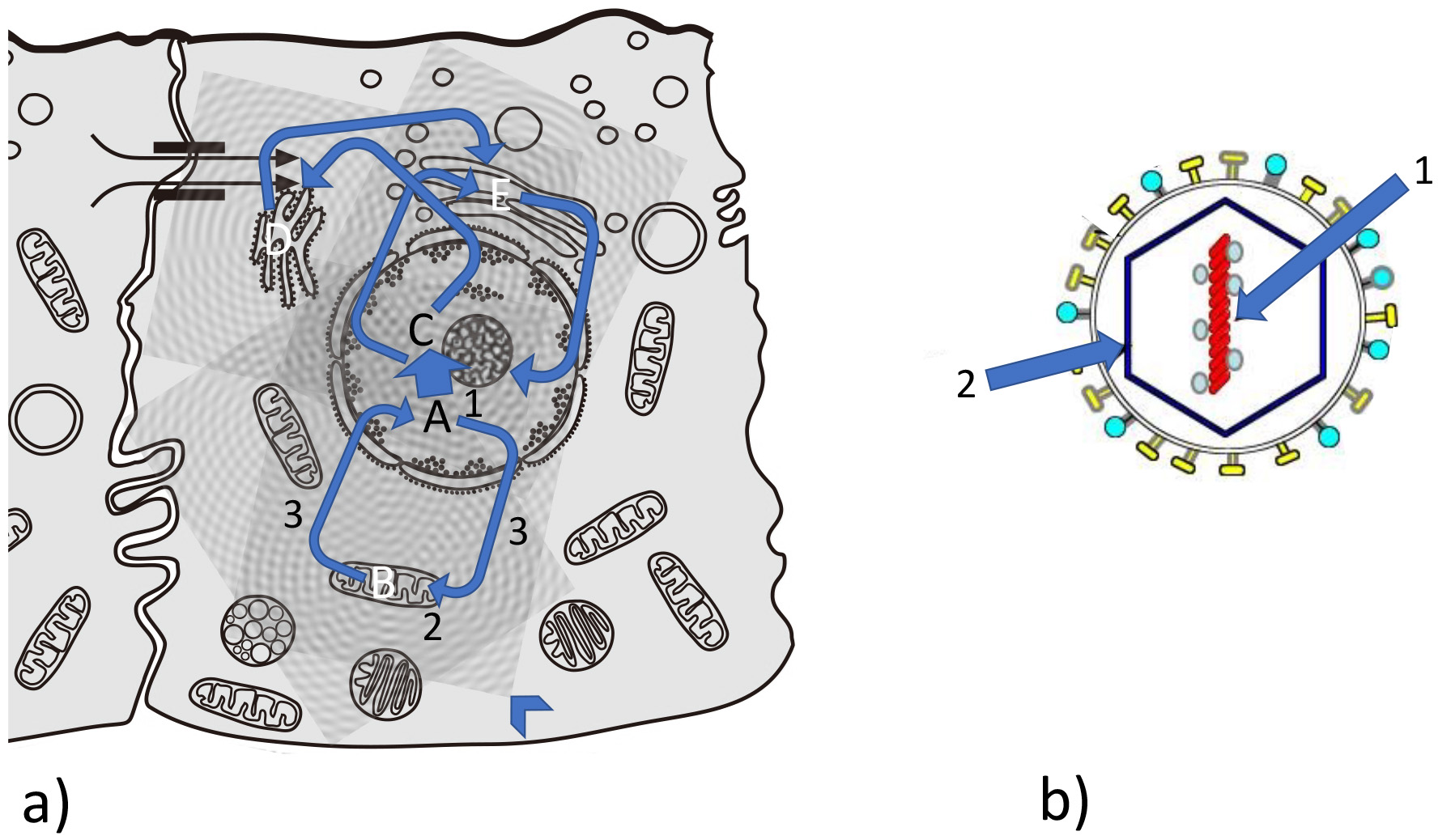 Fig. 2.
Fig. 2.Feedback loops between information and matter during evolution. (a) Complex interrelation between information/matter feedback loops in a eukaryotic cell. Example for information matter loops: A from nucleus to mitochondrion (B); C from nucleus to endoplasmic reticulum (ER) (D); from ER to Golgi apparatus (E) and from and to nucleus. Arrowhead: interference pattern of vibrational patterns coming from organelles and biomolecules (see Fig. 7). (b) Quasi-crystalline situation in a typical virus: information (1) (conserved in nucleic acid) and matter and structure (2) (capsid as well as phospholipid membrane and spikes) is without a self-mediated process (no 3).
This leads to topography as one of the crucial factors in the context of self-organizing structures. With adequate containment, all of the above-mentioned reactions are favored and reinforced by compartmentalization and by the process of “cavity resonance”. This can manifest itself as a substance that reflects from the wall of the compartment, by mechanical (phonon) waves in vibrations such as standing wave resonance, or by the reflection of chemical or electromagnetic waves [40]. In cell cultures, the environment provides the cells not only with mechanical signals and tissue-specific signaling, but it may also improve cell function and proliferation [41, 42, 43]. As an example, the in vitro differentiation of stem cells into hepatocytes is promoted by the extracellular matrix.
With respect to topography and evolution, some authors have suggested that porous material like clay has cavities of an appropriate size that can demarcate various internal processes [44]. These materials provide an ideal background for development of the first forms of life in a narrow sense [45]. This type of surrounding can lead to another feature that was common at the beginning of life on earth - coacervates [45]. Drobot et al. [46] showed that the process of phase separation in mixtures of oppositely charged polymers could provide a simple and direct route for building compartments via complex coacervation. This is important for primitive reactions where longer length RNA is retained, whereas transfer of lower molecular weight oligonucleotides is permitted. Even higher functional complexes are self-constituting as transcriptional droplets. These dissolve a few seconds or minutes after termination of the process by highly negatively charged RNA, thus causing self-regulation via a feedback mechanism [47]. Mediators can be drivers for evolution, as described in the quantum mechanical model of adaptive mutation proposed by McFadden and Al-Khalili [48, 49].
