1 Department of Obstetrics and Gynecology, The Second Affiliated Hospital of Xi’an Jiaotong University, 710004 Xi’an, Shaanxi, China
2 Department of Obstetrics and Gynecology, The First Affiliated Hospital of Xi’an Jiaotong University, 710063 Xi’an, Shaanxi, China
3 Department of Ultrasound, The Second Affiliated Hospital of Xi’an Jiaotong University, 710004 Xi’an, Shaanxi, China
Abstract
Current research on the characteristics of missed miscarriage (MM) in women over the age of 35 is limited. This study investigates the role of serum metabolites for MM among women in this age group.
This study included a total of 80 women over the age of 35 who experienced MM and 66 women over the age of 35 with healthy pregnancies, conducted between March 2021 and June 2022. General information, including age, gestational age, body mass index (BMI), gravidity, live birth, history of spontaneous miscarriage, drug or radiation exposure, occupation, and pregnancy-related complications, was collected. The recorded serum indicators included total cholesterol (TC), triglycerides (TG), high-density lipoprotein (HDL), low-density lipoprotein (LDL), lactate dehydrogenase (LDH), and gamma-glutamyl transferase (GGT). Potential risk factors for MM in the case-cohort were identified using univariate and multivariate logistic regression analyses. The diagnostic relevance of serum markers for MM was examined through receiver operating characteristics (ROC) curve analysis.
The incidence of MM increased with higher LDL and GGT values (p < 0.05). The area under the curve (AUC) was 0.709 for LDL and 0.792 for GGT. An LDL value >2.31 identified MM with a sensitivity of 72.5% and a specificity of 66.7%, while a GGT value >15.5 identified MM with a sensitivity of 78.8% and a specificity of 72.7%. However, the combined diagnostic accuracy of the two indicators was superior of that of either single index (AUC = 0.880, sensitivity = 92.5%, specificity = 72.7%; Z = 4.238, 2.813, p < 0.001, p < 0.01).
In women over the age of 35, serum LDL and GGT are high-risk factors for MM, each potentially playing a significant role in its diagnosis. The combination of these two markers may improve diagnostic accuracy. The preliminary results of this study warrant further investigation through a well-designed, multicenter prospective study.
Keywords
- missed miscarriage
- serum LDL
- serum GGT
- women over age 35
Missed miscarriage (MM), also referred to as silent or late miscarriage, occurs when the retained intrauterine products of conception do not spontaneously expel from the fetus or embryo [1]. It occurs in approximately 8–20% of pregnancies with a clinical diagnosis [2]. Multiple etiologic factors, including uterine abnormalities, endocrine disorders, parental chromosomal abnormalities, immunological factors, infections, hereditary thrombophilia, and environmental influences, have been identified as being associated with MM [3, 4]. However, its exact cause remains unknown. Recent studies have shown that the rate of spontaneous miscarriage increases with age, as does the incidence of stillbirth, while the rate of live births decreases [5, 6, 7]. With China’s new fertility policy, the proportion of older parents willing to have children is increasing [8]. Therefore, early identification of women at high risk of MM is crucial, especially for older women.
In the post-genomic era, where metabolomics has received increasing research attention, many serum metabolites have been identified as useful in diagnosing diseases. For example, Fei et al. [9] found that MM results in a significant metabolic disturbances, with glyceric acid, indole and sphingosine serving as promising metabolites for diagnosing MM. Li et al. [10] similarly reported that lactic acid and 5-methoxytryptamine concentrations in patients with recurrent spontaneous miscarriage differed significantly from those in women who underwent induced miscarriages during a normal pregnancy. Researchers also established a rat model of miscarriage to analyze metabolic profile changes between rats with recurrent spontaneous miscarriage and normal rats. The results showed that recurrent spontaneous miscarriage was closely related to arachidonic acid metabolism [11]. Taken together, these results suggest that metabolites in peripheral blood are closely associated with miscarriage. However, the association between other serum metabolites, such as total cholesterol (TC), triglycerides (TG), high-density lipoprotein (HDL), low-density lipoprotein (LDL), lactate dehydrogenase (LDH), gamma-glutamyl transferase (GGT), and MM in women over the age of 35, has been rarely reported. Thus, the current study aimed to investigate the relationship between these serum metabolites (TC, TG, HDL, LDL, LDH, and GGT) and MM in women over 35, with the intent of establishing a potential diagnostic reference in the future.
