1 Department of Obstetrics and Gynecology, Women’s Hospital Zhejiang University School of Medicine, 310006 Hangzhou, Zhejiang, China
2 Department of Gynecology, Ningbo NO.2 Hospital, 315000 Ningbo, Zhejiang, China
3 Zhejiang Provincial Clinical Research Center for Obstetrics and Gynecology, 310006 Hangzhou, Zhejiang, China
4 Traditional Chinese Medicine for Reproductive Health Key Laboratory of Zhejiang Province, Women’s Hospital, School of Medicine, Zhejiang University, 310006 Hangzhou, Zhejiang, China
†These authors contributed equally.
Abstract
During follow-up, some patients with endometrial hyperplasia (EH) progress to endometrial cancer (EC) while others diagnosed with EH experience pathological escalation following hysterectomy. When treating premenopausal women, it is imperative to consider reproductive function, especially if they wish to preserve fertility.
This study adhered to the Network Meta-Analysis extension of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting guideline. We screened the PubMed, Web of Science, Cochrane Library, and Embase databases to identify relevant studies published from inception through July 31, 2023. The methodological quality of the studies was evaluated using the Cochrane Collaboration’s tool for evaluating risk of bias. RevMan version 5.3 software, provided by the Cochrane Collaboration, was used for statistical meta-analysis.
A total of 45 studies were selected for final analysis, including 9 randomized controlled trials. We identified a pooled complete response (CR) rate of 0.82 [95% confidence interval (CI): 0.78–0.86] among premenopausal patients with EH undergoing fertility preservation therapy. In addition, we identified a pooled assisted reproductive technology (ART) utilization rate of 0.30 (95% CI: 0.10–0.49) among premenopausal patients with EH receiving fertility preservation therapy. The pooled pregnancy rate and pooled live birth rate were 0.30 (95% CI: 0.24–0.37) and 0.24 (95% CI: 0.17–0.30), respectively. Finally, we performed a subgroup analysis in to investigate the outcomes associated with atypical forms of EH.
Our analysis confirmed that fertility preservation in premenopausal patients with EH is effective. Following treatment, some patients achieved satisfactory fertility outcomes, while others required ART support. Despite these findings, natural conception remained the primary mode of conception.
The study has been registered on https://www.crd.york.ac.uk/prospero/ (registration number: CRD42023433030; registration link: https://www.crd.york.ac.uk/PROSPERO/view/CRD42023433030).
Keywords
- endometrial hyperplasia
- fertility preservation
- reproductive outcomes
- complete response
Endometrial hyperplasia (EH) is a hyperplastic endometrial lesion, involving irregular gland size, increased glands, and an increased glandular interstitial ratio [1]. EH can be divided into two distinct categories: EH without atypia and atypical endometrial hyperplasia (AEH), also referred to as endometrial intraepithelial neoplasia (EIN), which is known to be associated with a 1%–43% risk of malignant progression [2]. This two-tier model of EH was endorsed by the World Health Organization (WHO) Classification of Female Reproductive Tumors in 2014 [3].
Endometrial cancer (EC) represents one of the most significant tumors of the reproductive system in women, and represents the sixth most common form of cancer in women, with 417,000 new cases reported globally in 2020 [4]. Over the past 30 years, the overall incidence of EC has increased by 132% [5]. Most ECs occur after menopause, but as many as 14% occur before menopause (4% before the age of 40 years) [6]. During follow-up, some cases of EH can progress further to EC while some patients diagnosed with EH experience pathological escalation after hysterectomy, concurrent with the detection of EC. The primary prevention of EH in high-risk populations, as well as the timely detection and standardized treatment of women who have already been diagnosed with EH, are essential if we are to improve the overall prognosis and reproductive outcomes of these young patients. For any disease, primary prevention should be regarded as the most important aspect, and relevant measures can obtain maximum benefit by protecting health status with relatively little loss, including financial costs and physical pain.
There are several risk factors for EH, including obesity (especially the presence of abdominal fat), exogenous estrogen supplementation (unsupported or under-supported by progesterone), and polycystic ovarian syndrome (PCOS) [7, 8, 9, 10, 11, 12]. These risk factors apply to women of all ages, including both premenopausal and postmenopausal women. Women who have these risk factors but have not yet developed EH should regularly undergo endometrial screening. The primary prevention of EH is to control and standardize the treatment of the risk factors involved. Relevant measures include, but are not limited to, controlling the percentage of body fat (especially the consumption of abdominal fat and visceral fat) through exercise or by an appropriate diet, the careful use exogenous estrogens, and complete response outcome treatment of the intima with a sufficient amount and full cycle of progesterone if necessary [9, 13, 14, 15, 16, 17, 18]. In addition to these factors, attention should be paid to blood glucose (hyperinsulinemia) in patients who have been diagnosed with PCOS. The frequency of routine intima-related health examinations (mainly ultrasound) should also be increased [19].
Abnormal uterine bleeding (AUB) remains the primary clinical manifestation of EH, including infertility problems in women of reproductive age [20, 21]. Premenopausal women do have a regular menstrual cycle [22]; therefore, ultrasound examination, using endometrial thickness as the main evaluation criteria, is not applicable [19, 23]. There are currently no guidelines to recommend invasive testing in premenopausal women based on a single ultrasound report of a thickened endometrium [24, 25]. Therefore, endometrial ultrasonography for premenopausal women should pay special attention to endometrial blood flow and endometrial proliferation. In the presence of clinical symptoms, especially ultrasound abnormalities, once EH (or even EC) is suspected, endometrial biopsy is recommended for a definitive diagnosis in order to provide more timely treatment measures. The treatment measures for EH should be comprehensively considered according to the age of the patient and the atypia of the cells in the pathological report; in other words, whether the patient has AEH or EIN. Especially for pre-menopausal women, and particularly those with no reproductive history, it is also necessary to consider whether they have the intention to continue to have children after EH treatment, and undertake a comprehensive assessment to determine whether fertility preservation treatment is required [1].
Providing timely evaluation and treatment for existing fertility preservation protocols without compromising cancer treatment has become an important aspect of modern oncology care [26]. The same applies to EH, a precursor of EC. When considering fertility preservation for EC, it is also necessary to consider the impact of chemotherapy and radiotherapy on ovarian function [27, 28, 29, 30]; this is not a consideration for patients with EH.
