1 Department of Obstetrics and Gynecology, Yeouido St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, 07345 Seoul, Republic of Korea
2 Department of Obstetrics and Gynecology, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, 06591 Seoul, Republic of Korea
3 Department of Research Institute of Pharmaceutical Sciences, Seoul National University, 08826 Seoul, Republic of Korea
4 College of Pharmacy and Research Institute of Pharmaceutical Sciences, Woosuk University, 55338 Wanju, Republic of Korea
Abstract
Conservative uterine leiomyoma treatment options are limited. There is an unmet need for well-tolerated, non-invasive treatments that can be safely used long-term to reduce fibroid burden and symptoms. We investigated the efficacy of epigallocatechin gallate (EGCG) from green tea, in combination with vitamins D and B6, for fibroid reduction and improvement of symptoms.
In this single-center study, we enrolled Korean, reproductive age women aged 18–50 years with confirmed uterine leiomyoma, with fibroids ≥3 cm in the longest dimension. Participants received EGCG 150 mg, vitamin D3 25 μg/1000 international units (IU), and vitamin B6 5 mg in tablet form for 16 weeks, and were instructed to take 2 tablets each morning. Participants completed a pictorial bleeding assessment chart (PBAC) during menstruation, and uterine fibroid symptom (UFS) severity and quality of life (QoL) assessments before and after treatment. The primary endpoint was the change in largest fibroid volume at 16 weeks. Outcomes were compared using a paired t-test or Wilcoxon’s signed rank test based on satisfaction of the normality assumption.
After 16 weeks, 31/33 patients (93.9%) showed a reduced fibroid volume. Mean fibroid volume was significantly reduced by 12.7% (675.57 vs. 590.00 mm3, respectively; p < 0.001). Significant volume reductions were observed across all age groups, with the greatest reduction observed in the 41–50-year age group (–16.0%). While there was no significant improvement in mean PBAC scores, UFS-severity and UFS-QoL scores improved in 72.7% and 81.8% of patients, respectively, with significant improvements in mean scores compared to baseline (p = 0.002 and p = 0.008, respectively). No adverse events were reported.
The use of EGCG/vitamin D3/vitamin B6 represents an effective and well-tolerated non-hormonal and non-surgical treatment for reproductive age women with symptomatic uterine leiomyoma.
This study is registered on the Korean Clinical Research Information Service (CRIS) https://cris.nih.go.kr/cris/search/detailSearch.do?seq=30490&search_page=L (registration number: KCT0010798).
Keywords
- cholecalciferol
- epigallocatechin gallate
- leiomyomas
- uterine leiomyoma
- vitamin D3
Uterine leiomyoma is characterized by the presence of uterine leiomyomata (fibroids), the most common benign solid tumors of the uterus among women of reproductive age [1, 2]. The global prevalence is difficult to estimate because the disease is typically underreported, due to many cases being asymptomatic [3, 4]. Prevalence data range between 20%–80% of reproductive-age women, depending on the population, diagnostic methods, and study design used [4, 5].
Up to 30% of women with uterine leiomyoma have burdensome symptoms including pelvic or back pain, abnormal uterine bleeding, anemia, or bladder or bowel symptoms, which can impact psychosocial wellbeing and interfere with quality of life (QoL) and productivity [1, 2, 3]. Uterine leiomyoma is also associated with impaired fertility and poorer obstetric outcomes [1]. Goals of treatment are improvement of symptoms, sustained reduction in fibroid size, control of fibroid-related abnormal uterine bleeding, improved QoL, and maintenance of fertility if desired [1, 6]. A step-up approach is usual, including pharmacological treatments, minimally invasive medical therapies (including interventional radiology), and finally surgery (myomectomy or definitive hysterectomy) [1].
