Academic Editor: Baohong Zhang
Background: Animal-fats are rich in long-chain saturated fatty-acids, well known to induct diabetic distress among ingested insulin-insensitive individuals. In the current-study, bovine-fat was fed to selective mice breeds highly sensitized to heavy dietary lipid load. Methods: The later high fat diet (HFD) group indeed undergone diabetic-onset within weeks with a drastically altered feed-behavior pattern. It consumed more food, gained body mass, elevated homeostatic model assessment value and extensively glycosylated Hb transporters. Results: However, the hypothetical test drug (Cuminaldehyde or CA) with known therapeutic-potential worked-well to balance food efficiency-ratio and Hb- counts closer to control. The fat-soluble phytochemical mono-terpenoid (CA) promoted constitutive mono-hexose (glucose) consuming catabolic-cycles via mono-glycoprotein (insulin) signal-transduction. It resolved diabetogenic-upsurge of gluconeogenic-enzymes, reduced non-sugar (amino/fatty acids) utilization by restricting transamination/dephosphorylation and restored liver-glycogen reserves near to normal-group effectively at 10 mg/kg b.w dose. Conclusions: Hence, the nutraceutical-potential (anti-diabetes/transaminitis ability) of administered exogenous redox-active agent CA can be entertained for evoking therapeutic-heath in diabetic human-community.
Diabetes mellitus is a chronic metabolic disorder characterized by insufficient insulin secretion, or resistance to insulin action, or both. It is associated with abnormalities in carbohydrate, lipid and protein metabolism, which leads to hyperglycemia, hyperlipidemia, hyperinsulinemia and hypertension [1]. Approximately 425 million people are diabetic worldwide and this number is expected to increase to 629 million by 2045 [2]. Type-2 diabetes is more prevalent than type-1 diabetes, which is primarily associated with food intake and energy expenditure through diet and exercise respectively.
Humans, like other heterotrophic-organisms, are highly dependent on the
surrounding nutritional-supplements to conduct biochemical-reactions and
cellular-activities as programmed by the physio-anatomical body-system for
survival [3]. Unlike photoautotrophic-plants which consumes light to degenerate
H2O via dehydrogenation/oxidation in thylakoid-membrane, animals generate
proton-motive force (PMF) across mitochondrial-membrane through
precisely-regulated oxidative-phosphorylation of C
Most of the vital nutrients including exogenous redox-regulators are selectively
drawn from diet-input through dietary-tract in a low viscous chyme form. Most of
the absorbed materials are streamed through blood particles pumped by the
cardio-vascular network prior to the assimilation or excretion [6]. Most of the
chemical-messengers that govern complex metabolic-processes such as calorie
conversion and carbon assimilation inside the multicellular-mammalian body are
preliminarily directed through endocrine-glands. Pancreatic-organ comprises both
ducted and ductless dual (exocrine-endocrine) glands capable of secreting
duodenal digestive enzymes as well as trans-membranal inductive-hormones with
Two valid reasons for utilizing CA as redox-supplement are attributed to geographic resource-abundance and specific photorespiratory-resistance. India and China are the top-cultivators of cumin seeds, many of the traditional dishes prepared here carry its components as functional and/or aesthetic ingredient [10]. Nevertheless, the role of a strong secondary redox-carrier is mandatory for calming-down the destructive-effects of oxidative-reflux initiated by solar high-intensity electromagnetic-frequencies during the transformation of fruit to seed [11]. Thus, the literature seeks the correlative resemblance of chosen therapeutic-agent in curtailing/cushioning the deteriorative side-effects of ingesting surplus saturated-fats. Additionally, the neutralization/normalization effects of administered-drug on the diet-associated diabetic-distress are communicated in terms of anti-hyperglycemic, anti-insulinemic, and anti-transaminitis principles.
CA was purchased from Sigma Chemical Co. (St. Louis, MO) USA. All other chemicals and solvents used in this study were of the highest analytical grade, procured from HIMEDIA and S. D. Fine Chemicals (Mumbai, India).4. 1.
