1 Department of Reproductive Medicine Center, Changzhou Maternal and Child Health Care Hospital, Changzhou Medical Center, Nanjing Medical University, 213000 Changzhou, Jiangsu, China
2 Department of Gynecology and Obstetrics, Xuancheng City Central Hospital, 242000 Xuancheng, Anhui, China
3 Department of Pharmacy, Changzhou Maternal and Child Health Care Hospital, Changzhou Medical Center, Nanjing Medical University, 213000 Changzhou, Jiangsu, China
Abstract
In males with extremely severe oligospermia (MESO), single density gradient centrifugation (SDGC) has low sperm enrichment efficiency, making intracytoplasmic sperm injection (ICSI) challenging. This study aimed to determine whether double density gradient centrifugation (DDGC) can efficiently enrich sperm from MESO samples and whether these sperm are safe for clinical use.
MESO was defined as having ≤2000 motile sperm/mL of semen, whereas males with severe oligospermia (MSO) were defined as having 2000–10,000 motile sperm/mL. We compared sperm recovery between SDGC and DDGC in MESO samples and retrospectively analyzed in vitro fertilization (IVF) data from 39 MESO cases (sperm prepared using DDGC) and 78 MSO cases (sperm prepared using SDGC) collected from 2017 to 2023. The SDGC group served as the control group.
The results showed that the sperm recovery rate of DDGC was approximately three-fold higher than that of SDGC in MESO samples. We hypothesized that in normal semen samples, sperm aggregate into a pellet during centrifugation, enabling efficient enrichment by SDGC. In MESO, where sperm count is extremely low, sperm fail to form a pellet, leading to slower sedimentation and lower recovery rates with SDGC, thereby necessitating additional centrifugation. Importantly, sperm prepared by DDGC from MESO semen samples showed comparable in vitro and in vivo embryo developmental parameters to sperm prepared by SDGC. Interestingly, the DDGC group showed a significantly higher usable blastocyst formation rate compared to SDGC group (73.48% vs. 62.63%, p = 0.009).
In conclusion, DDGC can effectively enrich sperm from MESO samples, and no obvious adverse clinical outcomes were observed. The method is simple, requires no additional equipment, and may be considered as a routine sperm preparation technique for MESO in clinical use. However, the long-term safety of using DDGC for sperm preparation from MESO for ICSI still requires further confirmation, and more effective methods for sperm enrichment from MESO are needed.
Keywords
- double density gradient centrifugation
- embryo development
- intracytoplasmic sperm injection
- oligospermia samples
- sperm preparation
In the present study, males with extremely severe oligospermia (MESO) were defined as having
Commonly, due to its high sperm recovery rate, density gradient centrifugation (DGC) is widely adopted for sperm preparation in reproductive centers worldwide, including ours [4, 5]. In most cases, a single DGC (SDGC) is sufficient to obtain sufficient sperm for ICSI, despite a low recovery rate. However, for MESO, the sperm recovery rate using SDGC is very low, making it challenging to find morphologically good sperm for ICSI. We previously demonstrated that double DGC (DDGC) significantly increases the sperm recovery rate from poor-quality semen samples for conventional in vitro fertilization, including those with reduced motility, poor liquefaction, or excessive clots and fibers [6]. Thus, we speculated that DDGC may be a promising strategy for improving sperm recovery rate in MESO. However, whether DDGC may enhance sperm recovery rate from MESO for use in ICSI still needs further investigation.
Another concern is that the safety of using DDGC for ICSI in MESO remains unclear. Previous studies have indicated that a second round of DGC may introduce additional mechanical stress on sperm, compromising their quality and potentially impairing both in vitro and in vivo embryo development [7, 8]. Although we previously showed the clinical safety of using sperm prepared by DDGC in combination with swim-up (SU) procedure [6], there are two main differences between that study and the present one. First, the SU procedure itself can further select sperm [9]. In the present study, however, the extremely low sperm density in MESO prevented the use of SU procedure. Second, sperm from MESO may exhibit functional defects [10]. The additional mechanical stress caused by a second DGC step may further compromise these already “fragile sperm”, potentially impairing embryo development. Therefore, the safety of using DDGC-prepared sperm from MESO without a SU procedure for ICSI remains uncertain and warrants further investigation.