Non-steroidal anti-inflammatories may relieve pain, and hormonal contraceptives can improve bleeding patterns, but these do not reduce fibroid burden [6, 7]. Gonadotropin releasing hormone agonists (GnRHa) are commonly used to reduce fibroid size, abnormal bleeding, and pelvic symptoms, and may be used preoperatively to improve surgical outcomes [6, 7]. GnRH antagonists (GnRHant) have also recently shown robust effects on menstrual blood loss and QoL, but only modest effects on fibroid size [8]. GnRHa and GnRHant are prescribed for a limited time due to undesirable hypoestrogenic and metabolic side effects, must be combined with hormonal add-back therapy, and are not suitable for women who desire to conceive [8, 9]. Another agent, ulipristal acetate, which was known to reduce fibroid size and associated symptoms, has been suspended by the European Medicines Authority for uterine leiomyoma, due to a risk of severe liver injury [10, 11]. In the US, the Food and Drug Administration has not approved ulipristal acetate for similar reasons [12]. There is a pressing unmet need to expand the range of conservative treatment options.
Interest in green tea extracts is growing in both the nutritional and medical arenas. Epigallocatechin gallate (EGCG) is a type of catechin (a flavonoid enriched in green tea) which has demonstrated powerful antioxidant properties and acts as a selective inhibitor of catechol-O-methyl transferase, an enzyme highly expressed in human uterine fibroid tissue [13]. EGCG induces antiproliferative and apoptotic effects and inhibits fibrotic processes in uterine leiomyoma cells and murine models [13, 14, 15]. Human trials have also yielded promising results [16, 17]. For example, in a randomized, placebo-controlled trial conducted in Egyptian women aged 18–50 years with symptomatic fibroids, 4 months of treatment with green tea extract (containing 45% EGCG) resulted in a significant 32.6% reduction in total fibroid volume and reduced symptom severity, compared to placebo [16].
Evidence suggests vitamin D deficiency plays a significant role in the development and growth of fibroids [18, 19]. Women with vitamin D deficiency—including those of Black race, who have higher melanin levels placing them at greater risk of deficiency—are significantly more likely to develop uterine leiomyoma [18, 19]. In vitro studies have demonstrated the ability of vitamin D to inhibit fibroid growth by interfering with cell proliferation, extracellular matrix remodeling, DNA repair, signaling, and apoptosis regulation [20, 21, 22]. Clinical trials of vitamin D supplementation in women with uterine leiomyoma and hypovitaminosis D have shown mixed results [23, 24], but one study showed a decreased rate of progression to extensive disease, and less need for surgical or medical intervention, with vitamin D3 supplementation compared with controls [23].
Since both EGCG and vitamin D have shown promising inhibition of fibroid growth, interest in combining these as a potentially effective treatment for uterine leiomyoma is increasing. In addition, given the strong roles of inflammation and increased tryptophan metabolism in uterine leiomyoma growth [25, 26], there is rationale for the addition of vitamin B6—a known antioxidant and anti-inflammatory vitamin which has recently demonstrated effects on gene expression associated with inflammatory mediators and tryptophan-metabolizing enzymes [27, 28]. Commercial formulations containing EGCG in combination with vitamin D and B6 have recently become available over-the-counter in several countries, including the US, Italy, and Korea.
Five Italian studies have investigated this triple combination in reproductive age women with uterine leiomyoma, with most reporting significant decreases in fibroid size with no reported adverse events (AEs) [29, 30, 31, 32, 33]. In the present study, we sought to further explore the efficacy of this triple combination in reducing fibroid size and improving symptoms and QoL in a non-Caucasian cohort.
This prospective, single-center, single arm, interventional study was conducted at the Uterine Leiomyoma Center, Seoul St. Mary’s Hospital in Seoul, Korea, between March and September 2023.
Eligible participants were Korean reproductive age women between the ages of 18–50 years, with a prior diagnosis of uterine leiomyoma confirmed via abdominal or transvaginal sonography, magnetic resonance imaging (MRI), or computed tomography, who were not currently taking other treatments for uterine leiomyoma. To be included, the largest fibroid (without calcification) needed to measure at least 3 cm in the longest dimension.
