1 Department of Obstetrics and Gynecology, West China Second University Hospital of Sichuan University, 610041 Chengdu, Sichuan, China
2 Key Laboratory of Birth Defects and Related Diseases of Women and Children (Sichuan University), Ministry of Education, 610041 Chengdu, Sichuan, China
†These authors contributed equally.
Abstract
Ectopic pregnancy is a major early-pregnancy cause of maternal mortality, and hysteroscopy is the gold standard for uterine cavity assessment, offering direct visualization, accurate pathology, easy biopsy, and immediate therapeutic intervention. However, no studies have evaluated whether hysteroscopy improves subsequent pregnancy outcomes in infertile women with a prior ectopic pregnancy. This study aimed to evaluate the necessity of routine office hysteroscopy prior to the first embryo transfer in infertile women with a history of ectopic pregnancy, based on the hypothesis that hysteroscopy may assist in identifying intrauterine pathologies that could impact pregnancy outcomes.
We conducted a single-center retrospective cohort study including consecutive patients with a history of ectopic pregnancy at a university-affiliated hospital between January 2018 and December 2022. Patients were divided into two groups according to whether they underwent hysteroscopy prior to embryo transfer. Propensity score matching (PSM) was applied to balance baseline characteristics between the groups.
A total of 714 patients were included in the analysis. Following PSM, no significant differences in baseline characteristics were observed between the two groups. The clinical pregnancy rate was 58.26% in the hysteroscopy group and 53.22% in the non-hysteroscopy group (p = 0.397). Subgroup analysis revealed that patients diagnosed with and treated for chronic endometritis (CE) exhibited a higher spontaneous miscarriage rate (46.90%) and a lower live birth rate (25.00%) compared to the disease-free group (miscarriage rate 18.00%, live birth rate 45.61%), the endometrial polyps (EP) group (miscarriage rate 10.00%, live birth rate 52.31%), and CE + EP group (miscarriage rate 25.00%, live birth rate 44.26%).
Routine hysteroscopy prior to first embryo transfer in women with a history of ectopic pregnancy did not significantly improve clinical pregnancy rates. However, hysteroscopy proved valuable in identifying intrauterine abnormalities such as CE and EP, which were associated with adverse reproductive outcomes. Further prospective studies are warranted to determine whether targeted diagnosis and management of these conditions can improve live birth rates in this population.
Keywords
- hysteroscopy
- ectopic pregnancy history
- chronic endometritis
- endometrial polyps
- embryo transfer
Ectopic pregnancy is a critical gynecological emergency and a leading cause of maternal mortality in early pregnancy, affecting 1% to 2% of cases [1]. Ectopic pregnancy is defined as the occurrence of a pregnancy outside the uterine cavity, most commonly within the fallopian tube (96%) [2]. Despite numerous studies, many cases still lack identifiable risk factors. Established risk factors for tubal ectopic pregnancy include alterations in the tubal environment, impaired transport of the embryo through the tube, smoking, and advanced maternal age [3, 4]. The impact of various interventions for ectopic pregnancy on subsequent fertility outcomes has been inconsistent across studies. Most research has shown no significant difference in subsequent pregnancy rates, the risk of recurrent ectopic pregnancy, or time to next conception among expectant management, methotrexate (MTX) administration, and salpingectomy [5, 6, 7].
As there is a growing interest in reproductive outcomes for patients with a history of ectopic pregnancy who are undergoing assisted reproductive technology (ART), it is vital to understand the impact of each treatment method on pregnancy outcomes. This knowledge will help individuals with fertility concerns and a history of ectopic pregnancy choose the most appropriate therapeutic approach and preventative measures to reduce the risk of recurrence. Previous studies have explored various factors such as different embryo stages, transfer cycles, time to pregnancy, and treatments for ectopic pregnancies [8, 9, 10]. However, there is a gap in the literature regarding the use of hysteroscopy to assess the uterine environment before embryo transfer. Major uterine cavity abnormalities can be found in 10% to 15% of infertile women, with endometrial polyps (EP) being the most common acquired abnormality that could negatively affect fertility [11]. Another significant uterine abnormality is chronic endometritis (CE), a persistent inflammatory condition diagnosed histologically by the presence of plasma cells in the endometrium [12]. CE is linked to infertility of unknown etiology, repeated implantation failure, recurrent pregnancy loss, and maternal/neonatal complications [13, 14]. Approximately 30%–60% of infertile women with a history of repeated implantation failure (RIF) are found to have CE [15]. While evidence indicates the effectiveness of antibiotic administration to cure CE, concerns remain regarding its impact on reproductive outcomes [16]. Some researchers have shown that CE, even when successfully treated with antibiotics, is still associated with an increased risk of spontaneous miscarriage in women undergoing in vitro fertilization (IVF)/intracytoplasmic sperm injection (ICSI)-embryo transfer (ET) treatment [17].
