1 Department of Reproductive Medicine, Zhongda Hospital, School of Medicine, Southeast University, 210009 Nanjing, Jiangsu, China
2 Department of Obstetrics and Gynecology, Zhongda Hospital, School of Medicine, Southeast University, 210009 Nanjing, Jiangsu, China
†These authors contributed equally.
Abstract
Kisspeptin, a key regulator of the hypothalamic-pituitary-ovarian (HPO) axis through GnRH stimulation, is implicated in polycystic ovary syndrome (PCOS) pathogenesis via HPO axis dysregulation. Although follicular kisspeptin levels predict success in fresh IVF cycles, their prognostic value in PCOS patients undergoing frozen-thawed embryo transfer (FET) cycles remains unknown. This study investigated the correlation between serum kisspeptin levels on the day of embryo transfer and pregnancy outcomes in patients with PCOS undergoing FET cycles.
80 PCOS patients undergoing their first FET cycle were prospectively enrolled. Patients were divided into three groups based on the tertiles of serum kisspeptin levels on the day of embryo transfer. The relationship between serum kisspeptin levels and pregnancy outcomes was analyzed. Multivariate logistic regression analysis was conducted to adjust for potential confounders, and a restricted cubic spline model was employed to examine the dose-response relationship between kisspeptin levels and pregnancy outcomes.
There were no significant differences in baseline characteristics among the three groups, except for the basal follicle-stimulating hormone level (p < 0.001). Significant differences were observed among the three groups in terms of live birth rate, pregnancy rate, and clinical pregnancy rate. Multivariate logistic regression analysis revealed that the odds of live birth were significantly higher in the third tertile (T3) group (highest kisspeptin levels) than in the first tertile (T1) group (lowest kisspeptin levels). Restricted cubic spline analysis showed a significant dose-response relationship between serum kisspeptin levels and pregnancy outcomes. Additionally, serum kisspeptin levels were positively correlated with progesterone levels on the day of embryo transfer day, whereas no significant correlation was observed with estradiol levels.
In PCOS patients undergoing frozen-thawed embryo transfer cycles, higher serum kisspeptin levels were associated with improved pregnancy outcomes, suggesting that kisspeptin may serve as a useful biomarker for predicting pregnancy success.
Keywords
- frozen-thawed embryo transfer
- kisspeptin
- polycystic ovary syndrome
- pregnancy outcomes
Kisspeptin is a neuropeptide, primarily from the hypothalamus, that binds with the kisspeptin-1 (KISS1) receptor (also known as G protein-coupled receptor 54). Kisspeptin stimulates gonadotropin-releasing hormone (GnRH) secretion, thereby regulating the hypothalamic-pituitary-ovarian (HPO) axis and plays an important role in the female reproductive process [1, 2]. Clinical studies have shown that kisspeptin promotes oocyte maturation or ovulation by regulating GnRH secretion [3]. Ovarian kisspeptin also directly promotes oocyte maturation and ovulation. In addition, the kisspeptin/KISS1 receptor (KISS1R ) system in the endometrium and blastocyst participates in various physiological activities at the maternal-fetal interface and is critically involved in embryo implantation [4].
Dysfunction of the HPO axis is widely regarded as an important pathological mechanism of polycystic ovary syndrome (PCOS); the regulatory role of kisspeptin on GnRH secretion is particularly crucial. Studies have shown that patients with PCOS have higher levels of kisspeptin in their serum [5, 6], suggesting that excessive activation of the kisspeptin/KISS1R system may lead to enhanced HPO axis activity. One study has also shown elevated kisspeptin mRNA expression in the posterior hypothalamus of PCOS rat models [7]. These findings suggest the involvement of kisspeptin in PCOS pathogenesis and progression.
In the fresh embryo transfer cycle, the level of kisspeptin in the follicular fluid of the pregnancy group was significantly higher than in that of the non-pregnancy group. Serum kisspeptin levels around 8 days after gonadotropin stimulation, or on the day of ovum pick-up, were positively correlated with the corresponding serum estradiol (E2) levels and with the outcomes of in vitro fertilization/intracytoplasmic sperm injection (IVF/ICSI) treatment including serum human chorionic gonadotropin (hCG) levels [8]. Moreover, kisspeptin levels in early pregnancy predicted miscarriage and viable intrauterine pregnancy [9]. In frozen-thawed embryo transfer (FET) cycles, exogenous hormone replacement therapy may affect kisspeptin secretion, but the relationship between serum kisspeptin levels and pregnancy outcomes in FET cycles among PCOS patients remains unexplored. Therefore, the aim of the present study was to examine the association between serum kisspeptin levels on the day of embryo transfer and pregnancy outcomes, and the correlation between kisspeptin levels and E2 and progesterone levels in FET cycles of PCOS patients.
