1 Department of Obstetrics, Gynecology and Assisted Reproductive Technologies Unit, Yuzyıl Hospital, 34890 İstanbul, Turkey
2 Deparment of Obstetrics and Gynecology, Faculty of Medicine, Tekirdag Namik Kemal University, 59030 Tekirdag, Turkey
3 Department of Obstetrics and Gynecology, Ankara Medikal Park Hospital, Altunbaş University, 06680 Ankara, Turkey
4 Assisted Reproductive Technologies Unit, Momart Private Practise, 34890 İstanbul, Turkey
Abstract
Giving birth is one of the challenges that women of reproductive age encounter when their ovarian reserve has decreased or been lost. Platelet-rich plasma (PRP) may be advantageous for women experiencing a poor ovarian response (POR). To evaluate the efficacy of administering autologous PRP injections into the ovary in improving ovarian reserve, oocyte and embryo production, and live birth rates in patients with a poor prognosis.
The trial comprised 234 women in their reproductive years who had a diagnosis of POR and the Bologna criteria. These women received intraovarian PRP injections. The age range of the cases was between 30 and 44 years. Each ovary received a multifocal intramedullary injection of 3–4 mL of PRP. The effectiveness of PRP was evaluated in all patients, with a six-month follow-up to assess follicle-stimulating hormone (FSH), luteinizing hormone (LH), estradiol (E2), and anti-Mullerian hormone (AMH). An evaluation was conducted on the metrics of in vitro fertilization (IVF) results and indications of ovarian reserve.
Subsequent to PRP treatment, there was a rise in both the quantity of antral follicles count (AFC) and the concentration of AMH in the bloodstream. After receiving PRP injection, 21 women (9.0%) became pregnant without any intervention, 9 women (3.8%) were excluded in the study anymore, 192 women (82.0%) attempted IVF treatment with developing antral follicles, 4 of whom have premature ovulation and 12 women (5.1%) who did not have antral follicles did not need any more therapy. Out of the 188 women who had IVF, 126 (67.0%) successfully developed embryos and 106 of them had the embryos transferred. Among these, 42 (39.6% per transfer) achieved pregnancy, and 39 (36.8% per transfer) had a continuing pregnancy resulting in a live birth.
Autologous PRP injection into the ovary may be investigated as another experimental therapeutic option for women with POR.
Keywords
- intraovarian
- injection
- platelet
- rich
- plasma
Primary ovarian dysfunction is a medical disorder characterised by a substantial deterioration in ovarian function. This disorder has gained attention as a significant global public health issue. The primary challenges in reproductive science revolve around the suboptimal fertility outcomes experienced by women with poor ovarian reserves [1]. Despite the implementation of many strategies to increase the achievement rate of assisted reproductive technology (ART) in poor-risk persons the pregnancy rate in this population continues to be low. Although oocyte donation is gaining popularity, a multitude of women grapple with the concept of not having a child with their individual genetic material. One further obstacle to oocyte donation is the presence of ethical or religious restrictions in certain countries, which necessitate women to pursue experimental treatment instead of using donor oocytes.
Autologous platelet-rich plasma (PRP) is becoming increasingly popular in the field of ovarian rejuvenation and is being used as a regenerative treatment in various areas [2, 3]. PRP has been treated with growth factors and cytokines. Platelets play a promising function in regenerative therapy because they initiate cell proliferation and tissue development [3]. Platelet increase in a tissue induces tissue regeneration by generating proteins in reaction to cytokines and growth hormones, therefore reversing cellular damage and renewing the tissue [4]. PRP has been studied for its potential in treating infertility associated with ovarian insufficiency [5, 6, 7, 8].
Currently, there are no feasible therapy options for women with early ovarian insufficiency who desire to conceive using their own eggs. Studies conducted on both people and animals have indicated that intraovarian PRP therapy may have the ability to improve ovarian function and potentially increase the likelihood of conception in women with decreased ovarian reserve [2, 3, 4, 5, 6, 7, 8].