In the context of early life and artificial proto-cells, Fellermann et al. [50] studied whether micelles have the potential to serve as containers for proto-cells and whether they are able to grow in size and divide into daughter cells. They reported that “the ability of micelles to grow depends on the pathway new surfactants are provided: micelles grow only if new surfactants are provided faster than the monomers dissociate from the assembly”.
Lipid membranes can form in micelles or coacervates, thereby representing an evolutionary important step in the development of a typical cell membrane, as found from archaebacteria to eukaryotes [7, 51]. Overall, the presence of cell membranes paves the way for many bioelectric phenomena. Moreover, the arrangement of hydrophilic heads and hydrophobic tails in the lipid bilayer can prevent polar solutes (e.g., amino acids, nucleic acids, carbohydrates, proteins, and ions) from diffusing across the membrane, while mostly allowing the passive diffusion of hydrophobic molecules [52]. This also enables cells to control the movement of such substances via pores and gates (composed mainly of proteins) for ion transport, thus providing selective permeability (Figs. 1,2).
To build a eukaryotic cell, the principle of compartmentalization is expanded to the development of cell organelles with other specialized structures, such as the cytoskeleton and even finer sub-structures in the nucleus (Fig. 2). The Kyoto Encyclopedia of Genes and Genomes database contains the myriad of molecular reactions present in the cell, with all of the metabolic, genetic and general information pathways [53, 54]. Even the best cryo-electron microscope pictures show organelles and larger biomolecules as bare static structures and as components of a complicated machine [55, 56]. As stated by Nicholson [56]: “(the eukaryotic cell)… is a bounded, self-maintaining steady-state organization of interconnected and interdependent processes; an integrated, dynamically stable, multiscale system of conjugated fluxes, collectively displaced from thermodynamic equilibrium”.
To fulfill the interrelated processes of matter and information in eukaryotic cells, evolution has invented myriads of linker-structures to provide feedback mediation between matter and information. The related biophysical processes range from mechanical and phonon-like interactions to electromagnetic and even quantum mechanisms. In this regard, even small molecules represent a type of hub with not only structural and biochemical functions, but also involvement in many biophysical processes including phonon-like and vibrational mechanisms as well as electrical and quantum mechanisms (see below for details).
To examine the vast array of important linker-structures, one can study proteins such as integrins that serve as mediators between the extracellular and intracellular matrix, the intracellular cytoskeleton and the nuclear lamina. The latter anchors many nuclear membrane proteins to other complexes. With regard to information mediation, integrins are involved in DNA replication, transcription, chromatin organization, cell development, cell cycle regulation and others [57].
Integrins do not just serve as mechanical links for connecting the extracellular matrix to the cell membrane and cytoskeleton, but also serve as hubs between cells and their environment. A refined biomechanical regulation process allows integrins to sense and to respond to different physicochemical properties and signals in the environment, including rigidity, composition and spatial distribution. Thus, integrins are involved in major cell functions such as migration and cell division [58].
Within the intracellular matrix, the cytoskeleton and its motor proteins like dynein and kinesin have structural and cargo transporting functions [59]. However, they also mediate important informational aspects, as discussed in detail below.
The above-mentioned active and flowing processes in living cells are not found in viruses, which represent a strange phenomenon at the edge of life. However, viruses highlight the principles of this third component, since information and matter-energy also act in viruses. Viruses have all the information required to replicate, meaning they can build up all of the necessary proteins, nucleic acids and sugar moieties. Even energy is stored in the form of reactive molecules. However, a virus cannot replicate itself without help from outside in the form of prokaryotic or eukaryotic cellular machinery. Left alone in its surroundings, a virus is comparable to dead, non-living matter and is prone to slow degradation according to the laws of entropy, similar to all other nonliving matter. However, viruses are infectious agents that invade bacteria, such as bacteriophages, as well as eukaryotic cells, such as coronavirus [60, 61] (Fig. 2b).
A virus is a parasite that is reduced to the bare minimum but which is still able to replicate with the help of other organisms. Thus, it needs the above-mentioned processes to be performed by the host cells. These are borrowed functions that represent feedback loops involved in greater hierarchies and which are typical for cell-biological functions. The only remnants in viruses are the starting energies required for some processes. These are packed into pre-loaded molecules that allow docking onto the cell or injection of the infectious nucleic acids into the host. They are remnants of the third component that mediates between matter/energy and information.
As cells and multicellular organisms increase in size during evolution, fast
exchange processes that are mediated by electric charges, ion gradients and EMFs
(static electric fields, Ultra-Low Frequency EMFs) become more and more
important. Fast feedback loops can be established using these processes and are
much quicker than those mediated by mechanical pathways (random diffusion, and
directed diffusion through other forces), or by chemical or biochemical pathways.
To illustrate this, the speed of Brownian molecular movement in aqueous solutions
is about 0.1
Typical bioelectric phenomena are formed by ion gradients generated by passive
processes such as semi-permeable membrane barriers, and by channels and active
transporters, both of which lead to an unequal distribution of ions (see below).
The membrane potential is therefore a key characteristic of every living cell and
is the force behind many electrically-driven processes. Every cell type, not just
neural cells, generates a so-called resting membrane potential. Potassium and
sodium gradients are established by specific molecular mechanisms (“ion pumps”)
such as Na
Furthermore, in a multicellular and differentiated organism, the size of the
membrane potential is specific for each cell type and tissue (see below). During
evolution, membrane potential was probably the first guided control system
through the cell membrane. Electrically, the cell membrane acts like a Faraday
cage because the relatively high potential voltage across the membrane (0.05–0.1
V) together with a thickness of only 10 nm results in a potential gradient of
10
Direct EMFs produced by the cell membrane quickly fade away, typically with the square of the distance. They are overlayed by relatively strong electric fields from heart muscle (measured as ECG) and from other rhythmic sources of EMFs such as muscle tissues. These field-like feedback mechanisms can pre-sort body organization in the early embryo as well as the further development of organs at the most important steps [66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78].