At the baseline visit, participants received a 16-week supply of supplements containing green tea extract 330 mg (EGCG 150 mg), vitamin D3 (cholecalciferol) 25 µg/1000 international units (IU), and vitamin B6 (pyridoxine) 5 mg. Participants were instructed to take 2 tablets each morning for 16 weeks, beginning on the day of the first study visit. A 16-week supply of sanitary napkins was also issued to each participant, with instructions to use only the supplied napkins during the study period. Tampons or other brands of napkins were not permitted.
In addition, participants received printed copies of a modified Pictorial Blood-loss Assessment Chart (PBAC), derived from a validated pictogram for heavy menstrual bleeding [34, 35] (Supplementary Fig. 1), which they were instructed to complete on each day of menstruation at every napkin change, beginning on the first day of the next menstrual period after the baseline visit. If a woman was menstruating at the baseline visit, she was advised to start the PBAC on Day 1 of her next menstrual period. The PBAC instrument employed a pictorial scale to visually assist participants in scoring according to: (1) volume of menstrual bleeding (scale of 1–10, corresponding to 1 mL–15 mL); (2) estimated size of the observed blood clots when using the toilet (1 mL, 3 mL, 5 mL); and (3) estimated size of the observed blood clots on a sanitary napkin (1 mL, 3 mL, 5 mL). The sensitivity and specificity of the PBAC and modified versions of the PBAC are reportedly between 58%–99% and 8%–89%, respectively [34].
At the baseline visit, and again at the Week 16 visit, patients were required to complete a 37-question Uterine Fibroid Symptom and Health-Related Quality of Life (UFS-QoL) questionnaire [36] (Supplementary Fig. 2), to evaluate the severity of their menstrual symptoms and the impact of these symptoms on daily life and functioning. Patients scored each question on a scale of 1–5, 1 representing “none” and 5 representing “very often” or “very heavy”.
