Antibodies play a vital role in a variety of applications from diagnostics, imaging, and therapeutics. The stability of antibodies is one of the most important key attributes for its prolonged activity and functionality. Here, we demonstrate a detailed comparative study of the molecular stability of the rabbit immunoglobulin G (IgG) and chicken egg yolk immunoglobulin Y (IgY) at different pH, temperatures, and time points. The molecular stability of IgG and IgY is compared on the basis of its binding activity and conformational changes. The optimum temperature and time were found to be 30 °C, and 37 °C, up to 8 h for both IgY and IgG antibodies. With increasing temperature and time, IgG antibodies were found to be significantly less stable in comparison to IgY antibodies. IgY is almost twenty-fold more stable than IgG at 60° C for up to 8 hours. The extra domain present in the heavy chain of IgY plays a significant role in providing more molecular stability as compared to IgG under the above-mentioned experimental conditions. The results show that, as compared with IgG, the IgY are more stable, are less expensive to make in high yield and exhibit minimal conformational changes and hence are cost effective for use in a diverse array of purposes.
Chicken egg yolk immunoglobulin is a Y shaped heterodimer protein molecule that consists of a heavy and light chain, produced by the plasma cells in response to a pathogen invasion (1). Functionally, IgY have a similar function as IgG for providing defense against infectious agents (2). 75% of serum immunoglobulins consist of IgG making it the most abundant class of antibodies (3). IgG consists of two identical copies of light chain (κ or λ class) and heavy chain, and are linked together by a disulfide bond. IgG is also divided into subclasses on the basis of size of hinge region, position of interchain disulfide bonds and its molecular weight (3). However, there is a clear difference in the molecular structure of IgG and IgY antibodies (3–5). Structurally, these antibodies differ at the heavy chain where IgY comprised of higher molecular weight in comparison with IgG (68 kDa for IgY and 50 kDa for IgG). IgY has one extra constant domain (CH4) in its heavy chain, and also lacks a well-defined hinge region present in IgG. Immunological differences between avian IgY and mammalian IgG result in poor cross-reactivity of IgY (2).
Unlike IgG, IgY does not reacts with rheumatoid factors and thus does not activates the complement system (6). In immunoassays, rheumatoid factor (RF) is one of the major interference factors which interact with IgG and result in false–positive results which can be avoided with the integration of IgY antibodies, due to its non-reactivity with Rheumatoid Factor (7). Therefore, IgY has been applied successfully in diagnostic, and therapeutic research (8–11). IgY has a wide range of applications in the field of diagnostics such as detection of new protein spots (2, 12–16), plasma proteome analysis (17), improving the intensity of low-abundance proteins, and increasing the resolution. Additionally, IgY has many other advantages over IgG due to its non-invasive, and inexpensive method for the production of antibodies, which result in high yield at a low cost (18). Repetitive bleeding and pain to laboratory animals can be avoided as the IgY antibodies can be collected from the egg yolk (19–21). IgY was also recommended as an alternate of mammalian antibodies by the European Centre for the Validation of Alternative Methods (ECVAM) (22). In addition, long lasting and high titer of IgY produced in chicken eggs, reduces the requirement of frequent booster injections (23). Egg yolk also can stabilize the IgY at a higher temperature and low pH (24). According to Jacobson et al, IgY has 3-5 times higher immunogenicity, does not increase inflammatory cytokines, and 20 times the immunoglobulin concentration per unit. Moreover, IgY antibodies are non-toxic and have a rapid, highly specific, and local onset of action, which does not stimulate the human complement system, thus preventing non-specific inflammation (21).