In primitive multicellular systems, ionic channels from one cell to a neighboring cell may develop into the predecessor of hormone and nervous systems [79]. In vertebrates, the gap junction (GJ) components known as connexins arise at the 8-cell stage [79] and the GJs themselves help to convey electrical information. Later during evolution of the nervous system, specialized nerve and muscle cells developed the ability to “fire” an action potential, often regarded as the only electrical event in a biological cell. GJs form electric channels from one cell to another and are thus able to form large arrays of electrically coupled cells if these are in contact with each other [65] (Fig. 3). In the retina as well as in the developing brain, nerve cells receive electrical instructions to form their linking pattern. Here, slow electrical waves and microcircuits with electrical currents control the establishment of further connections. These electric currents are transmitted primarily to the neighboring cell membrane via GJs. Even the GJ-connected cells of retinal vessels are driven by this pre-formative electric system [80].
 Fig. 3.
Fig. 3.Eukaryotic (epithelial) cell with bioelectric relevant structures. (a) Schematic drawing of an epithelial cell; GJ and open arrowhead = Gap junction to the neighboring cell; arrows indicate ionic and molecular flow. (b) Detail indicated by arrow: membrane - integral channel with flow (tunneling) of ions in a “decoherence - sheltered” zone. (c) Detail indicated by arrow: polar water molecules oriented according to their charge towards electronegative actin filament.
The typical electrochemical synapses are formed later in the nervous system [65]. This also holds true also for autonomic nerves, which have a feedback mechanism between their sensory and motor parts via networks in the spinal cord. Furthermore, so-called ephaptic (electrical) coupling can occur in adjacent and accompanying nerves [81] (Fig. 4).
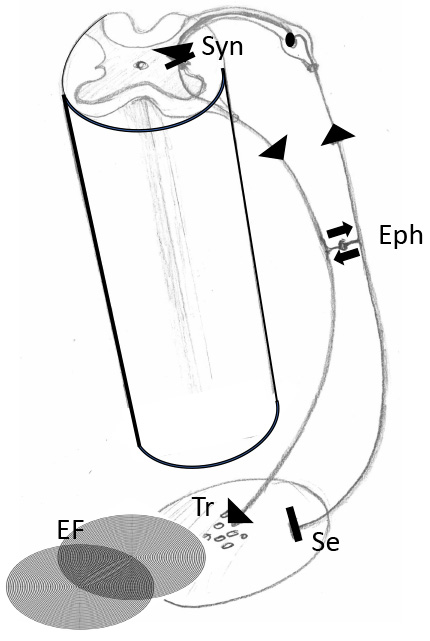 Fig. 4.
Fig. 4.Cooperation between feedback loops (reflex circuits) of the autonomic nerve system and local bioelectric fields. Segment of a spinal cord with transversal section showing the gray substance with sensory input from the periphery via spinal ganglion and connection (synapse) to an autonomic motoric nerve cell. Note that the sensory input comes from a tissue area and can be answered by release of transmitters of the autonomic nerve system. This reaction can interact with local bioelectric fields (EF). Eph, Ephapse, means direct connection between sensory and motoric nerve. Syn, synapse between afferent and efferent nerve fibers; Se, sensory input; Tr, transmitters of the efferent fiber; EF, local electric field.
Autonomic nerves are also actively involved in the development of internal organs through electric conduction along the classical pathways of nerve “cables” and transmitters. For example, the formation of ductal systems in glands is initiated by the neuropeptide VIP, which is released by nearby autonomic fibers. Ducts do not form within the glands if this peptide is removed by appropriate denervation [82, 83].
An interesting aspect of longer feedback loops is that nerve connections between the brain or brain stem and organs during the early embryo phase promote the expansion and differentiation of organs. For example, Levin et al. [84] showed that the arrangement of segments in the developing back muscles of Xenopus is disturbed if the connection to the brain was disrupted during development. In fact, nerve pathways that expand in the early embryonic phase towards muscles (somatosensory) and towards the skin (somatosensory) are involved in early maturation and further differentiation. Irregularities, including a tendency to form tumors, were found after connections to the head (brain) were severed [84]. This also raises the question of how “classical” nerve conduction pathways relate to the above-mentioned bioelectric phenomena.
As in embryologic development, rapid turnover occurs with many cell types in adult organisms [85] and hence fast and informative feedback is mandatory. For example, intestinal epithelial cells (inner lining of the intestine) must be replaced by the same number and type of cells within three days, while other tissues take two weeks (skin surface) or several months (liver cells). This turnover of cells also depends on circadian rhythms [86]. In addition, the membrane potential is an important key regulator in many cell types, with depolarization of the plasma membrane essential for the G2/M transition. As described by Blackinston [87]: “In the normally undulating course of the membrane potential, the beginning of mitosis is characterized by a spike in hyperpolarization before DNA synthesis, followed by a prolonged period of depolarization. This mechanism is seen from early cell divisions in embryogenesis until the normal division of differentiated tissues”.
GJ pathways can span longer distances with the help of phonon-photon coupling. Because everything in the body is in motion, this can elicit EMFs in cells (see below) via GJs, thus providing feedback between the connected parts. Mechanical stimulation may also elicit piezoelectric effects in the bone and so-called streaming potential whereby fluids with charged ions can move. This happens in all instances where the charged ions are separated by the movement. For example, muscle movement is transferred to connective tissues, tendons, and bones. Here, charge separation takes place in cells as well as in the intercellular substance [14]. In blood vessels, the arterial pulse and venous filling add to the “body motion” conduits where GJs can transport this electrical information. Phonon-photon (mechano-electrical) coupling is also possible here [14, 15, 20].