In the present study, we first compared sperm recovery efficiency between the SDGC and DDGC methods using MESO samples to determine whether DDGC could effectively enrich sperm from these samples. We then retrospectively analyzed in vitro fertilization data from 39 MESO cases (sperm prepared by DDGC, DDGC group) and 78 MSO cases (sperm prepared by SDGC, SDGC group) collected between 2017 and 2023 at the Reproductive Center of Changzhou Maternal and Child Health Care Hospital to determine whether sperm processed by DDGC from MESO samples are safe for clinical use. This study may be the first to provide a routine IVF sperm preparation method for MESO.
MESO was defined as having
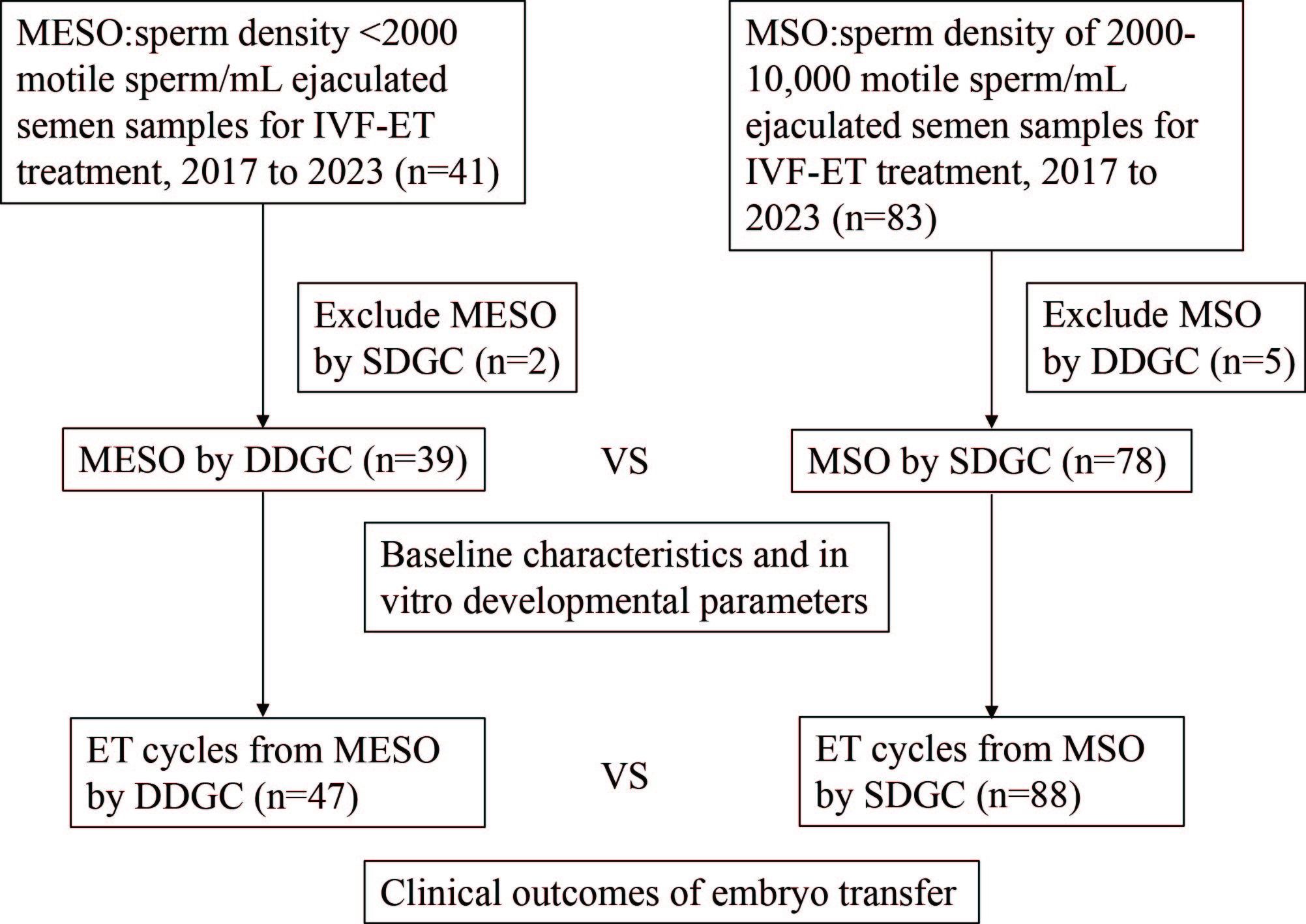 Fig. 1.