Studies were conducted to evaluate the stability of IgY under different acidic conditions, protease digestion, and heat. Chicken IgY was fairly stable under these conditions (25). The structural differences between the two immunoglobulins that may play a part in their different stabilities were inferred from their sequence of amino acids. To apprehend the stability of IgY, we proposed a detailed comparative study to compare the stability of IgY, and IgG antibodies at different pH, temperatures, and time points. Molecular stability and secondary structure of avian IgY was compared with it’s mammalian counterpart IgG based on binding affinity (via ELISA), and conformational change (via CD spectroscopy) at different temperatures, and time intervals. IgY antibodies were observed to be more stable which allows its application in different fields such as diagnostics, immunotherapy, and biomedical research (24, 25). Also, as an important therapeutic product, enhancement in thermal stability, and long half-life facilitates in long term storage and transportation (26).
Bovine serum albumin (BSA), anti-BSA antibodies (IgG and IgY), goat anti-rabbit IgG-HRP, goat anti-chicken IgY-HRP conjugate were obtained from Sigma Chemical Co. USA. 3,3′,5,5′-Tetramethylbenzidine (TMB) was procured from Bangalore Genei, India. Polystyrene ELISA plates were purchased from NUNC (Denmark). Chemical and reagents used for the preparation of buffers were all purchased from Sigma Chemical Co. USA unless otherwise stated.
Anti-BSA IgG and IgY antibodies (1 mg/ml) were treated with 0.1 M NaOH and 1N HCl separately, and pH was adjusted ranging from 11 to13 or 2 to7. Both antibodies were incubated for 1 h at 37 °C and later neutralized with 1x PBS, pH 7.4. The antibody activity was measured by ELISA.
Anti-BSA IgY and IgG antibodies were used at the concentration of 0.01 mg/ml and excited at 296 nm. The fluorescence intensity was measured by Hitachi F-4000 fluorescence spectrophotometer. The wavelength for the emission was analyzed at different concentrations of guanidine hydrochloride (Gu-HCl).
Antibody solution (0.1 mg/ml) in 1x PBS, pH 7.4 was heated at different time intervals (0, 2, 4, 8h) and at different temperatures (30 °C, 37 °C, 45 °C, 60 °C), and activity was measured with ELISA and circular dichroism (CD).
The activity of anti-BSA IgG and IgY was measured by indirect ELISA technique. The studies were done at different temperatures and time intervals (0, 2, 4, 8 h) also taken into consideration. In brief, BSA was coated on a 96 well Elisa plate (Nunc, Denmark) at a concentration of 5 µg/ml in carbonate buffer (50 mM, pH 9.6) by adding 100 µl/well and incubated overnight at 4 °C. After washing twice with 1x PBS, pH 7.4, the non-specific binding sites were blocked with 5% defatted skimmed milk in PBS using 200 µl/well and incubated at 37 °C for 2 h. PBS containing 0.05% Tween-20 (PBST) was used to wash the plates three times followed by washing with only 1x PBS, pH 7.4. The anti-BSA IgG and IgY antibodies, were pre-incubated at different temperatures viz. c and for different time intervals 2 h, 4 h and 8 h. 100 µl/well prepared in PBS containing 0.1% defatted skimmed milk, was added at various dilutions as required and incubated for 2 h at 37 °C. The plates were thoroughly washed thrice with PBST and finally with PBS. HRP labeled anti-IgG/IgY was used as a secondary antibody at a dilution of 1:10,000 and 100 µl/well was added into each well. Plates were incubated for 1 h at 37 °C followed by thorough washing, as in the earlier step to remove any non-specifically bound secondary antibody. The color was developed using TMB/H2O2 as a substrate (100 µl/well). The blue color developed, was stopped after 15 min using 50 µl/well 1N H2SO4 as a stop solution. The absorbance was taken at 450 nm using a BioTek microplate ELISA reader.
CD measurements were performed with JASCO 700 spectrometer coupled to a Peltier temperature controller. Spectra were taken at different temperatures (30 °C, 37 °C, 45 °C, 60 °C) at different time intervals of 0, 2h, 4h, and 8h. The antibody samples were dissolved in phosphate buffer saline to give a final concentration of 0.1 mg/ml. The result was expressed as mean residue ellipticity (θ), which is defined as MRE = 1000 x θ (observed) / C x L, where θ (observed) is observed ellipticity in degrees, C is the concentration of protein and L is path length in cm (25).