At baseline, patients provided medical and surgical history information. At baseline and at the Week 16 follow-up visit, all participants underwent clinical measurements, including vital signs, height, and body weight, and urinary human chorionic gonadotropin pregnancy tests were performed. Using transvaginal ultrasound, the number of fibroids was recorded at both visits; the size of the largest fibroid was measured, and the volume of that fibroid was calculated.
Safety was also assessed. Participants were encouraged to contact the study investigators at any time during the study period to report AEs of any severity.
The primary endpoint was change in volume of the largest fibroid after 16 weeks of treatment. Secondary endpoints included change in PBAC score over 16 weeks, change in UFS severity and health-related QoL per the UFS-QoL questionnaire, and safety.
Statistical analysis was performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA). Descriptive statistics (sample size, mean, standard deviation [SD], median, minimum, and maximum) were used to present baseline characteristics. After 16 weeks, change in the mean volume of the largest uterine leiomyoma was calculated overall and by pre-defined age group (18–30, 31–40, and 41–50 years); mean volume was estimated using the ellipsoid formula (Volume = length
A total of 34 reproductive age women were prospectively enrolled in this study. Baseline characteristics are shown in Table 1. The median age was 38.5 years (range, 27–48 years); 68% of patients were aged 40 years or under, and 32% were over 40. Before treatment initiation, the mean
| Characteristics | Subjects (n = 34) | |
| Age, years | ||
| Mean | 37.76 | |