Various techniques are used to detect abnormalities in the uterine cavity, including transvaginal ultrasonography (TVS), hysterosalpingography (HSG), saline hysterosonography, magnetic resonance imaging (MRI), and hysteroscopy. Hysteroscopy is considered the gold standard procedure for assessing the uterine cavity, and has the advantages of offering direct visual assessment, accurate pathological diagnosis, convenient biopsy procedures, and immediate therapeutic intervention [18]. To our knowledge, no study has investigated whether hysteroscopy administration enhances pregnancy outcomes in infertile women with a history of previous ectopic pregnancy. This study aims to determine whether performing a hysteroscopy to evaluate the uterine cavity before the first embryo transfer is necessary in patients with a history of ectopic pregnancy. By addressing this question, we aim to contribute to the clinical guidelines for the management of infertile women with a history of ectopic pregnancy.
We retrospectively reviewed the records of all women treated at the Reproductive Medicine Centre, West China Second University Hospital, Sichuan University for ART treatment between January 2018 and December 2022. Inclusion criteria for participation were patients with a history of tubal ectopic pregnancy, whether treated surgically or conservatively, who underwent their first cycle of fresh or frozen embryo transfer. The exclusion criteria included: (1) a previous ectopic pregnancy following ART, (2) the cycles involving preimplantation genetic diagnosis and screening; and (3) any abnormal ultrasound findings before embryo transfer that could impact the assessment of the uterine cavity. The rationale for these exclusions was to concentrate the study on the effects of hysteroscopy within a more homogeneous group, thereby reducing variability from factors unrelated to the condition of the uterine cavity. The study flow is presented in Fig. 1.
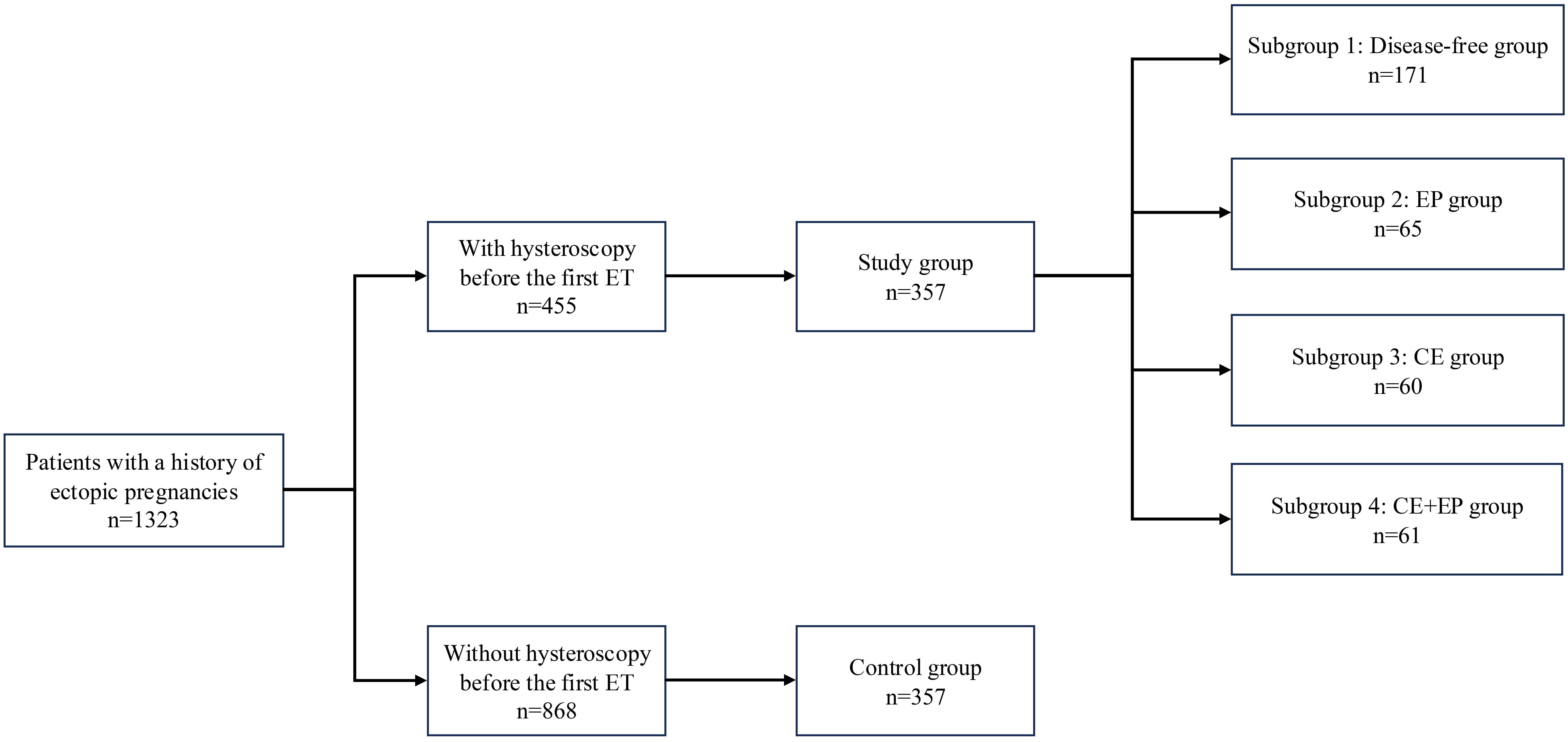 Fig. 1.
Fig. 1. Flowchart of the included population. ET, embryo transfer; EP, endometrial polyps; CE, chronic endometritis.