This prospective study was conducted at the Reproductive Medicine Center of
Zhongda Hospital, affiliated with Southeast University in Nanjing, China, between
December 2022 and June 2023. A total of 80 women diagnosed with PCOS, according
to the revised Rotterdam criteria (2004), were enrolled after rigorous screening.
The inclusion criteria were strictly defined as: (1) age
All clinical data, including demographic characteristics, reproductive history, and laboratory parameters, were systematically extracted from the electronic medical record system.
FETs were performed in artificial cycles using exogenous estradiol and
progesterone. In hormone replacement cycles, patients began taking estradiol
valerate H20160679 (Bayer Weimar GmbH & Co., Thuringia, Germany) or
17
Follicle-stimulating hormone (FSH), luteinizing hormone (LH), E2,
progesterone and total testosterone at menstruation were measured by
chemiluminescence immunoassay (UniCelTM Dxl 800, Beckman, CA, USA). On the
embryo-transfer day, 5 mL of peripheral venous blood were collected to test
kisspeptin level. Serum kisspeptin levels were quantified using ELISA kits
(Shanghai Jianglai Biological Co., Ltd., Shanghai, China). The cohort of 80 PCOS
patients undergoing their first FET cycles was stratified into three groups based
on serum kisspeptin tertiles on the embryo transfer day: the first tertile (T1)
group (
Pregnancy was defined as a blood
Continuous variables were described as mean and standard deviation if they were
normally distributed, otherwise, they were described as median and quartiles.
Analysis of variance or Kruskal-Wallis test were used for comparisons between
groups. Categorical variables were described as frequency and percentage, and
chi-square test was used to compare the difference among groups. Logistic models
were used for estimating the odds ratios (ORs) and their 95% confidence interval
(CI) of pregnancy, clinical pregnancy rate and live birth respectively, with
adjustments for the potential confounding factors, including female age, and body
mass index (BMI). A restricted cubic spline was drawn to explore the nonlinear
relationship between kisspeptin levels and outcome. Pearson correlation analysis
was used to explore the association between kisspeptin levels and progesterone or
E2 level. All statistical analyses were performed using R software (version
4.1.3, Boston, MA, USA), and results with p
A cohort of 80 PCOS patients undergoing their first FET cycles was prospectively
enrolled and stratified into three groups based on serum kisspeptin tertiles on
the embryo transfer day: the T1 group (
As summarized in Table 1, baseline demographic and clinical characteristics were
comparable across the three groups, with no significant differences observed in
age, BMI, anti-Müllerian hormone (AMH), antral follicle count (AFC), basal
LH, basal E2, total testosterone, thyroid stimulating hormone (TSH), or
E2 and progesterone levels on transfer day (all p
| T1 (n = 27) | T2 (n = 27) | T3 (n = 26) | p | |
| Age (years) | 31.04 |
32.56 |
31.50 |
0.437 |
| BMI (kg/m2) | 21.68 |
21.99 |
23.87 |
0.130 |
| AMH (ng/mL) | 7.50 |
7.33 |
6.98 |
0.801 |
| AFC | 13.89 |
16.76 |
16.32 |
0.325 |
| Basic FSH (mIU/mL) | 8.83 |
7.13 |
6.97 |
|
| Basic Estradiol (pg/mL) | 32.60 (22.92, 37.52) | 23.76 (6.92, 36.23) | 30.47 (18.76, 38.00) | 0.381 |
| Basic LH (IU/L) | 5.25 (4.08, 8.70) | 6.85 (3.38, 23.94) | 4.32 (3.42, 8.21) | 0.506 |
| Basic T (ng/mL) | 0.42 (0.36, 0.53) | 0.41 (0.29, 0.60) | 0.40 (0.27, 0.45) | 0.892 |
| TSH (µIU/mL) | 2.24 |
2.27 |
2.57 |
0.454 |
| Estradiol on the day of embryo transfer (pg/mL) | 534.33 (235.78, 1015.30) | 748.47 (467.85, 927.94) | 446.59 (179.55, 780.72) | 0.180 |
| P on the day of embryo transfer (ng/mL) | 6.11 (3.55, 10.43) | 6.98 (5.30, 9.45) | 7.59 (5.39, 8.69) | 0.645 |
Continuous variables are presented as mean and standard deviation (normally distributed) or median and quartiles (non-normal distribution). Analysis of variance or Kruskal-Wallis test were used for comparisons between groups. BMI, body mass index; AMH, anti-Müllerian hormone; AFC, antral follicle count; FSH, follicular stimulating hormone; LH, luteinizing hormone; T, testosterone; TSH, thyroid stimulating hormone; P, progesterone; T1, first tertile; T2, second tertile; T3, third tertile.