While some recent commentaries have raised doubts about the effectiveness of PRP as an additional therapy in fertility treatment due to limited research, the recent basic scientific studies discussed here highlight the need for more strict investigation into the potential benefits of PRP as a supplementary treatment for women with poor ovarian reserve (POR) and other ovarian disorders.
Our goal was to find out if injecting autologous PRP directly into the ovaries of women with POR would improve in vitro fertilization (IVF) outcomes and ovarian reserve metrics, given the paucity of research on the positive effects of PRP on ovarian parameters.
This study performed a retrospective observational analysis to examine the correlation between ovarian reserve measurements, IVF outcomes, and the administration of autologous PRP injections directly into the ovaries in women with POR.
A total of 234 women were included who applied to the Obstetrics and Gynecology Clinic and Assisted Reproductive Technologies Unit of Yuzyıl Hospital, İstanbul, Turkey. Local Ethics Committee was approval for this study (Number: 2024/609), and was designed following the principles outlined in the Declaration of Helsinki.
The selection of POR was based on the Bologna criteria, which include individuals who are over 40 years old or have additional risk factors for POR, those who have a history of detecting 3 or fewer oocytes with previous conventional stimulation protocols, and individuals who have undergone tests indicating lower ovarian reserve. The level of müllerian hormone should be below 1.1 ng/mL, or the antral follicle count should be less than 5 follicles. PORs are required to satisfy a minimum of two out of the three specified criteria [9].
An analysis was conducted on cases of PRP administered into the ovary from June 2019 to May 2023. The research was carried out at a private hospital located in Istanbul, Turkey. Patients who had a previous diagnosis of malignancy, undergone extensive pelvic surgery resulting in pelvic adhesions, were using anticoagulant medication that made injection of plasma inefficient, or had a present or past immunoglobulin A (IgA) deficiency were not eligible to participate in the treatment. Prior to the operation, all ladies granted written informed consent.
After the completion of the menstrual cycle, ten days elapsed. Before undergoing PRP treatment, follicle-stimulating hormone (FSH), anti-Müllerian hormone (AMH) and antral follicles count (AFC) were assessed.
The PRP was prepared using a T-lab autologous PRP kit (T-Biotechnology Laboratory, Bursa, Turkey) and centrifugation, following the manufacturer’s instructions [6]. Twenty milliliters of blood were given by each subject. They were then centrifuged for eight minutes with a force equal to 830 times the acceleration caused by gravity while yet being kept sterile. The layer of buffy coat was accessed with putting a 16 G needle, which was linked to a 5 mL syringe, into the tube. The growth factor-rich blood clot was not removed prior to drawing up PRP using a syringe. Following comparable processing, the initial tube produced approximately 3–4 cubic centimeters of platelet-rich plasma (PRP), whereas the second tube yielded around 6–8 cubic centimeters of PRP. The PRP solution was agitated for 30–60 seconds in a separate tube.
The intra-ovarian injection was administered in the operating room while the patient was under conscious anesthesia shortly after the preparation of PRP. The vaginal area was cleansed with a standard saline solution. Using a 17 G and 35 cm single lumen needle and transvaginal ultrasound for guiding, PRP was injected transvaginally into at least one ovary. Elevated platelet levels in a tissue enhance tissue regeneration by promoting protein production in response to cytokines and growth hormones, facilitating cellular damage repair and tissue rejuvenation. After the procedure, they were monitored in the recovery room for 90–120 minutes and then discharged. Following the administration of PRP injections, a period of eight weeks was allocated for anticipatory care in order to account for the possibility of spontaneous pregnancies or menstrual cycles in all female participants. If the menstrual cycle did not begin in eight weeks, it was stimulated artificially with a hormone treatment consisting of 2 mg of estradiol valerate and 0.5 mg of norgestrel (Cyclo progynova®; Bayer, Leverkusen, Germany), taken twice daily for five days. In the event that menarche was delayed in the subsequent cycle, the same procedure was used.