To account for potential confounding factors, we applied PSM using a multivariable logistic regression model based on age, body mass index (BMI), anti-Müllerian hormone (AMH) level, number of embryos transferred, and stage of embryos (whether they were cleavage-stage embryos or blastocysts). We also considered the type of treatment for ectopic pregnancy, which included salpingectomy, salpingostomy, or conservative treatment. This method enabled us to create pairs of patients with previous ectopic pregnancy, ensuring that those who underwent hysteroscopy were matched to those who did not, based on similar characteristics. The PSM was performed using 1:1 greedy nearest neighbor matching within a propensity score (PS) score range of 0.008 to balance the baseline characteristics and improve the comparability between groups.
In our study design, the patients in the study group were assigned to four subgroups based on hysteroscopic findings and pathological diagnosis: disease-free group, EP group, CE group, and CE plus EP (CE + EP) group.
Controlled ovarian hyperstimulation (COH) was performed using various protocols, including the gonadotropin-releasing hormone (GnRH) agonist long protocol, GnRH agonist short protocol, and GnRH antagonist protocol. These protocols were tailored to the patient’s age, ovarian reserve, and previous responses to ovarian stimulation. Human chorionic gonadotropin (hCG) (Lizhu Pharmaceutical Trading, Zhuhai, Guangdong, China) was administered when three or more follicles reached a diameter of 16–18 mm or greater. For patients at high risk for ovarian hyperstimulation syndrome (OHSS), hCG was used in combination with leuprolide acetate to trigger ovulation. The oocyte retrieval procedure was conducted 36 to 38 hours post-hCG administration, using transvaginal ultrasonography. Fertilization was achieved through conventional IVF or ICSI, and embryos were cultured until transfer on day 3, 5, or 6 following oocyte retrieval. The number of embryos transferred was determined according to the guidelines of the Chinese Society of Reproductive Medicine, with a maximum of two embryos per transfer [19]. All patients were evaluated postoperatively through a comprehensive follow-up program. A quantitative serum hCG level was determined two weeks post-embryo transfer. A subsequent transvaginal ultrasound examination was scheduled for five weeks post-transfer.