Significant statistical differences in live birth rate were observed among the
three groups (T1
| Pregnancy | Clinical pregnancy | Live birth | |
|---|---|---|---|
| T1 | 10 (37.0) | 9 (33.3) | 7 (25.9) |
| T2 | 14 (51.9) | 13 (48.1) | 9 (33.3) |
| T3 | 20 (76.9) | 18 (69.2) | 16 (61.5) |
| p | 0.013 | 0.032 | 0.021 |
After adjusting for potential confounds including age, BMI, AMH, AFC, and hormone levels (basal FSH, LH, E2, testosterone, and TSH), multivariate logistic regression analysis confirmed that patients in T3 group had significantly higher odds of live birth (adjusted odds ratio [aOR]: 3.83, 95% CI: 1.08–13.46) than did the reference group (T1) (Table 3).
| Pregnancy | Clinical pregnancy | Live birth | ||||
| Crude model | Adjusted model | Crude model | Adjusted model | Crude model | Adjusted model | |
| T1 | Ref | |||||
| T2 | 1.83 [0.62, 5.42] | 1.13 [0.20, 6.47] | 1.86 [0.62, 5.58] | 0.88 [0.14, 5.34] | 1.43 [0.44, 4.63] | 0.93 [0.19, 4.45] |
| T3 | 5.67 [1.71, 18.83] | 3.32 [1.05, 9.97] | 4.50 [1.42, 14.28] | 1.82 [1.02, 3.19] | 4.57 [1.42, 14.71] | 3.83 [1.08, 13.46] |
A restricted cubic spline showed that there was a significant dose-response relationship between kisspeptin levels and pregnancy outcomes. With the increase of kisspeptin levels, the pregnancy rate, clinical pregnancy rate, and live birth rate increased (Fig. 1).
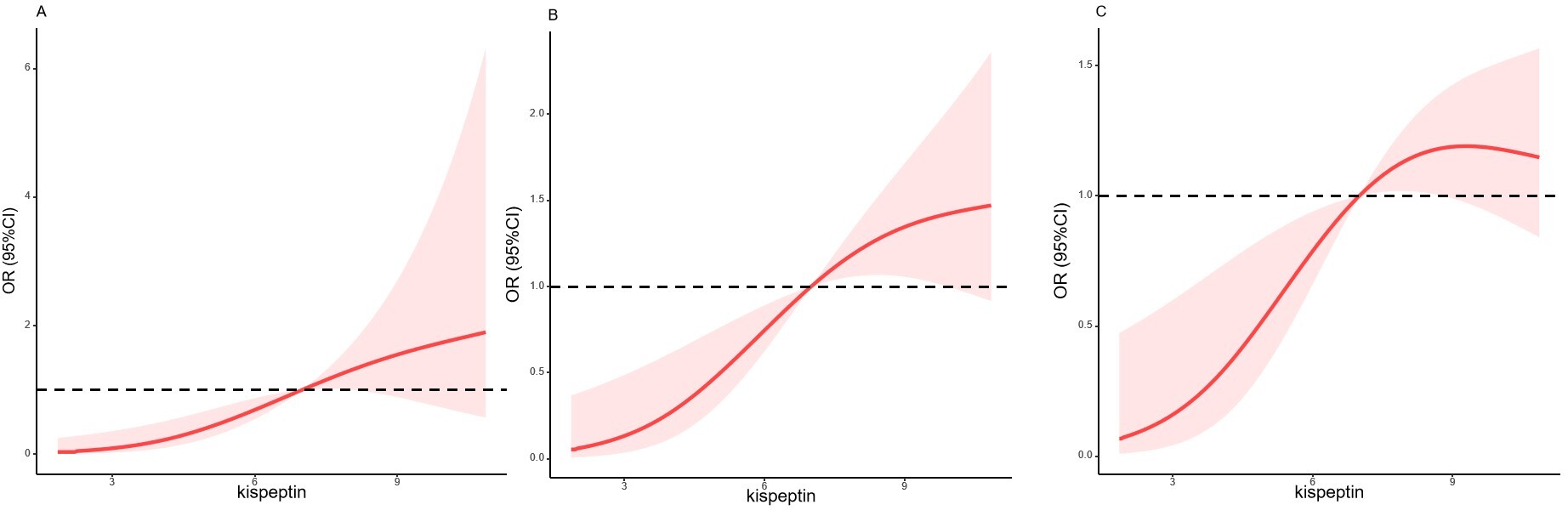 Fig. 1.