Fig. 1. Flowchart of data collection and analysis. IVF, in vitro fertilization; ET, embryo transfer; MESO, males with extremely severe oligospermia; MSO, males with severe oligospermia; SDGC, single density gradient centrifugation; DDGC, double density gradient centrifugation; n, number of cases.
(1) MESO cases with sperm density
(2) MSO cases with sperm density of 2000–10,000 motile sperm/mL of ejaculated semen samples that underwent IVF-ET treatment were included (n = 83).
(3) Semen samples from MESO prepared by SDGC were excluded (n = 2).
(4) Semen samples from MSO prepared by DDGC were excluded (n = 5).
Due to the extremely low concentration of motile sperm in MESO and MSO samples, motile sperm were counted manually. After liquefaction, a 10 µL aliquot of fresh semen was placed on a microscope slide and covered with a coverslip. The entire area under the coverslip was examined at 200
Fresh semen samples were collected from males after 2–5 days of abstinence. SDGC and DDGC were performed as previously described [6]. Briefly, for SDGC, semen samples were loaded onto a density gradient column consisting of 1 mL each of the lower layer and the upper layer (99264, FUJIFILM Irvine Scientific, Santa Ana, CA, USA). The lower layer consists of 90% suspended colloidal silicon dioxide particles, while the upper layer contains 50% suspended colloidal silicon dioxide particles. The tube was then centrifuged at 462 g for 15 min (Andreas Hettich GmbH & Co. KG, Nantong, Jiangsu, China). After centrifugation (370
Oocytes were retrieved 36–38 hours post-trigger with human chorionic gonadotropin (HCG). After ICSI, the injected oocytes were cultured in G1 medium (10128, Vitrolife, Kungsbacka, Sweden) for further development. At 18 h post-insemination (day 1 of culture), zygotic embryos were examined under a microscope. Zygotic embryos with two pronuclei were considered normally fertilized. On day 3 of culture, embryos were morphologically scored according to previously described criteria [11]. In this study, grade I and II embryos were defined as top embryos, whereas grade III embryos were defined as non-top embryos. Viable day-3 embryos were either frozen by vitrification, transferred, or cultured. For blastocyst culture, day-3 embryos were placed in G2 medium (10132, Vitrolife, Kungsbacka, Sweden). The outcomes of extended culture were examined on days 5 and 6. Blastocysts were scored using the Gardner system [11], and viable blastocysts were either vitrified or transferred.
Data were analyzed using SPSS software (version 21, IBM Corp., Chicago, IL, USA). Continuous data were first examined by the normality and lognormality test (Shapiro-Wilk test). Normally distributed data were compared using Student’s t-test and are expressed as mean
Our results showed that sperm enrichment for MESO using DDGC was approximately three-fold higher than that of SDGC when using the same semen samples (Fig. 2A). Compared with SDGC, DDGC significantly increased the sperm recovery rate in MESO samples (Fig. 2B), resulting in a significant increase in motile sperm density (Fig. 2C).