Office hysteroscopy was performed during the proliferative phase to diagnose and localize intracavitary lesions. All hysteroscopy treatments were conducted in the operating room by the same two physicians (Tianji Liao and Lijun Lin). Hysteroscopies employed a vaginoscopy approach under sedation, utilizing a 2.9-mm, 30-degree-angle hysteroscope with an external sheath of 4.4 mm diameter and offering inflow, outflow, and 5F working channels (Karl Storz, Tuttlingen, Germany). Saline solution (9% concentration) was employed to inflate the uterine cavity, with an expansion pressure approximating 100~120 mmHg. The procedure was performed with a 300-w light source with a high-definition digital camera/xenon bulb (Karl Storz™, Tuttlingen, Germany). All EP and CE were confirmed histologically. Syndecan-1 (CD138) immunohistochemistry (IHC) was conducted. CE diagnostic criteria consisted of
The clinical pregnancy rate was defined as the number of intrauterine gestations with fetal cardiac activity per IVF-ET cycle. A biochemical pregnancy was defined as a positive hCG level without a gestational sac. Any pregnancy loss occurring after the visualization of intrauterine gestation was considered a spontaneous miscarriage, and any birth after 28 weeks of gestation was considered a live birth. An ectopic pregnancy was defined as a pregnancy in which the fertilized ovum implants outside the uterine cavity.
Statistical analysis was performed using SPSS software (version 25.0 for Windows; SPSS Inc., Chicago, IL, USA). Non-normally distributed data was expressed as the median with interquartile range (Q1, Q3). Qualitative variables were presented as a case quantity (n) or percentage (%). Categorical data are described by the number of cases, including the numerator/denominator and percentages. A value of p
The clinical characteristics of the study and control groups before and following PSM are shown in Table 1. Both before and after PSM, baseline characteristics including age, BMI, gravidity, and treatment parameters were well-balanced between the two groups, confirming the effectiveness of the matching process.
| Before PSM | After PSM | ||||||
| Groups 1: Controls | Groups 2: Hysteroscopy | p value | Groups 1: Controls | Groups 2: Hysteroscopy | p value | ||
| (n = 868) | (n = 455) | (n = 357) | (n = 357) | ||||
| Age (years) | 31 (28, 34) | 31 (29, 34) | 0.689 | 31 (28, 34) | 31 (29, 34) | 0.744 | |
| BMI (kg/m2) | 21.84 (20, 24.03) | 21.64 (20.03, 23.63) | 0.397 | 21.78 (20.10, 23.97) | 21.76 (20, 23.63) | 0.542 | |
| Basal FSH (IU/L) | 6.55 (4.93, 8.20) | 6.70 (4.90, 7.90) | 0.617 | 6.50 (5.10, 8.21) | 6.70 (5.00, 7.90) | 0.863 | |
| Basal LH (IU/L) | 4.70 (3.10, 7.40) | 4.80 (3.30, 7.35) | 0.722 | 4.50 (3.20, 7.05) | 4.80 (3.30, 7.40) | 0.258 | |
| AMH | 2.90 (1.73, 5.00) | 2.93 (1.66, 4.56) | 0.658 | 2.99 (1.72, 5.08) | 3.02 (1.82, 4.64) | 0.871 | |
| Duration of infertility (years) | 1 (0, 2) | 1 (0, 2) | 0.943 | 1 (1, 3) | 1 (0, 2) | 0.064 | |
| Total dosage of Gn used (IU) | 2400 (1875, 2925) | 2250 (1875, 2775) | 0.036 | 2235 (1876, 2850) | 2325 (1837.50, 2775) | 0.593 | |
| Duration of Gn used (d) | 10 (9, 11) | 10 (9, 11) | 0.026 | 10 (9, 11) | 10 (9, 11) | 0.168 | |
| Peak E2 (nmol/L) | 2574.15 (1608.85, 3974.27) | 2548.80 (1544.45, 3829.37) | 0.610 | 2710.00 (1776.20, 4102.90) | 2648.70 (1614.40, 3855.70) | 0.086 | |
| Number of Oocytes retrieved | 10 (6, 15) | 10 (6, 14) | 0.551 | 10 (6.50, 16) | 10 (6.50, 14) | 0.144 | |
| Endometrial thickness of trigger day (mm) | 10 (8.80, 12) | 10 (8, 11.60) | 0.006 | 10 (12, 20) | 10 (8, 12) | 0.485 | |
| ET number of embryos | 1 (1, 2) | 1 (1, 2) | 0.011 | 1 (1, 2) | 1 (1, 2) | 0.217 | |
| Development stage of the embryo | - | - | - | - | 0.216 | ||
| Cleavage embryo | 59.68% (518/868) | 47.47% (216/455) | - | 60.22% (215/357) | 64.71% (231/357) | - | |
| Blastocyst | 40.32% (350/868) | 52.53% (239/455) | - | 39.78% (142/357) | 35.29% (126/357) | - | |
| Treatment of ectopic pregnancy | - | - | 0.056 | - | - | 0.233 | |
| Conservative treatment | 7.95% (69/868) | 6.81% (31/455) | - | 9.80% (35/357) | 6.44% (23/357) | - | |
| Salpingostomy | 34.22% (297/868) | 40.88% (186/455) | - | 36.70% (131/357) | 36.42% (130/357) | - | |
| Salpingectomy | 57.83% (502/868) | 52.31% (238/455) | - | 53.50% (191/357) | 57.14% (204/357) | - | |
Gn, gonadotropin; E2, estradiol; PSM, propensity score matching; BMI, body mass index; FSH, follicle-stimulating hormone; LH, luteinizing hormone; AMH, anti-Müllerian hormone.