Fig. 1.
Cubic spline for the association between kisspeptin levels and pregnancy outcomes. (A) The pregnancy rate. (B) Clinical pregnancy rate. (C) Live birth rate. OR, odds ratio; CI, confidence interval.
A significant dose response relationship existed between kisspeptin levels and
pregnancy outcomes. With an increase of kisspeptin levels, the pregnancy rate,
clinical pregnancy rate and live birth rate increased, p
Notably, correlation analysis revealed a significant positive association between serum kisspeptin levels and progesterone levels on the day of embryo transfer (R = 0.23, p = 0.044), although no such correlation was observed with estradiol levels (R = 0.18, p = 0.120) (Fig. 2).
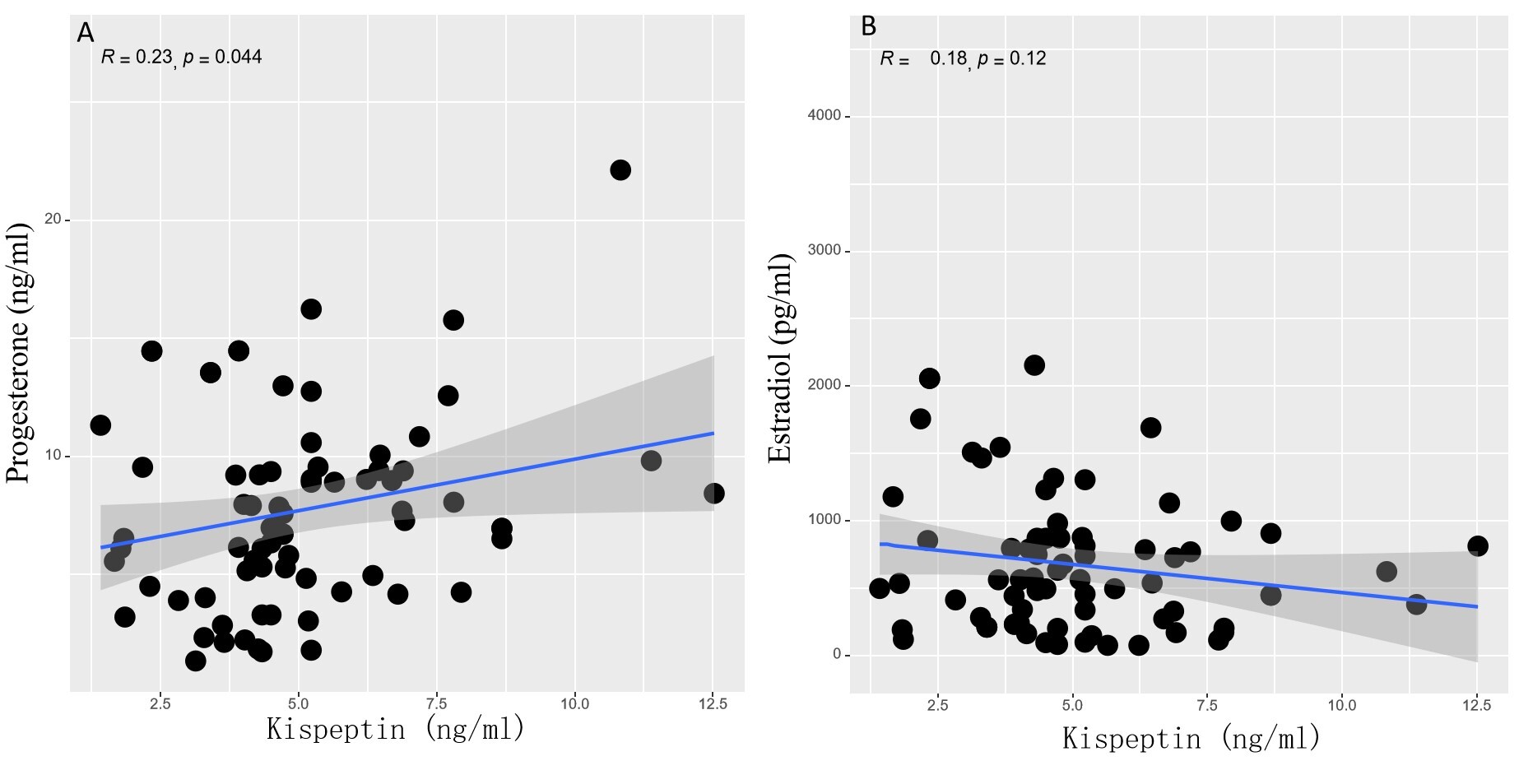 Fig. 2.
Fig. 2.
Association between kisspeptin levels and progesterone levels (A) or E2 levels (B) on the day of embryo transfer.
In this prospective study, significant differences were found in live birth rate, pregnancy rate, and clinical pregnancy rate among the three groups. After several covariates were adjusted, the live birth rates in highest tertile group were significantly higher than those of the lowest tertile group. Furthermore, the pregnancy rate, clinical pregnancy rate, and live birth rate increased as kisspeptin levels increased. There was a positive association between kisspeptin levels and progesterone. These results may suggest that kisspeptin plays a crucial role in endometrial receptivity and embryo implantation.
Factors associated with pregnancy outcomes in FET cycles included embryonic factors (cleavage stage embryos/blastocysts, number of transferred embryos, embryo quality), endometrial thickness, the woman’s age, and hormone levels on the day of embryo transfer [10]. If the success rate of embryo transfer can be determined by a single cut-off value of a single serum indicator, that would play an important role in guiding clinicians in adjusting the transfer plan. Our study reported that during the FET cycle in PCOS patients, serum kisspeptin levels on the embryo-transfer day were significantly positively correlated with live birth rate. The positive correlation found in our study corroborated the essential role of kisspeptin during embryo implantation; this had also been demonstrated in a previous study [11]. In that previous study, the serum kisspeptin expression level on hCG day was higher in the pregnant group than in the