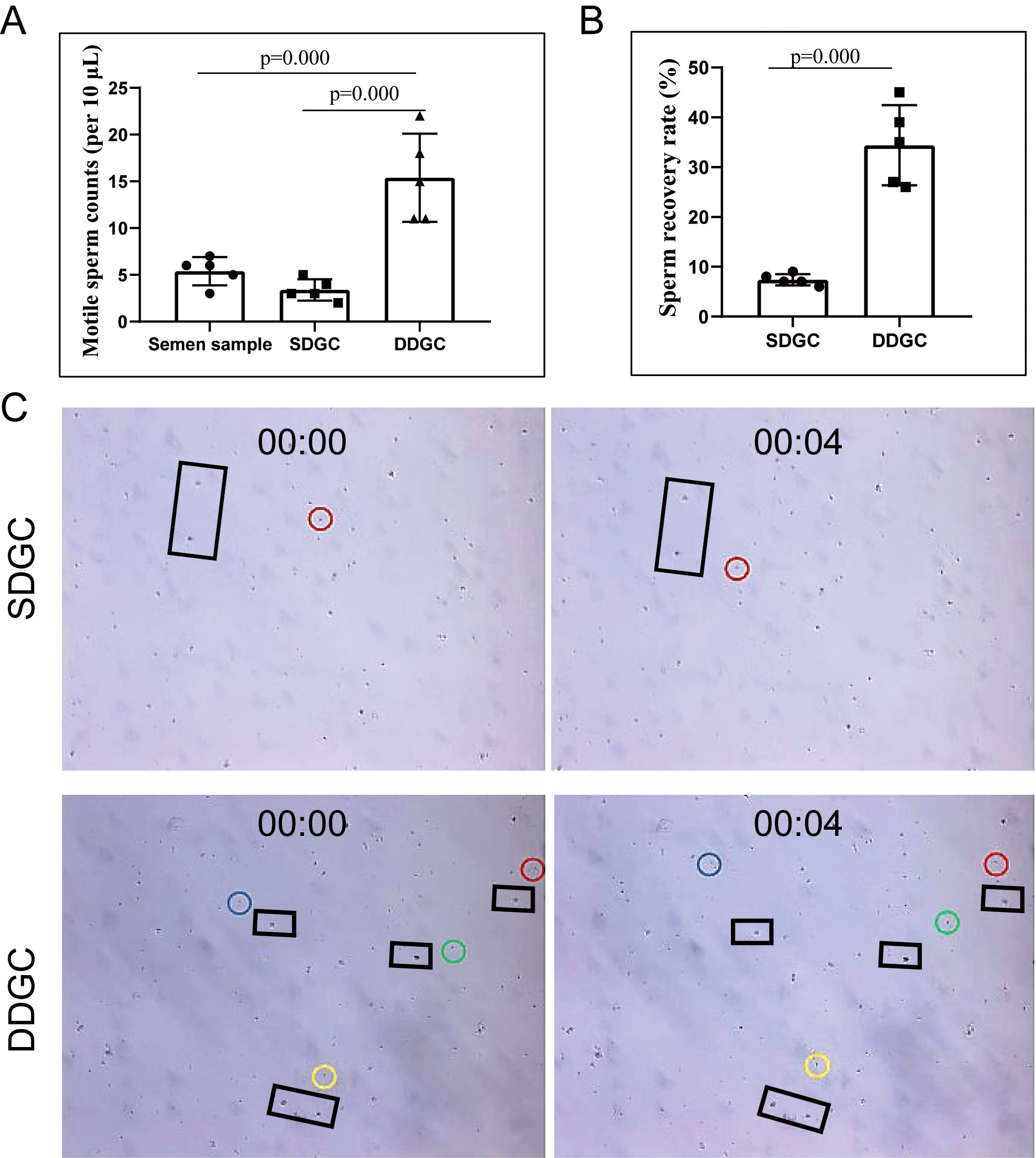 Fig. 2.
Fig. 2. DDGC increases sperm enrichment efficiency from semen samples of MESO patients. (A) Density of motile sperm in the original semen following SDGC and DDGC. (B) Sperm recovery rate following SDGC and DDGC treatment. (C) 10 microliters of semen samples following SDGC and DDGC treatment were smeared on slides and videotaped under a microscope. Photographs showing motile sperm at 0 second and 4 second. Circles indicate motile sperm, and black rectangles indicate reference objects. The same circle color indicates the same sperm. N = 5 for each group.
