1 Department of Obstetrics and Gynecology, Pusan National University School of Medicine, 49241 Busan, Republic of Korea
2 Biomedical Research Institute, Pusan National University Hospital, 49241 Busan, Republic of Korea
3 Department of Obstetrics and Gynecology, Pusan National University Hospital Infertility Center, Pusan National University Hospital, 49241 Busan, Republic of Korea
Abstract
Oocyte cryopreservation is a crucial technique in contemporary society since it provides a means for future fertility preservation at a time when delayed marriage and pregnancy are common. However, oocyte cryopreservation remains challenging because of the inherent vulnerability of oocytes becoming damaged during freezing and thawing. This study investigated the effects of uniform versus multi-gradient equilibration during vitrification on mitochondrial integrity and distribution in mouse oocytes.
We compared a conventional uniform equilibration method involving a 10-minute exposure to cryoprotective agents using a multi-gradient equilibration method that reduced the exposure time to 2.5 minutes. The survival rates of the vitrified oocytes and the mitochondrial fluorescence intensity and distribution were assessed using confocal microscopy.
The survival rates were not significantly different between the two methods. However, the multi-gradient equilibration method presented a higher mitochondrial fluorescence intensity and more uniform distribution, indicating better preservation of mitochondrial function.
These findings suggest that the multi-gradient equilibration method may offer a viable alternative to conventional vitrification that can reduce mitochondrial damage and potentially improve oocyte quality post-thawing.
Keywords
- oocyte cryopreservation
- multi-gradient equilibration vitrification
- mitochondria
Gamete cryopreservation is crucial for fertility preservation and gamete donation in humans. It is also important in animals for purposes such as breeding and conservation of endangered species. Oocyte cryopreservation was introduced to human assisted reproductive technology (ART) in the late 1980s [1]. However, the efficiency of oocyte cryopreservation at the time was quite low.
Subsequent advancements in vitrification techniques have greatly improved oocyte cryopreservation, and established protocols are currently in use. However, challenges persist in achieving satisfactory outcomes compared with fresh oocytes in terms of derived embryos and pregnancy rates [2, 3]. These difficulties arise from the unique characteristics of oocytes, which make cryopreservation more challenging than the cryopreservation of other cell types [4]. Oocytes have a larger cytoplasmic volume, higher water content, and a lower surface-to-volume ratio, rendering them more susceptible to ice crystal formation, osmotic shock, and toxicity from cryoprotective agents (CPA) during freezing and warming processes [5, 6, 7, 8].
Improved vitrification methods are essential for the efficient preservation of frozen-thawed (F/W) oocytes. Parameters such as the CPA exposure time and cooling rate during vitrification are critical [9], affecting post-thaw survival rates and potentially damaging mitochondria [7, 9, 10]. Recent studies have shown that reducing the CPA exposure time can enhance post-thaw survival rates and embryo development [11, 12, 13]. In a previous study, an ultrafast vitrification method was introduced that reduced the exposure time to the equilibration solution from 9 min (as used in conventional vitrification) to just 1 min. This modification was designed to minimize CPA exposure, thereby preserving intracellular organelle structures and achieving higher blastocyst formation rates [14].
However, ongoing research is being conducted to mitigate the rapid contraction caused by exposure to high-concentration CPA exposure [15, 16]. In this context, the current study explored an alternative method of sequentially increasing CPA concentrations rather than immediately exposing oocytes to high concentrations and assessed its impact on mitochondrial integrity during vitrification.
Therefore, this study aimed to compare a conventional method involving 10 min of equilibration using a uniform equilibration solution with an alternative method using a multi-gradient equilibration solution that reduced the equilibration time to 2.5 min. The evaluation focused on the survival rate of vitrified mouse oocytes as well as the fluorescence intensity and distribution of mitochondria.
All experimental procedures were approved by the Institutional Animal Care and Use Committee of Pusan National University Hospital (approval number PNUH-2022-202). Seven-week-old female B6D2F1 mice were obtained from Hana Corporation (Geumjeong-gu, Busan, Korea) and maintained in a controlled environment with regulated temperature and light conditions (12-hour light-dark cycle). The mice were provided ad libitum access to food and water.
A total of 150 oocytes were used in this study, with 50 allocated to each experimental protocol and fresh MII oocytes serving as the control group. To collect MII oocytes from the mice, 10 IU of pregnant mare serum gonadotropin (PMSG; 106911, Daesung, Gyeonggi-do, Korea) was administered intraperitoneally, followed by 10 IU human chorionic gonadotropin (hCG; 105112, Daseung) 48 h later to induce superovulation. The mice were euthanized using CO₂ gas, and oocytes were collected from the ampulla of the oviduct 15–16 h post-hCG injection. The cumulus-oocyte complexes were treated with 80 IU hyaluronidase to remove the cumulus cells. The denuded oocytes were washed twice with M2 medium (Sigma-Aldrich, St. Louis, MO, USA) and transferred to M16 medium (Sigma-Aldrich). Mature oocytes were classified as normal or abnormal based on their morphological characteristics assessed using a stereomicroscope. Only normal oocytes were used in the experiments, which were repeated three times.