As detailed in Table 2, pregnancy outcomes showed no statistically significant differences between the hysteroscopy and control groups after PSM. Clinical pregnancy rates were comparable between groups (58.26% vs. 53.22%, p = 0.397), as were biochemical pregnancy rates (p = 0.120) and live birth rates (p = 0.880). Notably, the spontaneous miscarriage rate remained significantly higher in the hysteroscopy group both before and after PSM (p = 0.031 and p = 0.042, respectively). Ectopic pregnancy rates were similar between groups.
| Outcomes | Before PSM | After PSM | ||||
| Groups 1: Controls | Groups 2: Hysteroscopy | p value | Groups 1: Controls | Groups 2: Hysteroscopy | p value | |
| (n = 868) | (n = 455) | (n = 357) | (n = 357) | |||
| Biochemical pregnancy rate | 61.75% (536/868) | 66.37% (302/455) | 0.097 | 60.78% (217/357) | 66.39% (237/357) | 0.120 |
| Clinical pregnancy rate | 54.61% (474/868) | 58.46% (266/455) | 0.180 | 53.22% (190/357) | 58.26% (208/357) | 0.397 |
| Spontaneous miscarriage rate | 18.14% (86/474) | 24.81% (66/266) | 0.031 | 14.21% (27/190) | 22.12% (46/208) | 0.042 |
| Live birth rate | 43.32% (376/868) | 41.98% (191/455) | 0.640 | 43.70% (156/357) | 43.14% (154/357) | 0.880 |
| Ectopic pregnancy rate | 2.53% (12/474) | 3.38% (9/266) | 0.084 | 3.68% (7/190) | 3.85% (8/208) | 0.932 |
Subgroup analysis revealed important differences in reproductive outcomes based on hysteroscopic findings (Tables 3,4,5). While baseline parameters were comparable across subgroups, the cured CE group demonstrated significantly worse outcomes. The spontaneous miscarriage rate was markedly elevated in the cured CE group (46.90%) compared to all other subgroups (disease-free: 18.00%, EP: 10.00%, CE + EP: 25.00%). Consequently, live birth rates were lowest in the cured CE group (25.00%). This association remained statistically significant after adjusting for confounding variables (adjusted odds ratio (OR) 0.348, 95% confidence interval (CI) 0.171–0.708).
| Characteristics | Disease-free group (n = 171) | EP group (n = 65) | CE group (n = 60) | CE + EP group (n = 61) | p value | |
| Age (years) | 31.00 (28.25, 33.00) | 31 (29.00, 34.00) | 30 (27.25, 34.00) | 31 (29.00, 33.00) | 0.725 | |
| BMI (kg/m2) | 21.86 (19.92, 23.57) | 21.39 (19.88, 23.73) | 21.675 (20.06, 24.08) | 22.22 (20.20, 23.73) | 0.690 | |
| Basal FSH (IU/L) | 6.90 (5.42, 8.12) | 6.5 (5.30, 8.37) | 6 (4.20, 7.65) | 6.4 (4.80, 7.90) | 0.150 | |
| Basal LH (IU/L) | 4.95 (3.32, 8.07) | 4.3 (3.20, 6.77) | 4.5 (3.51, 6.07) | 5.3 (2.70, 7.30) | 0.343 | |
| AMH | 3.26 (1.82, 5.09) | 2.77 (1.68, 4.305) | 2.67 (1.62, 4.66) | 3.24 (2.39, 4.66) | 0.319 | |
| Duration of infertility (years) | 1 (0, 2) | 1 (0, 3) | 2 (0, 3) | 1 (0, 2) | 0.494 | |
| Total dosage of Gn used (IU) | 2325 (1806.25, 2775) | 2287.5 (1875, 2850) | 2325 (1950, 2831.25) | 2175 (1725, 2850) | 0.728 | |
| Duration of Gn used (d) | 10 (9, 11) | 10 (9, 11) | 10 (9, 11) | 10 (9, 12) | 0.419 | |
| Peak E2 (nmol/L) | 2374.5 (1467.50, 3840) | 2718 (1633, 3900.25) | 2647 (1678.50, 3263.75) | 3046 (1873, 4415) | 0.200 | |