Baseline patient characteristics, including the percentage of primary infertility, infertility duration, paternal or maternal age, BMI, and ovulation induction protocols, were comparable between the two groups (Table 1). However, the ovarian reserve in the DDGC group was higher than in the SDGC group, as indicated by higher 17
| SDGC (n = 78) | DDGC (n = 39) | p | ||
| Primary infertility (%) | 49 (62.82) | 24 (61.54) | 0.893c | |
| Infertility duration (years) | 2.00 [1.00, 4.00] | 3.00 [2.00, 5.00] | 0.154a | |
| Age (years) | ||||
| Maternal | 31.00 [29.00, 37.00] | 31.00 [28.00, 35.00] | 0.117a | |
| Paternal | 30.00 [27.00, 34.00] | 29.00 [27.00, 33.00] | 0.351a | |
| BMI (kg/m2) | ||||
| Maternal | 22.90 [19.98, 25.20] | 22.40 [19.60, 24.60] | 0.728a | |
| Paternal | 25.20 [22.70, 28.30] | 25.05 [21,00 28.43] | 0.611a | |
| GnRH analogues (%) | 0.084c | |||
| Agonist | 63 (80.77) | 27 (69.23) | ||
| Antagonist | 8 (10.26) | 10 (25.64) | ||
| Mild stimulation | 7 (8.97) | 2 (5.13) | ||
| E2 on the trigger day (ng/L) | 2412.00 [1392.00, 4101.00] | 3008.00 [1583.00, 6233.00] | 0.157a | |
| Oocytes retrieved | 10.01 | 12.21 | 0.067b | |
| MII oocytes | 8.00 [4.75, 11.25] | 11.00 [6.00, 14.00] | 0.061a | |
| MII oocytes rate (%) | 661/781 (84.64) | 410/476 (86.13) | 0.468c | |
| Normal fertilization rate (%) | 488/661 (73.83) | 317/410 (77.32) | 0.199c | |
| Top day-3 embryo formation rate (%) | 326/488 (66.80) | 218/317 (68.77) | 0.560c | |
| Usable blastocyst formation rate (%) | 176/281 (62.63) | 169/230 (73.48) | 0.009c | |
Data are presented as the median [first quartile, third quartile], or count (percentage), or mean
a Mann-Whitney U test.
b Student’s t-test.
c Pearson’s chi-square test.
MII oocytes refer to mature oocytes. SDGC, single density gradient centrifugation; DDGC, double density gradient centrifugation; GnRH, gonadotropin-releasing hormone; E2, 17
Due to differences in embryo developmental potential, day-3 embryos and blastocysts were analyzed separately. Because of the higher number of oocytes retrieved in the DDGC group, more day-3 embryos were available for blastocyst culture, resulting in more blastocyst transfer cycles (Table 2). The number of embryos transferred, clinical pregnancy rate, abortion rate, and live birth rate were comparable between the two groups for both day-3 embryo and blastocyst transfers, respectively (Table 2). The offspring sex ratio and singleton pregnancy rate were also comparable between the two groups (Table 2). Interestingly, gestational age was longer and live birth weight was higher in the DDGC group than in the SDGC group for both singleton and twin births (Table 2). However, only the difference in gestational age for twin pregnancies between the SDGC and DDGC groups was statistically significant (Table 2).
| SDGC (n = 88) | DDGC (n = 47) | p | ||
| ET cycles (%) | ||||
| Day-3 embryos | 56 (63.60) | 16 (34.00) | 0.001b | |
| Blastocysts | 32 (36.40) | 31 (66.00) | ||
| Embryos per transfer | ||||
| Day-3 embryos | 2 [1.00, 2.00] | 2 [2.00, 2.00] | 0.126a | |
| Blastocysts | 1 [1.00, 1.00] | 1 [1.00, 2.00] | 0.556a | |
| Clinical pregnancy rate (%) | ||||
| Day-3 embryos | 27 (48.21) | 10 (62.50) | 0.313b | |
| Blastocysts | 24 (75.00) | 19 (61.29) | 0.243b | |
| Abortion rate (%) | ||||
| Day-3 embryos | 4 (14.81) | 1 (10.00) | 1.000c | |
| Blastocyst | 2 (8.33) | 1 (5.26) | 1.000c | |
| Livebirth rate (%) | ||||
| Day-3 embryos | 19 (33.93) | 9 (56.25) | 0.106b | |
| Blastocysts | 21 (65.63) | 17 (54.84) | 0.382b | |
| Pregnancies (%) | 0.505c | |||
| Singleton | 32 (80.00) | 23 (88.50) | ||
| Twin | 8 (20.00) | 3 (11.50) | ||
| Sex (%) | 0.537b | |||
| Female | 23 (47.90) | 16 (55.20) | ||
| Male | 25 (52.10) | 13 (44.80) | ||
| Gestational age (day) | ||||
| Singleton | 271 [262.30, 276.80] | 275 [265.00, 281.00] | 0.149a | |
| Twin | 242.5 [236.30, 253.30] | 260 [255.00, 265.00] | 0.049a | |
| Birth weight (g) | ||||
| Singleton | 3135 [2863.00, 3388.00] | 3400 [3140.00, 3520.00] | 0.064a | |
| Twin | 2350 [1933.00, 2438.00] | 2475 [2195.00, 2850.00] | 0.254a | |
Data are presented as the median [first quartile, third quartile], or count (percentage).
a Mann-Whitney U test.
b Pearson’s chi-square test.
c Fisher’s exact test.