During the last two decades a new type of GJ (electric synapse) communication bridge was discovered in nearly all cell types including vascular and epithelial cells, brain neurons and connective tissue cells such as fibrocytes and tenocytes, a type of fibrocyte located within tendon fibers [88]. These bridges are comprised of long elongations of the cell membrane together with parts of the cytoskeleton and are referred to as tunneling nanotubes (TNTs) [89]. They were long overlooked because of their minute size, with a circular cross-section of just 50 to 200 nm and a length of up to 1 mm. A network of mitochondria with electric conduction spreading via TNTs has also been reported [90, 91] (Fig. 5).
 Fig. 5.
Fig. 5.Cellular connections via tunneling nanotubes within general body fascia and in cell culture. (a) Part of the general body fascia: fibrocytes forming the connective tissue are depicted schematically. Processes with tunneling nanotubes (TNTs) can be found between the cells (arrow). (b) Microscopic picture of two cells connected by a TNT (arrow).
In summary, TNTs may represent a missing link for feedback controls that act over larger distances, such as the fascia or connective tissue surrounding larger conduits like vessels or nerve fascicles.
At the microscopic scale, mechanical and EMF coupling can also occur in the interior of a cell through the action of integrins [92, 93]. Here, the rhythmic push and pull is transferred to the cytoskeleton of the cell, as proposed by the “tensegrity” model [94, 95, 96]. These coupled systems represent a universal and quasi scale-invariant mechanical chain reaction [96]. Stretch-activated membrane channels like PIEZO [97, 98] can also be opened, eliciting concomitant changes in the resting potential. To close this loop, resting potential differences induced by membrane changes can also be propagated, for example by cell-to-cell GJ transfers.
Some workers have described a rich EMF world in the interior of the cell [15, 99]. However, only a few facts are available to build a comprehensive scientific understanding. It is known that mitochondria are characterized by a high membrane potential of about 140–180 mV [100] compared to 70–90 mV for the cell membrane [101]. “Nanopepples” are microspheres with a fluorescence probe. These were used to show that the membrane potential of mitochondria was spread over a larger distance than predicted. This was calculated using parameters for shielding and damping of the stochastic Brownian movement of random water molecules [102, 103].
In addition to their mechanical and transport functions, components of the cytoskeleton may serve as information conduits. Because of its negative charge, F-actin can transduce electric information and attract counterions [104, 105, 106]. Microtubules are also thought to be electric conduits, even superconducting [107] when permittivity and permeability parameters change periodically (Fig. 6). They have been suggested to function as sub-cellular memristors [108] or as memory-switching elements [109]. Microtubules have also been considered as one-dimensional crystals. Because microtubules lose all of their unique vibrational properties without the water channel, it has been suggested the latter controls all optical and electronic properties of the microtubule. This would allow water and ionic flows that travel as current waves, as in water-filled carbon nanotubes [99, 109, 110]. Therefore, as for macroscopic dimensions of the body mentioned above, vibrational modes are also characteristic of intracellular structures.
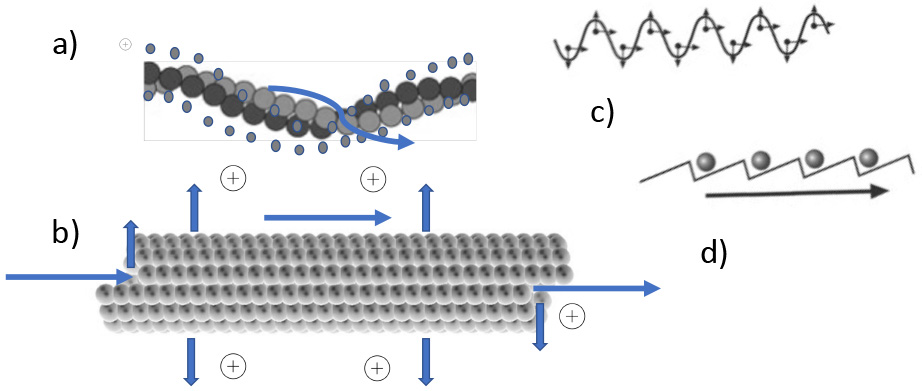 Fig. 6.
Fig. 6.Electric and topographical properties of cytoskeletal elements. (a) Actin filament with electropositive counterions. Cations are in a loosely bound state and can be moved by EMF (winding arrow). (b) Similar situation in the hollow (water filled) microtubules: Arrows depict in - out- and longitudinal movement. (c) Propagating EMF wave. (d) Attraction and rectifying of ionic movement (arrow) along a filament, induced by outside EMF due to molecular ratchets.
In almost all molecules, the side arms and other intermolecular bridges vibrate at specific frequencies. Electric charges are almost always present and are also a source of EMFs [111]. Therefore, reasonably large molecules can be characterized by Raman infrared spectroscopy whereby the specific photon energy is virtually absorbed through excitation of the molecular vibrations that come into resonance. Because of this absorption, the radiation spectrum is altered and hence the type of molecule can be identified. The larger the molecule, the more complex the vibrations and specific patterns observed [111, 112, 113] (Fig. 7).
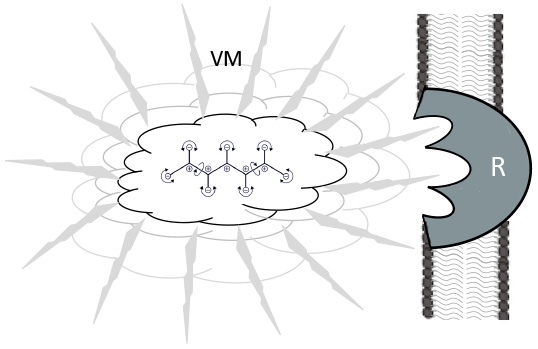 Fig. 7.