As mentioned earlier, AFC, serum AMH and FSH were reassessed between days 2 and 4 after the PRP therapy. Women who had an extra antral follicle since their initial examination commenced ovarian stimulation for IVF. Individuals whose AFC did not change underwent a further round of testing. If there was at least one follicle present in each ovary, no matter how many follicles there were compared to pre-PRP levels, stimulation was initiated in the next cycle. If there were no antral follicles observed in comparison to basal measures, but there was an FSH decline or AMH increase, the stimulation process was delayed until the subsequent period.
On the second or third day of the menstrual cycle, controlled ovarian hyperstimulation (COH) was initiated, which occurred spontaneously or was induced chemically. Starting from the third day of the menstrual cycle until the trigger day, all patients were administered subcutaneous FSH (GonalF®; Merck Serono, Darmstadt, Germany) at a dosage of 150 IU per day, along with human menopausal gonadotropin (hMG) (Merional®, IBSA, Lugano, Switzerland) at a dosage range of 75–150 IU per day. After confirming the presence of a fully developed follicle measuring 13–14 mm, a daily dose of 0.25 mg of Cetrotide (Merck Serono, Darmstadt, Germany) was administered until the day of triggering. To stimulate follicle development, 250 µg of recombinant chorigonadotrophin alfa (rHCG; Ovitrelle®; Merck Serono, Darmstadt, Germany) was injected when at least one leading follicle measured 18–19 mm. The process of extracting oocytes was conducted approximately 34–35 hours following the administration of rHCG. After four hours of retrieval, the process of removing the outer layer of the oocyte was finished, and all fully developed oocytes were gathered.
In fresh embryo transfer, daily administration of vaginal progesterone was used to support the luteal phase. This involved using either progesteron gel (Crinone 8%, Merck Pharmaceuticals, Wyeth Laboratories, St.Davids, PA, USA) twice a day or micronized progesterone capsules 200 mg (Progestan 200 mg capsule, Koçak Farma Pharmaceutical and chemical İndustry Inc., İstanbul, Turkey) three times a day, starting the day following oocyte retrieval. Individuals with frozen embryo transfers (FETs) received oral estradiol (Estrofem®; Novo Nordisk, Bagsværd, Denmark) for four days at a daily dosage of 4 mg, which was then raised to 6–8 mg each day to prepare the endometrium. If the thickness of the endometrial lining was than