Ethylene glycol (EG; 102466, Sigma-Aldrich) and dimethyl sulfoxide (DMSO; D2650, Sigma-Aldrich) were used as permeable CPA, whereas sucrose was used as a non-permeable CPA. Oocytes were equilibrated in Dulbecco’s phosphate-buffered saline (PBS; 14287080, Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) base medium containing 20% serum substitute supplement (SSS; 99193-12, lrvine Scientific, Inc., Santa Ana, CA, USA) and uniform and multi-gradient concentrations of EG and DMSO, respectively (Table 1). Following the conventional vitrification method, the uniform equilibration approach involved exposure to 7.5% DMSO (0.375 mL) and EG (0.375 mL) in base medium (SSS 1 mL, PBS 3.25 mL), which led to a rapid reduction in the oocyte cytoplasmic volume within approximately 40–60 s. This initial shrinkage was followed by CPA penetration into the cytoplasm, allowing the oocytes to gradually return to their original volume. In contrast, the multi-gradient equilibration method was designed to minimize rapid cytoplasmic shrinkage by sequentially exposing oocytes to increasing concentrations of CPA. The oocytes were exposed to 0.93% DMSO (0.046 mL) and 0.93% EG (0.046 mL) in base medium (SSS 1 mL, PBS 3.90 mL) for 30 s, followed by 1.87% DMSO (0.093 mL) and 1.87% EG (0.046 mL) in base medium (SSS 1 mL, PBS 3.81 mL) for 30 s, 3.75% DMSO (0.187 mL) and 3.75% EG (0.187 mL) in base medium (SSS 1 mL, PBS 3.62 mL) for 30 s, and 7.5% DMSO (0.375 mL) and 7.5% EG (0.375 mL) in base medium (SSS 1 mL, PBS 3.25 mL) for 1 min. Once the cytoplasmic volume reached its minimum, the oocytes underwent the vitrification solution (VS) process and were transferred to a base medium containing 15% DMSO (0.75 mL), 15% EG (0.75 mL), and 0.5 M sucrose (S1888-5006, Sigma-Aldrich) in base medium (SSS 1mL, PBS 2.5mL) for 1 min. During both the equilibration solution (ES) and VS processes, 300 µL drops were used per drop. The equilibrated oocytes were loaded into a Reprocarrier (Moduscience, Sejong, Korea) and stored in liquid nitrogen.
| Uniform equilibration | Multi-gradient equilibration | |||||
| DMSO | EG | Time | DMSO | EG | Time | |
| ES* | 7.5% | 7.5% | 10 min | 0.93% | 0.93% | 30 sec |
| 1.87% | 1.87% | 30 sec | ||||
| 3.75% | 3.75% | 30 sec | ||||
| 7.5% | 7.5% | 1 min | ||||
| VS* | 15% | 15% | 1 min | 15% | 15% | 1 min |
DMSO, dimethyl sulfoxide; EG, ethylene glycol; ES, equilibration solution; VS, vitrification solution.
*Base medium: Dulbecco’s phosphate-buffered saline + 20% serum substitute supplement.
To initiate the warming procedure, 1 M sucrose in the base medium was preincubated at 37 °C with 5% CO2 for 1.5 h. Frozen oocytes were thawed by immersing them in a 1 M solution on a 37 °C warm plate for 1 min, followed by 0.5 M solution at room temperature (RT) for 3 min. Finally, cells were transferred to PBS and incubated at RT for 6 min. The thawed oocytes were washed twice with M16 medium. F/W oocytes were cultured in M16 medium and incubated at 37 °C with 5% CO2 for 2.5 h, to facilitate recovery.
The survival rate of oocytes was assessed at 2.5 h after the warming process. Under high magnification, the oocytes were classified as viable or degenerated. Oocyte degeneration was identified using indicators such as cytoplasmic compaction, color darkening, widening of the perivitelline space, and the presence of cellular debris. The survival rate was calculated as the ratio of surviving oocytes to the total number of warmed oocytes and was subsequently compared across experimental groups. Fresh oocytes, which were not exposed to stress conditions, such as equilibration, vitrification, and warming, were used as controls, with their survival rate standardized to 1. The surviving oocytes were used for further experiments.