Fig. 7.Vibration effects of molecules in a ligand-receptor situation. Jittering and vibrating molecule (VM) by Brownian movement, surrounded by polarized water molecules. These are also propagating the IR - waves for resonant recognition (Cosic) of a membrane bound receptor (R), prior to the “classical” key and lock binding.
This is also the basis for “resonant recognition” [114, 115], which constitutes a resonance emission and a transmission back, as found in radio-frequency identification (RFID) tags [1, 113] (Fig. 7). The triggering force for these vibrations is thermodynamic movement at temperatures above absolute zero. In addition to this are local biochemical reactions that consume stored energy. Molecular vibration frequencies depend on the type of atom, the type of bond, the configuration of the molecular group, and the adjacent molecular groups [111]. Thus, every molecule is characterized by individual patterns, especially in the Terahertz and IR ranges. Interestingly, resonant recognition is prominent between molecules that are involved in a common functional context [112]. Vibration patterns can also induce phonon (soliton–like) wave trains of water and of other molecules to form filament-like structures [1]. In active enzymes centers, vibrational turnover rates of substrates are found in the 0.5 Hz to 1 MHz range [116, 117].
These findings lead to the concept of feedback between biomolecules, based on photons as mediators (bosons) of the EMF.
Photons in biological organisms are often called “biophotons”. However, these biophotons are not only bosons of visible light, but also of IR, THz and all other frequencies up to ULF-EMF. Such photons are also found in the undulating bioelectricity and ULF-EMF of the cell membrane and of internal cell processes. This often leads to a misunderstanding of the meaning of biophotons, due to the first measurements being made with photon multiplier tubes and mostly in the IR and visible light ranges [82, 118, 119, 120, 121, 122].
Photons also come into play as quantum exchange particles. They can emerge from any chemical and biochemical process for extremely short periods of time. However, quantum physics involves the action of virtual photons [123]. They cannot be seen or detected in any way because their existence violates the conservation of energy and momentum. Photon exchange is merely the “force” of the interaction, since interacting particles change their speed and direction of travel as they release or absorb the energy of a photon. Real photons are emitted when the movement of charges is not uniform, i.e., when they are accelerated or decelerated. Ultimately, all atom, ion and charged particle interactions (including in biology) are mediated by real or virtual photons. When two electrons interact, they exchange virtual photons. This means that when two electrons cross paths, they are not regarded as particles but rather as uniform folds in the electric field. Distortions of the respective folds and of the two electrons somewhat repel each other via mediation of the virtual photons [124].
EMFs and photon (including virtual) exchange are always present during biomolecular vibrations, regardless of how small they might be. According to recent findings, typical information transfer within the cell does not occur at the frequencies of visible light [82]. Nevertheless, there is more at play here, as mentioned above.
Indeed, measurements show that biophotons are detectable above the background of
thermal radiation emitted by tissues at their normal temperature [82]. Typical
Planck radiation at 37 °C has an average wavelength of about 9.55
In absolute terms, biological tissues typically produce radiant emittance in the
visible and ultraviolet frequencies that ranges from 10
Although there must be an extra source of photon production in living organisms, it is unclear whether this is solely from energetic molecular processes or from other phenomena such as the production of radical oxygen species [126, 127].
With respect to photon transduction, the intriguing properties of water must be
examined in addition to quantum phenomena (see below). Light suggests that living
systems are universally involved in EMF feedback loops at different scales with
the environment. With regard to sunlight, the coherence times of solar light as
well as the coherent areas of the sun’s radiation are reflected
in metabolic processes and in the topography of living systems. For example, the
spatial coherence area of sunlight was calculated to be about 3.67 -
All of the described features of living systems would not be possible without the medium of water and its special properties. Water occurs in two forms in the cell: as common bulk water and as bound water, which forms the majority. Water is bound to nearly every interface of internal structures, including ions, proteins, membranes and organelles.
Recently, cryo-electron microscopy revealed that the organelles and all of the components of a cell are densely packed with proteins, glycoproteins, lipids, nucleic acids and other “important” molecules (1% of volume). Nevertheless, water molecules fill the remaining gaps between and within these important molecules to occupy the remaining 99% of volume [52, 130]. As mentioned, most of this is bound water, not free (bulk) water. Bound layers [15] are well known in materials science, whereby the charged moieties of molecules form an “exclusion” zone by the bound water [131, 132, 133, 134, 135, 136]. This zone has a thickness of several water molecules and excludes not only bulk water molecules but also ions and other molecules. Bound water has a density of 0.97 instead of 1 for bulk water, much higher heat conductivity, dielectric-related frequencies of 2 GHz compared to 19 GHz [137], and significantly shorter NMR spin lattice and spin–spin relaxation times [138].
Bound or interfacial water therefore has a quasi–crystalline structure, with different electron conducting properties compared to “normal” water [3, 4, 5, 6, 65]. EMFs can thus be transduced for much longer distances than expected in bound water [131, 132, 133, 134, 135, 136, 137, 138, 139]. Due to its relatively fixed binding to the charged moieties, bound water can increase the “EMF antenna radius” of molecules, as well as the radius for vibrational interactions [1]. Furthermore, due to enlargement of the molecular “domains”, the reach out of molecular vibrations is also increased. Important molecules or complexes that are surrounded by bound water can therefore communicate with each other much faster than by random diffusion and the key-lock mechanism (Figs. 3,7).