Pregnancy outcomes were assessed by measuring blood
Continuous variables were initially assessed for normality of statistical distribution by graphical analysis and the Kolmogorov-Smirnov test. Normality test and plots were analyzed with Kolmogorov-Smirnov and Shapiro-Wilk test. Wilcoxon signed-rank test was used for non-normally distributed parameters. Data representing variables were displayed as frequencies and percentages. The data was represented as median (min-max). The data analysis was conducted using SPSS (SPSS-IBM 2.3, Inc., Chicago, IL, USA). The threshold for statistical significance was established at p
This study had a total of 234 women who were diagnosed with POR. The mean age of these women was 33.2
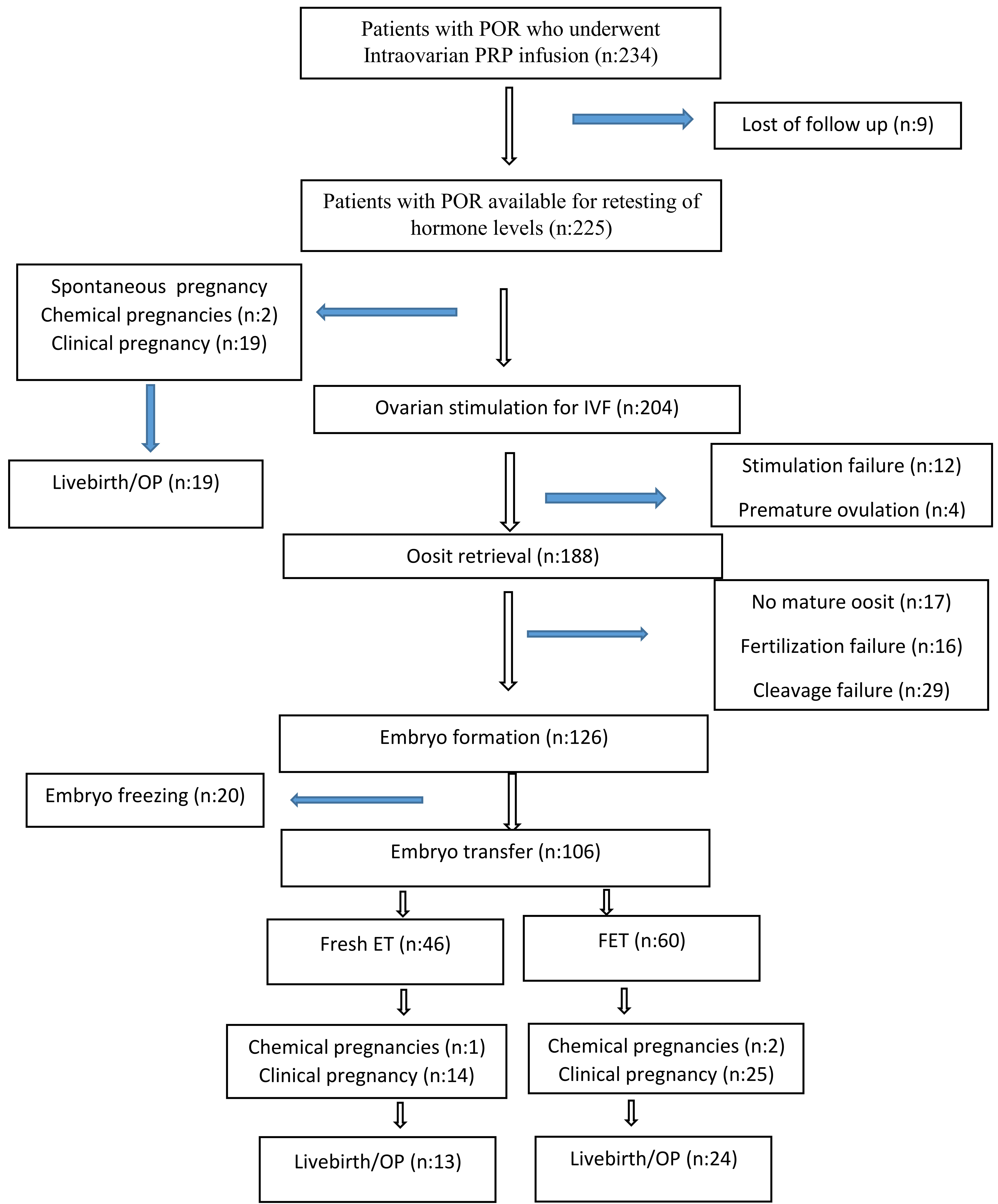 Fig. 1.
Fig. 1. Flowchart of the outcomes of intraovarian PRP infusion in all participants. POR, poor ovarian response; PRP, platelet-rich plasma; IVF, in vitro fertilization; OP, ongoing pregnancy; ET, embryo transfer; FET, frozen embryo transfer.
Intraovarian administration of PRP was performed on 234 women who have received a POR diagnosis earlier. Nine women lost to follow up after PRP treatment and 225 women were available for follow-up and comparison of pre and post treatment hormone levels. Out of the total number of women, 21 (9.0%) became pregnant without any assistance, while the rest 204 (87.2%) underwent COH and 188 (92.2%) underwent IVF. Additionally, 126 (67.0%) of the women successfully produced at least one cleavage stage embryo. A total of 42 women, accounting for 39.6% per transfer, experienced pregnancy following PRP therapy. Out of them, 37 women, representing 34.9% per transfer, successfully achieved continued pregnancy or live birth in just one cycle after the therapy (Fig. 1). With regard to pregnancy outcome, 21/234 (9.0%) of PORs had spontaneous pregnancy in response to PRP injection. Of those, 2 women (9.5%) had abortions after chemical or clinical pregnancy, whereas 19 pregnancies (90.5%) led to healthy live (47.6%) or ongoing pregnancy (42.9%). Out of the fifteen fresh ET pregnancies, two (13.3%) ended in miscarriage in the first trimester, six (40.0%) continued between weeks 12 and 36, and seven (46.7%) produced a live birth. Among the 27 FET pregnancies, 3 (11.1%) were terminated, 15 (55.6%) were still in progress between 14 and 34 weeks of gestation, and 9 (33.3%) finished with a live birth.