Current treatments for reproductive preservation in EH remain limited, and predominantly include progesterone therapy, which involves oral progesterone and the intrauterine placement of a levonorgestrel intrauterine sustained release system (LNS-IUS), and hysteroscopic lesions [31].
The vast majority of studies relating to fertility preservation for endometrial lesions have focused on patients with AEH and highly differentiated early-stage endometrioid endometrial carcinoma (EEC). Previous literature reviews and meta-analyses of the effects of fertility preservation therapy in EH patients only focused on AEH and were considered coincident with high-level EEC [32, 33, 34, 35, 36, 37].
Although the degree of cell differentiation in high-grade EEC is relatively high, this type of tumor is still malignant. No previous literature reviews or meta-analyses have focused on gynecological outcomes, such as endometrial complete response (CR) rate and obstetric outcomes (such as pregnancy and the live birth of offspring) in premenopausal women with precancerous EC lesions (i.e., EH) receiving fertility preservation therapy.
In the present study, we focused upon premenopausal patients with EH, including non-atypical EH and AEH. We reviewed previous studies on this type of patient receiving fertility preservation treatment and performed metanalysis on the effects of fertility preservation treatment on premenopausal patients with EH, especially those with strong fertility intention, including whether a satisfactory reproductive outcome was achieved.
This study followed the Meta-Analysis extension of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting guideline [38].
We screened the PubMed, Web of Science, Cochrane Library, and Embase databases to identify relevant studies that had been published from inception to the 31st of July 2023. Our literature searches featured a combination of subject-heading and keyword searches. Search terms included “fertility”, “endometrial hyperplasia”, “oral levonorgestrel”, “Levonorgestrel intrauterine sustained release system”, “LNS-IUS”, “metformin”, “medroxyprogesterone acetate” (MPA), “megestrol acetate” (MA), “progesterone”, “norethisterone” (NET), “dydrogesterone” and “reproductive”. The retrieved studies were limited to prospective studies, retrospective studies and randomized controlled trials (RCTs), with no language or site restrictions.
PubMed Search: ((FERTILITY) OR (reproductive)) AND (Endometrial hyperplasia); (“fertiles” OR “fertility”[MeSH Terms] OR “fertility” OR “fertile” OR “fertilities” OR (“reproduction”[MeSH Terms] OR “reproduction” OR “reproductions” OR “reproductive” OR “reproductively” OR “reproductives” OR “reproductivity”)) AND (“endometrial hyperplasia”[MeSH Terms] OR (“endometrial” AND “hyperplasia”) OR “endometrial hyperplasia”). Embase Search: (‘fertility’/exp OR fertility OR reproductive) AND (‘endometrial hyperplasia’/exp OR ‘endometrial hyperplasia’ OR (endometrial AND (‘hyperplasia’/exp OR hyperplasia))). Cochrane Library Search: ((FERTILITY) OR (reproductive)) AND (Endometrial hyperplasia). Web of Science Search: AB = ((FERTILITY OR reproductive) AND Endometrial hyperplasia) OR TS = ((FERTILITY OR reproductive) AND Endometrial hyperplasia).
The diagnostic criteria for EH was in accordance with the WHO Classification of Female Reproductive Tumors [39]. In addition, the specific diagnostic criteria were adjusted according to the year in which the published study was conducted. Literature published in languages other than Chinese and English were translated by a professional translator.
The inclusion criteria for meta-analysis were as follows: (1) the patients diagnosed with EH had been confirmed histologically; (2) the types of interventions included LNS-IUS or oral progesterone or other oral drugs (e.g., metformin); (3) the study included relevant outcomes for endometrial regression, and (4) premenopausal women.
The exclusion criteria were as follows: (1) the study design did not qualify as a controlled or observational study (thus excluding reviews, letters, and others); (2) the study did not meet the diagnostic criteria for EH (such as EC); (3) patients with comorbidities, such as severe kidney disease, or liver disease, and (4) postmenopausal patients.
Two evaluators independently searched the databases and identified relevant literature based on the inclusion and exclusion criteria. For each study retrieved, the evaluators recorded the name of the first author, the year of publication, the country in which the study was conducted, the methods used to preserve fertility function, treatment-time, follow-up time, the demographics of the subjects (sample size, age), and outcomes (primary and secondary results).
The methodological quality of the study was evaluated in accordance with the Cochrane Collaboration’s tool for assessing the risk of bias [40]. This evaluation included random sequence generation, allocation concealment, subject and intervention provider blinding, outcome evaluation blinding, outcome data integrity, selective outcome reporting, and other sources of bias. Disagreement was judged by discussion. In accordance with the Cochrane Collaboration Group criteria, we divided the retrieved studies into three categories: (1) a low risk of bias (in all areas); (2) an unclear risk of bias (in one or more key areas), and (3) a high risk of bias (in one or more key areas).
RevMan version 5.3 software (The Nordic Cochrane, Centre, Copenhagen) (https://revman.cochrane.org/), provided by the Cochrane Collaboration, was used for all statistical meta-analyses, and the relative risk (RR) difference and standard error (SE), along with the mean difference and 95% confidence interval (CI) were used as evaluation indices.
First, the Chi-squared test was used to assess heterogeneity; then the existence of heterogeneity (I2) was quantitatively analyzed (I2
We screened the PubMed, Web of Science, Cochrane Library, and Embase databases to identify relevant studies that had been published from inception to the 31st of July 2023. At the beginning of the study, we identified a total of 10,414 relevant articles based on the search strategy. After the two researchers independently read and screened the research data, a total of 44 studies [40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83] were selected for the final investigation. The literature screening process and results are shown in Fig. 1.
