Academic Editor: Hongwei Yao
The aim of this review is to highlight the beneficial attributes of flavonoids, a diverse family of widely-distributed polyphenolic phytochemicals that have beneficial cell and tissue protective properties. Phytochemicals are widely distributed in plants, herbs and shrubs used in traditional complimentary medical formulations for centuries. The bioactive components that convey beneficial medicinal effects in these complex herbal preparations are now being identified using network pharmacology and molecular docking procedures that identify their molecular targets. Flavonoids have anti-oxidant, anti-inflammatory, antiviral, antibacterial and anti-cancer properties that have inspired the development of potent multifunctional derivatised flavonoids of improved efficacy. The antiviral properties of flavonoids and the emergence of the severe acute respiratory syndrome (SARS-CoV-2) pandemic has resulted in a resurgence of interest in phytochemicals in the search for efficacious compounds that can prevent viral infection or replication, with many promising plant compounds identified. Promising semi-synthetic flavonoid derivatives have also been developed that inhibit multiple pathological neurodegenerative processes; these offer considerable promise in the treatment of diseases of cognitive decline. Clinical trials are currently being undertaken to evaluate the efficacy of dietary supplements rich in flavonoids for the treatment of virally-mediated diseases. Such trials are expected to identify flavonoids with cell and tissue protective properties that can be harnessed in biomedical applications that may serve as supportive adjunctive procedures to conventional anti-viral drug therapies against diseases such as COVID-19.
The aim of this review was to highlight the tissue and cell protective properties of flavones and chalcones as anti-viral compounds that prevent SARS-CoV-2 infection and replication through inhibition of key enzymes of the viral genome such as RNA-dependent RNA polymerase (RdRp), 3CL main protease (3CL Pro
Plants containing beneficial flavonoid polyphenolic antioxidant, anti-inflammatory neuroprotective compounds have been used in traditional complimentary medicine for thousands of years [4, 5, 6, 7, 8]. With the emergence of the SARS-CoV-2 pandemic, a search of compounds displaying SARS-CoV-2 inhibitory activity from literature searches of ScienceDirect, PubMed, Scopus, and Google Scholar databases in 2021 supplemented by data from articles in community surveys, case reports, and articles describing the use of antiviral herbal medicines in Traditional Chinese, Vietnamese, Thai and Indian Asian medicine has uncovered a number of promising plants and efficacious anti-viral compounds [6]. A total of 91 plant taxa contain anti-viral compounds with potency against SARS-CoV-2. Advanced screening and activity profiling of these compounds using in-silico computational docking simulations and X-ray crystallography have been undertaken, as well as assessment of their bioactivities in SARS-CoV-2-infected VERO cells. In vitro biochemical analyses of their enzyme inhibitory activities has further confirmed their potential. Network machine learning has also been employed to identify anti-viral compounds, their efficacy and molecular targets, and to search for foods rich in these compounds [9]. A large range of dietary phytochemicals have been identified; a few selected examples of these are presented in Table 1 (Ref. [6, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19]).
| Flavone sub-class | Flavonoid/chalcone examples | Food Source | Ref |
| Flavonol | (+) catechin/(-)epicatechin, epigallocatechin | Green and black tea | [10, 11] |
| Flavone | luteolin, rutin, chrysin, apigenin | red wine, capsicum, fruit skins, buckwheat | [12, 13] |
| Flavonol | kaempferol, quercetin, myrecetin, tamarixetin | red wine, onion, olive oil, red-black berries, grapefruit | [12, 13, 14] |
| Flavanone | naringin, naringenin, taxifolin, hesperidin | Citrus fruit flesh and skin | [15, 16, 17, 18] |
| Isoflavone | genistein, diadzin | Soybean | [19] |
| Chalcone | Panduratin A | Pomegranate, citrus flesh and peel, SE Asian medicinal herbs | [6] |
The chalcones and flavones are a diverse group of polyphenolic heterocyclic organic phytochemicals with roles in the defense of plants from parasites and are volatile scented insect attractants that promote pollination, provide flower colouration, protect plants from damaging UV radiation, and provide temperature stress properties to plants [20]. Flavone and chalcone compounds are useful therapeutic components in plant and herbal preparations that have been used in traditional Thai, Chinese, Ayurvedic and Australian First Nation medical practices for centuries [21, 22, 23]. Flavones and chalcones display anti-inflammatory activity through the inhibition of lipoxygenase (LOX) and cyclooxygenase (COX) activity, and regulate nitric oxide and prostaglandin tissue levels. They also suppress nuclear factor (NF)-
The structure and ring numbering systems of chalcones and flavones are shown in Fig. 1. Hybrid chalcone-flavone desmoflavans have also been identified (Fig. 1). Chalcones and flavones occur in plants as glycoside and aglycone forms. Two examples of these, namely rutin and hesperidin, are illustrated in Fig. 2g,k. The related Sofalcone and metochalcone are also shown (Fig. 2m,n). Dietary flavone and chalcone glycosides are converted to their aglycone forms when ingested and are then conjugated to glucuronic acid to form 7-O- and 3-O-glucuronate glycoforms. These are the forms that circulate in plasma. The 7-O-glucuronate glycoform is more bioavailable than the 3-O-glycoform. Hesperitin-7-O-glucuronate is an active pharmacologic flavonoid that exerts hypotensive, vasodilatory and anti-inflammatory effects on the endothelium, similar to the hesperetin aglycone however the 3-O-glucuronate glycoform is less active [28]. The increased bioavailability of hesperitin-7-O-glucuronate leads to an improved prevention of bone loss in ovariectomised rats [29].
 Fig. 1.
Fig. 1.
Comparison of the generic structures of chalcone and flavone showing their ring numbering system. The structures of flavonol and flavanone are also shown and cyclohexenyl chalcone (Panduratin) and the hybrid desmoflavan A and B. The reactive
 Fig. 2.
Fig. 2.
Flavone and Chalcone diversity. Structural depiction of the glycoside and aglycone forms of luteolin (a,b), baicalin and baicalein (c,d) and scutellarin and scutellarein (e,f). Structure of the glycoside form of quercetin (rutin) found in plant tissues (g) showing how it is converted to the aglycone form (h) when ingested and conjugated with glucuronate in plasma (i,j). The glycoside form of hesperidin (k) and its aglycone form, hesperitin (l) are also shown and two further licenced forms of hesperidin, metochalcone (m) and sofalcone (n). The rutinose (6-O-
Flavonoids are a diverse group of phytochemicals that have been grouped into families based on their structures (Fig. 3). Peterson [30] screened 72 flavonoids for their ability to interact with the SARS-CoV-2 3CLPro protease main active site using in-silico molecular docking. The 14 best inhibitors were listed (Fig. 4, Ref. [30]), with further studies confirming the inhibitory activity [24, 31, 32]. The IC
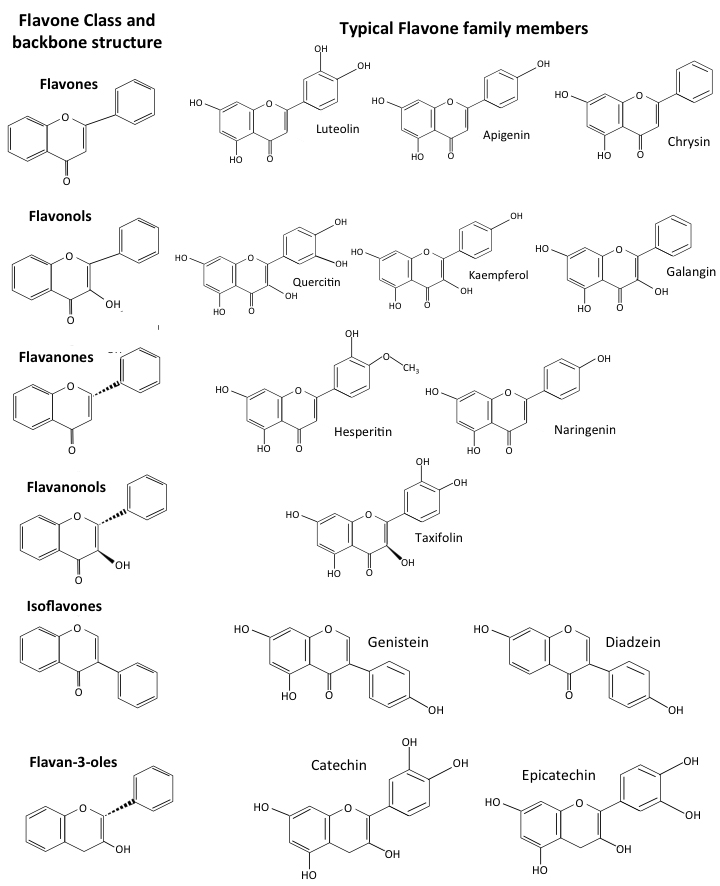 Fig. 3.