Upon analyzing the ovarian reserve data, we found that PRP treatment led to a measurable rise in the antral follicle count [median pre = 1 (min: 0 max: 2), median post 2 (min: 0 max: 5), p
| Before PRP | After PRP | p | ||
| Median (min–max) | Median (min–max) | |||
| AFC* | 1 (0–2) | 2 (1–5) | ||
| 1 (0–2) | 2 (0–5) | |||
| All ages (n = 225) | 1 (0–2) | 2 (0–2) | ||
| AMH* (ng/mL) | 0.06 (0.01–0.4) | 0.14 (0.01–0.4) | ||
| 0.02 (0.01–0.1) | 0.1 (0.01–0.3) | |||
| All ages (n = 191) | 0.06 (0.01–0.4) | 0.14 (0.01–0.4) | ||
| FSH* (IU/mL) | 21 (8–57) | 14 (7–23) | ||
| 21 (10–62) | 14 (7–25) | |||
| All ages (n = 225) | 21 (8–62) | 14 (7–25) | ||
| LH* (IU/mL) | 17 (7–45) | 14 (7–22) | ||
| 18 (8–43) | 14 (7–22) | |||
| All ages (n = 225) | 17 (7–45) | 14 (7–25) | ||
| E2* (pg/mL) | 18 (1–56) | 44 (12–87) | ||
| 21 (2–56) | 44 (12–87) | |||
| All ages (n = 225) | 18 (1–56) | 44 (12–87) | ||
AFC, antral follicle count; AMH, anti-Mullerian hormone; FSH, follicle-stimulating hormone; LH, luteinizing hormone; E2, estradiol; PRP, platelet-rich plasma; *, Wilcoxon signed rank test is used.
| A. Case Processing Summary | ||||||
|---|---|---|---|---|---|---|
| Cases | ||||||
| Valid | Missing | Total | ||||
| N | Percent | N | Percent | N | Percent | |
| AFC | 225 | 100% | 0 | 0% | 225 | 100.0% |
| FSH | 225 | 100% | 0 | 0% | 225 | 100.0% |
| LH | 225 | 100% | 0 | 0% | 225 | 100.0% |
| E2 | 225 | 100% | 0 | 0% | 225 | 100.0% |
| AMH | 191 | 84.9% | 34 | 15.1% | 225 | 100.0% |
| B. Tests of Normality | ||||||
| Kolmogorov-Smirnova | Shapiro-Wilk | |||||
| Statistic | df | Significance | Statistic | df | Significance | |
| AFC* | 0.339 | 225 | 0.739 | 225 | ||
| AFC** | 0.233 | 225 | 0.908 | 225 | ||
| FSH* | 0.195 | 225 | 0.863 | 225 | ||
| FSH** | 0.161 | 225 | 0.954 | 225 | ||
| LH* | 0.093 | 225 | 0.930 | 225 | ||
| LH** | 0.172 | 225 | 0.942 | 225 | ||
| E2* | 0.139 | 225 | 0.905 | 225 | ||
| E2** | 0.196 | 225 | 0.938 | 225 | ||
| AMH* | 0.222 | 191 | 0.663 | 191 | ||
| AMH** | 0.240 | 191 | 0.829 | 191 | ||
a, Lilliefors Significance Correction; df, degrees of freedom; *, before PRP injection; **, after PRP injection.