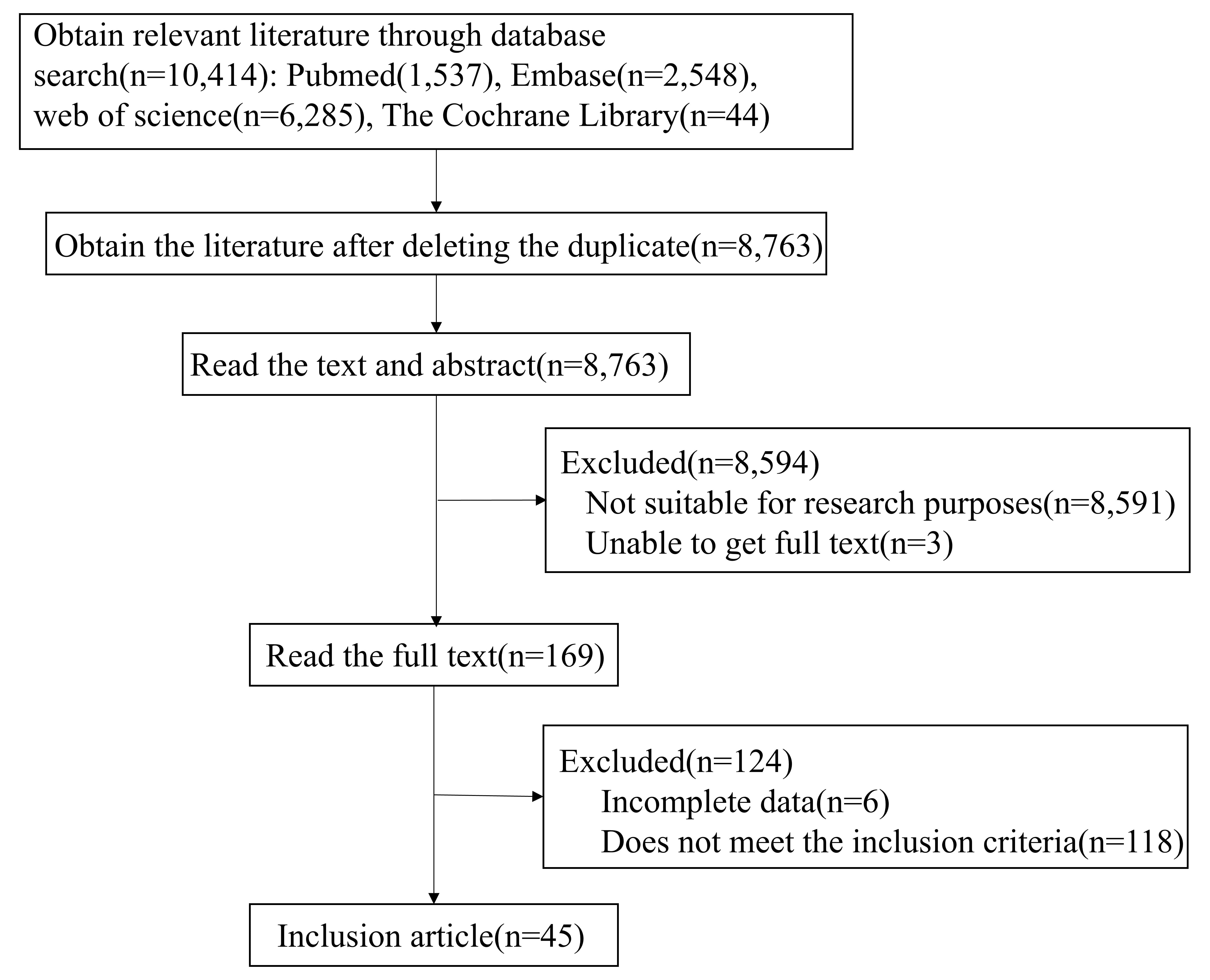 Fig. 1.
Fig. 1. Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram of study selection.
The basic features of the included studies are shown in Table 1 (Ref. [33, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83]) while the oncological and reproductive outcomes in pre-menopausal EH women with or without atypical EH are shown in Table 2 (Ref. [33, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83]). The quality of the literature was evaluated using the Cochrane risk of bias tool [39]; analysis showed that the main sources of potential bias were the blinding of subjects and intervention providers (Fig. 2). The included studies were published between 1997 and 2023. Nine of the studies were high quality RCTs [45, 47, 48, 50, 56, 60, 68, 69, 83].
| No. | First author | Year | Study type | County | Age (years) (95% CI) | Treatment type | Treatment time (months) | Follow-up time (months) | EH type |
| 1 | Mitsuhashi A [40] | 2019 | retrospective | Japan | 35.00 (26.00–44.00) | MPA plus metformin | 6.00 (3.00–18.00) | 57.00 (13.00–88.00) | AEH |
| 2 | Tabrizi AD [41] | 2014 | prospective | Iran | pre-menopausal | Metformin or MA | 3.00 | 3.00 | EH non-atypical |
| 3 | Simpson AN [42] | 2014 | prospective | Canada | 36.50 (26.00–44.00) | oral progestin | 9.50 (2.00–53.00) | 39.00 (5.00–128.00) | AEH |
| 4 | Yang B [43] | 2018 | retrospective | China | 33.00 (21.00–54.00) | oral MA or plus metformin | 6.00 | unclear | AEH |
| 5 | Yang B [44] | 2019 | retrospective | China | 32.00 (22.00–47.00) | MA, MA + metformin, LNG-IUD, Diane-35, MA + LNG-IUD | 6.80 | 13.50 (1.00–36.00) | AEH |
| 6 | Yang BY [45] | 2020 | RCT | China | 18.00–45.00 | MA or MA plus metformin | 16.00 | 24.00 | AEH |
| 7 | Ricciardi E [46] | 2012 | prospective | Italy | 30.00 (25.00–40.00) | MA or MPA | 12.00 | 4.00–24.00 | AEH |
| 8 | Behnamfar F [47] | 2014 | RCT | Iran | 38.40 | LNG-IUS or MPA | 3.00 | 3.00 | EH non-atypical |
| 9 | Sharifzadeh F [48] | 2017 | RCT | UK | pre-menopausal | MA or MA plus metformin | 3.00 | 3.00 | EH non-atypical |
| 10 | Brownfoot FC [49] | 2014 | retrospective | Australia | 37.00 | MPA or LNG-IUS | 12.00 | 24.00 (10.00–120.00) | AEH |
| 11 | Abu Hashim H [50] | 2013 | RCT | Egypt | pre-menopausal | LNG-IUS plus NET | 3.00–6.00 | 3.00–12.00 | EH non-atypical |
| 12 | Li H [51] | 2008 | prospective | China | 32.60 (27.00–38.00) | letrozole | 3.00 | 3.00 | EH non-atypical |
| 13 | Zhou H [52] | 2017 | retrospective | China | average 30.60 | GnRHa with LNG-IUD or GnRHa with letrozole | 4.50 | 18.70 (5.60–54.90) | AEH |
| 14 | Gallos ID [53] | 2013 | prospective | UK | Younger than 40.00 | LNG-IUD or oral progestogens | 6.00 | 58.80 (12.00–148.20) | EH non-atypical |
| 15 | Baek JS [54] | 2016 | retrospective | Korea | 33.00 (20.00–41.00) | MA or MPA | 3.00 (1.00–22.00) | 8.00 (7.00–11.00) | AEH |
| 16 | Bian J [55] | 2015 | prospective | China | Younger than 40.00 | LNG-IUS or non-LNG-IUS | 6.00 | 6.00 | EH non-atypical |
| 17 | Dolapcioglu K [56] | 2013 | RCT | Turkey | 43.50 (40.00–55.00) | MPA or LNG-IUS | 3.00 | 24.00 | EH non-atypical |
| 18 | Kim MK [57] | 2016 | prospective | Korea | 42.67 | LNG-IUS | 9.00 | 12.00 | EH non-atypical and AEH |
| 19 | Ushijima K [58] | 2007 | prospective | Japan | 20.00–39.00 | MPA with aspirin daily followed by cyclic estrogen-progestin therapy for 6 months or cyclic estrogen-progestin | 6.00 | 3.00–6.00 | AEH |
| 20 | Minig L [59] | 2011 | prospective | Spain | 34.00 (22.00–40.00) | LNG-IUD + GnRH analogue | 12.00 | 29.00 (4.00–102.00) | AEH |