Fig. 3.
Comparison of the structures of flavonoid forms showing the generic structures of flavone, flavonols, flavanones, flavanonols, iso-flavones and flavan-3- ols and representative members.
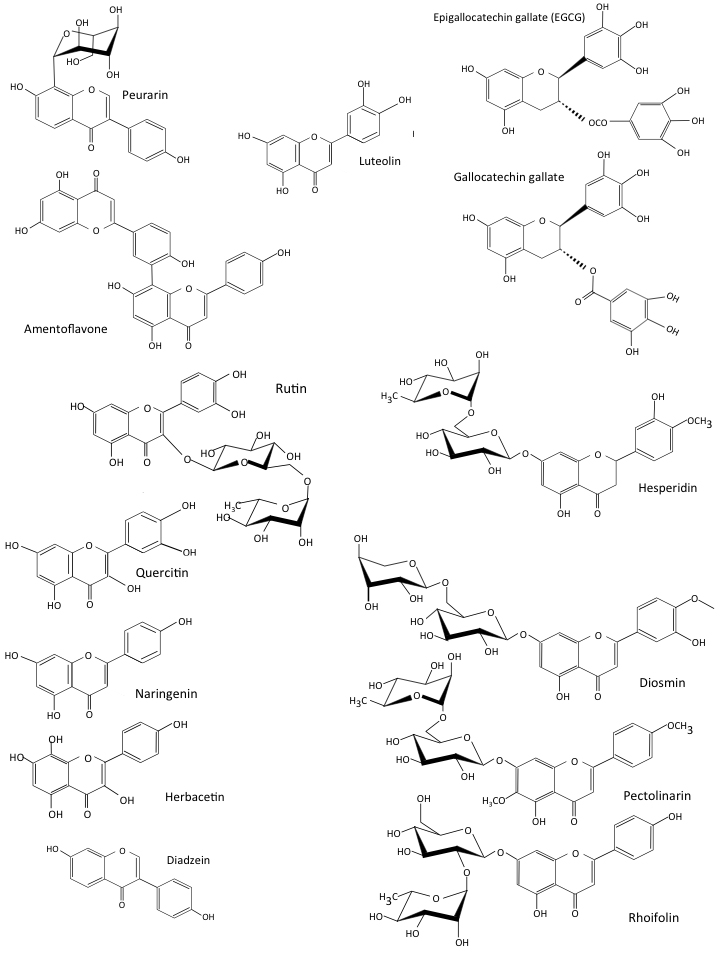 Fig. 4.
Fig. 4.
The 14 most inhibitory flavones identified by In-silico molecular docking procedures of 72 inhibitory COVID-19 flavonoids [30].
 Fig. 5.
Fig. 5.
Inhibitory flavonoids. Structures of selected flavonoids that display inhibitory activity for 3CLPro of CoV-2 (a) and their IC
Chalcones consist of a ketone composed of two aromatic rings linked by an aliphatic carbon bridge containing two unsaturated carbonyl residues [35]. Conjugated double bonds in these ring structures and a de-centralised Pi-electron system which can donate or accept outer shell electrons, make these compounds highly interactive. Natural and semi-synthetic chalcones and flavones are of considerable interest as therapeutic agents in biomedicine. The central unsaturated alpha and beta carbonyl residues in the chalcones which attach its two aromatic rings together are interactive with bioactive function-defining cysteine residues in proteins. Many of the flavonoids induce Nrf2 (nuclear factor erythroid-related factor-2) expression and are interactive with androgen and oestrogen receptors (ARs, ERs), peroxisome proliferator-activated receptor (PPAR-
Chalcone [(2E)-1, 3-diphenylprop-2-en-1-one] is an important scaffolding molecule amenable to derivatization with a diverse range of functional groups through varied linkage chemistries, making it a key intermediate in the synthesis of new and more efficient drugs that are of major importance in medicinal chemistry. Chalcone is considered a privileged structure and represents a template that can be used to synthesize compounds displaying a wide range of pharmacological activities, including anti-inflammatory, anti-microbial, anti-oxidant, anti-viral, anti-diabetic, anti-malarial and cytotoxic anti-tumor activities [38, 39]. Novel chalcones have been synthesised with CNS receptor interactive properties that equip them with anti-anxiety, anti-depression and analgesic properties [40]. Chalcones with vasodilatory properties [41], anti-hypertensive, anti-anginal, anti-arrhythmic and cardioprotective agents have also been developed.
SARS-CoV-2 3CLPro has major roles to play in viral replication and is inhibited to a variable degree by many natural plant compounds. These include biflavonoids [42], flavonoids [42, 43, 44], isoflavones [44], triterpenes [45, 46], phyto-sterols [47], lignans [46], indole alkaloids [44, 48, 49], glucosinolates [44], anthraquinones [50], phenanthrenes [51], phloro-tannins [52], chalcones [53], diaryl heptanoids [46], and propanoids [54]. Of all plant anti-viral studies that have been conducted, those on flavones and chalcones represent almost half of all studies so far conducted [reviewed in [55]]. It was beyond the scope of this review to examine all the aforementioned anti-viral phytochemicals (
Hesperidin methylchalcone, metochalcone and sofalcone are currently licensed for clinical use (Fig. 3). Hesperidin methylchalcone has vasodilatory properties and has been used to treat venous insufficiency for five decades. Metochalcone and Sofalcone are useful in the treatment of Helicobacter pylori-induced gastric inflammation and have been used for decades to treat gastritis and gastric ulcers in Japan [1, 2, 3].
Hesperidin is a member of the chalcone sub-category of plant flavanones, and is composed of an aglycone (hesperitin) linked to a disaccharide (rutinose). Hesperidin and hesperitin occur naturally in citrus fruits [56]. Hesperetin is reported to interact strongly with membranes; hesperidin may be sterically hindered in such interactions due to its rutinose side chain [57]. In a double-blind cross-over clinical trial in healthy volunteers, enzymatic removal of rutinose from hesperidin improved its bioavailability [58]. Rutinose is a 6-O-
Several metochalcone analogues display potent activity against drug resistant forms of Helicobacter pylori, inhibiting cellular adhesion and invasion of gastric epithelial cells. Metochalcone reduces H.pylori-induced gastric inflammation by reducing NF-
Sofalcone also has mucosal protective properties, inhibits growth of H. pylori and has been used to treat gastritis and gastric ulcers in Japan for decades. These protective properties stem from activation of the cytoprotective and anti-inflammatory nuclear factor-erythroid 2 (NF-E2) p45-related factor 2 (Nrf2)-heme oxygenase (HO)-1 pathway [68]. Sofalcone disrupts binding of the Kelch-like ECH-associated protein 1 (KEAP1), a cytosolic repressor of Nrf2 activation [68, 69] and increases VEGF via an Nrf2-HO-1 dependent pathway in gastric epithelial cells [70]. KEAP1 is a tumor and metastasis suppressor gene [71]. Sofalcone has been used to treat pre-eclampsia, where the cytoprotective and anti-inflammatory Nrf2-HO-1 pathway is induced in primary trophoblasts and human umbilical vein endothelial cells (HUVECs) [72]. Sofalcone promotes nuclear translocation of NF-E2 and transactivation of NF-E2 responsive genes, decreasing secretion of soluble fms-like tyrosine kinase-1 (sFlt-1) and endoglin by primary human trophoblasts. This potently suppresses endothelial cell dysfunction, blocks TNF
With the emergence of the coronavirus pandemics of the last five decades, plant extracts have been extensively screened in the search for phytochemicals that impede viral infection and replication [73]. Many plant flavones and chalcones display properties that block viral attachment to host cells while others specifically target enzymes responsible for viral replication. Hesperidin, quercetagetin, and myricetin are examples of phytochemicals that strongly bind to the active site of RdRp, inhibiting its enzymatic activity and viral replication [74, 75]. In-silico molecular binding studies have also identified a number of flavonoids that interact with the catalytic site of SARS-CoV-2 3 CL Pro inhibiting its enzymatic activity [24, 30, 31, 32] and Spike-ACE2 interaction. They can also inhibit helicase and topoisomerase [76, 77, 78, 79, 80, 81]. The RecQ helicase family (nsp13) unravel double-stranded DNA, producing ssRNA required for viral replication, transcription and translation. They also facilitate DNA repair from UV light damage through recombination processes that maintain genomic stability and integrity. A number of flavones that inhibit helicase also disrupt SARS-CoV-2 replication [82, 83, 84]. Anti-tumor studies with flavones have found many that inhibit topoisomerase I and II [85, 86, 87, 88].