Szent-Györgyi [140] states: “Each fiber of the living matrix, both outside and inside cells and nuclei, and the genetic material, is surrounded by an organized layer of water that can serve as a channel of communication and energy flow. While electrons flow through the protein backbone (electricity), protons flow through the water layer. Mitchell [141] referred to this proton flow as “proticity”. Various degrees of coupling between electron and proton flows are possible” [142].
Water molecules can form clusters amongst each other, as well as
proton-(Grothues mechanism), electron- and OH-transfer chains [143, 144]. Another
intriguing fact is the inherent property of water to form special domains. This
feature is related to the quantum physics of vacuum fluctuation: in an extremely
small dimension, virtual particles come and go even at zero degrees Kelvin in
what is known as the zero-point fluctuation (ZPF). This happens near the
so-called Planck length of 1.616
The ZPF movement can be transferred into water, since vacuum fluctuations can occur at any wavelength. Even at a wavelength of 100 nm (energy content 12 eV; water absorption maximum at 190 nm), water molecules can briefly be put into an excited state with a certain statistical probability. Although having only a very small effect on a single molecule, the excitation increases with an increasing number of water molecules. Above a certain amount, the effect is so strong that the water molecules pass into a new and energetically stable state and vibrate in time with the electromagnetic wave. This is no longer short-lived but is now stably connected to the water molecules in the area and called a coherent domain (CD) [147, 148, 149, 150]. The size of the CD is attuned to the wavelength of the stored wave, which is about 100 nm. It can be shown using femtosecond lasers [151, 152] that a short-lived electrical vacuum fluctuation can still be captured by a certain amount of matter. Hence, this fluctuation becomes a stable wave that makes the water molecules oscillate in coherence and in which the state is maintained by subradiance [153, 154]. Ions dissolved in water can also form coherent domains, even at low concentrations.
Furthermore, water can exhibit several excited states (Voeikov) and
“super-coherence” may occur up to the micron scale [155]. However, some
arguments against this have been raised. For example: “in liquid water, there
are 10
The quasi-crystalline order revealed by high-pressure cryo-microscopy is quite amazing [157]. Cells are filled to the brim with “important molecules” and contain much more than revealed by classical transmission electron microscopy. How are the connections between molecules made with 10–50,000 reactions occurring simultaneously within a eukaryotic cell? The classical chemical forces such as the Coulomb force and the weaker Van der Waals force are well known and add to the classical covalent bonds via electron orbitals [1].
But what are such orbitals? Here we enter the realm of quantum physics! Orbitals are spaces that describe the probability of an electron’s existence, and which exhibit intertwined forms in molecular reactions [158, 159, 160]. The uncertainty principle according to Heisenberg states that it is impossible to determine exactly where an electron is situated. This is better referred to as the “in-determinability relation” [161]. Importantly, quantum physics provides the most accurate and comprehensive explanation of most scientific phenomena. However, the terms “blur” and “uncertainty” have discredited the entire field of quantum physics in terms of its internal precision. For many years, it was believed that the classical (Newtonian and Einstein-extended) physics of “big” objects no longer applied to the observed phenomena of quantum physics. However, quantum physics can extend to objects of all sizes [124].
Quantum physics can be involved in effects that are mediated by photons over a great distance. If photons have established a quantum relationship between molecules (at the speed of light), then any action on this coupled system will cause an instantaneous change to the quantum state. This is the “pilot wave propagation” described by DeBroglie [162], where a quantum relationship is formed between the molecules, i.e., they are coupled. Incidentally, the pilot waves of light (evanescent photons) are used in “total internal reflection fluorescence” (TIRF) microscopy. Here, the evanescent photons of light “escape” into a range of 100–200 nm around the cover glass of the microscope preparation, thus delineating the outer layers of a cell.
There are some indications that the molecules of a living cell are also subjected to quantum physics. For example, electrons “tunnel” into biomolecules such as enzymes. Tunneling means that electrons can move beyond energy barriers, which according to classical physics they should not be able to skip. This fact has been confirmed many times, as described below. Another strange phenomenon in quantum physics is quantum superposition and the state referred to as entanglement. Superposition becomes shorter in time as an “object” becomes larger. A larger size also increases the probability that an object has contact with other surrounding systems, thus leading to a collapse of the state vector, or so-called decoherence. In living systems with a vast number of molecules in their surroundings, thermodynamics must also be kept in mind [163].
Thermodynamics determines that at room to body temperature, countless molecules perform random (stochastic) movements in accordance with conventional Brownian molecular motion [164]. Large molecular complexes and macroscopically living structures can also be subjected to the “bedrock” of quantum mechanical processes via the thermodynamic teeming of molecules [165]. This occurs because the molecules themselves have the aforementioned tendency towards self-organization and mutual bonding, as well as the ability to undergo molecular resonance (“molecular recognition”, see above [114, 115]).
In regard to the temperature of an organism, there must be an optimum between thermodynamic teeming (leading to decoherence) and the length of coherence. Molecules must not become too “hot”, otherwise many processes (e.g., enzymatic) would not be possible. However, at low temperatures the molecules would remain in coherence for too long and further development of living systems (self-organizing, dynamic, open systems; “dissipative systems”, see above [30]) would no longer be possible [165].
If a coherent or ordered measurement/query is carried out by bosons or electrons (e.g., by EMF rhythms), then such coherence can last longer despite and even with the help of the thermodynamic teeming zone [165]. Baranes et al. [166] used electron microscopy to control the quantum interactions between free electrons and light, thereby creating entanglement and bunching of light. Free electrons can control the second-order coherence of initially independent photonic states, even in spatially separated cavities that cannot directly interact. The authors suggested this also provides a new way to create entanglement at body temperature [167].
This means that “reference waves” of EMF could also be steered into feedback loops connected to “classical” processes, so that quantum mechanics can help to order information as well as energy patterns.