To measure the distribution and fluorescence intensity of mitochondria, frozen mouse MII oocytes were thawed for 2.5 hours and then incubated in MitoTracker™ Red (100 nmol/L, M7512, Invitrogen, Waltham, MA, USA) for 30 minutes at 5% CO2 and 37 ℃. Subsequently, the oocytes were mounted on slides, and live images of the mitochondria (red) were obtained using a laser-scanning confocal microscope (LSCM, Leica, Wetzler, Germany, TCS SP8). The fluorescence intensity was analyzed by defining the area of the fluorescent oocyte and counting the number of pixels within the region of interest (ROI). The mean values of all pixels in the ROI were summed and divided by the total number of pixels in the region to obtain the average fluorescence intensity. This analysis was conducted using the LAS X software (version 3.5.1, Leica). The distribution of mitochondrial fluorescence was classified as either uniformly distributed across the entire oocyte (normal) or clustered in the damaged regions (abnormal).
Statistical analyses were performed using R software (version 4.1.3; http://cran.r-project.org). One-way analysis of variance was used for comparisons. The chi-square test was used for categorical data. Continuous data are presented as mean
The survival rate of thawed oocytes was not significantly different from that of fresh oocytes based on the equilibration method. However, the survival rate was higher in the multi-gradient solution (96.1%) than in the uniform solution (92.6%).
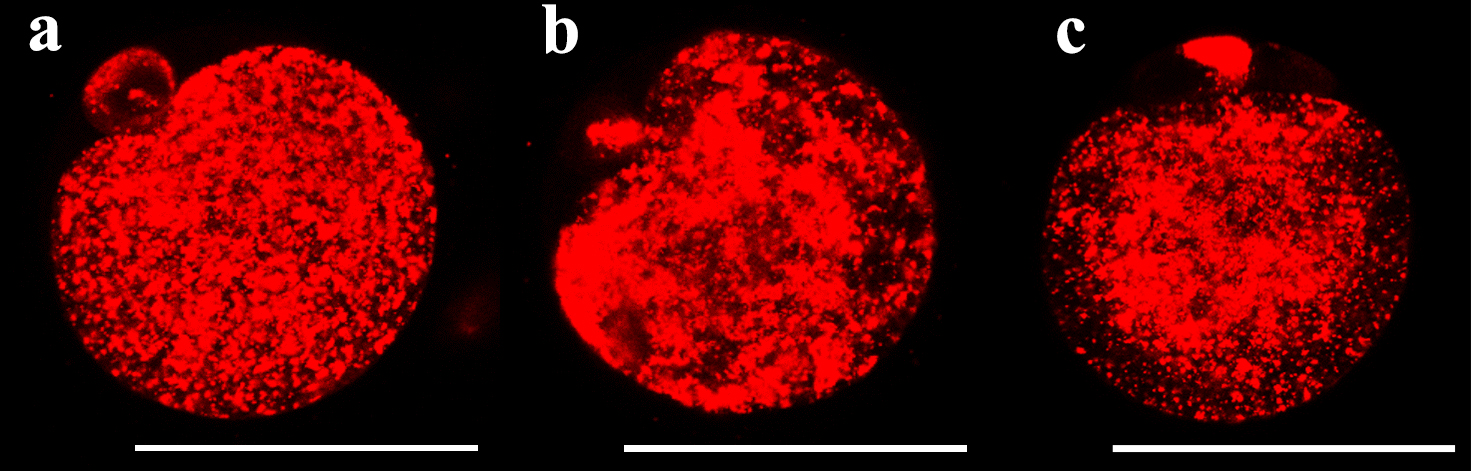
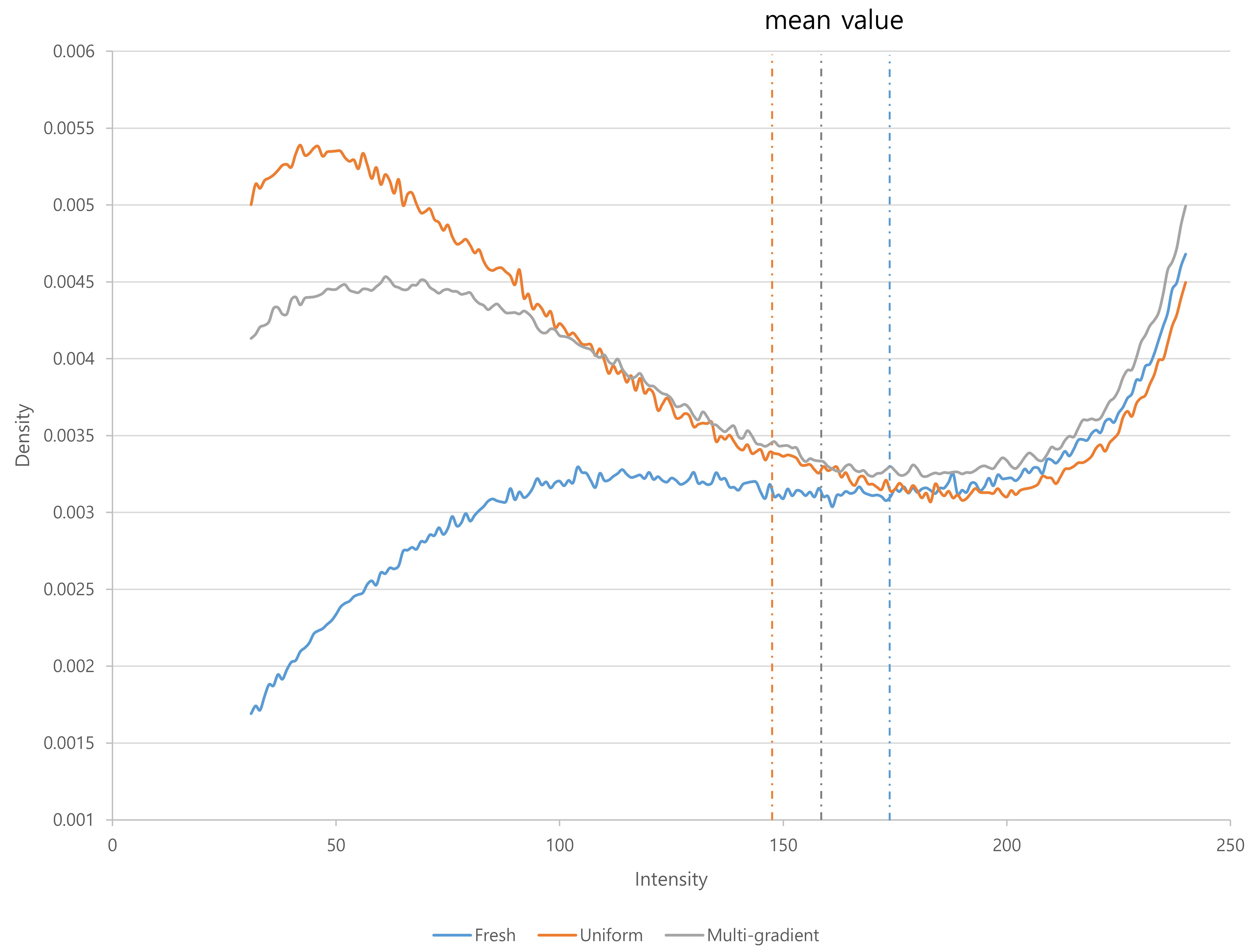
The mitochondrial fluorescence intensity was significantly different among the fresh oocytes, uniform solution, and multi-gradient solution groups (Fig. 1). The mean fluorescence intensity decreased in the uniform equilibration and multi-gradient equilibration groups compared to that of fresh oocytes. However, the mean value in the multi-gradient equilibration group was higher than that in the uniform equilibration group (uniform vs. multi-gradient: 148.57 vs. 164.13; p = 0.006) (Table 2). Fig. 2 illustrates the density patterns of the mitochondrial fluorescence intensity for each group. Although the density patterns of the experimental groups differed from those of the fresh oocytes, the density patterns of the multi-gradient equilibration group relatively more resembled those of the fresh oocyte group than those of the uniform equilibration group.
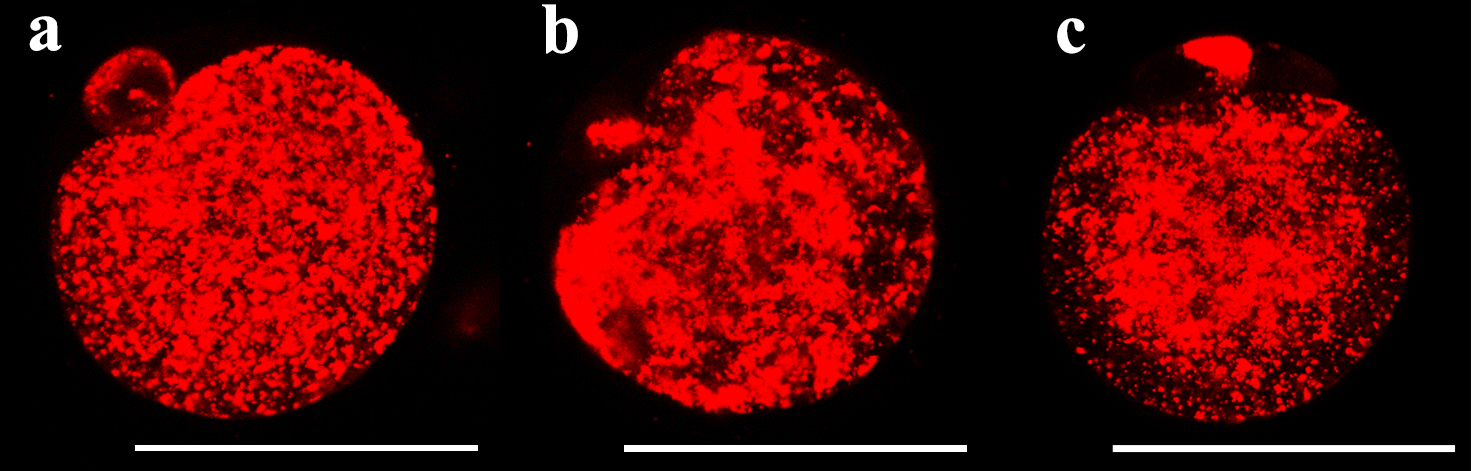 Fig. 1.