Figure 1 is the scheme which shows the experimental workflow, where chicken and rabbit immunoglobulins were used against BSA. The major experimental work is based on ELISA and CD spectra to measure the activity and conformation of IgY and IgG antibodies at different temperatures and time points.
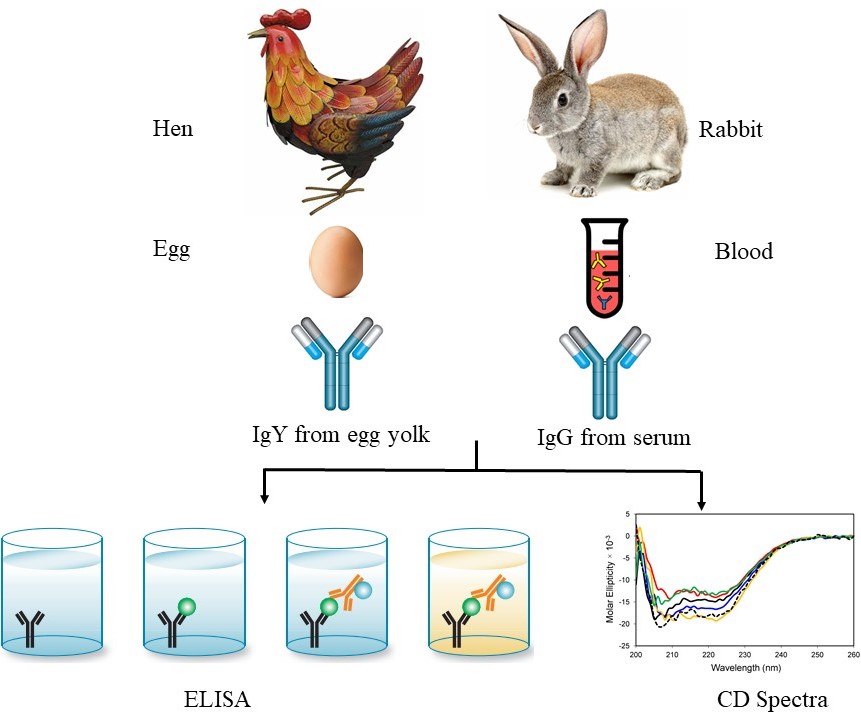 Figure 1
Figure 1Schematic representation of comparative study of stability antibody (IgG and IgY) with respect to their activity and conformational change at different temperature and time intervals; Indirect ELISA assay of antibodies to analyze the binding affinity of antibody at different temperature and time intervals. Absorbance was read at 450 nm using microplate ELISA reader; CD (Circular Dichroism) Spectroscopy of IgY and IgG at different temperature and time points.
The anti-BSA antibodies (IgG and IgY) activity was measured under alkaline and acidic treatment as shown in Figure 2A and B. It was observed that the activity of IgG antibody decreases after pH 8 under alkaline conditions. The pattern of antibody activity profile by ELISA showed a similar profile. Under acidic conditions, IgG showed a decrease in binding at pH 5 in comparison with IgY. The fluorescence emission spectra were also evaluated to observe the conformational changes in IgY and IgG antibodies in the concentration range of 0 to 5 M (Figure 2C). The IgG antibodies showed a maximum change in its spectra in the range of 2 to 3 M, while IgY antibodies were not affected in this range and remained stable. The conformational changes started to occur in a narrow range and very rapidly in IgG as compared to IgY antibodies.
 Figure 2
Figure 2(A) Binding activity of IgG and IgY antibodies at alkaline and (B) acidic pH; (C) Denaturation studies of anti-BSA chicken IgY and rabbit IgG antibodies using guanidine hydrochloride.
