1 Reproductive Center, Department of Obstetrics and Gynecology, West China Second University Hospital, Sichuan University, 610041 Chengdu, Sichuan, China
2 West China School of Medicine, Sichuan University, 610041 Chengdu, Sichuan, China
3 Key Laboratory of Birth Defects and Related of Women and Children of Ministry of Education, West China Second University Hospital, Sichuan University, 610041 Chengdu, Sichuan, China
Abstract
This review re-evaluates fertility-sparing surgery (FSS) in cervical cancer, synthesizing advancements in surgical precision, evolving indications, and sociodemographic factors influencing care access. It emphasizes the importance of multidisciplinary collaboration and equitable access to optimize both reproductive and survival outcomes for young patients.
A systematic search was performed across PubMed, Embase, and Web of Science for studies published in the past ten years, using key terms related to cervical cancer, FSS, oncologic and obstetric outcomes, and social determinants of health. Due to heterogeneity in study designs and outcome reporting, a narrative synthesis was conducted to analyze trends in surgical techniques, oncologic safety, obstetric outcomes, and sociodemographic disparities.
FSS encompasses procedures such as conization, radical trachelectomy (vaginal, abdominal, or minimally invasive), and neoadjuvant chemotherapy (NACT)- facilitated surgery, tailored to the tumor stage, size, and histology. Sentinel lymph node (SLN) mapping minimizes invasiveness during staging, while ovarian transposition (OT) preserves endocrine and reproductive function during pelvic radiotherapy. Multidisciplinary teams (MDTs) consider tumor biology, nodal status, and patient preferences to guide treatment decisions.
FSS offers cervical cancer patients with oncologic safety and fertility preservation, but its success relies on a multidisciplinary approach. Factors like surgical expertise, patient characteristics, and social determinants-like insurance coverage and access to care-significantly influence outcomes. Future research should prioritize improving multidisciplinary care and addressing disparities to enhance fertility preservation for all patients.
Keywords
- cervical cancer
- fertility preservation
- radical trachelectomy
- neoadjuvant chemotherapy
- medical decision
Cervical cancer ranks as the fourth most common cancer among women globally, with a notably younger age of onset compared to many other malignancies. Approximately 42% of cervical cancer patients are diagnosed before the age of 45 [1, 2]. With the rising trend of delayed childbearing, many women are diagnosed with cervical cancer before completing their family plans [3]. Despite concerns about infertility, many cancer survivors still wish to preserve their ability to have children, underscoring the growing demand for fertility-sparing treatment options [4, 5].
Since Dargent et al. [6] first reported radical trachelectomy (RT) with pelvic lymphadenectomy for early invasive uterine cervical cancer in 1987, fertility-sparing surgery (FSS) has become a viable option for women desiring to preserve their fertility. FSS offers a promising approach, allowing women with early-stage cervical cancer to retain fertility while maintaining oncological safety comparable to radical hysterectomy [7]. This includes procedures such as cone resection (excision of a cone-shaped cervical segment to remove precancerous or microinvasive lesions), simple trachelectomy, vaginal radical trachelectomy (VRT), abdominal radical trachelectomy (AbRT), robotic or laparoscopic radical trachelectomy (surgical removal of the cervix, upper vagina, and parametrium with uterine preservation) and surgeries following neoadjuvant chemotherapy (NACT), defined as preoperative platinum-based chemotherapy to downstage tumors and minimize surgical radicality. The aim of FSS is not only to preserve the ability to conceive but also to minimize the risks of preterm birth and miscarriage [8]. Beyond the surgical and oncological aspects, the decision to undergo FSS is also influenced by broader social and cultural factors. Traditional fertility expectations, familial roles, economic disparities, and healthcare accessibility can significantly shape patient preferences and clinical decisions. In low-resource settings, limited access to specialized surgical expertise and inadequate insurance coverage may restrict FSS availability, creating disparities in treatment options [9, 10].