Network machine learning has also been applied in the design of new SARS-CoV-2 drugs and the re-purposing of existing drugs for the treatment of SARS-CoV-2 [89, 90, 91, 92, 93]. Advanced computer software was developed to investigate molecular docking events in SARS-CoV-2 interactions with anti-viral compounds [94, 95, 96]. This methodology facilitated a systematic analysis of the interactive chemical determinants of anti-viral phytochemicals that determine SARS-CoV-2 spike glycoprotein interactions [97]. These functional interactive groups on phytochemicals can be modified to obtain a more efficacious anti-viral compound [98]. Chalcones and flavones are amenable structural templates for the synthesis of phytochemical libraries of varied structure to evaluate viral binding. AI-based computational simulation for drug design and large-scale inhibitor screening have also been applied to optimize such evaluations [99]. Homology modeling studies and in-silico studies employing advanced computational molecular docking software and x-ray crystallography have identified phytochemicals that interfere with the Spike glycoprotein interaction with the human ACE2 receptor [73]. The identification of TMPRSS2 (transmembrane serine protease 2), TMPRSS4 and furin cleavage sites in the Spike glycoprotein, which prime it for fusion with the host cell plasma membrane, have identified further targets of interest in anti-viral strategies. Phytochemical inhibitors of TMPRSS2 and furin have also now been identified [100].
Coronaviruses (CoVs) are enveloped viruses of the Nidovirales order, Coronaviridae family. Bats, dogs, cats and humans can all be infected with these viruses [101]. Seven species of CoVs have so far been identified, four of these produce relatively mild symptoms of the common cold [102]. Severe acute respiratory syndrome (SARS-CoV), Middle East respiratory syndrome (MERS-CoV) and SARS-CoV-2 induce high impact life-threatening diseases [102]. The appearance of the SARS-CoV pandemic in 2002–2003 resulted in 774 deaths and 8098 cases of infection in 26 countries. Ten years later, MERS-CoV emerged as a sixth coronavirus. Infections with this virus across 27 countries in the Middle East, Asia, North Africa and Europe resulted in 2040 infections and 712 deaths. The emergence of a seventh coronavirus (SARS-CoV-2) has lead to the COVID-19 global health pandemic. SARS-CoV-2 is closely related to SARS-CoV but is far more infectious and has significantly greater health consequences. As at 8 June 2022, more than 535 million SARS-CoV-2 cases and 6.3 million deaths in 223 countries had been reported (www.worldometers.info/coronavirus/). A highly infectious delta variant (B 1.617.2) of SARS-CoV-2 emerged in India in 2020 and rapidly became the dominant strain. On 24 November 2021, a further highly infectious SARS-CoV-2 variant (B.1.1.529/BA.1) was reported, which has had a significant global impact [103]. The World Health Organization Technical Advisory Group on SARS-CoV-2 Virus Evolution designated this B.1.1.529, the fifth coronavirus variant, and named it Omicron [104]. This is the most infectious form of SARS-CoV-2 so far identified. Of major concern are the 32 mutations in Omicron located within its Spike protein with 15 of these located in the receptor binding region [105]. The high infectivity rate of Omicron suggest that it uses an extensive range of cell surface binding sites in addition to the ACE2 receptor and neuropilin-1 (Nrp-1) on host cells to effect infection of host cells. Fig. 6 depicts the structure of a SARS-CoV-2 viral particle, its genomic organization and the open reading frames (ORFs) that encode non-structural proteins (Nsps) that have important roles to play in CoV-2 replication.
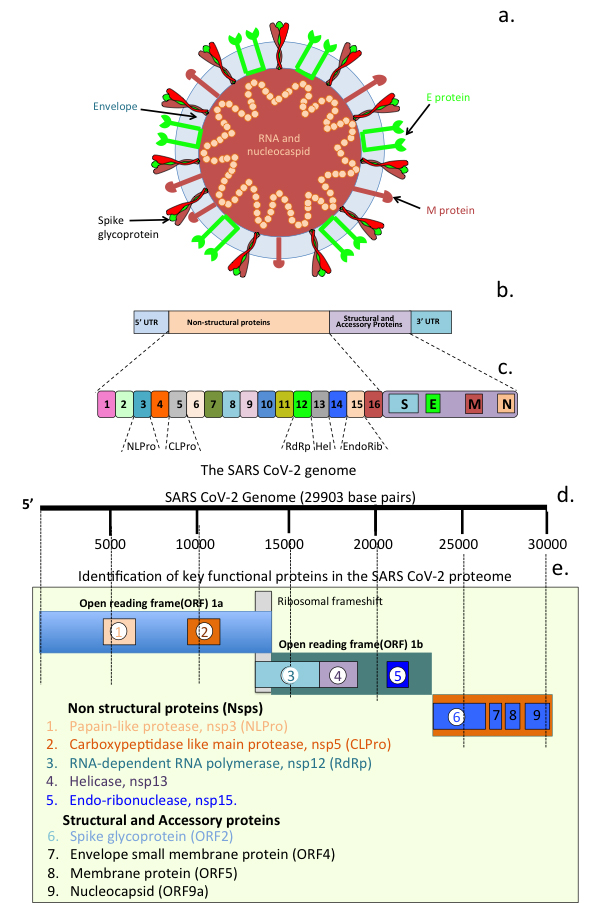 Fig. 6.
Fig. 6.
SARS Cov-2 structural organization and its genome. Schematic of a SARS-CoV-2 viral particle showing the structural organization of the nucleocapsid and viral RNA, viral envelope and Spike glycoprotein (a). Viral genomic organization (b) and open reading frames (ORFs) showing regions encoding the major non structural proteins (Nsps 1-6) and viral particle structural and envelope small membrane, membrane and nucleocapsid accessory proteins 7-9 (c).
In order to enter cells, viruses need to attach to and activate envelope glycoproteins by host cell proteases. Host cell surface TMPRSS2 plays a crucial role in the activation of SARS-CoV-2 spike protein, facilitating the rapid infection of these cells [106]. This activity of host cell proteases is essential for viral infectivity and constitutes a logical target for therapeutic intervention to prevent infection. Host cell entry is the first step in the viral life cycle with the Spike glycoprotein binding to host cell receptors, conformational reorganisation of the S1 sub-domain upon internal cleavages in this region by TMPRSS2 or furin facilitate the fusing of the viral membrane with the host cell plasma membrane to effect host cell entry. The SARS-CoV Spike protein is also the major target of the neutralizing antibody response of SARS-CoV-2 vaccines. ACE2 is the primary host receptor for SARS-CoV-2 and SARS-CoV however these related viruses have vastly different infection rates, suggesting the involvement of factors in addition to ACE2 that promote SARS-CoV-2 infection. Neuropilin-1 (Nrp-1) is another host cell receptor that SARS-CoV-2 uses for cellular attachment. Nrp-1 is processed by furin exposing a C-end rule motif (CendR) that binds to the SARS-CoV-2 spike protein and is internalised by endocytosis [107, 108, 109, 110]. The even greater infectivity of the Omicron CoV-2 variant is highly suggestive that this viral form may utilise additional host cell surface proteins to effect host cell infection that have yet to be identified.