Fig. 1. Fluorescent live cell images showing the mitochondrial fluorescence intensity according to equilibration methods. (a) fresh oocyte (b) uniform equilibration (c) multi-gradient equilibration. Scale bar = 58 µm.
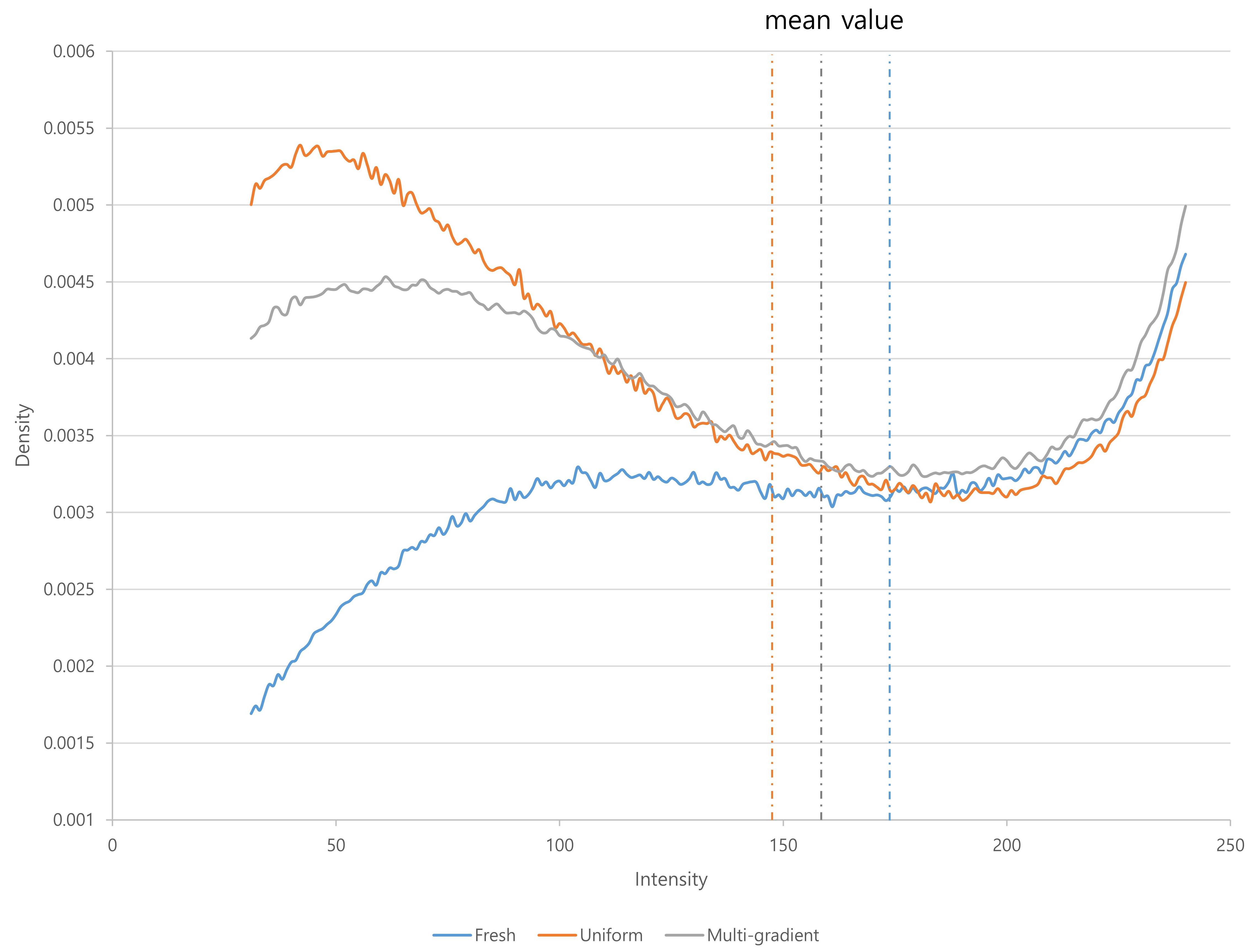 Fig. 2.