| 21 | Karimi-Zarchi M [60] | 2013 | RCT | Iran | 22.00–47.00 | MPA or MPA with LNG-IUD | 3.00 | 3.00 | EH non-atypical |
| 22 | Signorelli M [61] | 2009 | prospective | Italy | 32.00 (21.00–40.00) | danazole, GnRh analogue, natural progestin, MPA | 4.00 (3.00–9.00) | 98.00 (35.00–176.00) | AEH |
| 23 | El Behery MM [62] | 2015 | prospective | Egypt | 30.00–50.00 | Oral Progesterone or LNG-IUD | 6.00 | 6.00 | EH non-atypical |
| 24 | Koskas M [63] | 2012 | retrospective | France | 28.00–40.00 | Lynestrenol, megestrol acetate, medroxyprogesterone acetate, nomegestrol acetate, chlormadinone acetate | 3.00–6.00 | 14.00–84.00 | AEH |
| 25 | Yu M [64] | 2009 | retrospective | China | 29.90 | MPA, NET | 7.30 | 34.60 (7.00–114.00) | AEH |
| 26 | Mentrikoski MJ [65] | 2012 | retrospective | USA | 38.00 (25.00–39.00) | oral progestin or LNG-IUD | at least 6 | 7.00 | AEH |
| 27 | Chen M [66] | 2016 | retrospective | China | 32.00 (21.00–41.00) | MPA or MA | 8.00 (2.00–18.00) | 6.00 (3.00–24.00) | AEH |
| 28 | Mitsuhashi A [67] | 2016 | prospective | Japan | 35.00 (26.00–41.00) | MPA or plus metformin | 4.00–9.00 | 38.00 (9.00–66.00) | AEH |
| 29 | Ismail MT [68] | 2013 | RCT | Egypt | 35.00–50.00 | MPA or NET or LNG-IUS | 3.00 | 3.00 | EH non-atypical |
| 30 | Ohyagi-Hara C [33] | 2015 | retrospective | Japan | 34.20 (22.20–43.90) | MPA | 12.00 | 39.20 (3.40–153.80) | AEH |
| 31 | Ozdegirmenci O [69] | 2011 | RCT | Turkey | 30.00–57.00 | MPA, LYN and NET | 3.00 | 6 | EH non-atypical |
| 32 | Pashov AI [70] | 2012 | prospective | Russia | 28.90 | LNG-IUS with GnRHa | 3.00 | 48.37 | AEH |
| 33 | De Marzi P [71] | 2015 | retrospective | Italy | 36.58 (23.00–43.00) | MA | 3.00 | 25.00 (8.00–37.00) | AEH |
| 34 | Giampaolino P [72] | 2019 | retrospective | Italy | 35.1 | LNG-IUS | 1.00–6.00 | 1.00–6.00 | AEH |
| 35 | Tamauchi S [73] | 2018 | retrospective | Japan | 34.00 (19.00–45.00) | MPA | 6.50 (2.50–15.80) | 13.00 (4.00–32.00) | AEH |
| 36 | Lee SY [74] | 2010 | retrospective | Korea | 39.10 (25.00–46.00) | LNG-IUS | 4.50 (3.00–9.00) | 3.00–30.00 | EH non-atypical and AEH |
| 37 | Shan B [75] | 2013 | prospective | China | 30.00 (18.00–38.00) | MA | 6.00 | 17.00–54.00 | AEH |
| 38 | Pronin SM [76] | 2015 | prospective | Russia | 33.00 (28.00–42.00) | LNG-IUS | 6.00 | 17.00 (1.00–45.00) | AEH |
| 39 | Acosta-Torres S [77] | 2020 | retrospective | USA | 35.00 (30.00–38.50) | Progestin alone or with metformin | 4.00 (3.40–7.40) | 28.40 (17.20–61.60) | AEH |
| 40 | Randall TC [78] | 1997 | retrospective | USA | 34.30 (25.00–39.00) | MA or MPA | 9.00 (3.00–18.00) | 40.10 (7.00–79.00) | AEH |
| 41 | Leone Roberti Maggiore U [79] | 2019 | retrospective | Italy | 35.10 | LNG-IUS | 6.70 | 76.40 | AEH |
| 42 | Shan W [80] | 2014 | prospective | China | 28.00–43.00 | MA or MA plus metformin | 12.00 | 12.00 | AEH |
| 43 | Wheeler DT [81] | 2007 | retrospective | USA | 34.00 (24.00–47.00) | oral progestin or progesterone, or LNG-IUS | 3.00–6.00 | 11.00 | AEH |
| 44 | Yang YF [82] | 2015 | retrospective | China | 33.00 (24.00–39.00) | MA, MPA, LNG-IUS, NET | 6.00 (3.00–13.00) | 52.00 (8.00–78.00) | AEH |
| 45 | Zhou R [83] | 2015 | RCT | China | 30.40 (20.00–40.00) | oral progestin | 6.00 (1.00–41.50) | 32.50 (10.00–92.00) | AEH |
AEH, atypical endometrial hyperplasia; EH, endometrial hyperplasia; GnRHa, gonadotropin-releasing hormone analogue; LNG-IUS, levonorgestrel-releasing intrauterine system; LNG-IUD, levonorgestrel-releasing intrauterine device; MA, megestrol acetate; MPA, medroxyprogesterone acetate; LYN, lynestrenol; NET, norethisterone acetate; RCT, randomized controlled trial.