nonpregnant group. Another study investigated the expression profiles and functions of kisspeptin and KISS1R in the reproductive tissues of imprinting control region mice, and showed that kisspeptin and KISS1R were expressed in both female gametes and mouse reproductive tissues [12]. Another study has shown decreased expression of kisspeptin/KISS1R in women with recurrent spontaneous abortion [13], thus suggesting that kisspeptin may play an important role in embryonic implantation.
The kisspeptin system may regulate fertilization and reproduction in mammals. A prospective cross-sectional study found that patients with high estradiol levels on trigger day of the ovarian stimulation in IVF cycles had a high mRNA expression level of endometrial kisspeptin and leukemia inhibitory factor (LIF) [14]. LIF is regarded as one of the key markers for endometrial receptivity, and there was also a strong positive correlation between the expressions of kisspeptin and LIF in the above-mentioned study.
Contrary to our results, one prospective case-control study reported that serum kisspeptin level 14 days after FET could not be used to distinguish between miscarriage and ongoing pregnancy [15], and another previous study found that serum kisspeptin levels were negatively correlated with the number of retrieved oocytes and pregnancy rates in IVF cycles [16]. The possible disagreement between the present study and the two previous studies could be due to differences in the study sample and the small sample size. Our study subjects were women with PCOS undergoing IVF. A previous study has analyzed the serum kisspeptin level in PCOS patients, and pooled data showed that kisspeptin levels were significantly higher in women with PCOS [2]. The elevated basal FSH levels observed in the lowest kisspeptin T1 group in the present study supported the idea that abnormal kisspeptin secretion results in HPO-axis dysfunction, which impairs the pulsatile secretory mode of LH and FSH. A meta-analysis revealed that serum kisspeptin levels in PCOS patients were not only significantly higher than those in the control group but also positively correlated with the serum concentrations of AMH, testosterone, and dehydroepiandrosterone [17]. These findings suggested that abnormal secretion of kisspeptin in PCOS patients represents a complex interplay between HPO-axis dysfunction and peripheral ovarian steroidogenic activity.