Despite significant advancements in fertility-preserving cervical cancer treatments, critical gaps remain in understanding the multifactorial determinants influencing patient selection and optimizing outcomes for diverse patient groups. This review integrates the latest surgical techniques, oncologic and obstetric outcomes, and sociocultural considerations to provide a comprehensive perspective for clinical decision-making. By systematically evaluating the safety and efficacy of FSS and highlighting emerging trends in personalized treatment (Table 1, Ref. [11]), this article aims to guide clinicians in balancing cancer survival with reproductive aspirations, ultimately enhancing patient-centered care in cervical cancer management.
| Cervical cancer stage | Surgical approach | Obstetric outcomes [11] | Additional influencing factors |
| IA1 | |||
| IA2–IB1 | |||
| + lymph node evaluation (SLN/PLND) | |||
| IB2 | |||
| + RT (vaginal/abdominal/MIS) | |||
| + PLND | |||
| Selected IB2 or IIA1 patients (tumors |
*FSS should not be performed in patients with nodal involvement; small cell
neuroendocrine carcinoma, gastric-type adenocarcinoma, adenoma malignum; or when
the tumor is
FSS, fertility-sparing surgery; SLN, sentinel lymph node; PLND, pelvic lymphadenectomy/pelvic lymph node dissection; NACT, neoadjuvant chemotherapy; RT, radical trachelectomy; ART, assisted reproductive technologies; FIGO, International Federation of Gynecology and Obstetrics; MIS, minimally invasive surgery.
A systematic search was performed across PubMed, Embase, and Web of Science databases for studies published in the past ten years, using key terms related to cervical cancer, fertility-sparing surgery, neoadjuvant chemotherapy, and various outcomes such as oncologic safety, recurrence rates, pregnancy rates, obstetric outcomes, and sociodemographic factors (Fig. 1). Boolean operators (AND/OR) were employed to refine the search, e.g., (“Uterine Cervical Neoplasms” [MeSH Terms] OR “Cervical Cancer” [Title/Abstract]) AND (“Fertility Preservation” [MeSH Terms] OR “fertility-sparing surgery” [MeSH Terms]). Studies were further identified through manual screening of reference lists in relevant