Fig. 2. Density patterns of mitochondrial fluorescence intensity according to equilibration methods.
| Fresh oocytes (n = 50) | Uniform equilibration (n = 50) | Multi-gradient equilibration (n = 50) | p value | |
| Pixel sum (gray) | 15,830,496.98 (2,909,528.59) | 13,174,689.80‡ (3,720,840.96) | 13,784,776.50‡ (3,180,029.42) | 0.001 |
| Sum processed Pixel in ROI | 89.54 (13.87) | 87.70 (14.37) | 84.92 (15.43) | 0.296 |
| ROI area (kpixel) | 89.58 (13.89) | 87.61 (14.40) | 84.77 (15.42) | 0.274 |
| Mean value (gray) | 176.32 (21.65) | 148.57‡ (24.64) | 164.13‡† (29.88) |
Data are presented as the mean (standard deviation).
A p-value
Abbreviations: ROI, region of interest.
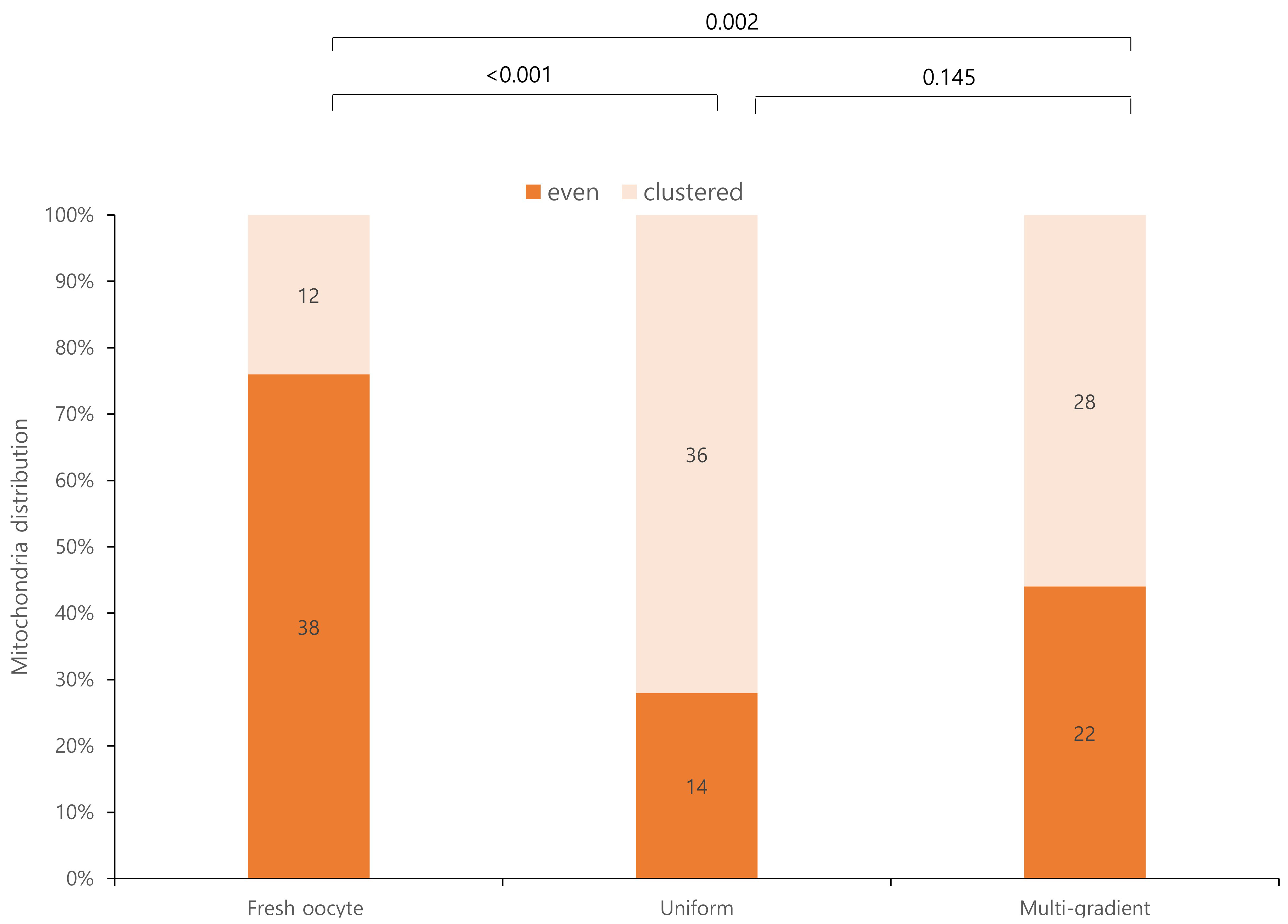
Mitochondrial distributions differed significantly between fresh oocytes and the experimental groups (Fig. 3). Fresh oocytes exhibited a more uniform mitochondrial distribution than the experimental groups. Although the mitochondrial distribution was not significantly different between the two experimental groups, the multi-gradient equilibration group exhibited a more even mitochondrial distribution. Specifically, 22 oocytes in the multi-gradient group exhibited an even distribution compared to 14 oocytes in the even equilibration group, although this difference was not statistically significant.