Another way to create quantum mechanical processes is between molecules in protected zones, such as calcium channels. The ions are cooled down (e.g., by interacting with negative channel protein moieties) so much within the channel that they undergo quantum mechanical coupling even at body temperature, as with Bose-Einstein condensation. It has been shown that an ion in a channel behaves more like a delocalized (propagated) wave that is held in the delocalized quantum state for longer [168, 169, 170]. By opening and closing the channels, EMFs can connect with the quantum-coherent ions that travel through the channels [171, 172] (Fig. 3).
As stated by Kim et al. [173]: “…at its most fundamental level of particles, atoms and molecules, biology, life, like everything in the world, is governed by quantum laws. For example, the structure of electronic orbitals and chemical bonds, what are sometimes referred to as examples of “trivial quantum mechanics”, are as central to structural biology as they are to chemistry or physics; yet structural biology is not usually considered to be a branch of quantum biology”.
The tunneling effect as it occurs in enzymes, for example, represents a quantum
mechanical process that is very effective at short distances up to the submicron
scale. In these dimensions, tunneling provides a reliable transfer of protons or
electrons at speeds faster than light, although the exact mechanism remains
unknown [174, 175]. The short length of the hydrophobic gate in quantum tunneling
provides sufficient time before decoherence occurs, since the distance has been
shown to correlate inversely with the time of de-coherence [176]. Superluminal
quantum tunneling allows the turnover rate in enzymes to be increased by up to
10
Recently, the proton pathway in the membranes of myelin sheets and in mitochondrial membranes was traced using fluorescence indicators to follow proton movement. A more lateral rather than transversal proton translocation was found, as postulated by Mitchell’s chemiosmotic theory [178, 179].
Microtubules have also been claimed to be a site for tunneling processes. Here, a bundle of thirteen microfibrils with stacked disulfide bonds should allow delocalization of a common electron orbital down the entire length of the microtubule. This delocalization represents a temperature independent tunneling phenomenon that is regulated as a Josephson junction with Cooper pairs of electrons [180, 181]. Cooper pairs (e.g., electrons) are “particles” that have passed the threshold where they bind. This is despite the so-called Pauli Exclusion Principle that states two particles cannot occupy the same quantum state, meaning they are not entangled. This can occur at very low temperatures and underlies the phenomenon of electric superconductivity (Fig. 6).
Another example for mediating the information in DNA and conveying it to
repressor or restriction enzymes was recently proposed [182]. In view of the
extremely rapid cooperation between proteins and DNA, this process is likely to
rely on quantum signatures, especially because proteins find their consensus
sequence on DNA so quickly. Here, entanglement between
Besides such quantum signatures and tunneling, other examples of quantum phenomena in organisms include photosynthesis and navigation by birds. There are clearly no feedback loops within an organism that has been described as characteristic of living systems. However, they show that a warm and wet organism is able to perform complicated quantum processes and to read classical pathways. The mechanism of bird navigation is based on the production of radical oxygen species elicited by blue light within the retina of the eye. The radicals are produced in photoreceptors (cones) located in the two hemispheres of the eye bulbs. Flavoprotein cryptochrome has been suggested as the receptor molecule [183, 184] because the absorption of photons leads to radical production and the formation of triplets. The unpaired spin is susceptible to magnetic field effects such as the inclination of the earth’s magnetic field. The orientation of the receptors in the two hemispheres is ideal for detecting the magnetic vector orientation. This information is then transmitted from the photoreceptors by the optic nerve to the visual system of the brain. In addition, the magnetic intensity appears to be perceived by magnetite-based receptors located in the beak region and transmitted by the ophthalmic branch of the trigeminal nerve, which ends in the trigeminal brainstem nuclei [184].
A crucial question is how far quantum interactions can reach. Moreover, what types of mediating processes are able to maintain a “living state” in organisms?
The electric properties of microtubules in the cell and the property of wave propagation in such periodically aligned structures has already been mentioned. Considering the complex behaviors of unicellular organisms like amoebae, Penrose and Hameroff argued that although such organisms do not have a nervous system, they actively move towards food and avoid obstacles that are in the way. These authors further speculated that microtubules are not only conventional electric conduits but also have the ability to perform quantum calculations in a kind of proto-consciousness. In their initial work they attributed quantum calculations to the subunits (tubulin proteins) of the microtubules [185, 186]. The now modified Orch Or (orchestrated objective reduction) model [187, 188, 189] uses entangled dipole states that act collectively between the electron clouds of aromatic amino acid rings within the microtubules. These electron clouds work only via femtometer-sized changes in conformation that occur because of nuclear shifts. The molecules are located within “sheltered” apolar pockets and are thus protected from immediate decoherence.
A coherent oscillating system has also been discussed in relation to microtubules and may interact with electrons on delocalized orbitals, thereby storing energy [63].
It has even been claimed that microtubules have superconductive properties, whereby the exchange of information by Josephson oscillations is postulated to explain the processing of information between tubulin subunits [107]. Josephson oscillations are the periodic, macroscopic phase-induced collective motions of quantum condensates (e.g., Bose-Einstein condensates) normally seen at cryogenic temperatures [190].
Quantum calculations are assumed to occur within protected and shielded regions, meaning that non-polar pi resonance regions are shielded from charges in the polar cytoplasmic environment [191]. Posner molecules are perfect candidates for this. These molecules are spherical calcium phosphate nanoclusters that can protect the nuclear spin of qubits in the phosphorus atom, thus enabling the storage of quantum information in the direction of membrane proteins or microtubules [192]. Such systems can easily resonate and are shielded from the warm and noisy surrounding of the cell. With relatively few elements that are “slightly shiftable”, such as the spin of electrons, coherence times of many minutes or even longer are possible.