Anti-BSA IgG and IgY antibodies were incubated at different temperatures (30 °C, 37 °C, 45 °C, and 60 °C) for 2, 4, and 8 h. The activity of antibodies was measured by ELISA (Figure 3 and 4). As shown in Figure 3 and Figure 4, the activity of IgG and IgY at 30 °C and 37 °C, were the same up to 8 h. With an increase in the temperature from 37 °C to 45 °C, and further upto 60 °C, the activity of IgG antibodies was found to be stable up to 2 h. After 4 h and 8 h, a decrease in the activity was observed of the IgG antibody. On the other hand, the IgY antibodies remained stable at 20-fold higher temperatures up to 60 °C.
 Figure 3
Figure 3Antibody stability was measured with indirect ELISA. (i) IgG (ii) IgY at different temperatures and time intervals. Increasing the temperature upto 60 °C for 2 h does not have any impact on the activity of IgG but with increase in the time from 2 h to 8 h, stability of IgG decreases significantly as compared to IgY.
 Figure 4
Figure 4Stability at different time (2, 4, and 8 h) and temperatures of IgG vs IgY viz. 30 °C, 37 °C, 45 °C, and 60 °C. Figure (A and B) shows that IgG and IgY were very stable at 30 and 37 °C up to 8 h. (C) At 45 °C up to 2 h, IgG and IgY were stable but on increasing time from 4 to 8 h at 45 °C, the binding of IgG antibodies as compared to IgY were found to decrease. (D) Further increase in temperature at 60 °C from 2 to 8 h, there was a drastic decrease in binding of IgG antibodies.
The conformational changes in IgG and IgY antibodies were evaluated as shown in Figure 5. The secondary structures of proteins (IgG and IgY) were determined in the far as well as near UV region (190-250 nm). Thus, by using temperature as a function to measure the ellipticity at 206.5 nm, changes in the α helix, ß turn and random coil contents were monitored. At this wavelength, a positive shift in ellipticity was observed with increase in ß turns, whereas a negative shift was observed with an increase in α helix and random coil contents. The CD spectra as shown in Figure 5, for IgG and IgY indicates a change in the overall secondary structure of the antibody (more in case of IgG as compared with IgY) with an increase in temperature (30° C, 37 °C, 45 °C, 60 °C) and time (2 h, 4 h, and 8 h). At 30 °C and 37 °C, the spectrum indicated the presence of primary alpha coils. The maximum negative shift observed indicated a helical secondary structure. Significant changes in secondary structures were proved by the CD spectra of the IgG and IgY, when there was an increase in the temperature w.r.t time. It was observed that the mean residual ellipticity (MRE) changed towards the positive direction as we increased the temperature from 37 to 60 °C, which was indicative of the increase in β-turns. Very little change was observed when the incubation was given at 30 °C and 37 °C and did not show much deviation in CD spectra of both IgG and IgY. Thus, much of the structural changes arose only at higher temperatures for prolonged period.