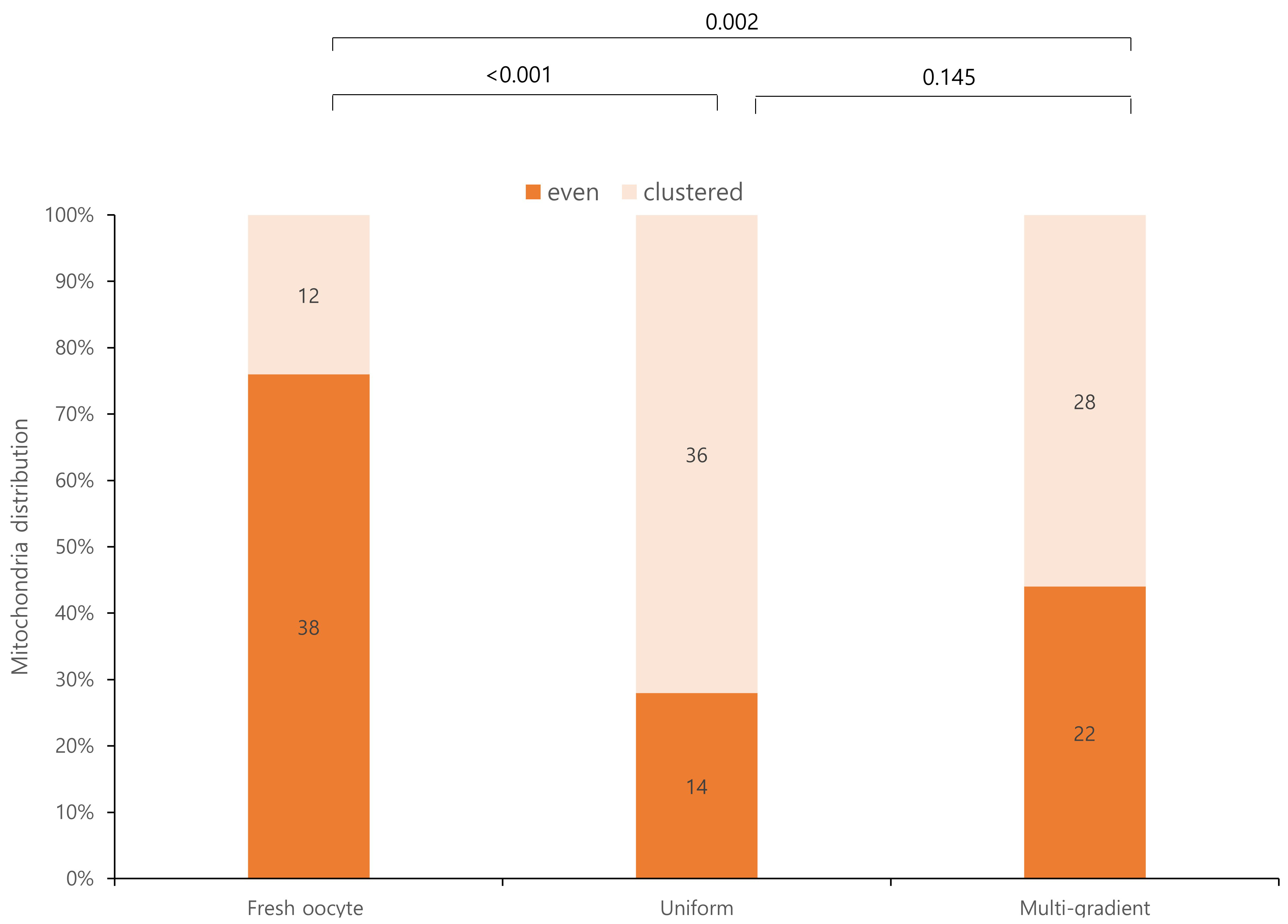 Fig. 3.
Fig. 3. Mitochondrial distribution according to equilibration methods.
In this study, the multi-gradient equilibration method did not show a statistically significant difference in survival rates compared with the uniform equilibration method. However, the mean intensity values of mitochondrial fluorescence were higher with multi-gradient equilibration, indicating enhanced mitochondrial activity. Furthermore, mitochondrial density patterns per intensity were more similar to those observed in fresh oocytes when using the multi-gradient equilibration method than when using uniform equilibration. Additionally, the mitochondrial distribution was more even with the multi-gradient equilibration method than with the uniform equilibration method. These findings suggest that the multi-gradient equilibration method better preserves the mitochondrial function and distribution, reflecting a state closer to that of fresh oocytes.
Uniform equilibration vitrification is currently the most widely used method for oocyte cryopreservation [17]. Although it demonstrates superior oocyte survival rates compared to slow freezing, concerns have been raised regarding damage to cellular organelles due to prolonged exposure to high concentrations of CPA. This damage has been reported in various organelles, including the mitochondria, endoplasmic reticulum, and spindle apparatus [18, 19, 20, 21].