| Number of Oocytes retrieved | 9 (6, 14) | 10 (6.25, 15.75) | 9 (7, 12) | 12 (7, 16) | 0.255 | |
| Endometrial thickness of trigger day (mm) | 10 (8, 12) | 12 (10, 12) | 10 (8, 12) | 12 (10, 14) | ||
| ET number of embryos | 1 (1, 2) | 1 (1, 2) | 1 (1, 2) | 1 (1, 2) | 0.746 | |
| Development stage of the embryo | 0.177 | |||||
| Cleavage embryo | 56.14% (96/171) | 60.00% (39/65) | 43.33% (26/60) | 47.54% (29/61) | ||
| Blastocyst | 43.86% (75/171) | 40.00% (26/65) | 56.67% (34/60) | 52.46% (32/61) | ||
| Treatment of ectopic pregnancy | 0.210 | |||||
| Conservative treatment | 7.60% (13/171) | 4.62% (3/65) | 1.67% (1/60) | 9.84% (6/61) | ||
| Salpingostomy | 34.50% (59/171) | 47.69% (31/65) | 33.33% (20/60) | 32.79% (20/61) | ||
| Salpingectomy | 57.90% (99/171) | 47.69% (31/65) | 65.00% (39/60) | 57.37% (35/61) | ||
Baseline characteristics of patients undergoing hysteroscopy before the first embryo transfer, stratified by physiological results.
Abbreviations: ET, embryo transfer; CE, chronic endometritis; EP, endometrial polyps.
| Outcomes | Disease-free group (n = 171) | EP group (n = 65) | CE group (n = 60) | CE + EP group (n = 61) | p value |
| Biochemical pregnancy rate | 65.50% (112/171) | 69.23% (45/65) | 61.67% (37/60) | 70.50% (43/61) | 0.716 |
| Clinical pregnancy rate | 58.48% (100/171) | 61.54% (40/65) | 53.33% (32/60) | 59.02% (36/61) | 0.824 |
| Spontaneous miscarriage rate | 18.00% (18/100) | 10.00% (4/40) | 46.88% (15/32) | 25.00% (9/36) | 0.001 |
| Live birth rate | 45.61% (78/171) | 52.31% (34/65) | 25.00% (15/60) | 44.26% (27/61) | 0.013 |
| Ectopic pregnancy rate | 4.00% (4/100) | 5.00% (2/40) | 5.00% (2/40) | 0.00% (0/36) | 0.738 |
Reproductive outcomes of patients undergoing hysteroscopy before the first embryo transfer, stratified by physiological results.
| Characteristics | Adjusted OR | 95% CI | p value | |
| Age (years) | 0.965 | 0.900–1.036 | 0.324 | |
| BMI (kg/m2) | 0.998 | 0.917–1.087 | 0.969 | |
| Basal FSH (IU/L) | 1.091 | 0.997–1.193 | 0.057 | |
| Basal LH (IU/L) | 0.993 | 0.968–1.018 | 0.566 | |
| AMH | 0.952 | 0.859–1.055 | 0.346 | |
| Duration of infertility (years) | 0.952 | 0.857–1.058 | 0.364 | |
| Total dosage of Gn used (IU) | 1.000 | 0.999–1.000 | 0.321 | |
| Duration of Gn used (d) | 1.007 | 0.796–1.273 | 0.956 | |
| Peak E2 (nmol/L) | 1.000 | 1.000–1.000 | 0.419 | |
| Number of Oocytes retrieved | 0.992 | 0.932–1.056 | 0.812 | |
| Endometrial thickness of trigger day (mm) | 1.158 | 0.953–1.408 | 0.141 | |
| ET number of embryo | 3.641 | 1.882–7.044 | ||
| Development stage of the embryo | 2.125 | 1.607–4.235 | 0.032 | |
| Treatment of ectopic pregnancy | 0.359 | |||
| Salpingectomy | 1.000 | |||
| Conservative treatment | 0.720 | 0.439–1.182 | 0.194 | |
| Salpingostomy | 1.161 | 0.471–2.857 | 0.746 | |
| Different of pathological findings | 0.015 | |||
| Disease-free group | 1.000 | |||
| EP group | 1.288 | 0.653–2.309 | 0.525 | |
| CE group | 0.348 | 0.171–0.708 | 0.004 | |
| CE + EP group | 0.773 | 0.397–1.507 | 0.450 | |
Abbreviations: OR, odds ratio; CI, confidence interval.