ET, embryo transfer.
Considering the differences in baseline characteristics, we adjusted for confounding factors, including female age, type of infertility, female BMI, number of oocytes retrieved, number of embryos transferred, and embryo stage, to evaluate whether DDGC for MESO affects clinical outcomes. Our results showed that there were no significant differences in clinical pregnancy rate, abortion rate, or live birth rate between DDGC and SDGC groups (Table 3). Detailed analyses are provided in Supplementary Tables 1–3.
| SDGC (MSO) | DDGC (MESO) | p | |
| Clinical pregnancy rate | Ref (1) | 0.701 (0.283–1.736) | 0.443 |
| Abortion rate | Ref (1) | 0.850 (0.131–5.516) | 0.865 |
| Live birth rate | Ref (1) | 0.984 (0.422–2.294) | 0.970 |
Adjusting for female age, type of infertility, female BMI, oocytes retrieved, number of embryos transferred, stage of embryos.
In normal semen samples with sufficient motile sperm, centrifugation produces a compact sperm pellet due to centrifugal force, leading to accelerated sedimentation and a high recovery rate with SDGC (Fig. 3). In contrast, MESO samples have a low sperm count, making it impossible to for a pellet (Fig. 3). As such, it leads to slower sedimentation and lower recovery rate (Fig. 3). Therefore, a second DGC is necessary to effectively enrich sperm from MESO (Fig. 3). Our current data indicate that sperm prepared using DDGC do not result in adverse clinical outcomes.
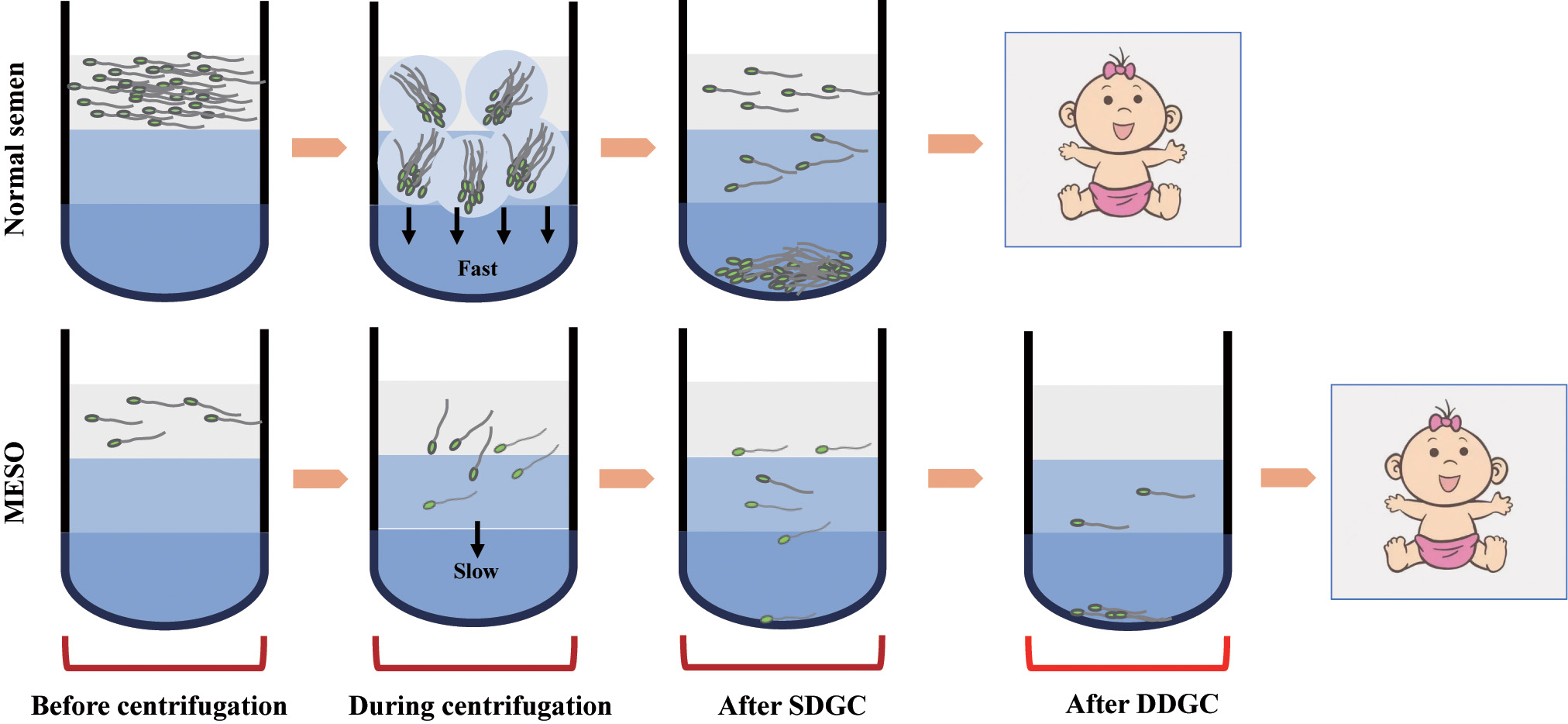 Fig. 3.