Among the crucial organelles in oocytes, mitochondria play a vital role in maturation by providing adenosine Triphosphate (ATP) for critical processes, such as transcription, translation, and meiosis, while also maintaining intracellular Ca2+ homeostasis and regulating cell death and signaling pathways [22, 23, 24, 25, 26]. When oocytes are exposed to high concentrations of permeant CPA, the intracellular Ca2+ levels increase. This premature increase in Ca2+ can cause partial exocytosis of cortical granules and hardening of the zona pellucida [27, 28]. Additionally, prolonged opening of mitochondrial permeability transition pores due to Ca2+ overload can lead to the production of reactive oxygen species, further Ca2+ release, loss of mitochondrial membrane potential, reduced ATP content, and the release of cytochrome C, culminating in DNA damage and apoptosis. Ultrastructural damage to the mitochondria can lead to decreased oocyte quality and is closely related to reduced embryonic developmental potential [14, 29].
The multi-gradient equilibration method involves a stepwise approach in which the CPA concentration is gradually increased through a series of equilibration steps. Based on our previous findings [14], which highlighted the benefits of reducing the CPA exposure time, we devised this approach by setting the exposure time to 2.5 minutes and introducing stepwise CPA concentration changes. Although research on this method is scarce, one notable study by Zhu et al. [15] evaluated the effects of multi-gradient equilibration vitrification in mice and compared two approaches for gradually increasing the CPA concentration: the multi-gradient equilibration method and the droplet merge method. Their findings demonstrated that the multi-gradient equilibration method was superior to the droplet merge method in maintaining oocyte survival, spindle morphology, and embryonic development. This advantage is likely due to the fact that, unlike the multi-gradient equilibration method, the droplet merge method may result in inconsistencies in CPA concentration within the droplets, leading to less predictable outcomes. In contrast, the present study compared the multi-gradient equilibration method with the uniform equilibration vitrification method, which is one of the most widely used approaches for oocyte cryopreservation. The multi-gradient equilibration method examined by Zhu et al. [15] aligns closely with the approach used in this study, as both share the fundamental principle of gradually increasing the CPA concentration, although the specific concentrations and exposure times of the equilibration solutions (ES) differed between the two studies. Our study provides novel insights by examining these methods from a mitochondrial perspective. We demonstrated that the multi-gradient equilibration method preserved the mitochondrial function and ultrastructure while maintaining oocyte survival rates. This finding underscores the potential advantages of the multi-gradient equilibration method over the uniform equilibration method. However, further investigation is warranted to evaluate other critical aspects, including spindle morphology and normal embryonic development rates, which are essential for successful downstream embryological outcomes.
Although the precise mechanisms by which the multi-gradient equilibration method enhances oocyte survival rates while preserving the function and structure of cellular organelles remain unclear, it has been hypothesized that the gradual introduction of CPA minimizes osmotic shock and cellular toxicity. This process likely allows the cells to adapt more effectively to CPA exposure. Moreover, the relatively short CPA exposure time associated with this method may further contribute to the improved outcomes.
Various strategies have been investigated to address CPA toxicity and osmotic stress-related damage to cellular organelles. For example, shortening the CPA exposure time and increasing the temperature during the equilibration stage have shown promising improvements over conventional vitrification techniques [11, 12, 13, 14]. As the global emphasis on fertility preservation increases, particularly oocyte cryopreservation, owing to delayed childbearing, it is imperative to move beyond the methods used for embryo freezing. The optimization of oocyte vitrification presents challenges and opportunities for both clinicians and researchers. These efforts have the potential to not only redefine existing standards of practice but also open new directions for improving fertility preservation strategies.
One limitation of this study was that it used mouse oocytes rather than human oocytes. However, mouse oocytes share several key characteristics with human oocytes, including similar reproductive cell developmental processes and
The multi-gradient equilibration method is a viable alternative to the uniform equilibration vitrification technique. Although no significant differences in oocyte survival rates were observed, this method effectively reduced mitochondrial damage and preserved the ultrastructure, making it a promising approach for improving cryopreservation outcomes.
During the preparation of this work, the authors used ChatGPT to check spelling and grammar. After using this tool, the authors reviewed and edited the content as needed and took full responsibility for the content of the publication.
Data sharing is available upon request.
EY and JC designed the study. EY and JC performed the experiments. HJ, HL, and JJ provided advice on data collection and analysis. HL analyzed the data. EY, JC, HJ, HL, and JJ wrote the manuscript. All authors contributed to the editorial changes in the manuscript. All the authors have read and approved the final version of the manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
All experimental procedures were approved by the Institutional Animal Care and Use Committee of Pusan National University Hospital (approval number PNUH-2022-202). This study was carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Every statistical analysis performed in this study was discussed professionally, in consultation with the Department of Biostatistics, Biomedical Research Institute, Pusan National University Hospital.
This research received no external funding.
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.