Animals were maintained as per the National Guidelines and Protocols approved by
the Institutional Animal Ethics committee (Reg. No. 160/ 1999/CPCSEA, Proposal
number: 1166 in 2017). Healthy male C57BL/6J mice (21 days old weighing 18–20 g),
obtained from Biogen Bangalore were used in this study and maintained in clean
polypropylene cages at Rajah Muthiah Medical College and Hospital, Annamalai
Nagar, Chennai under standard humidity (65–70%) and temperature (23
The normal group was provided with a standard pellet diet, which is commercially obtained from the Institutional Animal Ethics Committee of Rajah Muthiah Medical College and Hospital, Annamalai Nagar and had a fat composition of 4.2%. The HFD experimental group was fed with beef tallow-based high fat diet (carbohydrate 34.5 g, fat 35.8 g, protein 17.7 g, vitamins 1.8 g, minerals 6.8 g and fiber 3.4 g) [12]. Weekly blood samples were collected by pricking the tail using a needle after a 4 h fast and the blood glucose was measured using a glucometer. On the 8th week, animals with a blood glucose of more than 180 mg/dL were considered to be diabetic and were used for the experiment.
The animals selected for the study were categorized into following hexa-groups with a individual group consisting six animals each. CA was administered through oral gavage.
Group I: Normal or control mice under above-mentioned standard diet, Group II: Mice fed with standard diet for 105 days and then with test-drug Cuminaldehyde for 35 days. Group III: Mice fed with diabetogenic HFD for 105 days. Group IV: diabetic HFD mice with administered CA (2.5 mg/kg body weight) as therapeutic-dose A for the last 35 days. Group V: diabetic HFD mice administered with CA (5 mg/kg b.w) as TD B for the last 35 days. Group VI: HFD diabetic mice administered with CA (10 mg/kg b.w) as TD C by an oral gavage for the last 35 days. At the end of the experimental period, mice were allowed to fast overnight. Then, the mice were sacrificed by cervical dislocation and blood was collected by cutting the jugular vein into heparinized glass tubes. Plasma was isolated from blood samples and stored at 4 to 8 ºC. Liver tissue was washed in ice-cold isotonic saline and blotted with a filter paper. A portion of the tissue was weighed, homogenized in 0.1 M Tris-HCl buffer (pH 7.4) and the tissue homogenate was used for carbohydrate metabolic enzyme and hepatic glycogen estimations.
Plasma glucose was estimated by the method of Trinder [13]. Hemoglobin (Hb) was estimated by the method of Drabkin and Austin [14]. Glycosylated hemoglobin (HbA1c) was gauged by the method of Sudhakar and Pattabiraman [15] respectively. Plasma insulin was measured by the method of Burgi et al. [16].
For oral glucose tolerance test, animals have fasted overnight and blood glucose was estimated using On-Call Plus blood glucose test strips at various time periods (30, 60 and 120 min) after giving the oral glucose load (2.0 g/kg b.w). Blood glucose value before giving glucose load is considered as 0-minute value. Results are expressed as mg/dL.
Insulin sensitivity was assessed by computing homeostatic model assessment (HOMA) by the method of Matthews et al. [17].
Liver function markers including alanine aminotransferase (ALT), aspartate aminotransferase (AST) and alkaline phosphate (ALP) were measured using biochemical-assay kits procured from Spinreact, Spain using Semi-Automated Biochemistry Analyser System Frankel and Kind and King’s method [18, 19].
Hepatic glucokinase activity was assayed by the method of Brandstrup et al. [20]. Glucose-6-phosphate dehydrogenase activity was assayed by the method of Bergmeyer [21]. The activity of glucose-6-phosphatase was assayed by the method of Koide and Oda [22]. Fructose-1,6-bisphosphatase activity was measured by the method of Gancedo and Gancedo [23]. Phosphoenolpyruvate carboxykinase (PEPCK) activity was measured by the method of Bentle and lardy [24]. Glycogen content in liver and their primary metabolic enzymes was estimated by the method of Morales et al. [25], Golden et al. [26] and Shull et al. [27].
Liver histopathological hematoxylin and eosin staining (H&E) study were done by the method of hine [28]. For histological examination, a portion of the liver was fixed in 10% neutral buffered formalin embedded in paraffin, sectioned and stained with H&E dye. Then semi-thin sections (0.5–1 microns) were prepared by using LKB ultramicrotome. Selected sections were stained with toluidine blue, examined with a light microscope and photographed.