| Median (range) | 38.5 (27‒48) | |
| Median (IQR) | 38.5 (35‒41) | |
| Age group, n (%) | ||
| 18–30 years | 6 (17.64) | |
| 31–40 years | 17 (50.00) | |
| 41–50 years | 11 (32.35) | |
| Marital status, n (%) | ||
| Married | 8 (23.53) | |
| Unmarried | 26 (76.47) | |
| Occupation status, n (%) | ||
| Working | 30 (88.24) | |
| No occupation | 4 (11.76) | |
| Parity, n (%) | ||
| 0 | 29 (85.29) | |
| 1 | 3 (8.82) | |
| 2 (5.88) | ||
| BMI (kg/m2) | ||
| Mean | 22.62 | |
| Median (min, max) | 21.45 (2.73‒61.62) | |
| Median (IQR†) | 21.5 (19.49‒24.61) | |
| BMI category (kg/m2) | ||
| 4 (11.76) | ||
| 18 (52.94) | ||
| 4 (11.76) | ||
| 8 (23.53) | ||
| Number of uterine fibroids, n | ||
| Mean | 5.06 | |
| Median (min, max) | 3.5 (1‒22) | |
| Median (IQR) | 3.5 (1‒6) | |
| Uterine fibroid volume, mm3 | ||
| Mean | 677.87 | |
| Median (range) | 671 (73‒1709) | |
| Median (IQR) | 664 (265‒956) | |
| PBAC‡ | ||
| Mean | 93.64 | |
| Median (range) | 86 (20‒160) | |
| Median (IQR) | 86 (60‒126) | |
| UFS§ severity score | ||
| Mean | 17.18 | |
| Median (range) | 16 (8‒29) | |
| Median (IQR) | 16 (13‒22) | |
| UFS-QoL¶ | ||
| Mean | 55.85 | |
| Median (range) | 51.5 (30‒104) | |
| Median (IQR) | 51 (38‒72) | |
†IQR, interquartile range; ‡PBAC, Pictorial Blood-loss Assessment Chart; SD, standard deviation; §UFS, Uterine Fibroid System; ¶UFS-QoL, Uterine Fibroid Symptom and Health-related Quality of Life Questionnaire.
During the 16-week study period, 33/34 (97%) participants were compliant in taking the interventional supplement, completing the PBAC while menstruating, and completing the UFS-severity and UFS-QoL assessments at baseline and Week 16. These patients were included in the present analysis. The remaining patient was lost to follow-up.
During the 16 weeks, 22/33 participants (66.7%) had 4 menstrual periods; 7 (21.2%) had 3 periods, 3 (9.1%) had 5 periods, and 1 (3.0%) had 2 periods (Supplementary Table 1).