Fig. 3. Schematic model of sperm enrichment from semen samples of normal males and MESO patients by density gradient centrifugation. Due to the extremely low sperm density in semen samples from MESO patients, the “agglomeration effect” of sperm is absent, making SDGC ineffective for sperm enrichment.
MESO is a rare clinical condition, affecting approximately 0.6% of couples undergoing IVF at our reproductive center. Obtaining adequate motile sperm from MESO samples is crucial for the success of ICSI. In this study, we demonstrated that DDGC significantly increased the sperm recovery rate from MESO samples, meeting the requirements for ICSI. Importantly, no adverse effects on in vitro or in vivo embryo development were observed when using DDGC-prepared sperm from MESO.
In normal semen samples with adequate sperm counts, sperm cells aggregate to form a pellet during centrifugation, which accelerates sedimentation and allows enrichment using SDGC. In contrast, in semen samples from MESO, a low sperm count prevents the pellet formation, leading to a very slow sedimentation rate and ineffective enrichment by SDGC. Although SDGC is widely used for sperm enrichment, it fails to produce satisfactory results in MESO cases. Consistent with our previous study [6], the present study showed that DDGC significantly increased the sperm recovery rate from MESO samples, yielding post-purification sperm densities more than three-fold higher than pre-purification levels, providing adequate sperm for ICSI.
A potential concern is whether additional centrifugation could damage the sperm, thereby compromising the embryo developmental potential. To avoid the potential failure of sperm acquisition from MESO using SDGC, our center routinely uses DDGC for sperm enrichment in these cases. However, SDGC is still used for sperm enrichment in MSO cases at our reproductive center. Therefore, SDGC for MSO was used as the control in this study. The general condition of the couples was comparable, although the ovarian reserve was smaller in the SDGC group than in the DDGC group. Since previous studies, including ours, have reported that ovarian reserve does not affect oocyte quality [12, 13], this difference is unlikely to introduce bias. We found that the normal fertilization rate and top-quality day-3 embryo formation rate were similar between the SDGC and DDGC groups. Surprisingly, the usable blastocyst formation rate was significantly higher in the DDGC group than in the SDGC group. We observed that maternal age was lower in the DDGC group, although not statistically significant. Therefore, the higher usable blastocyst formation rate in the DDGC group may be due to younger female age. Overall, these results indicated that DDGC in MESO patients does not impair in vitro embryo developmental potential compared to SDGC in MSO patients.
It has been reported that semen quality is not associated with clinical pregnancy rates [14, 15]. However, poor semen quality has been reported to be associated with pregnancy loss [16, 17]. If the additional round of DGC truly damaged sperm in MESO samples, the DDGC group would be expected to show lower clinical pregnancy and live birth rates, along with a higher abortion rate. However, the clinical pregnancy rate, live birth rate, and abortion rate were comparable between the SDGC and DDGC groups, even after adjusting for confounding factors. Interestingly, gestational age and birth weight were higher in DDGC group for both singleton and twin births, with gestational age in twin pregnancies being significantly longer (p
Although increased mechanical stress has been reported to damage sperm organelles and membranes [7, 8, 22, 23, 24], it has also been shown that simultaneous removal of the sperm plasma membrane and acrosome before ICSI improves oocyte activation and embryonic development [25], indicating a central role of sperm DNA. We hypothesize that an additional round of centrifugation is unlikely to cause sperm DNA damage, as DNA is highly compacted within the sperm head. This hypothesis is further supported by a previous study showing that over-centrifugation of mouse sperm did not affect fertilization rates [26]. Additionally, it is reasonable to deduce that if sperm membrane integrity were compromised by the additional DGC, sperm motility would likely be affected. During ICSI, only morphologically normal sperm with good motility are selected for injection. Therefore, even if additional DGC increases the proportion of damaged sperm, the sperm selected for injection is unlikely to be damaged.