The study was conducted from March 1, 2021, to June 30, 2022. We selected women between 7 and 12 weeks of pregnancy who visited the Department of Obstetrics and Gynecology at the Second Affiliated Hospital of Xi’an Jiaotong University. These participants were divided into an MM group and a healthy pregnancy group based on their pregnancy outcomes. The common inclusion criteria for both groups were as follows: (1) age over 35; (2) natural conception with a single fetus; and (3) intrauterine pregnancy. The inclusion criteria for the MM group were as follows: (1) crown-rump length
A total of 162 pregnant women were screened for participation. Participants with the following characteristics were excluded from the analysis: (1) missing body mass index (BMI) values (n = 5); (2) missing serum lipid information (n = 6); and (3) internal medical or surgical disorders (n = 5). After screening, 146 participants were included in the final analysis. The study inclusion flow chart is shown in Fig. 1.
 Fig. 1.
Fig. 1. Flowchart of participant inclusion. BMI, body mass index.
All patient data were obtained from the hospital’s electronic medical record system. The indicators analyzed in this study included demographic information and laboratory data. General information included age, gestational age, BMI, gravidity, live birth, spontaneous miscarriage, history of drug or radiation exposure, occupation (unemployed, clerk, professional/technical personnel), and pregnancy complications (e.g., asymptomatic uterine microfibroids or small ovarian cysts). Laboratory data included TC, TG, HDL, LDL, LDH, and GGT.
For all participants, fasting blood samples (5 mL) were drawn from the antecubital vein after the diagnosis was confirmed, and collected in tubes without anticoagulant. All samples were immediately centrifuged at 3000 rpm for 5 min at 4 °C, and the serum was separated from the blood clot to minimize the time samples were kept in the lab. This was done after the samples had been incubated at room temperature for 5 minutes. Serum indicators were simultaneously analyzed using an automated biochemical analyzer (version AU5800, Beckman Coulter Inc., Brea, CA, USA).
The data’s conformity to a normal distribution was evaluated using the Kolmogorov-Smirnov test. Continuous variables with a normal distribution were presented as means
A total of 146 women were included in this study. Based their pregnancy outcomes, they were divided into two groups: the MM group (n = 80) and the healthy pregnancy group (n = 66). The general characteristics of all participants are presented in Table 1. No significant differences were observed between the two groups in terms of age, gestational age, BMI, gravidity, number of spontaneous miscarriages, or history of drug or radiation exposure (p