The data obtained were analyzed by one‑way analysis of variance (ANOVA) and
Duncan’s multiple comparison tests to assess the significance of individual
asymmetries between the control and treatment groups using a computer‑based
software SPSS version 17 (SPSS, Chicago, IL, USA). The significance was considered at
the level of p
Feed-behavior alteration induced by the diet inclusive of fat-rich animal-based (bovine) substances was characterized by comparing temporal food intake rate and initial body weight gain in HFD-group with normal-group (Table 1). Moreover, the effective therapeutic dose (TD) of test-drug (Cuminaldehyde) was formulated after evaluating FER, HOMA, HbA1c and Hb readings along with feed-rate and mass-gain in test-groups. Feeding the diet rich in animal-fat indeed increased food intake and body weight, whereas, administering plant-based fat-soluble drug dose-dependently decreased food consumption, weight gain, HOMA, HbA1c and improved FER as well as Hb levels significantly in test-groups as presented in Table 1. This information was correlated with the graphical distribution obtained with the oral glucose tolerance test depicted in Fig. 1. From the data, the effective TD was found to be 10 mg/kg body weight due to its shared insignificant criterion with control group.
| Groups | Food intake (g/mice/day) | Initial Body weight (g) | Final Body weight (g) | FER |
HOMA | HbA1c (mg/g of Hb) | Hb (g/dL) |
| Normal | 3.22 |
22.01 |
33.11 |
0.290 |
4.20 |
0.581 |
10.52 |
| Normal + cuminaldehyde 10 mg/kg b.w | 3.22 |
22.11 |
34.25 |
0.265 |
3.80 |
0.580 |
10.48 |
| HFD | 3.36 |
23.10 |
46.08 |
0.146 |
21.00 |
1.82 |
6.92 |
| HFD + cuminaldehyde 2.5 mg/kg b.w | 3.31 |
22.06 |
42.17 |
0.164 |
14.81 |
1.54 |
7.40 |
| HFD + cuminaldehyde 5 mg/kg b.w | 3.30 |
23.10 |
40.20 |
0.192 |
10.81 |
1.10 |
8.90 |
| HFD + cuminaldehyde 10 mg/kg b.w | 3.28 |
23.50 |
38.54 |
0.218 |
5.70 |
0.68 |
9.40 |
| Values are mean | |||||||
 Fig. 1.
Fig. 1.Effect of cuminaldehyde on oral glucose tolerance test (OGTT).
Values were means
The feature of effective TD (10 mg/kg b.w) was once again validated to eliminate chaos in the empirical results obtained with experimental animal models. The obtained plasma glucose values and insulin levels of test-group supplemented with 10 mg/kg b.w of CA was closest to the range of control group (Table 2). Hence, the above-mentioned statement not only informs about hyperglycemic and diet-associated diabetogenic effects of preferring fatty animal-based meal, it also confirms the hypoglycemic and insulin-resistance reverting effects of CA input. Therefore, further reports on biochemical/histopathological analyses will be consisting of 10 mg/kg b.w of CA alone without including 2.5 and 5 counterparts in the attempt to concise the content of current treatises.