Table 2 [6, 22, 27, 65, 84, 111, 112, 113, 114, 115, 116, 117, 118, 119, 120, 121, 122, 123, 124, 125, 126, 127, 128, 129, 130, 131, 132, 133, 134, 135, 136, 137, 138, 139, 140, 141, 142, 143, 144, 145, 146, 147, 148, 149, 150, 151, 152, 153, 154, 155, 156, 157, 158, 159, 160, 161, 162, 163, 164, 165, 166, 167, 168, 169, 170, 171, 172, 173, 174, 175, 176, 177, 178, 179, 180, 181, 182, 183, 184, 185, 186, 187, 188, 189, 190, 191, 192, 193, 194, 195] reviews the properties and mode of action of a selected number of naturally-occurring plant flavones, chalcones and analog derivatives.
| Compound | Properties/Mode of Action | Ref |
| Naturally occurring flavones/chalcones | ||
| Phenolic compounds | Inhibition of SARS 3CLPro activity, cell ular anti-oxidant, anti-inflammatory activity. Panduratin A inhibits SARS-CoV-2 infection at pre-entry and post-infection phases. Multi targeting chalcones show promise in the treatment of AD. | [6, 22, 65, 111, 112, 113, 114, 115] |
| Panduratin A | ||
| Flavonoids | ||
| Chalcones | ||
| Quercetin | Block RNA dependent RNA polymerase activity, inhibit SARS-CoV-2 cell entry. Quercetin inhibits ACE2 enzymatic activity. Molecular docking studies show rutin binds to SARS-CoV-2 M |
[27, 113, 116, 117, 118] |
| Rutin | ||
| Myrcetin | Interference with the ATPase activity of nsp13 inhibitis helicase activity, viral replication and SARS 3CLPro enzymatic activity. Analog aglycone and glycoside forms of scutellarin have therapeutic anti-viral properties. | [84, 113, 119] |
| Scuttellarein | ||
| Glycirrhizin | Inhibition of SARS-CoV-2 adsorption to host cells through interactions with Spike protein antagonises host cell ACE2 interactions, tissue anti-oxidant, anti-inflammatory activities | [120, 121, 122, 123, 124] |
| Quercetin | Inhibition of SARS-CoV-2 3CLPro enzymatic activity, Spike protein interactions blocks viral host cell entry, inhibition of nsp15 endoribonuclease atttenuates viral replication. Molecular docking studies show Catechin targets 3CLpro, CTSL, RBD of S protein, NSP6 and nucleocapsid protein. CoV-2: Spike ACE2 interactions inhibited. | [125, 126, 127, 128, 129, 130, 131, 132, 133, 134] |
| Epigallocatechin | ||
| Gallate Gallocatechin | ||
| Catechin | ||
| Chrysin | Chrysin has anti-oxidant and immunomodulatory properties. Inhibits NFkB pathway as a PPAR |
[135, 136, 137] |
| Kaempferol | Inhibition of movement of metabolites through viral 3a ion channels inhibits viral replication | [138] |
| Luteolin | Binding to Spike protein inhibits viral attachment to host cells, also displays inhibitory activity against SARS-CoV-2 3CL pro. Has anti-oxidant activity, inhibits MAPK, NFκB pathways, reduces COX-2, TNF |
[139, 140, 141, 142] |
| Kaempferol, luteolin | Kaempferol and luteolin have monoamine oxidase inhibitory activity therapeutic agents in neurodegenerative disorders | [143, 144, 145] |
| Hesperidin/hesperitin | Vasodilatory, used to treat stress induced H.pylori gastric ulcer, ulcerative colitis, gastric/mucosal infections. Supports innate and acquired immune responses, binds to SARS-CoV-2 3CL pro, blocks CoV-2 entry into host cells. Promising agents for treatment of neurodegenerative disorders. | [2, 146, 147, 148, 149] |
| Induces Nrf2 and tissue protection. | ||
| Licochalcone B | Multifunctional, inhibits A |
[150, 151, 152, 153] |
| Flavokawin | Suppresses NF-κB-mediated inflammation and cancer | |
| Butein | An anti-oxidant flavonoid, hepato-protective, anti-tumour activity against a range of cancer types | [154, 155, 156] |
| Xanthoangelol | Anti-oxidant, anti-inflammatory, anti-cancer, anti-bacterial properties, neuroprotective. Induces apoptosis in neuroblastoma and leukemia tumour cells | [157, 158, 159] |
| Scutellarin | Multifunctional phenolic herbal flavonoid, interacts with SARS-CoV-2 3CL pro and endoribonuclease (NSP15) to disrupt viral replication. | [160, 161] |
| 4-Hydroxyderricin | Produced by Angelica keiskei, anti-tumour activity through induction of Caspase mediated apoptosis of leukemia cells | |
| Cardamonin | Anti-oxidant, anti-inflammatory chalcone used in the treatment of gastric, colonic and breast cancer | [162] |
| Isoliquiritigenin | Antiinflammatory, anti-oxidant, anti-cancer, hepato- and, cardio protective, potent MAO inhibitor, has potential in the treatment of neurodegenerative disorders. identified as a bioactive component of the Chinese herbal Qing Fei Pai Du decoction, used to treat COVID-19 and fatty liver disease. | [163, 164, 165, 166] |
| Naringenin | Anti-oxidant, anti-cancer, suppresses allergic asthma, cholinesterase inhibitor. Inhibits ERK and NFκB pathway COX-2, iNOS, TNF |
[167, 168, 169, 170, 181, 172] |
| Analog Chalcone/Flavone derivatives | ||
| Tris chalcones | A novel class of fluoro-substituted tris-chalcone AChE and BuChE inhibitors, K |
|
| Bis chalcones | Carbonic anhydrase inhibitors | [173] |
| Chalcone metal co-ordination complexes | Metallopharmaceuticals have improved efficacy through enhanced pharmacokinetic pharmacodynamics. Carbonyl, hydroxyl, phenolic oxygen in heterocyclic chalcone ring facilitate metal coordination. Cu (II)-cardamonin, is a potent antitumour agent, induces DNA damage, microtubule disruption, ROS inducing apoptosis, activation of caspase-3/7, PARP cleavage. Downregulation of Mcl-1 inhibits Akt signalling. Platinum (IV) chalcones are cytotoxic in Cisplatin resistant tumour cells, mitochondrial membrane collapse, induces apoptosis, intracellular ROS in tumour cells. | [174, 175, 176, 177, 178, 179, 180] |
| Ferulic acid –O-alkylamines | Anti-oxidant, impressive inhibitor of BuChE, inhibits and disaggregates self-induced A |
[181] |
| Dimethylamino chalcone-O-alkylamines | Impressive dimethylamino chalcone-O-alkylamines multifunctional compounds, inhibit/disaggregate A |
[180, 182, 183] |
| 4-hydroxy-chalcones, bis-chalcone ethers | antioxidant, LOX, AChE inhibitory activity, potent inhibitors of lipid peroxidation multifunctional compounds for treatment of AD. | [184] |
| chalcone-O-carbamates | inhibits AChE/BChE, MAO-A/MAO-B, A |
[160, 161] |
| Scutellarein-O-alkylamine analogs | Multifunctional, metal chelating, anti-oxidant, inhibits self-induced, Cu(2+) and AChE-induced A |
[160, 161] |
| Halogenated coumarin-chalcones | MAO, AChE, BuChE, and BACE-1 inhibitor, non-toxic to Vero cells up to 100 |
[185] |
| Derivatised Hesperitin analogs | Improved inhibition of AChE, selectivity for BuChE, inhibits self-induced A |
[149, 186, 187] |
| Structure based anti-viral drugs targeting the SARS-CoV-2 main protease active site | Inspired by anti-viral inhibitory activities of flavones and chalcones through virtual screening of ChEMBL database*. Compounds 11a, 11b target CoV-2 MPro active site. X-ray crystallography shows C-terminal aldehyde groups of 11a and 11b covalently attach to the Cys 145 moeity in MPro catalytic dyad, potent anti-virals 11a 100% and 11b 96% inhibition of 3CL MPro at a concentration of 1 |
[33] |
| Selenium chalcones | Anti-tumour, inhibit tubulin polymerisation, thioredoxin reductase. potent anti-cancer agents, anti-viral properties. Ebselen has potent anti-bacterial activity against MDR C. difficile targets the transpeptidase Ldt Mt2 protease, acts synergistically with Remedesivir to eradicate SARS-CoV-2 and MDR bacterial infections in long COVID disease. | [188, 189, 190, 191, 192, 193, 194, 195] |
| Abbreviations used: ACE2, Angiotensin converting enzyme-2; AChE, Acetylcholinesterase; AD, Alzheimer’s disease; A |
||
The neuroprotective properties of chalcones and flavones have been attributed to their anti-oxidant and anti-inflammatory properties and ability to induce Nrf2 expression [61, 64]; flavonoids also induce neurogenesis and neural differentiation [196]. Besides having an ability to induce Nrf2 (Fig. 7, Ref. [197]) [198], flavonoids regulate the production of inflammatory mediators, inhibit endothelial activation and the NLRP3 inflammasome and toll-like receptors (TLRs). Flavones also counter mitochondrial dysfunction [199] in neurodegenerative disorders [200].