| No. | First author | EH non-atypical | CR | ART | Pregnancy | Live birth | AEH | CR | ART | Pregnancy | Live birth |
| 1 | Mitsuhashi A [40] | - | - | - | - | - | 21 | 21 | - | - | - |
| 2 | Tabrizi AD [41] | 43 | 34 | - | - | - | - | - | - | - | - |
| 3 | Simpson AN [42] | - | - | - | - | - | 19 | 18 | - | - | - |
| 4 | Yang B [43] | - | - | - | - | - | 148 | 141 | 22 | 25 | - |
| 5 | Yang B [44] | - | - | - | - | - | 120 | 112 | - | 21 | - |
| 6 | Yang BY [45] | - | - | - | - | - | 123 | 79 | - | - | - |
| 7 | Ricciardi E [46] | - | - | - | - | - | 14 | 11 | 9 | 4 | - |
| 8 | Behnamfar F [47] | 55 | 44 | - | - | - | - | - | - | - | - |
| 9 | Sharifzadeh F [48] | 42 | 30 | - | - | - | - | - | - | - | - |
| 10 | Brownfoot FC [49] | - | - | - | - | - | 42 | 32 | 2 | 4 | 4 |
| 11 | Abu Hashim H [50] | 120 | 86 | - | - | - | - | - | - | - | - |
| 12 | Li H [51] | 5 | 5 | - | - | - | - | - | - | - | - |
| 13 | Zhou H [52] | - | - | - | - | - | 12 | 12 | 0 | 0 | |
| 14 | Gallos ID [53] | 34 | 30 | - | - | - | - | - | - | - | - |
| 15 | Baek JS [54] | - | - | - | - | - | 18 | 16 | 4 | 3 | 2 |
| 16 | Bian J [55] | 190 | 117 | 171 | 64 | 53 | - | - | - | - | - |
| 17 | Dolapcioglu K [56] | 102 | 76 | - | - | - | - | - | - | - | - |
| 18 | Kim MK [57] | 32 | 30 | - | - | - | 6 | 6 | - | - | - |
| 19 | Ushijima K [58] | - | - | - | - | - | 17 | 14 | - | - | - |
| 20 | Minig L [59] | - | - | - | - | - | 20 | 19 | 2 | 11 | 0 |
| 21 | Karimi-Zarchi M [60] | 40 | 33 | - | - | - | - | - | - | - | - |
| 22 | Signorelli M [61] | - | - | - | - | - | 10 | 1 | 0 | 8 | 7 |
| 23 | El Behery MM [62] | 100 | 88 | - | - | - | - | - | - | - | - |
| 24 | Koskas M [63] | - | - | - | - | - | 14 | 13 | 5 | 6 | 5 |
| 25 | Yu M [64] | - | - | - | - | - | 17 | 14 | 13 | 7 | 3 |
| 26 | Mentrikoski MJ [65] | - | - | - | - | - | 7 | 5 | - | - | - |
| 27 | Chen M [66] | - | - | - | - | - | 16 | 12 | 9 | 9 | 6 |
| 28 | Mitsuhashi A [67] | - | - | - | - | - | 17 | 16 | 11 | 11 | 6 |
| 29 | Ismail MT [68] | 90 | 88 | - | - | - | - | - | - | - | - |
| 30 | Ohyagi-Hara C [33] | - | - | - | - | - | 11 | 9 | 2 | 5 | 7 |
| 31 | Ozdegirmenci O [69] | 82 | 80 | - | - | - | - | - | - | - | - |
| 32 | Pashov AI [70] | - | - | - | - | - | 13 | 13 | 0 | 0 | 0 |
| 33 | De Marzi P [71] | - | - | - | - | - | 20 | 13 | 1 | 6 | 5 |
| 34 | Giampaolino P [72] | - | - | - | - | - | 55 | 51 | 0 | 10 | 10 |
| 35 | Tamauchi S [73] | - | - | - | - | - | 30 | 26 | 8 | 11 | 7 |
| 36 | Lee SY [74] | 11 | 11 | - | - | - | 1 | 1 | - | - | - |
| 37 | Shan B [75] | - | - | - | - | - | 12 | 9 | 0 | 0 | 0 |
| 38 | Pronin SM [76] | - | - | - | - | - | 38 | 32 | 0 | 5 | 4 |
| 39 | Acosta-Torres S [77] | - | - | - | - | - | 54 | 41 | 7 | 9 | 9 |
| 40 | Randall TC [78] | - | - | - | - | - | 17 | 16 | 2 | - | - |
| 41 | Leone Roberti Maggiore U [79] | - | - | - | - | - | 28 | 25 | 2 | 6 | 5 |
| 42 | Shan W [80] | - | - | - | - | - | 16 | 8 | - | - | - |
| 43 | Wheeler DT [81] | - | - | - | - | - | 18 | 12 | - | - | - |
| 44 | Yang YF [82] | - | - | - | - | - | 37 | 34 | - | - | - |
| 45 | Zhou R [83] | - | - | - | - | - | 13 | 9 | 4 | 4 | - |
ART, assisted reproductive technology; CR, complete response; EH, endometrial hyperplasia.