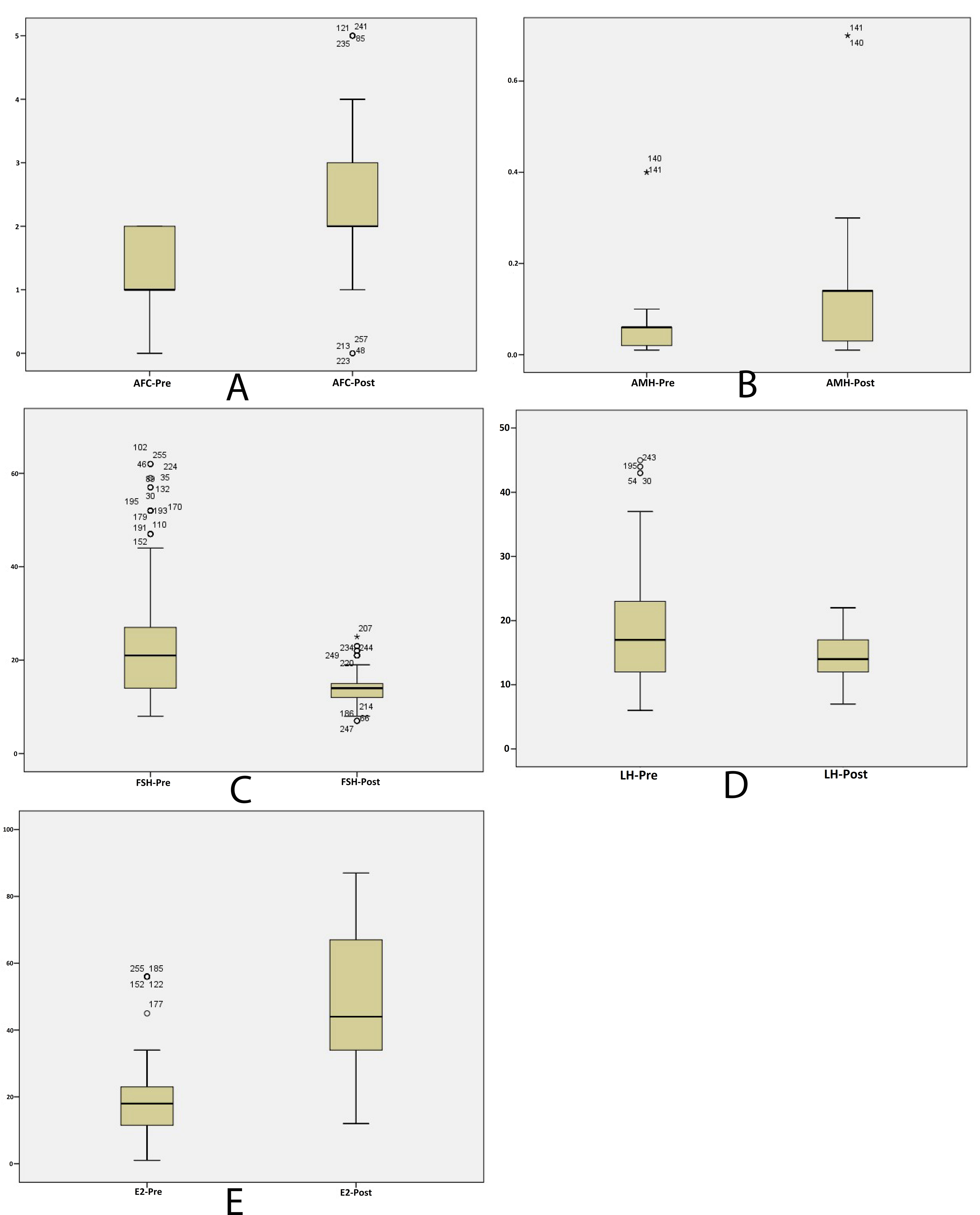 Fig. 2.
Fig. 2. Serum levels of prior to PRP injection, 2 months post-PRP injection. (A) Serum levels of AFC; antral follicle count; (B) AMH, anti-Mullerian hormone; (C) FSH, follicle-stimulating hormone; (D) LH, luteinizing hormone; (E) E2, estradiol. The boxes’ ends are the upper and lower quartiles, so the box covers the interquartile range (25th to 75th percentile). The boxplot’s horizontal line reflects the median value. The whiskers range from 5% to 95%. *: p
 Fig. 3.