| Groups | Glucose (mg/dL) | Insulin (µU/mL) |
| Normal | 89.16 |
19.22 |
| Nomal + cuminaldehyde (10 mg/kg b.w) | 88.49 |
17.36 |
| HFD | 216.24 |
39.41 |
| HFD + cuminaldehyde 2.5 g/kg b.w | 190.30 |
31.42 |
| HFD + cuminaldehyde 5 mg/kg b.w | 151.23 |
29.07 |
| HFD + cuminaldehyde (10 mg/kg b.w) | 104.42 |
22.15 |
| Values were means | ||
Persistence of insulin-insensitivity in HFD-group under/over-expressed
glycolytic/gluconeogenic effector enzymes as represented in Table 3,4
respectively. The impact of heavy-calorie ingestion severely down-regulated chief
glucose utilizing enzymes of the cytosol popularly known as glucokinase and
glucose-6-phosphate dehydrogenase. The pathogenic over-expression caused by
animal-fat mediated feed-behavior attenuation accumulated remarkably high amounts
of Glucose 6 phosphatase (0.53
| Groups | Glucokinase ( |
Glucose 6 phosphate dehydrogenase (nmol of NADPH formed/min/mg protein) |
| Normal | 0.40 |
4.45 |
| Nomal + cuminaldehyde (10 mg/kg b.w) | 0.39 |
4.29 |
| HFD | 0.15 |
2.49 |
| HFD + cuminaldehyde (10 mg/kg b.w) | 0.30 |
3.80 |
| Values were means | ||
| Groups | Glucose 6 phosphatase ( |
Fructose 1,6- bisphosphatase ( |
PEPCK (µmoles of glucose phosphorylated/h/mg protein) |
| Normal | 0.17 |
0.50 |
2.46 |
| Normal + cuminaldehyde (10 mg/kg b.w) | 0.18 |
0.49 |
2.43 |
| HFD | 0.53 |
1.19 |
3.90 |
| HFD + cuminaldehyde (10 mg/kg b.w) | 0.25 |
0.65 |
2.84 |
| Values were means | |||
The upsurge of aspartate and alanine transaminase activities along with
phosphomonoester dephosphorylation via upregulation of AST, ALT and ALP enzymatic
machineries respectively in the HFD-group (Fig. 2) suggested the presence of the
disordered liver state. Uptake of excess non-sugar biomolecules in terms of
amino/fatty acids to compensate the energy/carbon-skeleton needs of compromised
glycolytic/tri-carboxylic acid cycles were diagnosed accordingly during the
diabetic-onset. This error-prone diversion/dissipation (alternative) pathway
simultaneously took-place with disrupting hepatocytes of mice undergoing
diabetic-distress as illustrated in Fig. 3. Although, addition of tiny CA dose
(10 mg/kg b.w) as an oral-supplement significantly (p
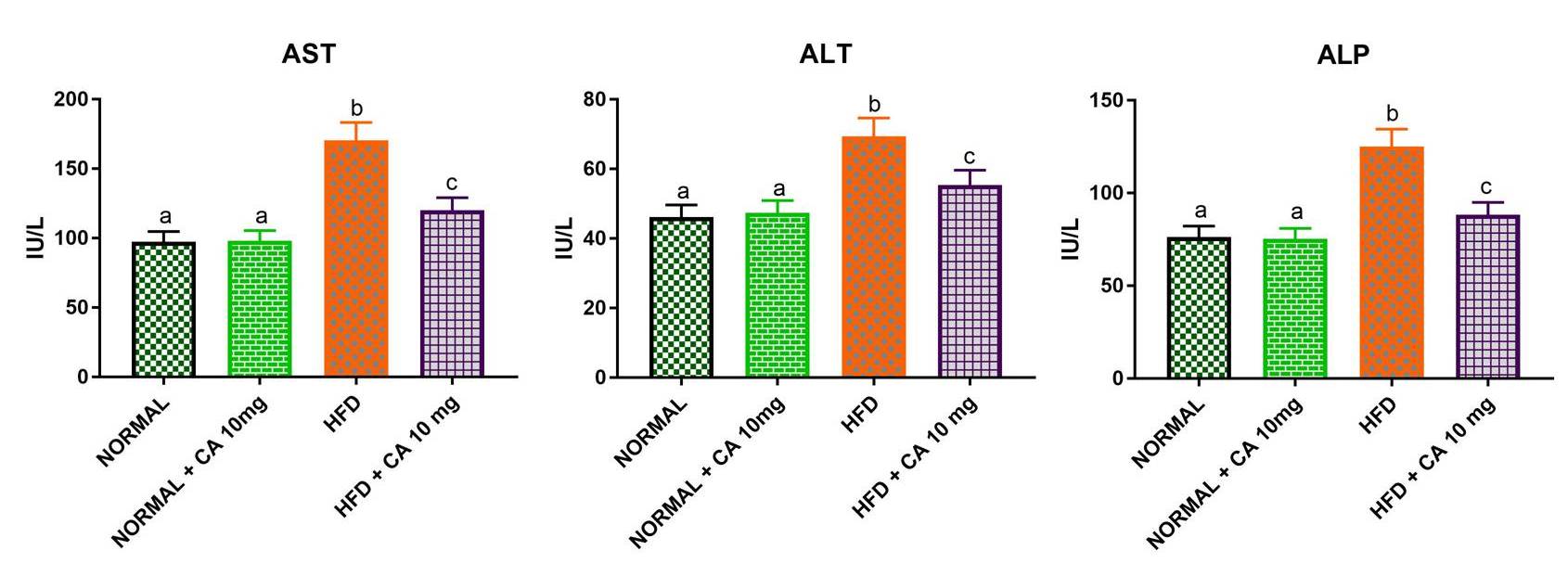 Fig. 2.
Fig. 2.Effect of cuminaldehyde on liver function markers (ALT, AST, and
ALP) in normal and experimental mice. Values were means
 Fig. 3.