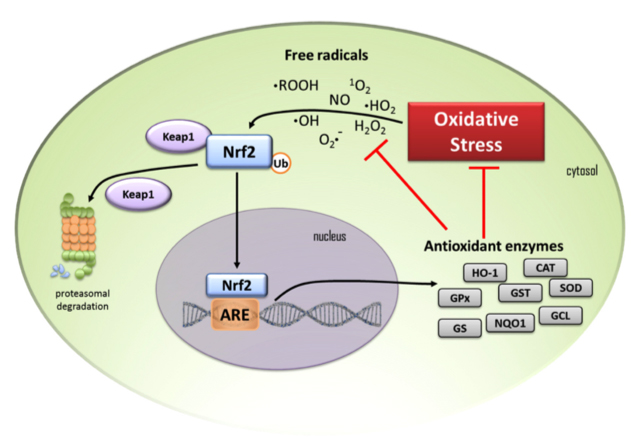 Fig. 7.
Fig. 7.
Schematic depiction of a cell and the Nrf 2 cell signalling pathway showing the anti-oxidant enzymes that are induced by oxidant stress. (1) Under homeostatic conditions cytosolic Nrf2 transcription factor is maintained at low levels by proteasomal degradation under control of the Keap1 protein complex. (2) When cells are exposed to oxidative stress free radicals result in the release of Nrf2 from Keap1 to escape proteasomal degradation and it translocates to the nucleus where it binds to the oxidant response element (ARE) and anti-oxidant enzymes are induced. (3) These include heme oxygenase-1 (HO-1), glutathione peroxidase (GPx), glutathione-S- transferase (GST), superoxide dismutase (SOD), catalase (CAT), glutathione reductase (GR), NAD(P)H quinone oxidoreductase (NQO1), glutamine-cysteine ligase (GCL) and glutathione synthetase (GS). These enzymes diminish oxidative stress on the cell and reduce free radical levels. (4) Black arrows in the schematic depict activation pathways, (5) red T-bars signify the blocking steps induced by transcription of anti-oxidant enzymes. Figure reproduced from [197] by open access Creative Common CC BY license.
Licochalcone A and B from liquorice root (Glycyrrhiza glabra or Glycyrrhiza inflata) are bioactive anti-tumour chalcones [201] that up-regulate the Nrf2 anti-oxidant pathway [202] and attenuate neuronal injury in a rat model of stroke [153]. Licochalcone B is neuroprotective, inhibits amyloid
Quercetin is neuroprotective, enhances neuronal viability, promotes neurogenesis [203, 211] and can modulate/inhibit a number of cell signaling pathways including Nrf2, PON2 (paraoxonase-2), JNK (c-Jun N-terminal kinase), TNF-
Chrysin exhibits anti-oxidative effects on dopaminergic neurons in PD by increasing Nrf2 expression [208], reduces neuron NO levels intracellularly and regulates neuronal anti-oxidant pathways. Chrysin promotes dopaminergic neuronal survival by upregulating the activation of myocyte enhancer factor 2D (MEF2D), suppresses the upregulation of c-caspase and Bax and downregulates the anti-apoptotic protein Bcl 2 and enhanced neuronal survival through production of neurotrophic factors. Chrysin’s anti-inflammatory properties increase dopamine levels through inhibition of monoamino-oxidase B activity restoring behavioral deficits in animal models of PD [213].
Oxidative stress and inflammation are major contributors to the pathogenesis of neurodegenerative diseases. Catechins are powerful antioxidants with free radical scavenging properties that have roles to play in the management of neurodegenerative diseases. Catechins modulate cellular processes mediated through NF-
Luteolin’s anti-oxidant, anti-inflammatory properties and ability to induce Nrf2 are neuroprotective [214, 215] and counter neuroinflammation following brain trauma [216] downregulating the TLR4/TRAF6/NF-
Myrcetin has beneficial properties in the treatment of cerebral ischemia and AD and has multifunctional properties regulating the expression of Hippo, MAPK, GSK-3
Apigenin’s antioxidant properties regulate redox cell signaling pathways involving NF-
In-vitro, in-silico and x-ray crystallographic studies show EGCG exerts anti-oxidative health benefits to neural tissues [205]. Surface plasmon resonance and computational docking simulations demonstrate EGCG’s direct binding to pro-inflammatory chemokines blocking the recruitment of inflammatory cells into tissues, regulating inflammatory diseases [223]. EGCG also inhibits amyloid plaque formation in AD and aggregation of A
Genistein modulates pathogenic events in neurodegeneration and is neuroprotective, attenuates amyloid-beta-induced cognitive impairment in rats in an in-vivo model of A
Cardamonin induces Nrf-2 expression and its neuroprotective anti-oxidant enzyme systems [229], attenuates inflammation and oxidative damage in IL-1 stimulated chondrocytes in OA [230] and significantly up-regulates seleno- anti-oxidant enzymes induced by Nrf2 [231].
Hesperidin’s anti-oxidant, anti-inflammatory and neuroprotective properties are useful in the treatment of neurodegenerative conditions [232] and memory impairment in AD, PD, MS, and ALS. Hesperidin glycoside and its aglycone form, hesperitin, have been developed into multifunctional derivatives of higher efficacy [233]. A multi-tier flavonone screening protocol employing molecular docking for BACE1 inhibitory, and anti-amyloidogenic and antioxidant activities have demonstrated hesperidin derivatives as potent AD therapeutics [234].
Hesperidin is a high affinity BACE1 inhibitor completely inhibiting BACE1 at a concentration of 500 nM and provides complete inhibition of amyloid fibril formation [234, 235]. Inhibition of BACE1 by hesperidin acts upstream of the APP processing that generates A
Cerebral ischaemic injury and degenerative pathology in AD are linked, hesperidin down-regulates Bcl-2, Akt/PI3K protecting against A
Intracerebroventricular injection of hesperetin 24 hours after injection of A
NF-E2-related factor 2 (Nrf2) is a master regulator of numerous cytoprotective genes [241, 242]. After translation, the Nrf2 protein is rapidly degraded by the ubiquitin-proteasome system in the cytoplasm [243]. Kelch-like ECH-associated protein 1 (Keap1) is a component of the Cullin 3 (CUL3)-based E3 ubiquitin ligase complex and controls the stability and accumulation of Nrf2 (Fig. 7). Table 3 (Ref. [197, 225, 226, 227, 228, 229, 230, 231, 232, 233, 234, 235, 236, 237, 238, 239, 240, 241, 242, 243, 244, 245, 246, 247, 248, 249, 250, 251, 252, 253, 254, 255, 256, 257, 258, 259, 260, 261, 262, 263, 264, 265, 266, 267, 268, 269, 270, 271, 272, 273, 274, 275, 276, 277, 278, 279, 280, 281, 282, 283, 284, 285, 286, 287, 288, 289, 290, 291, 292, 293, 294, 295, 296, 297, 298, 299, 300, 301, 302, 303, 304, 305, 306, 307, 308, 309, 310, 311, 312, 313, 314, 315, 316, 317, 318, 319, 320, 321, 322, 323, 324, 325, 326, 327, 328, 329, 330]) illustrates the diversity of flavonoids that up-regulate Nrf2 to exert a cell and tissue protective effect.