After 16 weeks of treatment, 31/33 patients (93.9%) showed a reduction in fibroid volume, with a magnitude of shrinkage ranging between –3 and –487 mm3. The remaining two patients had increased volumes of +38 and +4 mm3, respectively (Fig. 1). Compared to baseline, the mean volume of the largest fibroid was significantly reduced (675.57
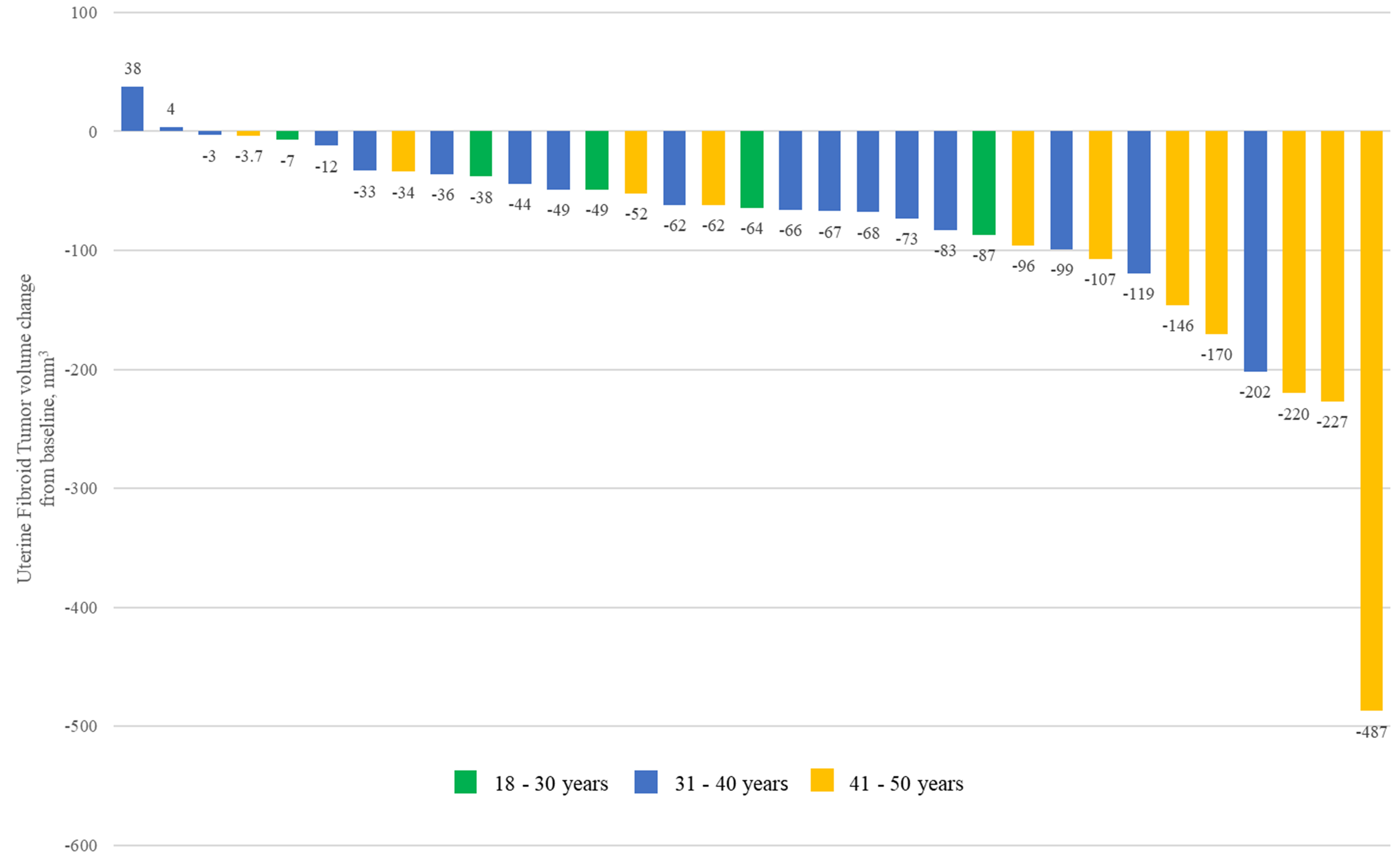 Fig. 1.
Fig. 1. Waterfall plot of fibroid volume change from baseline to Week 16 in 33 participants.
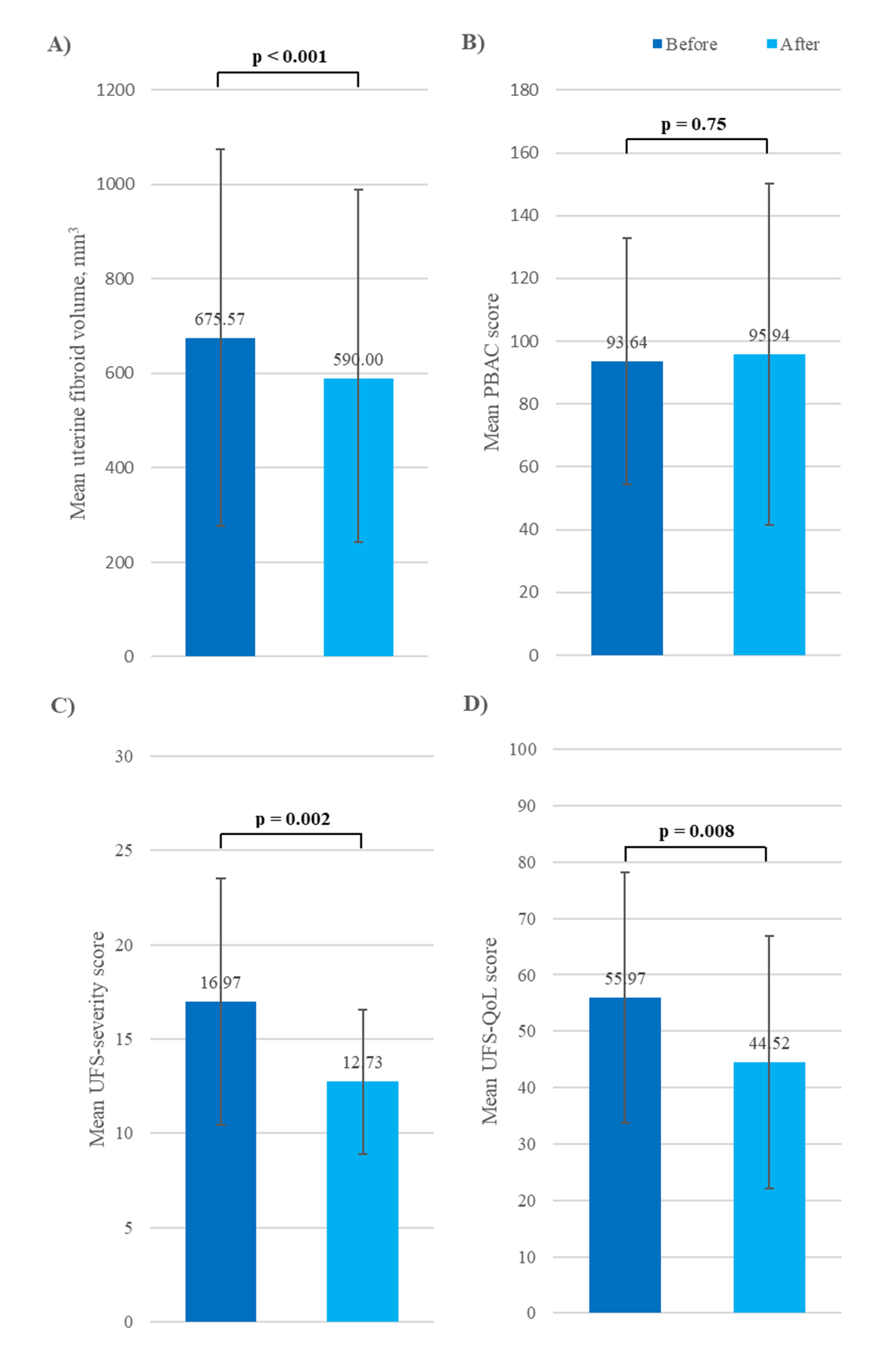 Fig. 2.
Fig. 2. Change from baseline in mean (A) fibroid volume, (B) PBAC score, (C) UFS-severity score, and (D) UFS-QoL score in patients with uterine leiomyoma. PBAC, Pictorial Bleeding Assessment Chart; UFS, Uterine Fibroid Symptom; QoL, quality of life.
| Fibroid volume, mm3 | Before treatment (Baseline) | After treatment (16-week visit) | Mean difference in volume | Percentage reduction from baseline (%) | p-value | |
| Overall population | ||||||
| Mean | 675.57 | 590.00 | –85.57 | –12.7 | ||
| Median (IQR) | 664.0 (265.0–956.0) | 579.0 (223.0–851.0) | −64.0 (−99.0 to −36.0) | −14.2 (−18.5 to −5.0) | ||
| Age 18–30 years | ||||||
| Mean | 363.00 | 314.00 | –49.00 | –13.5 | 0.021‡ | |
| Median (IQR) | 261.0 (154.0–265.0) | 216.0 (138.0–223.0) | −49.0 (−64.0 to −38.0) | −14.6 (−18.5 to −8.8) | ||
| Age 31–40 years | ||||||
| Mean | 591.83 | 535.83 | –56.00 | –9.5 | ||
| Median (IQR) | 601.5 (434.0–784.5) | 500.5 (405.8–739.2) | −55.5 (−71.8 to −33.2) | −9.4 (−14.9 to −4.5) | ||
| Age 41–50 years | ||||||
| Mean | 982.57 | 825.50 | –157.07 | –16.0 | 0.005† | |
| Median (IQR) | 1045.0 (769.2–1172.2) | 821.5 (652.5–1089.2) | −126.5 (−207.5 to −70.5) | −16.1 (−21.6 to −10.0) | ||
IQR, interquartile range; SD, standard deviation.