To the best of our knowledge, our study is the first to focus on sperm enrichment from MESO semen samples. Although emerging sperm preparation techniques such as microfluidic sperm sorting, electrophoretic sperm selection, and magnetic-activated cell sorting (MACS) are gaining attention [27, 28], these methods aim to isolate healthier sperm while minimizing mechanical stress, rather than improving sperm recovery rates [27]. While MACS has demonstrated recovery rates comparable to or exceeding those of conventional methods like DGC [29], its efficiency depends heavily on forward sperm progression [30], raising uncertainty about its suitability for processing MSO or MESO semen samples. Similarly, electrophoretic sperm selection has been reported to achieve only half of the recovery rate of DGC [31]. A study combining SU or DGC with MACS have shown improved sperm quality, but also a significant reduction in total sperm count and in the proportion of rapidly progressive spermatozoa [32]. Although advanced techniques may enhance sperm quality, their impact on IVF outcomes remains inconclusive [33]. Moreover, these methods are used for conventional semen samples rather than MESO, and formal studies on sperm enriching from MSO or MESO are lacking.
Another strength of the present study is that DDGC has been routinely used for sperm enrichment from MESO at our reproductive center. After implementing DDGC, concerns about failing to obtain sufficient sperm from MESO for ICSI are eliminated. In addition, DDGC does not require additional equipment and is a simple method that meets clinical requirements.
The present study presents several limitations. First, due to its retrospective nature, there are possibly important confounding factors that may not have been considered. Second, due to the low incidence of MESO, the sample size in the present study was limited, which may affect the reliability of the results we obtained, such as the abortion rate. Third, the sperm concentration differed between the SDGC and DDGC groups. However, we believe this difference may not affect the overall conclusion. It is unlikely that the sperm quality in MESO is superior to that in MSO. The observation that sperm from MESO, which are unlikely to be of better quality than those from MSO, showed similar clinical outcomes after DDGC to MSO processed by SDGC further supports the safety of using sperm from MESO processed by DDGC. Fourth, due to the very low sperm density, we were unable to measure sperm membrane integrity, acrosome status, or DNA fragmentation before and after DDGC. Therefore, it remains uncertain whether the additional centrifugation force may introduce further mechanical stress. Lastly, although we did not observe any adverse effects of DDGC in our study, the long-term safety of offspring conceived using this method still needs to be confirmed.
In conclusion, our study demonstrated that DDGC can effectively enrich sperm from MESO semen samples. Importantly, DDGC did not appear to compromise either in vitro or in vivo embryo development, supporting the preliminary safety and feasibility of this method. These findings hold significant clinical value as they provide a simple, efficient, and safe method for sperm enrichment for MESO. Therefore, DDGC could potentially be adopted as a routine sperm preparation method for MESO in IVF centers. Nevertheless, the long-term safety of offspring conceived via DDGC remains to be established, and the development of more effective methods for sperm enrichment from MESO is still needed.
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
XD, YW and LC designed the research study. LL, QW and XL performed the research. LL, QW, XY and YW analyzed the data. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
All couples included in the study read and signed the informed consent forms. This study was approved by the Ethics Committee of Changzhou Maternal and Child Health Care Hospital 2023[45]. All treatments were performed in strict accordance with the Declaration of Helsinki for Medical Research.
Not applicable.
This work was supported by the Cutting-edge technology project (QY202405), and the Changzhou Municipal Health Commission Youth Science and Technology Project (QN202241).
The authors declare no conflict of interest.
During the preparation of this work, the authors used ChatGPT-3.5 to check spelling and grammar. After using this tool, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
Supplementary material associated with this article can be found, in the online version, at https://doi.org/10.31083/CEOG43582.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.