systematic reviews. The eligibility of studies was initially assessed based on titles and abstracts, applying predefined inclusion and exclusion criteria to exclude irrelevant articles. Non-original studies, non-human studies, and publications in languages other than English or Chinese were excluded. The remaining studies were reviewed independently by researchers SZ and MMZ. Due to heterogeneity in study designs and outcome reporting, a narrative synthesis approach was used to analyze trends in surgical techniques, oncologic safety, obstetric outcomes, and sociodemographic disparities (Table 2, Ref. [3, 12, 13, 14, 15, 16]).
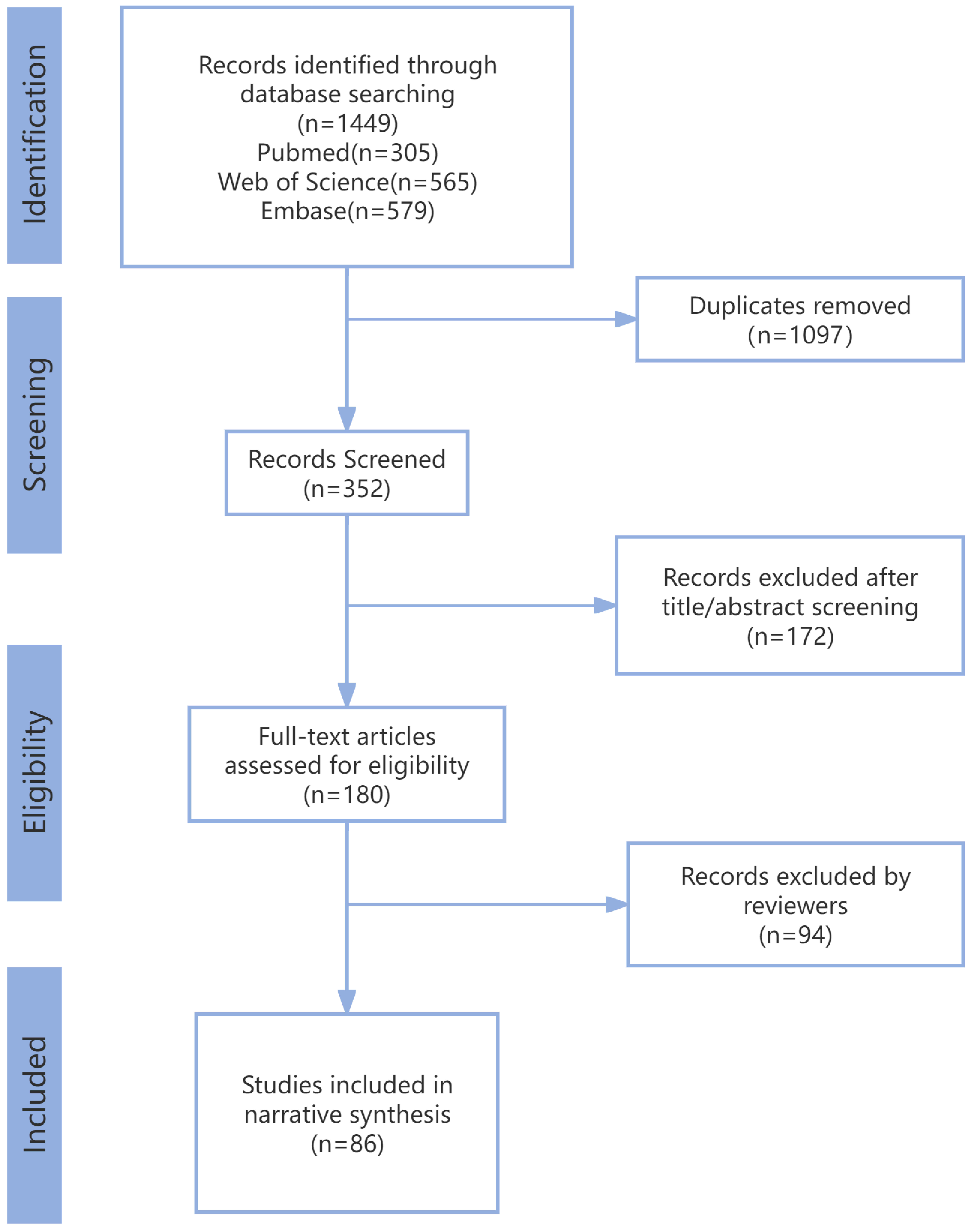 Fig. 1.
Fig. 1.
Flow diagram of study selection process.
| Study (author/year) | Methodology | Sample size | Key findings |
| Wright et al. (2010) [12] | Retrospective cohort study | 1409 | Women |
| Cui et al. (2018) [13] | Retrospective cohort study | 15,150 | Younger women and those more recently diagnosed were more likely to undergo trachelectomy while Medicaid recipients (RR = 0.39; 95% CI: 0.28–0.54) and the uninsured (RR = 0.67, 95% CI: 0.45–1.00) were less likely to undergo trachelectomy. |
| Markt et al. (2018) [14] | Retrospective cohort study | 13,698 | Insurance status and cancer treatment mediate the association between race/ethnicity and cervical cancer survival. |
| Machida et al. (2020) [15] | Retrospective cohort study | 393 | Less-ideal candidates had approximately four-fold higher recurrence risk and cancer mortality compared with ideal candidates*. |
| Jorgensen et al. (2023) [16] | Retrospective cohort study | 4521 | Fertility-sparing treatment was more likely among young patients overall, and of those in racial and ethnic minority groups. ART use was low and was associated with non-Hispanic White race and ethnicity, younger age (18–35 years), and private insurance. |
| Slama et al. (2023) [3] | Retrospective cohort study | 733 | Nonradical fertility-sparing cervical procedures were not associated with a higher recurrence risk compared to radical procedures for tumors |
*Ideal candidates were defined to have a tumor size of
OR, odds ratio; RR, relative ratio; CI, confidence interval.