Quantum calculation properties have been ascribed to Ca
To prepare quantum calculations and to have a read out, such resonating systems must have a “reference beam” like a corresponding EMF frequency. Only then can information be transferred into “harder” classical systems like action potentials. In nerve cells and in the aromatic amino acids of microtubules, collective oscillations were found in networks of London-force dipoles (among the pi electron resonance clouds of aromatic amino acids) in the range of 480 to 700 THz [200]. These frequencies may be transposed down to the MHz range and a final read out may occur via the frequencies of action potentials, as measured by EEG [57]. Therefore, only relatively few and extremely small (atoms, electrons, etc.) elements are useful in quantum relationships [165, 193, 194].
With regard to brain nerve cells, the nonlinearity of the synaptic system (i.e., “quantal” delivery of transmitter vesicles) and the large number of metastable states of the brain helps with quantum processes. As for the cell interior, processes such as stepwise electric conduction in actin and microtubule filaments, as well as molecular (Brownian) ratches, can help to convert subtle changes into patterns that are better suited for reliable read out [1] (Fig. 6). Due to thermal movement, stochastic resonance is also able to strongly amplify weak signals [1]. Ultimately, a layered system of quantum processes is followed by decoherence and read out into classical (e.g., EMF) systems, followed by a repeat phase of quantum calculation and decoherence. Görnitz [124] refers to this feedback process as the “layered architecture of reality” [201] (Fig. 8). Similarly, Salari et al. [202] asks: “What is the meaning of classicality when a large or complex system (as a quantum system) collapses to become a classical entity while the components (atoms or molecules) are still quantum mechanical?”.
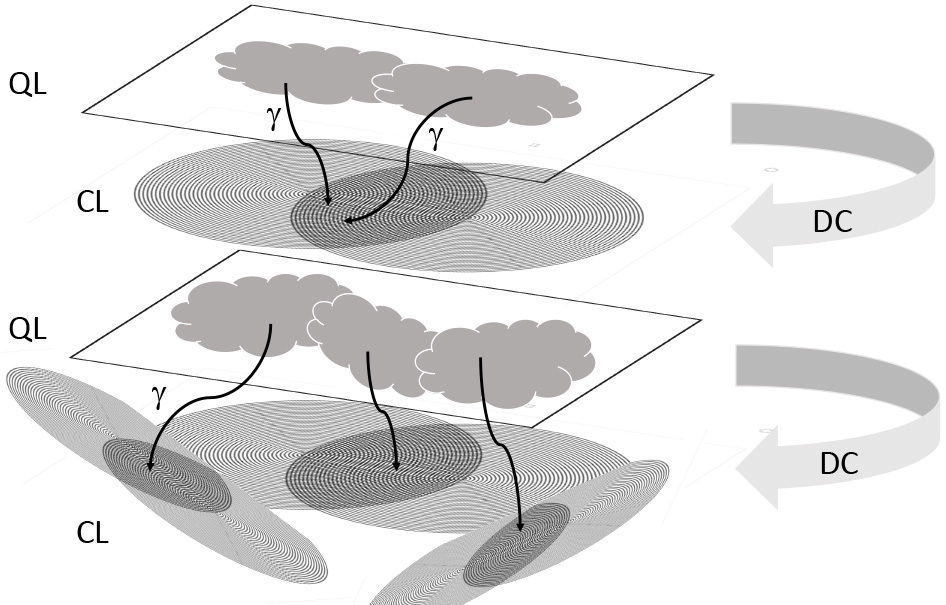 Fig. 8.
Fig. 8.Layered architecture of information processing between quantum
processes and decoherence into “classical” EMF patterns. Subtle processes at a
quantum “layer” (QL) are followed by decoherence (DC) and read out by photons
or bosons in general (
All of these processes are perhaps fundamental not only for the brain but also for other organs and organ systems, presumably connecting them with each other. However, much more research work is still needed on this topic.
From our present understanding, it appears that many exchange processes occur at different levels of information and matter. This begins with the deepest level of quantum fluctuations and the resulting vacuum EMF, and proceeds to molecular vibrations and related EMFs. With regard to cell organelles and the cell, information for feedback can spread within the cell and then via cell-cell connections or widespread EMFs to reach entire organs and organ systems. This occurs in conjunction with the well-known classical EMF pathways of the nervous system, both in the somatic and autonomous branches.
For brain and body awareness, Jerath et al. [203] proposed a model of layered oscillatory architecture for EMF frequencies. This architecture constitutes a global bioelectric continuum, rising to the emergence of consciousness. Quantum processes in the cell as well as ionic and plasma oscillations of water generate fields that interact with other electric fields. These EMFs can be associated with membranes, biopolymers and water polarization coherence domains [148, 149, 150, 151, 152, 153, 154, 155, 156, 157, 158, 159, 160]. In this context, Geesink and Meijer [6] proposed the model of a semi-harmonic pattern of biologically significant EMF frequencies that organize local and non-local states by quantum entanglement [204]. In view of the myriad of processes that occur simultaneously in a cell, this must be a concerted action (similar to interference patterns) and should not represent crowded, non-coordinated teeming.
In summary and upon consideration of all the findings discussed above as well as the statements regarding “quantum hypotheses”, it is possible that electrical activity and photons (including virtual ones, i.e., all bosons in general) “bring everything together” [173]. This could occur via a hierarchy of feedback loops in the brain, thus forming the basis for consciousness and providing a “whole body feeling” (super-coherence [150]).
With regard to the proposed mediators of feedback loops in living systems,
bosons per definition mediate information and forces between fermions,
which are the basic building blocks of matter. The notion of bosons as the third
component and interaction partner highlights the beauty of fundamental knowledge
in physics. The relationship between information and matter in quantum physics
was calculated by Görnitz [124]. A photon is represented by 10
RHWF laid out the manuscript design and made all the writing as well as the figures.
Not applicable.
Not applicable.
This research received no external funding.
The author declares no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