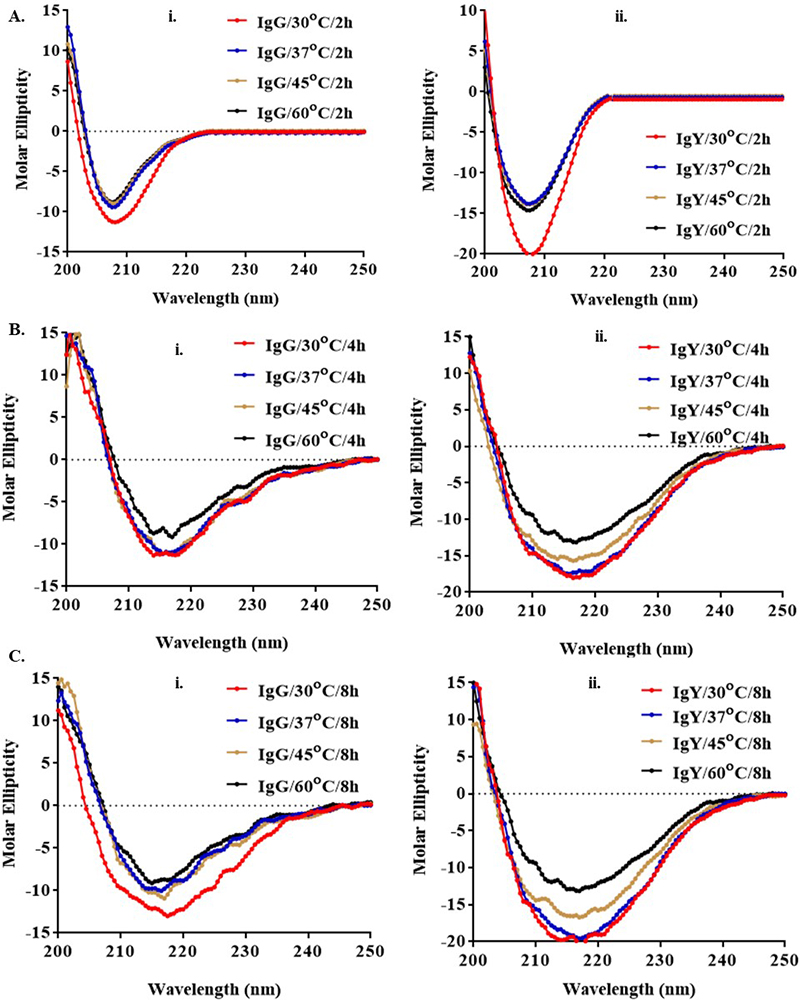 Figure 5
Figure 5CD spectroscopy of (i) IgG and (ii) IgY antibodies at different time (2, 4, and 8 h) and temperatures (30 °C, 37 °C, 45 °C, and 60 °C). Alpha helix shows a decrease while there is an increase in β-turns as the temperature increased. Overall helical backbone was disrupted only to a small extent in case of IgY as compared to IgG and it is the random coil, turns or alpha helical structures that give rise to the observed changes in the CD spectra of the proteins (30 °C used as a control).
Different stability parameters of chicken IgY and rabbit IgG antibodies were measured on the based on their binding activity and conformational changes as shown in Figure 6. Although the stability of chicken IgY and rabbit IgG immunoglobulins was similar, chicken IgY showed higher stability under alkaline and acidic pH. Anti-BSA antibodies were pre-incubated at different temperatures (30 °C, 37 °C, 45 °C, 60 °C), and for different time intervals (4-8 h). The antibodies were analyzed using ELISA assay to check the rate of activity at higher temperature and CD spectra for the change in the percent of alpha and beta-helix. Although the stability of IgG and IgY was found to be similar at 30 and 37 °C with a further increase in temperature and time it leads to a decrease in the activity of IgG while there was no significant effect on the activity and conformation of IgY. A decrease in the activity of IgG indicated the change in the conformational antigen binding sites of IgG at higher temperature.