| Variables | MM (n = 80) | Healthy pregnancy (n = 66) | p-value | ||
| Age (year) | 36.00 (35.00, 37.75) | 36.50 (35.00, 39.00) | –0.972 | 0.331 | |
| Gestational age (day) | 75.00 (66.00, 84.00) | 71.50 (59.00, 90.00) | –0.775 | 0.438 | |
| BMI (kg/m2) | 0.145 | 0.986 | |||
| Underweight ( | 12 (15.00) | 9 (13.64) | |||
| Normal (18.50–23.90) | 38 (47.50) | 31 (46.97) | |||
| Overweight (24.00–27.90) | 16 (20.00) | 13 (19.70) | |||
| Obese ( | 14 (17.50) | 13 (19.70) | |||
| Gravidity (times) | 1.624 | 0.444 | |||
| 1 | 13 (16.25) | 7 (10.61) | |||
| 2 | 25 (31.25) | 18 (27.27) | |||
| 42 (52.50) | 41 (62.12) | ||||
| Live birth (times) | 14.416 | ||||
| 0 | 25 (31.25) | 4 (6.06) | |||
| 55 (68.75) | 62 (93.94) | ||||
| Spontaneous miscarriage (times) | 4.426 | 0.109 | |||
| 0 | 28 (35.00) | 33 (50.00) | |||
| 1 | 27 (33.75) | 21 (31.82) | |||
| 25 (31.25) | 12 (18.18) | ||||
| Drug or radiation exposure | 0.001 | 0.980 | |||
| No | 68 (85.00) | 56 (84.85) | |||
| Yes | 12 (15.00) | 10 (15.15) | |||
| Occupation | 17.404 | ||||
| Unemployed | 12 (15.00) | 30 (45.45) | |||
| Clerk | 40 (50.00) | 25 (37.88) | |||
| Professional/technical | 28 (35.00) | 11 (16.67) | |||
| Pregnancy complications | 18.781 | ||||
| No | 32 (40.00) | 50 (75.76) | |||
| Yes | 48 (60.00) | 16 (24.24) | |||
BMI, body mass index; MM, missed miscarriage. The Pearson
TC, TG, HDL, and LDH levels were similar between the groups (p
| Variables | MM (n = 80) | Healthy pregnancy (n = 66) | t/z | p-value |
| TC (mmol/L) | 4.87 | 4.52 | 1.947 | 0.053 |
| TG (mmol/L) | 1.35 (0.99, 1.99) | 1.30 (0.97, 1.77) | –0.348 | 0.728 |
| HDL (mmol/L) | 1.50 | 1.49 | 0.297 | 0.767 |
| LDL (mmol/L) | 2.56 | 2.18 | 4.269 | |
| LDH (IU/L) | 153.50 (135.50, 174.75) | 149.00 (135.75, 163.25) | –1.078 | 0.281 |
| GGT (U/L) | 22.50 (16.25, 29.00) | 11.50 (9.00, 16.25) | –6.059 |
MM, missed miscarriage; TC, total cholesterol; TG, triglycerides; HDL, high-density lipoprotein; LDL, low-density lipoprotein; LDH, lactate dehydrogenase; GGT, gamma-glutamyl transferase. Student’s t-test was used to compare normally distributed continuous variables, which were represented as means
In the univariate analysis, the number of live births, occupation, pregnancy complications, LDL, and GGT levels were significantly associated with increased risk of MM (p
| Variables | B | S.E. | Wald | p-value | OR (95% CI) | |
| Constant | –4.013 | |||||
| Live birth (times) | ||||||
| 0 | 1 | |||||
| –1.719 | 0.681 | 6.377 | 0.012 | 0.179 (0.047–0.680) | ||
| Occupation | ||||||
| Unemployed | 1 | |||||
| Clerk | 1.201 | 0.562 | 4.559 | 0.033 | 3.322 (1.104–10.003) | |
| Professional/technical | 1.935 | 0.630 | 9.420 | 0.002 | 6.922 (2.012–23.810) | |
| Pregnancy complications | ||||||