Ectopic pregnancy is a significant health concern among women of reproductive age, and its management can profoundly impact future fertility. Our retrospective study provides insights into the role of hysteroscopy in influencing pregnancy outcomes among patients with a history of ectopic pregnancies undergoing IVF treatment. To our knowledge, this study represents the first report on the impact of hysteroscopy on clinical reproductive outcomes in infertile women with a history of ectopic pregnancies.
Our study initially demonstrated that the utilization of hysteroscopy had an impact on pregnancy outcomes among patients with a history of ectopic pregnancies. The administration of hysteroscopy benefits patients without CE. The overall ectopic pregnancy rate in our cohort closely aligns with previously published data on similar populations, further supporting the generalizability of our findings [24]. Interestingly, although the hysteroscopy group showed a non-significant increase in biochemical and clinical pregnancy rates relative to the control group, there was a significantly higher rate of spontaneous miscarriage in the hysteroscopy group, ultimately resulting in comparable live birth rates between groups. A likely explanation for this observation is that CE serves as an important confounding factor. Our subgroup analysis demonstrated that CE was associated with the high rate of pregnancy loss in the hysteroscopy group, a finding that is consistent with a previous study [25]. Overall, our results indicate that routinely performing hysteroscopy in all patients with a history of ectopic pregnancy offers limited clinical benefit. An individualized strategy, emphasizing the detection and treatment of CE, may be more effective in enhancing reproductive outcomes for these patients.
Our data shows that patients with confirmed endometritis, diagnosed through hysteroscopy, experience the highest rate of pregnancy loss. Despite empirical antibiotic treatment aimed at reducing or eliminating plasmacyte infiltration within the endometrial stroma, the pregnancy loss rate in this subgroup remains the highest. This indicated that the current antibiotic approach might be insufficient for addressing endometritis. This finding is consistent with previous studies that have established CE as a significant risk factor for adverse reproductive outcomes [25, 26].
Several factors may explain why CE patients experienced persistently high pregnancy loss rates despite treatment. First, antibiotic resistance is a serious global medical problem in treating infectious diseases. A comprehensive survey of 3449 infertile women with RIF and three or more failed IVF-ET cycles found resistance to first-line 14-day oral doxycycline treatment in 21.2% of CE cases [27]. Our findings suggest that this resistance problem may be even more pronounced in patients with prior ectopic pregnancy, potentially due to more complex underlying pathophysiology or previous antibiotic exposures. As a result, it has been suggested that, alongside doxycycline, a 14-day course of a combination of levofloxacin lactate and metronidazole may be beneficial [20]. However, multidrug-resistant chronic endometritis CE (MDR-CE) is becoming an emerging concern in clinical management [12]. CE was resistant to two courses of combined oral antibiotic treatments (levofloxacin lactate and metronidazole) in 11.0% of cases [20]. This resistance pattern may partially explain why our hysteroscopy group, despite CE detection and antibiotic treatment, continued to demonstrate elevated pregnancy loss rates. On the other hand, a growing number of studies demonstrated that antibiotic treatments could enhance the clinical outcomes; this is only the case if follow-up biopsy confirms the successful eradication of CE [28]. Second-look hysteroscopy may be considered a potential option, despite the lack of conclusive evidence supporting its effectiveness in improving reproductive outcomes. An urgent need exists to establish universal diagnostic criteria that integrate histopathology, hysteroscopy, and microbiome analysis to address unanswered questions surrounding CE. Based on the results of our study, the pregnancy loss rate in patients with chronic endometritis is extremely high. Based on our results and the substantial gap between treatment intention and pregnancy outcomes, we strongly recommend that CE patients undergo second-look hysteroscopy to verify complete inflammation resolution before attempting conception. This approach, while requiring further validation through randomized controlled trials, represents a logical next step given the consistently poor pregnancy outcomes observed in our treated CE patients.