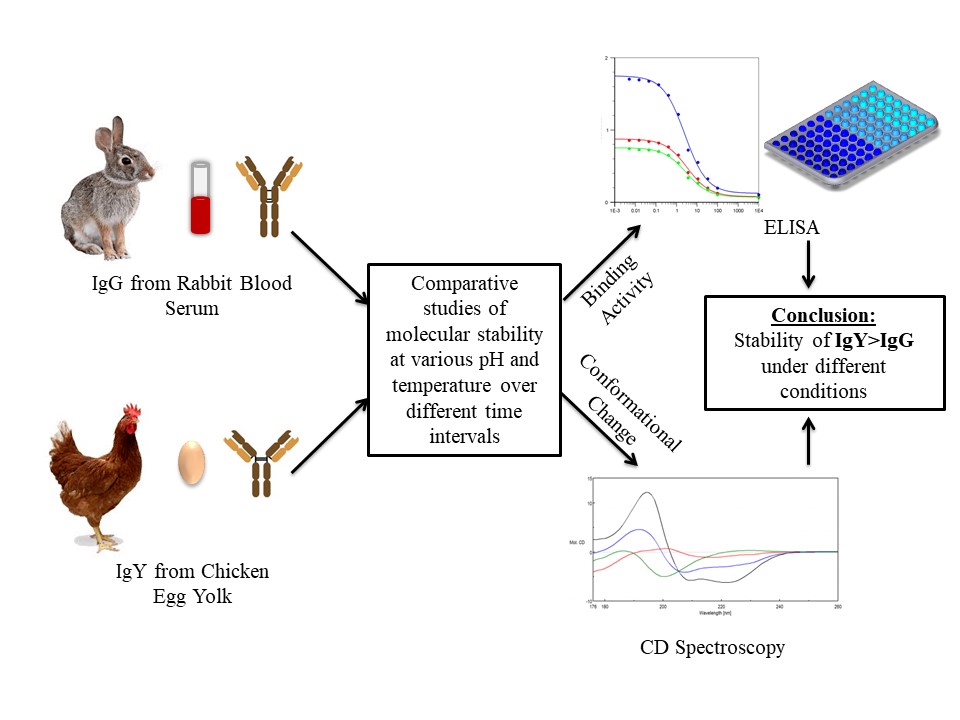 Figure 6
Figure 6The comparative study of the stability of antibody IgG and IgY at different temperatures and pH for varying time periods. Avian IgY antibody has more molecular stability than its mammalian counterpart IgG under different conditions.
Although the mechanisms behind protein denaturation by heat and conformational changes are thought to be different. Heat and guanine hydrochloride-denaturation showed the conformation of IgY to be more unstable then IgG. These results confirmed that the conformation stability of chicken IgY was higher than that of rabbit IgG in any kind of treatment, signifying that the inclusive stability of the chicken IgY molecule was higher than that of the rabbit IgG. From the result of CD spectra of the IgG and IgY the significant changes in secondary structures were observed when there was an increase in the temperature with respect to time. It was observed that the overall helical backbone of IgG was more disrupted in comparison to IgY. Hence from the above data, we concluded that avian IgY is more stable when compared to its mammalian counterpart, IgG. The experimental information about the structural properties that provide stability to IgY is unknown. Also, the structural factors that provide stability to IgG and IgY are unknown, as antibodies are bulky complex biomolecules with heterogenous peptides containing heavy and light chains. With the obtained data, we conclude that chicken anti-BSA IgY antibodies are more stable and can tolerate harsh conditions such as acidic, alkaline, and heat. IgY antibody is important for the welfare of immunized animals due to its non-invasive production as well as simple egg collection. Additionally, isolation of IgY from egg yolk is a simple and fast procedure in comparison to IgG, therefore IgY technology can be used as a good alternative to conventional IgG polyclonal antibody production in animal.
There is no conflict of interest among the authors. The authors are thankful and acknowledge the research grant from Science and Engineering Research Board (SERB-ECR/2016/000075), New Delhi, India and Department of Biotechnology (DBT-Biocare-BT/PR18069/BIC/101/574/2016), New Delhi, India for providing support in carrying out this research work. The authors would also like to extend their sincere appreciation to researchers supporting project at King Saud University for funding this research (RSP-2020/29). The present work was done completely at Amity Institute of Biotechnology, Amity University, Noida, India. The corresponding author is working presently at National Institute of Biotechnology, Hyderabad, India.
IgG
Immunoglobulin G
Rheumatoid factor
European Centre for the Validation of Alternative Methods
Bovine serum albumin
Immunoglobulin Y
Enzyme-Linked Immunosorbent Assay
Phosphate Buffer Saline
Guanidine hydrochloride
Hydrochloric Acid
Sodium hydroxide
3,3’,5,5’- Tetramethylbenzidine
Horseradish peroxidase