| No | 1 | |||||
| Yes | 1.070 | 0.446 | 5.763 | 0.016 | 2.916 (1.217–6.985) | |
| Serum indicators | ||||||
| LDL (mmol/L) | 1.117 | 0.410 | 7.417 | 0.006 | 3.057 (1.368–6.831) | |
| GGT (U/L) | 0.078 | 0.025 | 9.535 | 0.002 | 1.082 (1.029–1.137) | |
OR, odds ratio; 95% CI, 95% confidence interval; LDL, low-density lipoprotein; GGT, gamma-glutamyl transferase; S.E., standard error.
The diagnostic potential of the two serum indicators was further assessed using ROC analysis, and the AUC was calculated to assess their accuracy in diagnosing MM. The AUC for LDL was 0.709, while the AUC for GGT was 0.792. An LDL value
| Variable | AUC | S.E. | 95% CI | Cut-off value | Sensitivity (%) | Specificity (%) | p-value | |
| Lower | Upper | |||||||
| LDL (mmol/L) | 0.709 | 0.044 | 0.623 | 0.794 | 2.31 | 72.5 | 66.7 | |
| GGT (U/L) | 0.792 | 0.039 | 0.715 | 0.868 | 15.5 | 78.8 | 72.7 | 0.005** |
| Combine | 0.880 | 0.030 | 0.821 | 0.940 | - | 92.5 | 72.7 | |
AUC, area under the curve; 95% CI, 95% confidence interval; LDL, low-density lipoprotein; GGT, gamma-glutamyl transferase. * indicates that the AUC value of LDL was compared with the combined AUC value, Z = 4.238, p
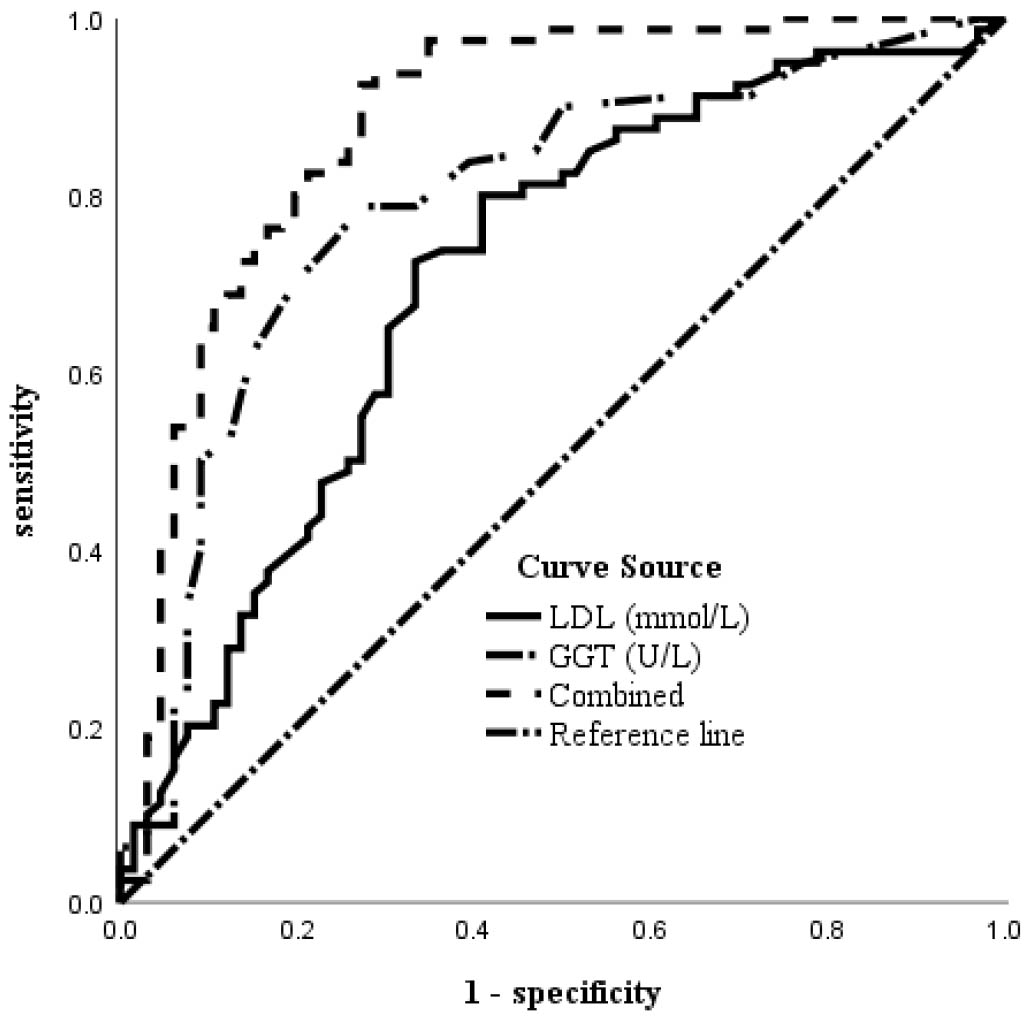 Fig. 2.
Fig. 2. ROC curve of serum LDL, GGT, and the combined diagnosis of MM in women above age 35. LDL, low-density lipoprotein; GGT, gamma-glutamyl transferase; ROC, receiver operating characteristics; MM, missed miscarriage.
Clinically, patients with MM may remain asymptomatic, making early detection challenging without regular ultrasound monitoring and sequential beta-human chorionic gonadotropin (
In our study, univariate analysis revealed statistically significantly differences in serum levels of LDL and GGT between women with MM and those with healthy pregnancies. Multivariate logistic regression analysis further showed a positive correlation between the incidence of MM and elevated levels of LDL and GGT. Using ROC curve analysis, we found that the AUC for LDL was 0.709, with a sensitivity of 72.5%, and a specificity of 66.7%. The AUC for GGT was 0.792, with a sensitivity of 78.8%, and a specificity of 72.7%. However, the AUC of the combined ROC increased to 0.880, surpassing that of either individual marker, with a sensitivity of 92.5%, and a specificity of 72.7%. Although the optimal cut-off values for LDL and GGT remained within the normal range, these levels were significantly higher in women over the age of 35 who experienced MM. These results suggest that elevated serum LDL and GGT levels are risk factors for MM in women over the age of 35. Furthermore, serum LDL and GGT may play a crucial role in the diagnosis of MM, with their combination improving diagnostic accuracy compared to either marker alone.