† Normal‑distribution groups (overall, 31–40 year and 41–50 year) analyzed with paired t‑test.
‡ Non‑normal group (18–30 year) analyzed with Wilcoxon signed‑rank test.
Over the study period, changes in PBAC scores for individual patients varied widely, from +106 to –126 points. Seventeen patients (51.5%) had a decreased score (indicating improvement in estimated blood loss), 15 (45.5%) had an increased score (indicating worsening), and one patient’s score was unchanged (Supplementary Table 1). Overall, the mean estimated blood loss was 95.94
To shed further light on bleeding patterns, we looked at the first question in the UFS-QoL symptom severity questionnaire (“My bleeding amount has increased”), which showed wide individual patient variability post-treatment, consistent with the PBAC analysis. Twelve participants indicated a decrease in bleeding volume, 11 reported an increase, and 10 indicated no change (data on file).
Mean UFS-severity and UFS-QoL impact scores were significantly improved (lowered) from baseline after 16 weeks of treatment (p = 0.002 and p = 0.008, respectively; Fig. 2C,D). At Week 16, 24/33 patients (72.7%) reported improvement in symptom severity scores, while 27/33 (81.8%) reported improved QoL scores, compared with baseline (Supplementary Table 2).
During the study, no AEs were reported by any patient.
Currently, non-surgical options for women with uterine leiomyoma are limited, and available hormonal options can be associated with undesirable side effects. Noninvasive treatment methods are preferable over surgical methods wherever possible, to reduce the risk of morbidity and complications associated with surgical intervention. In addition, along with the increased incidence of fibroids among women desiring children later in life, surgical interventions such as myomectomy frequently necessitate a cesarean section at delivery, with corresponding increases in morbidity risks and medical expenses. There is a great unmet need to expand the range of non-surgical treatment choices.
To our knowledge, our study is the first of its kind conducted in Asian patients. We demonstrated that treatment with EGCG, vitamin D3, and vitamin B6 was well-tolerated and effective in reproductive age women with uterine leiomyoma. This combination resulted in a significant reduction of approximately 13% in mean fibroid volume, suggesting the promising clinical potential of using these agents to reduce fibroid size.
Our results are consistent with the five aforementioned Italian studies, all of which used formulations, dosages, and treatment durations that were comparable to our study. Those studies reported greater reductions in fibroid volume, including –37.9% [30] (mean age 43.2
Measurement of patient-reported outcomes is highly important in gynecological disease, particularly when symptoms substantially impact a patient’s day-to-day wellbeing and functioning. In our results, the reduction in fibroid volume appeared to correspond with the observed significant improvement in mean uterine leiomyoma symptom severity and QoL scores, both at the cohort level and at the individual level—which is an important finding, given the appreciable impact uterine leiomyoma imposes on QoL. The UFS-QoL tool used in our study is a validated and responsive method for assessing symptoms and QoL specifically in women with uterine leiomyoma [36], and was able to demonstrate disease-specific patient-reported improvements in a range of symptoms such as abnormal uterine bleeding and cycle patterns, pelvic pain, frequent urination, and fatigue. Specific QoL improvements were also reported in terms of physical discomfort, restrictions in daily activities, travel, and exercise, and the psychosocial burden of disease including depression/anxiety, frustration, embarrassment, uncertainty, low sexual desire, and lack of motivation.
Consistent with our findings, the Egyptian study (which also used the UFS-QoL questionnaire) reported a significant 32.4% reduction in symptom severity and improved QoL scores over 4 months with EGCG compared to placebo [16]. The Italian studies that included QoL assessments employed different tools (the Short Form 36 or an unnamed tool), with mixed results. While one study failed to demonstrate significant changes in health-related QoL [29], two reported significant improvements [31, 33], with two also reporting significant improvements in symptom severity including pelvic pain and fatigue [30, 33].