It is interesting that we found that the serum kisspeptin level on the embryo-transfer day was related to the progesterone level on that day, but not to the E2 level. However, E2 has been regarded as the key regulator of the expression of kisspeptin [18]. Elevated serum E2 levels in women undergoing IVF were also proposed to affect the expression of kisspeptin mRNA in the endometrial stroma during the late secretion phase (5 days after oocyte retrieval) [19]. The present study used estrogen replacement therapy to maintain serum E2 concentrations within a relatively stable physiological range. That range was significantly lower than the high E2 levels caused by multiple follicle development during ovulation induction cycles, which may not enhance kisspeptin expression. In addition, progesterone may interrupt estrogen-kisspeptin signaling, making kisspeptin secretion more dependent on progesterone [20]. Our finding of the positive correlation between kisspeptin levels and progesterone levels on the day of embryo transfer suggested that kisspeptin may enhance endometrial receptivity through progesterone-mediated pathways. This result was consistent with the reported expression of KISS1R in the endometrium and its involvement in decidualization processes [4].
The uterus needs to be in a relatively relaxed state to facilitate embryo implantation. Kisspeptin has a relaxing effect on uterine contractions in pregnant rats [21], so kisspeptin may create a suitable microenvironment for embryo implantation by inhibiting uterine contractions. The use of kisspeptin-54 as an ovulation trigger could lead to an increase in the expression of embryo implantation markers (e.g., LIF) in the endometrium of the mouse [22]. By doing so, kisspeptin-54 may improve embryo implantation in IVF. In the IVF cycle, after kisspeptin was used to trigger oocyte maturation, the serum gonadotropin profile was closer to that of the natural cycle [23]. Therefore, kisspeptin may help to prepare the ovarian environment for steroidogenesis, especially the synthesis of progesterone required for embryo implantation.
Although our findings are promising, several limitations should be acknowledged. First, the single-center design and relatively small sample size (n = 80) may have limited the generalizability of our results, particularly given the variability of PCOS phenotypes. Second, the observational nature of this study precluded establishing causal relationships between kisspeptin levels and pregnancy outcomes. Third, there was a lack of continuous kisspeptin measurement throughout the FET cycle, making it impossible to monitor the dynamic changes of kisspeptin during the hormone replacement cycles.
Higher serum kisspeptin levels on the day of embryo transfer were associated with better pregnancy outcomes in PCOS patients undergoing FET cycles.
The observed dose-response relationship between kisspeptin levels and pregnancy outcomes suggested that kisspeptin may serve as both a prognostic marker and potential therapeutic target in this patient population. Further research is needed to elucidate the underlying mechanisms and clinical applications of these findings.
The data that support the findings of this study are available on request from the corresponding author, Xia Zhao, upon reasonable request.
LL and XZ designed the research study. DX performed the research. MC and YL provided help and advice on acquisition of data, LL and DX analyzed the data. DX, MC, YL and XZ wrote the manuscript and revised it critically for important intellectual content. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
The study was carried out in accordance with the guidelines of the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of Zhongda Hospital, School of Medicine, Southeast University (2022ZDSYLL452-P01). All patients gave their informed consent for inclusion before they participated in the study.
The authors thank all the staff members of the Department of Reproductive Medicine of Zhongda Hospital of Southeast University for their support and cooperation. The authors also appreciate Zhang Lanyu’s contribution to data analysis.
Zhongda Hospital Affliated to Southeast University Jiangsu Province High-Level Hospital Construction Funds [GSP-ZXY07]. The Key Research Project on Ideological and Political Education and Teaching Reform for Postgraduate Studies in 2024, Southeast University [No. 5024002403].
The authors declare no conflict of interest.
The author’s native language is not English. Therefore, during the preparation of this work, we used DeepSeek to improve the English expression. After using this tool, the authors reviewed and edited the content as needed and takes full responsibility for the content of the publication.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.