Fig. 3. Normality test and plots of AFC, AMH, E2, FSH, LH. Serum levels of AFC (antral follicle count): A1-before treatment, A2-after treatment; AMH (anti-Mullerian hormone): B1-before treatment, B2-after treatment; E2 (estradiol): C1-before treatment, C2-after treatment; FSH (follicle-stimulating hormone): D1-before treatment, D2-after treatment; LH (luteinizing hormone): E1-before treatment, E2-after treatment.
204 women underwent COH. Following PRP treatment, 21 individuals experienced spontaneous pregnancies, while 19 successfully attained continuing pregnancies resulting in live births. Out of all the participants, 204 individuals (87.2%) had COH. Among those who were stimulated, 188 women (92.2%) underwent oocyte extraction. However, 16 individuals (7.8%) were unable to undergo oocyte retrieval. This was either because their stimulation failed (n = 12) or because they experienced premature ovulation (n = 4) (Fig. 1).
Among the 204 women who had COH, 119 of them showed an increase in the number of antral follicles that were pre-existing, ranging from one to five at most. A total of 32 women had an equal amount of antral follicles before and after undergoing PRP administration, while 53 women had no antral follicles at first but had at least one after PRP.
A minimum of one embryo at the cleavage stage was obtained from 126 women, accounting for 61.8% of those who underwent stimulation. Subsequently, either a fresh embryo transfer or cryopreservation was carried out. The embryos had an A/B morphology. The mean number of eggs obtained during oocyte retrieval was 1.92
A total of 106 individuals had embryo transfers. Fresh embryos were implanted in 46/106 (43.4%) patients who underwent embryo transfer; frozen-thawed embryos were deposited in 60/106 (56.6%) patients, and pregnancies were produced in 15/46 (32.6%) fresh embryos and 27/60 (45%) frozen-thawed embryos and 37 (34.9% per transfer) achieved ongoing pregnancy/live birth (OP/LB).
Among the 234 women included in the present study, 21 of them achieved spontaneous pregnancy within a period of four months after undergoing PRP treatment. Our findings indicate that the administration of PRP into the ovary by a skilled practitioner is deemed to be secure. Throughout the observation period, the individuals did not exhibit any premature or subsequent problems, such as infection, bleeding, or blood clot formation.
This study targeted to determine the effects of directly injecting autologous PRP into the ovaries on markers of ovarian reserve and the outcome of pregnancy in POR women. There were 234 ladies who underwent PRP injections directly into their ovaries. Out of the total, 56 women (23.9%) experienced successful live births or sustained implantations, either through natural means or after undergoing IVF. Additionally, 20 women (8.5%) were able to keep cryo-preserved embryos. An individual intraovarian PRP injection significantly augmented the quantity of oocytes and embryos in women diagnosed with POR. Furthermore, the administration of a solitary PRP injection led to a notable elevation in the levels of AMH and estradiol in the individuals participating in our study.
The process of aging is linked to a drop in the number of natural eggs, a reduced production of eggs, and an increased likelihood of failure in ART, ultimately leading to a decrease in the rate of successful pregnancies. Intravaginal administration of PRP can effectively improve ovarian function in women with POR or those experiencing perimenopause [5, 6, 10, 11]. Previous research has showed the efficacy of PRP in rejuvenating tissues and promoting healing in various medical fields [12]. Furthermore, several obstetric and gynecological studies have investigated the impact of PRP injections in the uterus and ovaries. Sills et al. [13] were pioneers in documenting the administration of PRP into the ovary. Four patients with diminished ovarian reserve (DOR) experienced improvements in test markers after receiving intraovarian PRP treatment [13]. According to Hosseini et al. [14], the addition of PRP to the culture significantly enhanced the development of early antral follicles, suggesting that this approach could be advantageous for stimulating follicular growth. Aflatoonian et al. [5] found that 47% of PORs experienced spontaneous pregnancy after receiving PRP injections. Cakiroglu et al. [6] conducted a study to examine the impact of autologous PRP intraovarian injections on measures of ovarian reserve, responsiveness to stimulation, and live births in women with premature ovarian insufficiency (POI). A total of 311 women, aged between 24 and 40 years, who met the European Society of Human Reproduction And Embryology (ESHRE) criteria for POI, received intraovarian PRP injections. PRP therapy caused a rise in AFC and serum AMH levels. Out of the 23 women, 7.4% became pregnant naturally, while 64.8% developed antral follicles.