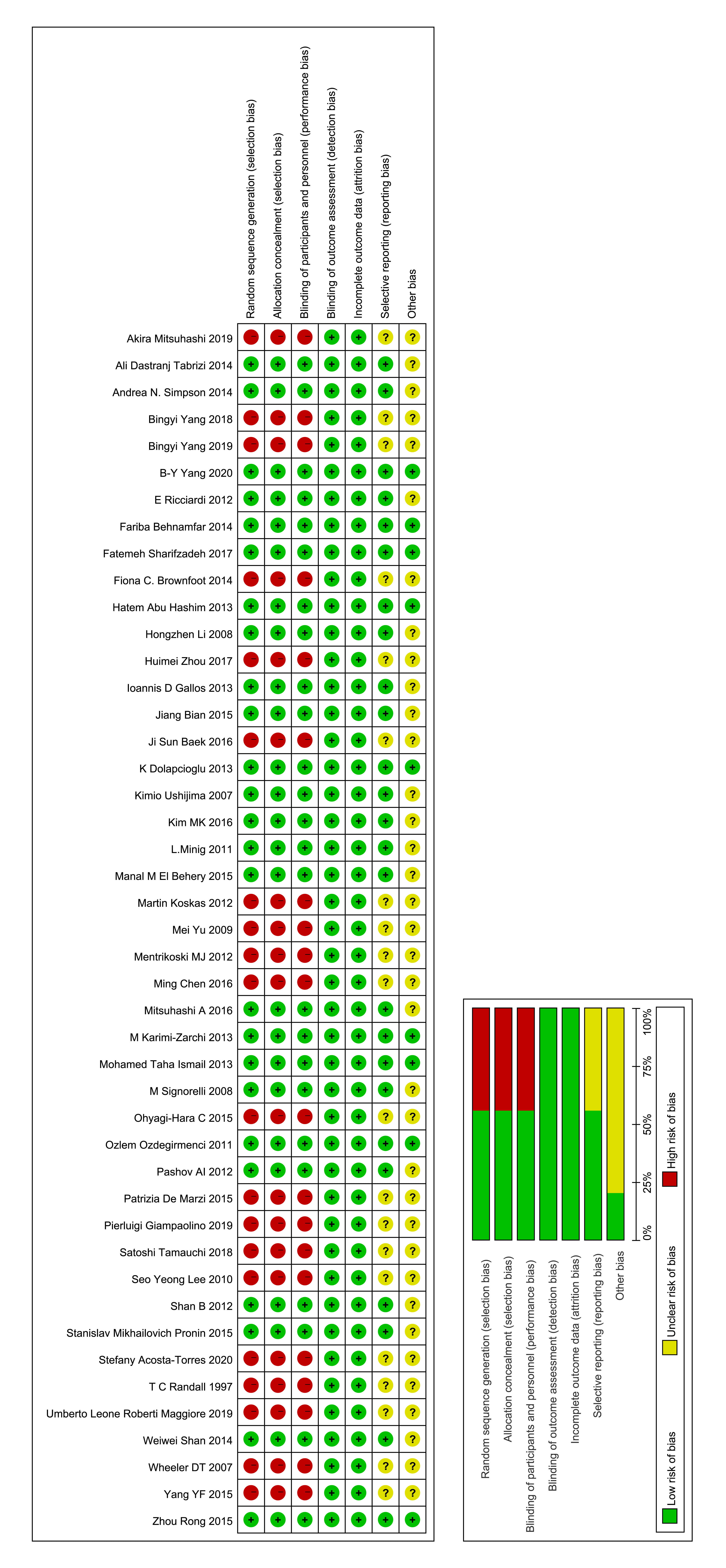 Fig. 2.
Fig. 2. Risk of bias and the clinical applicability of the included studies.
Fig. 3 shows the pooled CR in premenopausal EH women with or without atypical EH who were treated with fertility-preservation therapy. There was a pooled CR rate of 0.82 (95% CI: 0.78–0.86) in premenopausal EH patients receiving fertility preservation therapy. Since endometrial cell atypia is related to treatment effect, we conducted subgroup analysis on whether EH has atypical. The pooled CR rate of EH patients without atypical EH was 0.83 (95% CI: 0.75–0.90); in comparison, the pooled CR rate for patients with AEH was 0.81 (95% CI: 0.76–0.87).
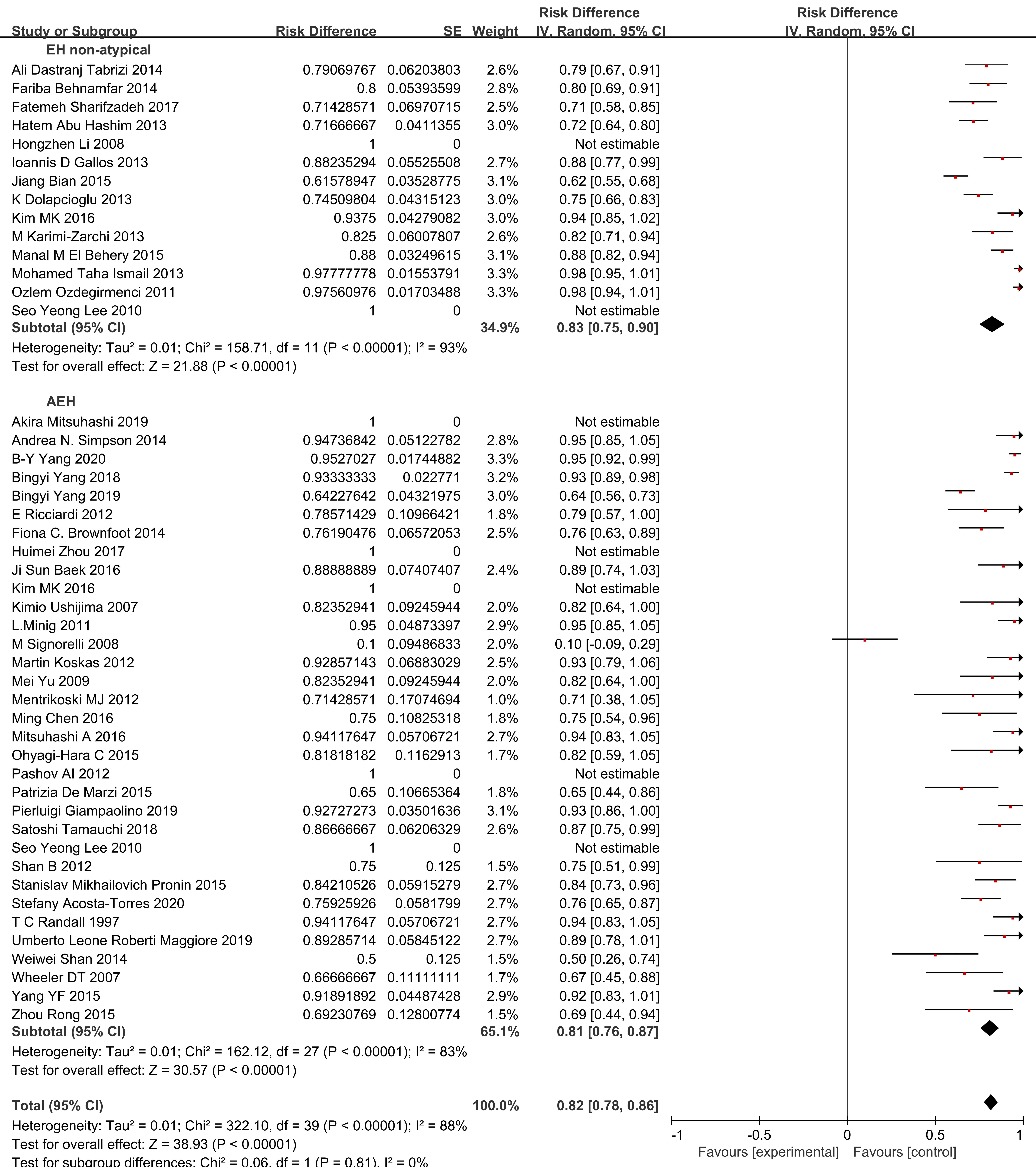 Fig. 3.