| Flavonoid | Ref | Flavonoid | Ref |
| Acacetin | [244] | Licocalchlcone A. | [245] |
| Apigenin | [246] | Liquiritin | [247] |
| Artocarmitin B | [248] | Limonin | [249] |
| Baicalein | [250] | Luteolin | [251, 252, 253, 254, 255, 256, 257, 258] |
| Baicalin | [259, 260, 261] | Malvidin-3-O-Glucoside | [262] |
| Biochanin A | [263, 264] | Morin | [265] |
| Cardamonin | [266, 267] | Naringenin | [268, 269] |
| Cynaroside | [270] | Natural/synthetic chalcones | [271] |
| Chrysin | [272, 273] | Neobavaisoflavone | [274] |
| Chrysoeriol | [275] | Nobiletin | [276] |
| Cyanidin-3-glucoside | [277] | Orientin | [278] |
| Daidzein | [279] | Peurarin | [280, 281] |
| Dihydromyrecetin | [282] | Phloretin | [263] |
| Epigallocatechin Gallate | [283] | Pinocembrin | [284, 285] |
| (-)-Epicatechin | [286] | Pinocembrin-7-methylether | [287] |
| Formononetin | [288] | Punicalagin | [289] |
| Galangin | [290, 291, 292, 293] | Quercetin | [294, 295, 296, 297, 298] |
| Gallocatechin | [299] | Scutellarin | [300] |
| Genistein | [301, 302, 303] | Silychristin A | [304] |
| Hesperidin | [305, 306] | Silymarin | [307] |
| Hesperitin | [308, 309, 310] | Theaflavin | [311, 312, 313] |
| Hyperoside | [314] | 6,7,4’-Trihydroxyflavanone | [315] |
| Icariin | [316, 317, 318] | Vitexin | [319] |
| Icaritin | [320] | Wogonin | [321, 322] |
| Kaempferol | [323, 324, 325, 326, 327] | Xanthohumol | [328, 329] |
| Kushenol | [330] |
Diversely-substituted 4-hydroxy-chalcones and a series of bis-chalcone ether derivatives with antioxidative properties, lipoxygenase (LOX) and AChE inhibitory activity are potent in vitro inhibitors of lipid peroxidation and potential new multifunctional AD compounds [184]. Multifunctional 4-hydroxy chalcones inhibit self-induced A
Dimethylamino chalcone-O-alkylamines derivatives inhibit A
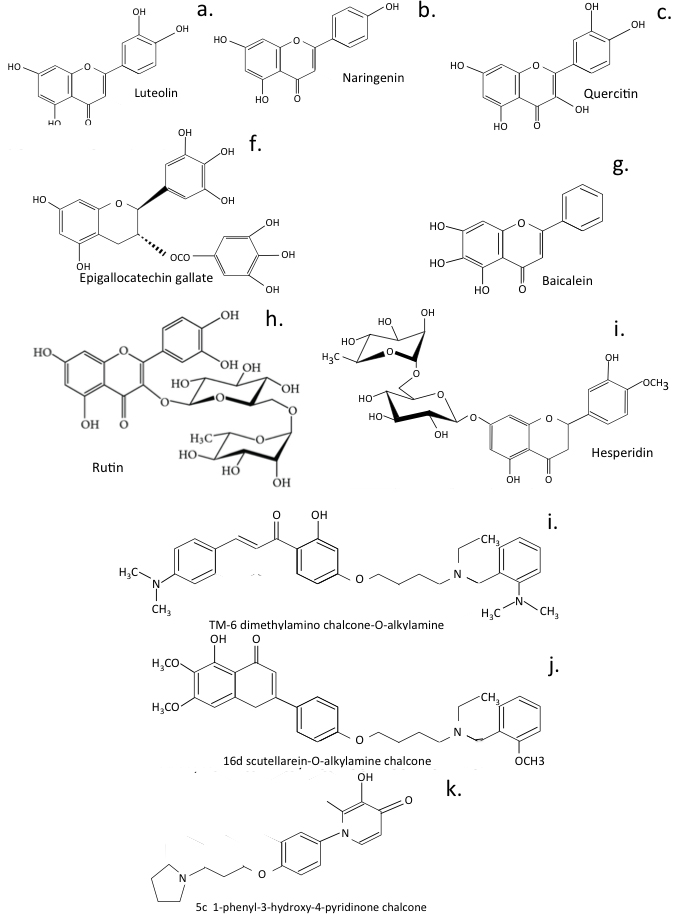 Fig. 8.
Fig. 8.
Naturally occurring flavonoids and multifunctional flavonoids developed from for the treatment of neurodegeneration.
Anti-oxidant chalcone-O-carbamates are multitargeting compounds that inhibit AChE/BChE and MAO-A/MAO-B, A
Scutellarein-O-alkylamine analogs have metal chelating properties, anti-oxidative activity, and inhibit self-induced, Cu
Ferulic acid-O-alkylamines are anti-AD agents with impressive inhibitory activity against BuChE, inhibition/disaggregation of self-induced A
Halogenated coumarin-chalcones inhibit MAO s, AChE, BuChE, and BACE-1. Compound CC2 potently inhibited MAO-B (IC
Monoamine Oxidase inhibitors regulate monoamine neurotransmitters, oxidative stress, A
Hesperetin derivatives are AChE dual-site inhibitors displaying strong inhibitory activity against AChE, high selectivity for BuChE and inhibit self-induced
A series of 7-O-1, 2, 3-triazole hesperetins inhibit BuChE, are anti-neuroinflammatory, and neuroprotective. Compound a8 (7-O-((1-(3-chlorobenzyl)-1H-1,2,3-triazol-4-yl)methyl)hesperetin) displayed excellent anti-BuChE inhibitory activity (IC
7-O-amide hesperetins inhibit BuChE and are neuroprotectors. Compound 7c (7-O-(4-(morpholinoethyl)-acetamide) hesperetin) was the most effective BuChE inhibitor (IC
Traditional Chinese herbal preparations have been used for centuries in complementary alternative medicine [336, 337]. Attempts have been made to better understand their chemical components to determine if they can be applied in Western medicine. Claims have been made that herbal medications can successfully combat COVID-19 infections [338, 339]. Network pharmacology, molecular docking and in-vitro cell based investigations have identified a number of active components in these herbal preparations that could potentially provide a therapeutic effect [340, 341]. Chinese herbal preparations used to treat AD have also undergone similar assessments to identify their active therapeutic components and their molecular targets.
LeZhe is purported to be a nerve calmative detoxifying antipyretic agent useful in the prevention and treatment of age dependent AD [342]. Network pharmacology and molecular docking studies have been employed to identify LeZhe’s active components and their molecular targets and these have been evaluated in PC12 primary hippocampal neural cultures where injury had been induced using A
Chaihu Shugan San (CSS) is another well-known herbal antidepressant used in traditional Chinese medicine. Modern pharmacological and clinical evidence indicate that CSS could also be beneficial in the treatment of cognitive dysfunction in AD. Active compounds in CSS have been screened using the Traditional Chinese Medicine Systems Pharmacology database. Compound-related targets retrieved using the SwissTarget Prediction database facilitated the identification of major depressive disorder (MDD)-related targets The CSS compounds were examined in cumulative unpredictable mild stress (CUMS) mice. Molecular docking analyses determined the binding affinities of the bioactive CSS compounds [344]. Elucidation of multi-target mechanisms of action for CSS using network pharmacology analysis identified a total of 152 active compounds, 520 predicted biological targets and 160 AD-related targets [345] regulating PI3K-Akt, MAPK and HIF signaling pathways. Pre-treatment of neural cell cultures with CSS reduced A
Qing Fei Pai Du and Ma Xing Shi Gan anti-viral decoctions used to treat COVID-19 and AD in Traditional Chinese Medicine are of considerable complexity, however molecular networking of mass spectrometry data has been used to identify a number of bio-active flavone and chalcone compounds present in these formulations [346]. Hesperidin, glycyrrhizic acid, baicalin, baicalein, naringin, phillyrin, quercetin, luteolin, kaempferol, licochalcone B and mangiferin were all present [346]. Further studies are required to fully decipher all the therapeutic bioactive component combinations in these formulations and their pharmacological interactions.
Highly active chalcone metal co-ordination complexes were originally developed to treat drug resistant solid tumours. Metallopharmaceuticals have amplified therapeutic modulatory pharmacokinetic and pharmacodynamic properties against cell receptors [178]. Carbonyl, hydroxyl, phenolic oxygen in heterocyclic ring structures in chalcones and flavones have excellent chelating properties on the preparation of metal coordination complexes. These have improved therapeutic and catalytic activities that have found successful application in the treatment of drug resistant tumours but have not been extensively examined for their anti-viral properties. Platinum(IV) complexed chalcones have potent anti-tumour activity and low cytotoxicity, inducing G2/M phase arrest and apoptosis in A549 cancer cells. Collapse of mitochondrial membrane potential, elevated expression of apoptosis-related proteins and reactive oxygen species all contribute to inhibition of tumour growth [177]. Metal coordination complexes prepared with chalcones and flavones represent a novel area of application in anti-viral development that needs to be explored further. Zinc not only inhibits the SARS-CoV-2 Mpro with nanomolar affinity, but also inhibits viral replication [347]. Furthermore, the natural ionophore quercetin increases the anti-viral potency of Zn
While selenium is a non-metal, it can also inhibit viral replication. Ebselen is an active seleno-organic anti-viral against zika, influenza A, HCV, and HIV-1, and SARS-CoV-2 [194, 349]. Selenium interacts with thiol groups in proteins and this may represent a mechanism whereby it inhibits SARS-CoV-2 Mpro activity and viral replication [350]. Selenium-substituted chrysin and quercetin, developed as anti-cancer agents, also display anti-viral properties that need further evaluation (Fig. 9).