Over 60% of cervical cancer cases are diagnosed at a localized stage, for which surgery offers curative potential [17]. Fertility-sparing treatment (FST) comprises a variety of surgical procedures, differing in the extent of paracervical tissue removal and surgical techniques, each of which can lead to distinct perinatal outcomes [11, 18]. The selection of the surgical approach should invariably prioritize optimal oncological outcomes whilst ensuring the preservation of adequate functional cervical tissue, a fundamental prerequisite for successful pregnancy [11, 19, 20, 21].
Surgical management of cervical cancer varies depending on the stage and extent
of the disease, with both non-radical and radical approaches offering different
benefits and challenges. Non-radical surgical approaches for early-stage cervical
cancer are focused on preserving the patient’s fertility and minimizing
complications, with two primary techniques being conization and simple vaginal
trachelectomy (SVT). Conization or SVT are less radical options for select
patients, including those with stage IA1 disease with lymphovascular space
invasion (LVSI) and IB1 disease with tumors
Radical vaginal cervix removal is typically recommended for tumors in the IA2 to
IB1 (tumors
For stage IA2 and more advanced cervical cancers, radical
trachelectomy should include lymph node assessment, as lymph node involvement is
a significant prognostic factor and can influence treatment decisions [27].
Sentinel lymph node (SLN) mapping has revolutionized surgical staging by
minimizing invasiveness while maintaining diagnostic accuracy, particularly for
tumors
For patients with locally advanced cervical cancer (tumors
For patients with locally advanced cervical cancer (LACC), the standard
treatment includes external beam radiation therapy (EBRT)
For patient’s ineligible for uterine preservation, fertility preservation relies
on integrated approaches combining OT and ART to safeguard endocrine function and
future reproductive potential [41, 42]. Surgically relocates ovaries
The oncological safety of FSS varies by histological subtype. A retrospective
study by Zusterzeel et al. [50] (n = 132) reported divergent recurrence
rates: 20% for adenosquamous carcinoma (ASC), 12.5% for adenocarcinoma (AC),
and 4.2% for squamous cell carcinoma (SCC) after VRT, with a median recurrence
time of 21 months. In contrast, a multicenter cohort study (n = 733) with a
median follow-up of 72 months found no significant association between histology
(70% SCC, 24% AC) and recurrence, identifying tumor size
Current guidelines permit FSS for SCC, AC, ASC, and clear-cell carcinoma, provided lymph node (LN) status is negative [31]. However, neuroendocrine carcinomas, adenoma malignum cell types, LN-positive tumors, and rare variants (e.g., gastric-type AC) are excluded due to aggressive behavior and poor prognosis [51]. While some studies suggest heightened vigilance for AC/ASC cases—particularly those with LVSI or deep stromal invasion—the absence of prospective subtype-specific data necessitates individualized risk-benefit assessments by MDT. Emerging molecular profiling (e.g., human papillomavirus (HPV) integration patterns, programmed death-ligand 1 (PD-L1) expression) may refine selection criteria, enabling histology-agnostic approaches tailored to tumor microenvironment dynamics [52, 53].
FSS enables pregnancy in approximately 55% of stage I cervical cancer patients,
with live birth rates reaching 70% across surgical modalities [11]. However, FSS
significantly elevates obstetric risks. The risks of different fertility and
pregnancy-related complications are affected by the heterogeneity of women after
FSS, the radicality of the procedure, and the amount of damage done to
paracervical tissue and uterine artery ligation. Hence, the incidence of preterm
birth and miscarriage varies significantly with surgical radicality, with
reported preterm birth rates ranging from 38% to 76.5% and early miscarriage
rates between 20%–23% [11, 54]. While first-trimester miscarriage rates
post-FSS (8%–10%) remain comparable to the general population [55, 56],
second-trimester miscarriage rates after radical vaginal trachelectomy (RVT)
double this baseline (8%–10% vs. 4%–5%) [55, 57]. A population-based study
linking the California Cancer Registry with birth and hospital discharge data
revealed that among 4087 women diagnosed with cervical cancer, only 118 (2.9%)
conceived following FSS, highlighting the impact of treatment on fertility.