Circulating LDL particles, rich in cholesterol, have the ability to penetrate the vessel wall and undergo oxidation to form oxidized LDL (ox-LDL) [14]. Ox-LDL can activate and impair vascular endothelial cells, thereby enhancing their pro-adhesion properties of endothelial cells and promoting the monocyte recruitment. This process leads to the accumulation of inflammatory, lipid-rich macrophages and pro-inflammatory lymphocytes in arterial intima, ultimately leading to the formation of atherosclerotic plaque [15]. Although the role of LDL in miscarriage remains unclear, some research suggests that acute atherosis and diffuse lipid infiltration may occur in the placenta, potentially serving as definitive indicators of placental dysfunction [16]. Furthermore, altered lipid metabolism may impact placental lipid transport and fetal development [17]. Animal studies have substantiated the efficacy of statins in mitigating severe pregnancy complications, such as recurrent miscarriages and preeclampsia, which are often linked to the impact of hyperlipidemia on placental function [18]. In recent years, numerous studies have focused on dyslipidemia in the field of assisted reproduction, yet few have examined dyslipidemia in natural pregnancies. In a retrospective study, Cai et al. [19] examined 2011 women who underwent in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI) with fresh embryo transfer. Their findings indicated that LDL had the most significant negative impact on reproductive outcomes, resulting in increased rates of miscarriage, reduced live birth rates, and decreased conception rates. Research from human IVF studies also indicates a negative correlation between LDL levels and the number of normally fertilized oocytes. Conversely, no significant correlation has been found between LDL levels and the morphological characteristics of embryos [20]. Furthermore, dyslipidemia significantly decreases the biochemical pregnancy rate, embryo implantation rate, and live birth rate in individuals undergoing donor egg treatment [21]. Our research reveals that, in natural pregnancies, the pregnancy outcomes of women over 35 years of age are associated with LDL levels.
The liver enzyme GGT is essential for glutathione metabolism ; its deficiency causes oxidative stress and increases cellular vulnerability to oxidative damage [22, 23]. GGT expression increases in response to oxidative stress as part of an adaptive mechanism [24]. Recent studies have shown that GGT is a key marker of oxidative stress. Indeed, this enzyme can trigger pro-oxidant processes, which play a crucial role in tumor development and cell growth [25]. Miscarriage has been associated with oxidative stress; decreased antioxidant function during pregnancy can lead to spontaneous miscarriage [26]. Research indicates that excessive oxidative stress on the placental tissues is a unifying pathophysiological mechanism underlying the various causes of early pregnancy loss [27]. Oxidative stress plays a role in vascular endothelial injury, angiogenesis, and the establishment of adequate blood supply to the embryo, all of which are critical for implantation and pregnancy success [28]. In a retrospective study, Fang et al. [2] found that the serum marker GGT was associated with apoptosis and oxidative stress, with significantly elevated levels of GGT in women with MM. Several studies have demonstrated that elevated GGT levels in the early stages of pregnancy are independent risk factors for both preeclampsia and gestational hypertension [29]. These studies suggest that serum GGT is associated with pathological pregnancies. To our knowledge, our present study is the first to demonstrate that elevated LDL and GGT levels in natural pregnancies may serve as diagnostic indicators for MM in women over the age of 35.
A limitation of this study is its retrospective design, which may have introduced bias into the results. In addition, since all patients and healthy pregnant women were enrolled from the same center, the results may be center-specific and not generalizable. The sample size was also small; however, we intend to continue collecting patient records for further investigation. Notably, we did not compare the indices related to MM in young women.
In summary, serum LDL and GGT are high-risk factors for MM in women over the age of 35. These markers may also play a crucial role in the diagnosis of MM, with their combination potentially improving diagnostic accuracy compared to either marker alone. These preliminary results warrant further investigation in a well-designed multicenter prospective study.
The data sets analyzed during the current study are not publicly available due to [The data that has been used is confidential.] but are available from the corresponding author on reasonable request.
NZ designed the research study and wrote the first draft of the manuscript. YMH and MYM collected and analyzed the data, and contributed to the interpretation of the results and critical revision of the manuscript for important intellectual content. XNL and YHQ participated in study implementation and manuscript revision. JFW designed the study and significantly revised the paper. All authors have read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
The study was conducted in accordance with the Declaration of Helsinki, and the research protocol was approved by the Ethics Committee of the Second Affiliated Hospital of Xi’an Jiaotong University (Ethic Approval Number: 2023267), and all of the participants provided signed informed consent.
The authors are grateful to all the participants, nurses, doctors, and staff who contributed to this study. The authors thank AiMi Academic Services (https://www.aimieditor.com.cn) for English language editing and review services.
This research received no external funding.
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.