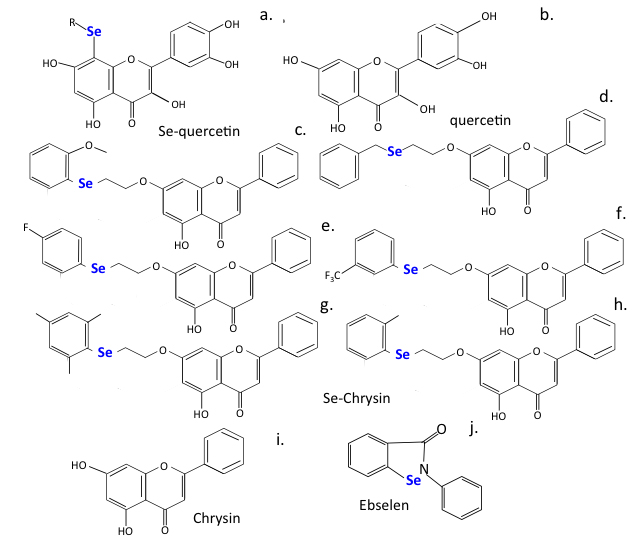 Fig. 9.
Fig. 9.
Selenium substituted flavonoids of improved efficacy.
Critically ill COVID-19 patients suffering from acute respiratory distress syndrome (ARDS) show lung injury and haemolysis. Heme is a prosthetic group crucial for the function of the oxygen-trapping haemoglobin and the energy-producing cytochromes of the electron transport chain of mitochondria. Haemolysis generates free heme in ARDS patients promoting adhesion molecule expression, leukocyte recruitment, vascular permeabilization, platelet and complement activation, thrombosis, and fibrosis. Heme is degraded by the anti-inflammatory enzyme heme oxygenase-1 (HO-1) generating biliverdin/bilirubin, iron/ferritin, and carbon monoxide. Free heme contributes to many of the inflammatory aspects of critically ill COVID-19 patients, thus induction of HO-1 may be protective and a therapeutic target in COVID-19 patients reducing long-term fibrotic changes in lung tissues [351]. HO-activity not only degrades injurious heme, but its effector molecules possess anti-oxidative and anti-inflammatory properties of potential benefit to ARDS patients [352]. 4-Anilinoquinolinyl chalcone upregulates HO-1 expression and has beneficial anti-inflammatory and anti-oxidant properties typical of the chalcone family [353]. Novel chalcones display anti-inflammatory and anti-oxidant effects in-vitro and after LPS induced acute lung injury [354]. Studies are warranted with these chalcone derivatives for the treatment of long COVID-19 disease [355]. Promotion of tissue fibrosis in COVID-19 infections results in fibrotic changes in liver and lung tissues [356] and leads to long-term pulmonary fibrosis and associated breathing difficulties. A number of chalcones (Panduratin A) present in Thai and Chinese herbal medicines [6, 357] and identified in pomegranate [358] display beneficial anti-inflammatory properties and reduce tissue fibrosis in a similar manner to Pirfenidone [359, 360], a long-standing anti-fibrosis medication. These are worthy of further investigation in the management of long COVID-19 disease.
A screen of extracts from 122 Thai traditional medicinal plants for anti-viral and specifically anti-SARS-CoV-2 compounds identified Panduratin A from Boesenbergia rotunda. This plant is also known as Chinese keys, finger-root, lesser galangal or Chinese ginger and is found in SE Asia and China. Panduratin-A (2,6-dihydroxy-4-methoxyphenyl)[(1R,2S,6R)-3-methyl-2-(3-methylbut-2-en-1-yl)-6-phenylcyclohex-3-en-1-yl]methanone) is a potent non-toxic anti-inflammatory chalcone that strongly inhibits NO (IC
The cost of dementia in Australia in 2016 was estimated at $14.25 billion and is escalating [370] with an increased incidence of AD and dementia in global ageing populations [371]. In 2010, the cost of treating dementia in the USA was estimated at $200 billion. The COVID-19 pandemic has had a disproportionately negative impact on people affected by AD and dementia. Individuals affected with dementia may have a reduced capacity to understand and comply with pandemic health care restrictions and thus potentially represent a spreader risk for COVID-19 infection [372]. With present day AD/dementia patient numbers of 47 million projected to triple by 2050 compounded by the impact of the present day COVID-19 pandemic there is a clear need to develop therapeutics that target oxidative stress, neuroinflammation, cholesterol metabolism, amyloid plaque formation, and adverse regulatory effects on neurotransmitters and vascular factors to combat this progressive and debilitating neurodegenerative disorder.
Of particular concern are the cognitive deficits that have been reported in patients who have recovered from COVID-19 respiratory disease. This includes an inability to concentrate and a fogging of thought processes impairing concentration for tasks at hand and the solving of problems and feelings of long-term anxiety and insecurity [373, 374, 375, 376, 377]. Particularly disturbing are emerging reports of COVID-19 causing a reduction in IQ in children. Long-term fatigue associated with long covid patients impacts on the development of neuro-psychiatric disorders [378, 379, 380]. AD is the sixth-leading cause of death and is present in 70% of all cases of dementia. The global burden of AD is expected to accelerate from 26.6 million cases in 2006 to 106.8 million by 2050, estimated worldwide costs of dementia were US$ 604 billion in 2010 so this projected increase in the number of AD and dementia patients will make a significant impact on healthcare resources.
Secondary bacterial infections have been observed in long COVID disease and this may involve MDR bacterial strains. The attainment of bacterial antibiotic resistance is a serious healthcare problem [381] and one that has been acknowledged by the WHO with their publication of the dirty dozen list of MDR pathogenic bacteria [http://www.who.int/news-room/detail/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-areurgentlyneeded (accessed 12 January 2018)]. MDR bacterial infections have been compounded by the COVID-19 pandemic and the emergence of the MDR strains of Clostridium difficile and Mycobacterium tuberculosis in long COVID bacterial infections [382, 383, 384, 385, 386]. Inappropriate administration of antibiotics to long COVID-19 patients despite the fact that this is not a bacterial infection may be inappropriate even when these are administered as a preventative measure against potential secondary bacterial infections that may occur and may actually result in these patients acquiring troublesome antibiotic resistant bacterial strains [387]. Antibiotic resistance is a serious problem and a major public health concern. Multi drug resistant Mycobacterium tuberculosis bacterial strains causing tuberculosis (TB) have emerged in the COVID-19 pandemic. These may not be responsive to any antibiotic currently available, leading to lethal pneumonia as a secondary respiratory infection of COVID-19, although other organs can also be effected including the brain, 150,000 TB infections are reported annually with lethal consequences in 40% of these patients [388, 389, 390, 391]. Clostridium difficile has also emerged during the COVID-19 pandemic as an additional MDR gut bacterium with serious health impact. Some positive developments have also emerged on how to combat such infections. Phage therapy is a therapeutic which is proving effective against a number of MDR bacteria and secondary infections occurring with COVID-19 [392, 393, 394, 395, 396, 397, 398]. Ebselen has been used as an anti-cancer, anti-bacterial and anti-viral SARS-CoV-2 main protease inhibitor [189]. Ebselen has potent anti-bacterial activity against antibiotic resistant C. difficile where it targets the transpeptidase Ldt Mt2 protease [188, 189, 190] and can act synergistically with the CoV-2 replication inhibitor Remedesivir to eradicate both SARS-CoV-2 and MDR bacterial infections [191]. Flavonoids are active against MDR bacteria and are a promising and underappreciated reservoir to counter antibiotic resistance. The antimycobacterial and anti-inflammatory activities of substituted chalcones have also been used in the development of anti-tuberculosis therapeutic treatments [399]. Flavonoids have been widely utilized in traditional medical practices to combat bacterial infections [17, 399, 400] with some approaches focusing specifically on how to combat MDR bacteria [400, 401, 402, 403, 404]. Combination therapies with antibiotics [405] and approaches examining how the antiviral and immunomodulatory properties of flavonoids can be harnessed in the treatment of respiratory diseases have also been examined [406].