Notably, squamous cell carcinoma accounted for 63.2% of these cases, followed by
adenocarcinoma at 30.8%. Compared to both the general population and cervical
cancer patients who conceived before their diagnosis, FSS patients exhibited
significantly higher odds of preterm birth before 37 weeks, with rates of 21.5%
versus 9.3% (OR: 2.66, 95% CI: 1.38–5.10) and 12.7% (OR: 1.88, 95% CI:
1.01–3.57), respectively, while the risk of preterm birth before 32 weeks did
not significantly increase. Furthermore, neonatal morbidity was notably higher in
FSS patients than in cervical cancer controls (15.9% vs. 6.9%, OR: 2.53, 95%
CI: 1.16–5.54), although no significant differences were observed in fetal
growth restriction, stillbirth, cesarean delivery, or maternal morbidity. The
Fertility Sparing Surgery in cervical cancer patients outside controlled trials
(FERTISS study), encompassing 44 centers in 13 countries, revealed stark
disparities between non-radical and radical FSS: pregnancy success rates were
63.2% for non-radical procedures versus 25.7% for radical trachelectomy
(p
NACT may reduce surgical invasiveness by shrinking tumor volume, thereby minimizing cervical/uterine isthmus resection and lowering preterm birth risk compared to upfront radical surgery [25]. However, its gonadotoxic potential necessitates careful ovarian reserve assessment pre-treatment [48]. Pre-treatment oocyte/embryo cryopreservation is advised to mitigate gonadal toxicity, though ovarian stimulation carries theoretical risks of cancer dissemination [45, 60]. For patients requiring hysterectomy, gestational surrogacy—achieving 66.7% ongoing pregnancy rates—remains the sole option in regions permitting it [61]. Ovarian tissue cryopreservation (OTC) is limited by malignancy recurrence risks, particularly in non-squamous histology (e.g., stage IIB adenocarcinoma) [62].
Post-FSS complications including cervical stenosis, cervical incompetence, and diminished ovarian reserve affect 25–50% of patients. Nevertheless, subsequent ART demonstrates favorable reproductive outcomes [16], with 53% of women achieving at least one pregnancy through interventions such as cervical dilation, intrauterine insemination, or in vitro fertilization, necessitating pre-surgical counseling by reproductive endocrinologists and high-risk obstetricians [11, 25, 26, 63, 64, 65]. To better evaluate post-FSS pregnancy outcomes and guide clinical counseling, a Japanese study established a clinical prediction model, the Subsequent Pregnancy Index (SPI) score—a stratification tool incorporating age, marital status, and ART utilization—to evaluate expected pregnancy efficacy following fertility-sparing trachelectomy [66]. Despite established guidelines and emerging advances in pregnancy outcome prediction models, inadequate preoperative counseling persists, highlighting the urgent need for standardized MDT protocols to address anatomical, hormonal, and psychosocial barriers.
FSS achieves survival outcomes equivalent to
radical hysterectomy in rigorously selected early-stage cervical cancer patients,
but deviations from strict eligibility criteria exponentially increase recurrence
risks, necessitating precise multidisciplinary evaluation. A study of 1409
IA1-stage patients demonstrated no survival difference between conization and
hysterectomy (HR = 0.65, 95% CI: 0.23–1.47), with 40% undergoing conization
[12]. Similarly, among IA2-IB2 stage patients in the U.S. National Cancer
Database (2004–2014), trachelectomy and hysterectomy showed comparable 5-year
survival (5.2% vs. 6.0% mortality; HR = 1.24, 95% CI: 0.70–2.22) [13].