Fig. 3.Photomicrographs of hematoxylin and eosin stained section of
mice liver (100
| Groups | Glycogen (mg/100g tissue) | Glycogen synthase ( |
Glycogen phosphorylase ( |
| Normal | 66.01 |
640.10 |
580.29 |
| Normal + cuminaldehyde (10 mg/kg b.w) | 68.03 |
635.10 |
576.09 |
| HFD | 30.00 |
450.22 |
630.10 |
| HFD + cuminaldehyde (10 mg/kg b.w) | 50.02 |
560.09 |
600.30 |
| Values were means | |||
Out of innate and acquired metabolic disorders (type I and II diabetic mellites respectively), the later condition (T2D) occurs presumably due to improper dietary-selection, which primarily affects micro/macro-vasculature (neuro endocrine and cardiovascular-networks) of humans/mice hosts [29]. Compromised hypo-glycemic hormonal-signaling could cause insulin-receptor (IR) dysfunction in cellular-surface/membrane, as a consequence, it omits vital sugar-uptake in a viable/respiring-cell. Committed dimeric-autophosphorylation of IR cum irreversible catabolic-dehydrogenation of glycosyl-subunits via kinase/oxidoreductase pathway(s) are important for the uptake and utilization of glucose. Thermo-homeostatic multi-cellular hydro-chemical medium has high fidelity in facilitating systemic-connectivity and transcellular-communication bio-responses for promoting cum progressing of cellular-operations [30]. Pancreatic-cells are capable of sensing external cum internal status of the body with its dual exocrine-endocrine recepotocytes via gastrointestinal tract and cardio-vascular network respectively [31]. Caloric-density (light vs heavy) seems to be the major determining factor for metabolic-threshold (free/fatty ergogenic kinetics) maintenance in the chosen mammalian animal-model of the current study. Catabolic-perturbation thus took-place because of the disturbance projected/propelled by highly-saturated fatty-components transiting in GI tract/CV network could be the most crucial contributor for pathogenic-transformation of proximate-organs participating in the circulatory, secretory and excretory processes. Altered feed-behavior along with adulterated energy-exchange patterns in the affected individuals of diet-associated diabetic-distress implies negative-effects of consuming animal-based meal which comprises larger-amounts of long-chain saturated fatty-acids capable of proliferating hydro-peroxidation.
Glucose is the primary fuel/nutrient metabolite for many life domains ranging from prokaryote to eukaryote [32]. The six-carbon biomolecule (hexose pyran) is vastly abundant in this planet and so many heterotrophic organisms including mammals mainly depend on this monosaccharide moiety for caloric cum carbonic needs. The cycles that utilize glucose (metabolic-fate) is said to be constitutive because of the overwhelmed demand in cellular respiratory compartments for assuring metabolic-flow/continuum. In plants, the photo-synthetic pathway fixes CO2 to produce this particular sugar via ribulose-bis-phosphate carboxylase/oxygenase (rubisco), surplus sugars are converted to starch (storage polysaccharide) for later utilization. In animals (especially with endocrine system), excess glucose is spontaneously converted into highly branched glycogen and stored in the liver/muscle tissues under finely regulated biochemical signaling cascade. However, during metabolic stress conditions, the bio-conversion and storage of glucose seem to be partially ceased or improperly monitored. Diet-associated diabetic distress (T2D) or non-insulin dependent diabetes mellitus (NIDDM) is popularly known to cause the above-mentioned prolonging-pathogenic response in humans with logarithmic global-prevalence [33]. Ingestion of saturated fats (fatty/heavy calorie-meal) was identified and inferred as a causative factor for T2D/NIDDM among humans and genetically manipulated lab mice.
The animals fed (HFD-group) with rich bovine-fat diet indeed developed signs and symptoms of NIDDM, which includes temporally increased body weight, food intake (polyphagia) and post-prandial blood glucose/insulin levels than the control group. It significantly brought down the levels of plasma hemoglobin (Hb) simultaneously by increasing its glycated version (HbA1c) and homeostatic assessment (HOMA) value used to forecast insulin-resistance [34]. This confirms the development of diabetic-distress in-terms of hyperglycemia and hyperinsulinemia among affected animals grown on periodic HFD pattern. In the physiological condition, glucokinase and glucose 6 phosphate dehydrogenase (G6P-DHase) utilizes glucose by means of spontaneous oxidative-phosphorylation steps [35]. However, the pathological (animal-fat fed) mice group expressed abnormally low levels of constitutive sugar utilizing enzymes. Additionally, the overexpression of gluconeogenic enzymatic machineries such as glucose 6 phosphatase, fructose 1,6- bisphosphatase and phosphoenolpyruvate carboxykinase supports prevailing pathogenicity in the HFD-population.