Clostridium difficile (now renamed as Clostridioides difficile) is a problematic bacterium that has recently attained antibiotic-resistant status. Antibiotic resistant Clostridium difficile spore-forming bacteria are frequently found in the bowel. Infections with C.difficile are lethal in 30% of patients. Faecal transplant therapy has been used to treat these infections [407, 408, 409, 410, 411, 412]. This is a phage-mediated therapy that is used to treat antibiotic resistant bacterial infection and is a useful approach harnessing protective aspects of the human microbiome; a 80% cure rate is reported for faecal transplant therapy [407, 408, 409, 410, 411, 412]. While faecal transplantation is a highly effective modern development in Western medicine it is not a new technique. In traditional Chinese medicine, Ge Hong in the 4th century used faecal transfer as a therapeutic approach for the treatment of chronic diarrhea. In the 16th century, another famous Chinese physician, Li Shizhen, described the use of fresh or fermented faecal products, called “yellow soup” to treat severe diarrhea, fever, pain and constipation [413]. A series of publications have appeared in Western medical circles advocating this treatment [188, 414, 415, 416, 417] and guidelines on this methodology have also been published [410, 411]. A faecal enema may be a more acceptable route of administration for phage therapy rather than “yellow soup”.
Dietary supplements or diets rich in flavonoid and chalcone components may be of benefit in the treatment of long COVID disease and neurological disorders [418, 419]. A recent study comparing the impact of diet versus drugs on cellular metabolism found nutrition had a much stronger impact than drugs on many cellular processes [420]. This pre-clinical study showed that diet could be more powerful than drugs in keeping conditions like diabetes, immune dysfunction, stroke and heart disease at bay. Diet is a powerful medicine, involving nutrient-signaling pathways that affect the gut microbiome [421, 422, 423]. The formation of a healthy microbiome in early childhood, is important to the establishment and maintenance of health in later life. Studies have suggested that COVID-19 may impact the microbiome composition and diversity, increasing the incidence of allergic and autoimmune disorders, especially in children [424]. The full impact of the gut microbiome on the attainment of tolerance to certain foods and the neurological pathways that train innate immune responses is, however, incompletely understood. Dietary flavonoids have been shown to interact with the microbiome [425] and the gut microbiome has emerged as a key conduit in mental health and a promising target for interventions [426, 427, 428]. Dietary flavones and chalcones can have important cell regulatory and tissue protective properties positively impacting on a number of diseases, many studies have shown how flavones and chalcones can impact diabetes, liver fibrosis, cancers and bacterial infections and these can also be beneficially regulated by dietary control [11, 429, 430, 431, 432]. Pre-clinical studies have also shown that neurological disorders such as AD, PD, ALS, MS and autism can also benefit from dietary flavones and chalcones and related compounds which regulate mitochondrial activity and pathways that can generate oxidative stress. Dietary components need to be taken seriously in the overall scheme of improving and maintaining a healthy cellular metabolic environment in tissues. There therefore is a scientific basis to the use of superfoods rich in flavonoid dietary components to positively aid in tissue protection and cellular functions that maintain tissue homeostasis and combat disease. Nutrient-sensing pathways influence metabolic health and aging, offering the possibility that diet might be used therapeutically. For example, dietary composition powerfully impacts on the hepatic proteome, not only on its metabolic profile [420] but on fundamental processes such as mitochondrial function and RNA splicing. This also needs to be considered in other tissue contexts in health and disease and in the specific context of viral infection could represent a supportive adjunct to conventional anti-viral therapeutic treatments.
Flavonoid supplements have emerged as possible approaches in the treatment of COVID-19 and neurodegeneration based on their cell and tissue protective properties as already discussed.
Flavonoid supplements have emerged as putative nutritional or therapeutic adjunct approaches for the treatment of COVID-19 [24] and neurodegeneration based on their antioxidant, antiviral, anti-inflammatory, immunomodulatory effects and ability to promote a healthy gut microbiome [212, 433]. Flavonoid-modifying enzymes are encoded in gut bacteria however little is known of the active flavonoid components that they generate from dietary flavonoids and polyphenolic compounds and how these exert disease prevention and beneficial effects on the health of tissues.
Intestinal microbiota can indirectly modulate airway physiology and immunity. COVID-19 patients have been observed to exhibit a specific imbalance in their gut microbiome closely associated with CoV-2 disease pathophysiology [433]. Rebalancing the intestinal microbiome using probiotics has been suggested as an effective therapeutic approach against COVID-19.
Lactobacillus plantarum, Bifidobacterium longum and Lactococcus lactis ssp. lactis, exhibit robust anti-infective properties against respiratory RNA viruses [434]. Furthermore, L. plantarum is capable of expressing viral antigens including the spike protein of SARS-CoV-2 and is capable of inducing protective immune responses in the gut and respiratory tract and of modulating innate and adaptive immune responses. This has led to L-plantarum being suggested as a potential adjuvant delivery system for the development of SARS-CoV-2 oral vaccines [435]. The gut microbiome is influenced by dietary flavonoids and these can have disease modifying health promoting benefits. Dietary polyphenolic compounds have beneficial properties on the gut microbiome and feed-on effects on neurodegenerative disorders through the gut-brain axis. Hesperidin has been used clinically for decades due to its anti-inflammatory gut mucosal protective and anti-bacterial properties against Helicobacter pylori which can produce ulcers in the colon and stomach [436, 437]. Myrecetin [438], kaempferol [439], naringin [440], quercetin [441, 442] and luteolin [443] beneficially modulating the colon microbiome. Flavonoids thus have a number of beneficial health promoting properties exerted through the gut-lung, gut-liver and gut-brain axes [444, 445, 446, 447, 448, 449, 450]. Functional screening of metagenome and genome libraries has also been employed to detect flavonoid-modifying enzymes that generate bioactive components from dietary flavonoids [451] and bacterial species that convert dietary flavonoids have been identified [450]. However this is an emerging area and much more research is required to better understand the health promoting properties of flavonoids and polyphenolic substances delivered by the gut-lung and gut-brain axes, this may represent a new therapeutic frontier [451, 452, 453, 454, 455, 456]. A number of recent studies have shown the potential of dietary flavonoids to treat neurodegenerative conditions [457, 458], depression, anxiety and cognitive dysfunction [459, 460, 461, 462] and Alzheimer’s disease [463, 464, 465, 466, 467, 468].
Chinese traditional medicine is claimed to effectively alleviate COVID-19 disease symptoms, delay disease progression and reduce death rates however much more research is required to de-mystify their therapeutic effects and the active components responsible for their purported effects [469, 470]. The herbal formulations used in Chinese complementary medicine are complex mixtures of bioactive compounds and attempts are now being made to identify individual bioactive components and their molecular targets [469]. Oral administration of the Chinese herbal medicine Qingfei Paidu decoction regulates plasma TNF-
This review has documented the beneficial health-promoting and tissue-protective attributes of dietary flavones and chalcones and the semi-synthetic multifunctional analog derivatives that have been developed from them. Plant and herbal formulations containing flavonoids have been used in traditional Chinese, Thai, Ayurvedic and Australian First Nation alternative medicinal practices for many generations and some of their bioactive components and molecular targets are now being deciphered using network pharmacology. Flavones and chalcones have antioxidant, anti-inflammatory, anti-viral and anti-bacterial health promoting properties that combat SARS-CoV-2 infection, long COVID disease and neurodegeneration. Flavonoids not only obstruct the Spike ACE-2 interaction to restrict infection but also target key enzymes essential for viral replication. Multifunctional flavonoid derivatives have been designed to target multiple targets with high binding efficiency including molecular targets responsible for neural changes reported in long COVID disease. Flavonoids also induce Nrf2 expression with tissue and cell protective properties addressing aspects of long COVID disease such as inflammation and hemolysis which release injurious free heme into tissues. Ebselen, a Selenium substituted cysteine reactive antioxidant phytochemical has been used as an anti-bacterial, anti-viral SARS-CoV-2 Main protease inhibitor and is also active against MDR C. difficile secondary infections that have emerged in long COVID disease. Ebselen synergises with the CoV-2 replication inhibitor Remdesivir to eradicate both SARS-CoV-2 and MDR bacterial infections. Flavonoids are thus versatile multifunctional therapeutics and can be prepared with varied novel structures that can potentially target emerging new SARS-CoV-2 variants and may be used in combination with conventional anti-viral drug therapies to improve health and well-being.
JM conceived the study, JM and MMS both wrote the original draft and edited subsequent versions. Both authors approved the final version of the manuscript.
Not applicable.
Not applicable.
This research was funded by the Melrose Personal Fund, Sydney, Australia.
The authors declare no conflict of interest.