Fig. 3. Meta-analysis of complete response (CR) in women with endometrial hyperplasia with or without atypical hyperplasia (including subgroup analysis). EH, endometrial hyperplasia; AEH, atypical endometrial hyperplasia.
Fig. 4 shows the reproductive outcomes in premenopausal EH women with or without atypical EH who were treated with fertility-preservation treatment. There was a pooled assisted reproductive technology (ART) rate of 0.30 (95% CI: 0.10–0.49) in premenopausal EH patients receiving fertility preservation therapy. The pooled pregnancy rate and pooled live birth rate were 0.30 (95% CI: 0.24–0.37) and 0.24 (95% CI: 0.17–0.30), respectively.
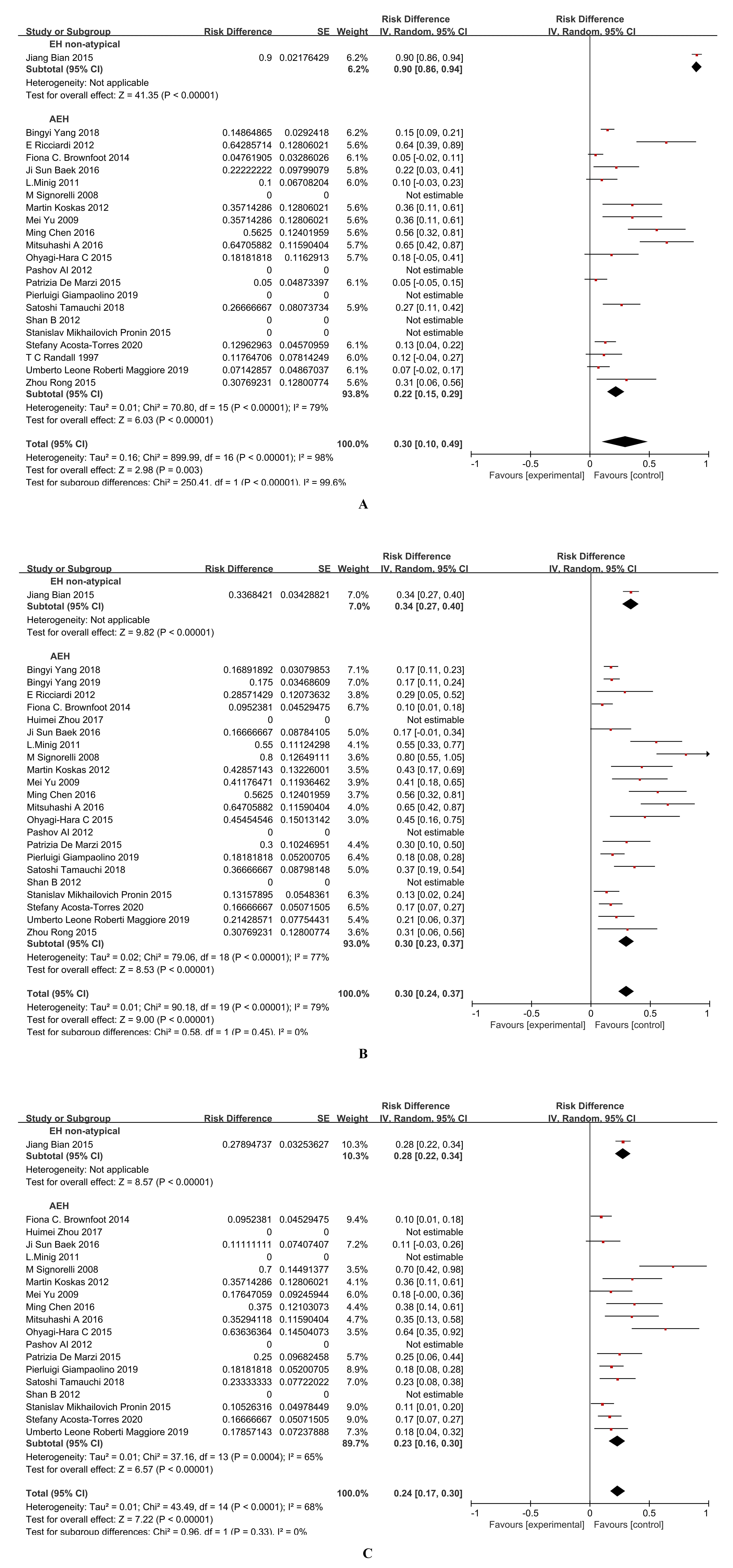 Fig. 4.
Fig. 4. Meta-analysis of reproductive outcomes for women with endometrial hyperplasia with or without atypical hyperplasia (including subgroup analysis). (A) Assisted reproductive technology (ART). (B) Pregnancy. (C) Live birth.
Since only one article reported the obstetric outcomes of atypical EH, we only conducted subgroup analysis on the reproductive outcomes of patients with AEH. There was a pooled ART rate of 0.22 (95% CI: 0.15–0.29) in premenopausal patients with AEH receiving fertility preservation therapy. The pooled pregnancy rate and pooled live birth rate were 0.30 (95% CI: 0.23–0.37) and 0.23 (95% CI: 0.16–0.30), respectively.
Fig. 5 shows an adjusted funnel diagram, including the endometrial CR rate, ART rate, pregnancy rate and live birth rate. All studies shown on the funnel maps were symmetrically distributed with respect to the vertical line, thus indicating that there were no significant small-sample effects or publication bias.
 Fig. 5.