In our cohort, mean PBAC scores indicated that the amount of vaginal bleeding was comparable after versus before treatment. The first question in the UFS-QoL symptom severity questionnaire, “My bleeding amount has increased”, showed individual patient variability. Twelve participants indicated a post-treatment decrease in bleeding volume, 11 reported an increase, and 10 indicated no change (data on file). This is consistent with the mixed findings reported in other studies of EGCG/vitamin D/vitamin B6 that measured bleeding volume. Among the Italian studies, one showed no improvement in ‘menstrual flow intensity’ [29], while two demonstrated significant improvements in heavy menstrual bleeding [30, 32]. The Egyptian study also showed significant improvement in bleeding patterns after 4 months of EGCG alone compared with placebo [16], using a comparable pictogram tool to our present study [16, 35]. Our findings may have been confounded by women who were menstruating during the first visit but failed to exclude this bleeding and wait for their next menstruation before commencing participation.
Our study had several strengths and limitations. The design had a strong rationale and the cohort included a wide age range. In addition, we achieved 97% compliance with the PBAC and QoL questionnaires. Limitations included the small sample size, the lack of a placebo or control comparator, and the lack of follow-up visits between the first and last visits to check and encourage adherence; nevertheless, our participants’ compliance was excellent. We also measured only the largest fibroid in each participant, but not total fibroid volume, and did not distinguish between multiple and single uterine leiomyomas. In addition, the use of transvaginal ultrasound is reported to have lower sensitivity for detection and measurement of fibroids, and slightly greater room for error, compared with MRI [38]. This study’s sample size was limited and may not have been sufficient to detect all treatment effects, such as changes in bleeding volume. Future studies with larger cohorts are needed to validate and expand upon these findings. Blood loss is difficult to quantify in an outpatient setting. While the PBAC assessment chart is a highly subjective measure of blood loss, a recent systematic review of various measurement methods concluded that pictogram-style tools are most appropriate because they achieve a good balance between ease-of-use for patients and validated accuracy in clinical and research settings [34]. The sensitivity and specificity of the PBAC and modified versions of the PBAC can range from 58%–99% and 8%–89%, respectively [35].
In conclusion, the combination of EGCG, vitamin D3, and vitamin B6 represents a promising and well-tolerated non-hormonal and non-surgical treatment for reproductive age women with symptomatic uterine leiomyoma, and may be presented to women as an appropriate therapy option during shared decision-making. Global interest in these combined agents is ongoing; larger-scale randomized controlled trials in different populations, including Asian populations, are warranted to further validate these encouraging results.
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
IS, JS, YC and MK designed the study, SL provided statistical analysis, IS and JP analyzed the data. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
This study was conducted in accordance with the ethical principles of the Declaration of Helsinki and Korean national laws, and was approved by the Catholic University of Korea Seoul St. Mary’s Hospital Institutional Review Board (Study Number, KC22HISI0774) before commencement. All participants provided written informed consent to participate.
Medical writing and editorial assistance were provided by Geri Skidmore of PharmaMED, LLC., UT, USA in accordance with International Society for Medical Publication Professionals Good Publication Practice (ISMPP GPP) 2022 updated guidelines (https://www.ismpp.org/gpp-2022).
Han Kook Pharmed Co., Ltd., South Korea provided Myostat Plus® tablets and funded the cost of sanitary napkins, printing, statistical analysis, Clinical Research Coordinator’s personnel costs, and editorial assistance for this manuscript. No monetary compensation was offered to any study investigators.
All listed authors meet the International Committee of Medical Journal Editors (ICMJE) authorship criteria (https://www.icmje.org/icmje-recommendations.pdf). This study received research support from Han Kook Pharmed Co., Ltd., as detailed in the Funding section. Han Kook Pharmed Co., Ltd. was not involved in the preparation or publication of this manuscript. None of the authors have any personal financial disclosures or other relevant conflicts of interest to declare.
Supplementary material associated with this article can be found, in the online version, at https://doi.org/10.31083/CEOG39094.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.