The natural progression of EP is still not well understood, but they are commonly found in infertile women. It has been hypothesized that EP may impair endometrial receptivity and subsequently influence ART outcomes [29]. Hysteroscopic polypectomy was associated with a higher C-reactive protein (CRP) rate and an increased total number of pregnancies [30]. In our study, EPs were identified in 18.21% of 357 patients with a previous history of ectopic pregnancy in this study. While our study found that patients with EP had higher live birth rates compared to the CE group, the clinical implications of this finding remain uncertain due to the absence of an untreated EP control group. This limitation prevents us from definitively attributing the improved outcomes to polypectomy versus the inherently better prognosis of EP compared to CE. The exact impact of EPs on fertility remains unclear, with most studies suggesting that pregnant women diagnosed with them should be managed expectantly [31]. However, clinical data on this topic are contradictory.
Regarding clinical decision-making, whether EP identified on hysteroscopy should always be surgically removed or can be managed conservatively remains unclear. In practice, polypectomy is generally recommended if EP is found before FET or ovarian stimulation for IVF, while management during ovarian stimulation is individualized based on polyp characteristics, embryo number, reproductive history, and clinic outcomes [32]. Our study found that hysteroscopy may be valuable for identifying EP in women with a history of ectopic pregnancy, but we cannot definitively conclude that polypectomy always improves outcomes without a randomized controlled trial comparing surgical removal versus conservative management. The evidence supporting the removal of EP is limited, and the best course of treatment must be determined by a carefully planned randomized controlled trial.
The primary strength of our study lies in the status as the first investigation into the administration of hysteroscopy in women with a previous history of ectopic pregnancy conducted at a single center. However, as with all clinical studies, our research has several limitations. The most notable drawback is its retrospective design, which limits the strength of our conclusions. While we matched multiple factors and found that the groups were generally similar in terms of age, BMI, number of transferred embryos, and types of embryos, unidentified confounding variables cannot be ruled out. Furthermore, the relatively small sample size may limit the generalizability of our results. Based on our findings, hysteroscopy appears most valuable for detecting specific uterine pathology (such as CE and EP) rather than universally improving reproductive outcomes in all patients with previous ectopic pregnancy. The clinical pregnancy rates did not differ significantly between groups, suggesting that routine hysteroscopy should not be universally recommended at this time. Regarding the CE subgroup, the underlying causes of the increased spontaneous miscarriage rate and decreased live birth rate remain unclear, including factors such as pre-existing microbial invasion, inadequate antibiotic administration, or potential direct effects of antibiotic treatment itself. Therefore, while our results suggest potential diagnostic value of hysteroscopy in identifying intrauterine abnormalities, we emphasize that further prospective, randomized controlled trials with larger sample sizes are essential before implementing this practice universally. Future studies should specifically compare outcomes in patients with detected pathology who receive treatment versus those managed conservatively, and establish clear criteria for when hysteroscopy would be most beneficial in this patient population.
In summary, our findings indicate that routine hysteroscopy before embryo transfer did not significantly improve most reproductive outcomes in patients with a history of ectopic pregnancy. However, hysteroscopy proved valuable in identifying CE, which remained associated with poor outcomes despite treatment. These results highlight the need for further research to determine optimal management strategies for CE, including the potential role of second-look hysteroscopy and prolonged antibiotic therapy. Our study underscores the importance of refining both diagnostic criteria and treatment protocols for CE in the context of IVF.
The data used in this study will be available upon reasonable request from the corresponding author.
LX, TJL, and LJL validated the results and conducted the investigation. LX carried out the formal analysis. TJL curated the data. LX and TJL drafted the original manuscript. WH contributed substantially to the study design, furthermore, reviewed and edited the manuscript, and supervised the project. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
The study was carried out in accordance with the guidelines of the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of West China Second University Hospital of Sichuan University (approval number: 2024-245). All patients undergoing ART treatment in our center provided written informed consent to use their medical records for research purposes. Additionally, all patients were registered in the data management system, which stores the medical information of patients seeking to conceive through ART.
We would like to express our thanks to everyone who helped us in the process of writing this manuscript. Thanks to all the peer reviewers for their opinions and suggestions. The authors thank all the patients included in this study and the staff of the Department of Reproductive Medicine of West China Second University Hospital of Sichuan University.
This research received no external funding.
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.