Fig. 5. Funnel plot. (A) Complete response (CR). (B) Assisted reproductive technology (ART). (C) Pregnancy. (D) Live birth. RD, relative distance.
A total of 45 previous studies (including prospective studies, retrospective studies, and high-quality RCTs) were included in this study. Two studies included non-atypical EH and AEH, 12 studies focused on non-atypical EH only, and 31 studies focused on EH and AEH only. The study participants ranged from 18 to 57 years-of-age, and fertility preservation treatments included oral progesterone preparations (such as MPA/MA/NET) or the placement of an intrauterine LNG-IUS. The treatment period ranged from 1 to 53 months, and the follow-up period ranged from 1 to 176 months. The overall intimal pooled CR rate of premenopausal EH patients was 0.82 (95% CI: 0.78–0.86). The pooled CR rate of EH patients without atypical EH and AEH were 0.83 (95% CI: 0.75–0.80) and 0.81 (95% CI: 0.76–0.87), respectively. For reproductive outcomes (including ART, pregnancy, and live births), the overall pooled rates were 0.30 (95% CI: 0.10–0.49), 0.30 (95% CI: 0.24–0.37), and 0.24 (95% CI: 0.17–0.30), respectively. Since only one study reported the fertility outcome of atypical EH, we did not analyze the fertility outcome of this type of EH patients after undergoing fertility preservation therapy. For premenopausal AEH patients treated with fertility preservation, the fertility outcomes (including the pooled ART rate, the pooled pregnancy rate, and the pooled live birth rate) were 0.22 (95% CI: 0.15–0.29), 0.30 (95% CI: 0.23–0.37), and 0.23 (95% CI: 0.16–0.30), respectively.
Many drugs were described by the papers included in the present analysis. Different progesterone analogues exert differing metabolic pathways in the human body, which may provide patients with different degrees of side effects. The chemical name for MPA is pregn-4-ene-3, 20-dione, 17-(acetyloxy)-6-methyl-, (6
Previous literature reviews on the use of fertility preservation therapy in women with EH focused only on patients with AEH, including those with early-stage EEC [32, 34, 35, 36]. Although this specific cohort cannot represent the entire EH population, it can still partially indicate that fertility preservation therapy is effective for EH patients who wish to retain their fertility. The present study subclassified patients according to endometrial atypia; our AEH analysis generated data that were similar to those reported by previous studies. With regards to premenopausal women with non-atypical EH, it was evident that fertility preservation was effective with regards to the intima, and the comprehensive CR rate was close to that of patients with AEH. However, only one article has been published relating to the reproductive outcome of non-atypical EH [68]. This particular publication reported that the fertility outcomes (including ART, pregnancy and live birth) of EH patients without atypical after fertility preservation treatment were 0.90, 0.34, and 0.28, respectively; these rates were slightly higher than those of patients with AEH following fertility preservation treatment. However, further research is required to identify any statistical differences between these two types of patients.
In addition to utilizing the pregnancy rate and live birth rate as the main evaluation indicators, the health status of the offspring is also an issue that must be considered in terms of eugenic care. None of the studies with reported reproductive outcomes included in this meta-analysis reported health problems associated with their offspring. No previous study investigated whether the presence of maternal EH can exert an impact on the growth, development and long-term health of the offspring. However, it should be noted that some of the high-risk factors associated with EH disease will affect the growth and development of offspring and their long-term health, especially with regards to PCOS, hyperinsulinemia, diabetes, and obesity [93, 94, 95, 96].
There are some limitations to this study that need to be considered. For example, this study only conducted meta-analysis on the gynecological outcome (CR rate) and obstetric outcomes (ART rate, pregnancy rate, live birth rate) of patients with EH following fertility preservation treatment, and did not conduct subgroup analysis on other factors associated with infertility, such as ovulation abnormalities or tubal factor infertility. In addition, we did not perform a comparative evaluation of different treatment modalities, such as drug types, medication modalities, or specific treatment regiments, and did not compare different pregnancy assistance regimens that may have been received by patients receiving ART. Therefore, our findings cannot provide recommendations for the treatment of patients with EH, including the treatment of EH and ART treatment. Furthermore, due to the content and quality limitations of the published literature, the results of the present study should be adopted cautiously. It is still necessary to investigate the fertility outcomes of the heterotypic EH population after receiving fertility preservation therapy. Finally, we must consider the final fertility outcomes of patients with EH as such outcomes will inevitably be associated with a range of influencing factors. Further research should include multi-center RCTs with strict designs and long follow-up periods in order to determine the best treatment plan.
According to the present meta-analysis, the use of fertility preservation treatment for premenopausal patients with EH was effective. Approximately, four-fifths of patients achieved complete endometrial regression after a certain cycle of treatment. After treatment, some of the patients achieved satisfactory fertility outcomes, some of which needed to be supported by ART technology, although natural conception was still the main method of conception. Fertility preservation treatment for women with EH who still wish to have children still needs to be confirmed. However, based on the studies included in our meta-analysis, we are unable to identify an optimal treatment plan. Furthermore, additional research is required to determine whether there was any difference in efficacy, including endometrial outcomes and satisfactory fertility outcomes, when compared between patients with AEH and non-atypical EH. Appropriate studies are still needed, especially those related to the fertility outcomes of patients with non-atypical EH.
AEH, atypical endometrial hyperplasia; ART, assisted reproductive technology; AUB, abnormal uterine bleeding; CI, confidence interval; CR, complete response; EC, Endometrial cancer; EEC, endometrioid endometrial carcinoma; EH, endometrial hyperplasia; EIN, endometrial intraepithelial neoplasia; I2, heterogeneity; LNS-IUS, levonorgestrel intrauterine sustained release system; LNG-IUD, levonorgestrel-releasing intrauterine device; MA, megestrol acetate; MPA, medroxyprogesterone acetate; LYN, lynestrenol; NET, norethisterone; PCOS, polycystic ovarian syndrome; PRISMA, the Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RCTs, randomized controlled trials; SE, standard error; RD, relative distance; WHO, world health organization.
The data that support the findings of this study are available on request from the corresponding author.
JW, YY and RJ designed the research study. JW and YY performed the research. JW and YY analyzed the data, TW made substantial contributions to the interpretation of the data. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Not applicable.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest.
Supplementary material associated with this article can be found, in the online version, at https://doi.org/10.31083/CEOG39051.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.