Oral-supplementation of plant-based fat-soluble monocyclic-terpenoid cumin-aldehyde (CA) provided to HFD-subjects exhibited anti-glycemic/insulinemic activity and enhanced food efficiency ratio (FER) closer to the control-counterpart. It stepped-up the bio-activity of sugar-utilizing glucokinase/glucose-phosphate-dehydrogenase enzymes which involves in producing vital intracellular redox-potentials such as NADH/NADPH respectively via glycolysis/HMP-shunt. Surprisingly [36], the selected-dose TD C (10 mg/kg b.w) of supplementary-drug (CA) suppressed the upsurge of above-mentioned gluconeogenic effector-enzymes including transaminases (ALT/AST) and phosphatases (ALP family) in the blood-serum. The latter statement provides the evidence that the administered-drug in test-group (HFD-diet + CA) is profoundly capable of counteracting the developing demand to consume non-sugar components for partially fulfilling current caloric cum carbonic pre-requirements. Nevertheless, the conversion of surplus monomeric glycosyl-monosaccharides into storable multimeric pyranosyl-polysaccharides was favored by the implemented dose-effective drug devoid of any observable metabolic toxicity. The previous study preferred 5 mg/kg b.w of CA as effective therapeutic-dose for treating streptozotocin-induced diabetic-distress [37]. In the current study with HFD design, we recommend a slightly higher concentration of CA i.e., 10 mg/kg b.w because of its insignificance with normal-group and efficacy with abnormal-group.
Pancreatic-organ plays a crucial role in governing basal metabolic-rate/fate and maintaining homeostatic-threshold by monitoring gastrointestinal-tract and cardiovascular-network via collective hydrolytic enzymes and selective signaling hormones represented as exocrine detectors and endocrine effectors respectively. The inter-connecting cum trans-communicating signals for conjugating and mediating peripheral tissues to promote uptake, utilization or storage of fuel (glucosyl-subunits) according to the energy consumptive/conservative demand by provoking commitment and completent duo. This dual-purpose gland located near duodenum seems more sensitive to the caloric-density of ingested-material, highly saturated bovine fat imposed deleterious side-effects in the body of mice with insulin-insensitive diabetic-onset. The latter condition lowered food-efficiency ratio and hemoglobin levels simultaneously by enhancing post-prandial blood-glucose, insulin, and glycated-hemoglobin partial-pressures. The load exerted from fatty-diet created deteriorative stress in committed oxidation/phosphorylation reactions of constitutive energy assimilative/transformative processes of chemo-respiration. The availability of surplus transaminases implies intensive leakage of high-energic (caloric) keto-acids and extensive breakage of aspartyl-alanyl amino-acyl (carbonic) derivatives. The demand shifted towards gluconeogenic cycle rather than the glycogenic pathway suggests the chaos in respiratory-redox balance due to improper feed-behavior pattern of pathogenic subjects. However, the induced redox-imbalance can be counteracted with the introduction of fat-soluble redox-carriers containing phenyl-nucleus and carbonyl-terminal for complementing electron-shuttling and proton-pumping across membranal-matrix/space interface respectively. The prophylaxis promoted via exogenous photo-respiratory redox-potentials of monocyclic-terpenoid cuminaldehye (CA) most probably participated in the replenishment of endogenous redox-adapters and restored glucose-catabolizing enzymes. Therefore, pathological-complications such as hyperglycemia, hyperinsulinemia, and transaminitis can be profoundly prevented by preferring plant-based semi-oxidized fat-soluble redox-couplers such as CA in required amounts (10 mg/kg b.w) orally to establish effective therapeutic dose-action.
CA, Cuminaldehyde; FER, food efficiency ratio; Hb, hemoglobin; HbA1
KD and PL designed the study, supervised the data collection, analyzed the data, KD interpreted the data and prepare the manuscript for publication, PE, PR, SJ, SB and TDSB supervised the data collection, analyzed the data and reviewed the draft of the manuscript. All authors have read and approved the manuscript.
Ethical approval was obtained from the Ethics Committee of the Ethics Committee of the First Affiliated Rajah Muthiah Medical College and Hospital, Annamalai University, Annamalai Nagar, Animals were maintained as per the National Guidelines and Protocols approved by the Institutional Animal Ethics committee (Reg. No. 160/ 1999/CPCSEA, Proposal number: 1166 in 2017).
Not applicable.
This research received no external funding.
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