However, a Japanese nationwide cohort (n = 393) stratified patients into ideal
candidates (tumor
The success of fertility-sparing surgery (FSS) and patient decision-making are shaped by a complex interplay of institutional resources, surgical expertise, and individual preferences. A study showed that in centers ranked in the top 10% (performing 30% of cervical surgeries), the relative risk of short-term perioperative complications was reduced by 65% when cervical trachelectomy was performed, which may also influence the pregnancy outcomes of FSS [67]. Surgical approaches—vaginal, abdominal, or robotic—depend on patient-specific factors (e.g., anatomy, body habitus), surgeon proficiency, and institutional capabilities. Robotic trachelectomy, for instance, may offer advantages such as reduced blood loss and faster recovery [68], but its higher cost and limited accessibility often restrict its use to specialized centers, disproportionately affecting socioeconomically disadvantaged populations [69].
Patient preferences for FSS are further influenced by disparities in counseling quality and access to multidisciplinary care. While some patients prioritize uterine preservation despite higher obstetric risks, others may opt for definitive surgery due to concerns about recurrence or limited access to long-term surveillance. Notably, institutional experience with SLN mapping varies regionally: SLN biopsy is preferred in high-resource settings, whereas full pelvic lymphadenectomy remains common where SLN expertise is limited [70]. These variations emphasize the imperative for standardized training programs and referral networks to ensure equitable access to advanced techniques. MDT must therefore integrate surgeon experience, institutional capabilities, and patient-specific factors (e.g., body habitus, tumor location) when tailoring FSS strategies, prioritizing referral to high-volume centers whenever feasible.
The uptake of FSS and ART varies significantly across sociodemographic groups,
reflecting complex interactions between age, insurance status, race/ethnicity,
and geographic accessibility [16]. In IA1-stage cervical cancer, women aged
In contrast, ART utilization post-FSS reveals persistent racial inequities: minority patients face lower ART adoption rates, partly mediated by Medicaid’s exclusion of fertility services (covering 30% of Black and 25% of Hispanic vs. 15% of White patients) [71]. Geographic disparities in ART access, prevalent nationally (30% lack local clinics), were absent in California due to high clinic density and state-mandated partial insurance coverage [72, 73]. However, rural-urban gaps in FSS access remain mitigated by the feasibility of procedures like electrosurgical excision procedure (LEEP)/conization in low-resource settings [16]. These findings underscore that equitable fertility preservation requires addressing both structural inequities (insurance reform, ART clinic distribution) and cultural competence in counseling.
FSS has advanced significantly, offering cervical cancer patients a viable option to preserve fertility while ensuring oncologic safety. However, the success of FSS—encompassing both oncologic outcomes and reproductive prognosis—depends on a range of factors. Crucially, a multidisciplinary approach is essential, with comprehensive evaluation from gynecologic oncologists, pathologists, and fertility specialists ensuring the most appropriate treatment for each patient. Factors such as the surgeon’s expertise, institutional resources, and the choice of surgical technique all influence obstetric outcomes, including preterm birth and miscarriage rates. Beyond the clinical and surgical aspects, patient-specific factors, such as age and individual health conditions, must also be considered in treatment planning. Furthermore, social determinants like ethnicity, insurance coverage, and geographic access to specialized care can create disparities in treatment outcomes. These factors highlight the need for coordinated efforts across medical, social, and policy levels. Future research should focus on optimizing multidisciplinary care, addressing healthcare access disparities, and improving fertility preservation strategies, to ensure that all women have equitable opportunities for successful fertility preservation after cervical cancer treatment.
SZ conceptualized the study, developed the framework, and led the drafting and revising. MMZ contributed to conception of the work, literature research, writing, and revising. YYZ contributed to the literature research for this work, supervised the project, reviewed the manuscript, and ensured alignment with journal requirements. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Not applicable.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest.
During the preparation of this work the authors used ChatGpt-3.5 in order to check spell and grammar. After using this tool, the authors reviewed and edited the content as needed and takes full responsibility for the content of the publication.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.