Panda et al. [15] conducted a comprehensive analysis of the utilization of PRP in individuals with POI and POR diagnoses. The researchers found that there were positive changes in the outcomes of IVF and the characteristics of ovarian reserve. In addition, Pacu et al. [16] observed that treatment with PRP increased the AFC and levels of AMH, while decreasing levels of FSH and LH. Additionally, Melo et al.’s study [17] examined the results of giving 83 women with limited ovarian reserve a direct PRP injection into their ovaries. The PRP group had higher pregnancy rates both biochemically and clinically. According to a study by Farimani et al. [18], patients identified with the POSEIDON criteria had a pregnancy rate of 14.6%. In another study with 40 participants, live birth rates were compared between women treated with PRP and controls after low-dose ovarian stimulation and IVF. Patients who underwent PRP therapy experienced an increase in implantation and live birth rates, indicating a growing trend in this direction [19].
In a recent study, Cakiroglu et al. [7] investigated the potential benefits of intraovarian PRP injections on ovarian paramaters and the results of IVF for POR women. 510 women were administered intraovarian PRP injections. Following this surgery, the ovarian reserve characteristics showed improvement thus that the maintained implantation/live birth rate was ~13% and the pregnancy rate was ~20%.
Growth factors and chemokines are biologically active molecules that facilitate the activation of follicles and their passage through various phases of development. Certain substances found in PRP have been linked to the activation of follicles following intraovarian injection. These substances include platelet-derived growth factor, transforming growth factor beta, insulin-like growth factor, platelet-derived epidermal growth factor, basic fibroblast and vascular endothelial growth factor [20, 21]. Additionally, platelet-generated mediators can enhance oxygen supply, correct ovarian hypoperfusion, and eliminate reactive oxygen species (ROS), all of which promote follicle repair. The hypothesis suggests that the impact is linked to the restoration of mitochondrial function, which leads to ploidy rescue in blastocysts [22]. This theory is supported by Sills et al.’s report [23] of a successful 46, XY pregnancy through IVF after injecting PRP into the ovaries of a patient who had previously experienced 5 unsuccessful IVF attempts due to 20 genetically defective embryos.
Patients diagnosed with POR had superior indicators of ovarian reserve and experienced enhanced production of oocytes and embryos, as evidenced by multiple case studies and prospective investigations. These studies have documented a significant number of successful live births in women who had previously had ineffective therapy. Although there are multiple methods for generating and delivering PRP, and no consensus on the optimal approach, the advantageous effects of PRP are still a matter of debate in the literature.
For to these findings, we suggest that the rejuvenation is facilitated by the administration of exogenous PRP therapy. As indicated before, PRP includes a high concentration of growth factors, which can help with follicle development, ovulation, and oocyte production. Injecting PRP directly into the ovaries restored the balance of hormones such as FSH, LH, AMH, and E2, leading to successful conception and live births. Moreover, improvements in IVF parameters may be linked to enhancements in both the quantity and quality of oocytes.
Initially, this study conducted a retrospective analysis, evaluating the results of patients against their baseline conditions. Consequently, randomization and an independent control group are absent from our investigation. Furthermore, the rates of pregnancy after transferring embryos could not be definitively ascertained due to spontaneous conceptions among some women and loss to follow-up among others, as well as the storage of cryopreserved embryos.
The results of this study revealed that administering autologous PRP with a single injection directly into the ovaries had a beneficial impact on patients with POR. By administering PRP directly into the ovaries, women were able to conceive a child using their own oocytes. This approach has the potential to be a cost-efficient and time-efficient therapeutic technique for clinical therapy in the future. Injecting PRP into the ovary is a promising and successful therapy option for patients with a poor prognosis.
During the preparation of this work, the authors used ChatGpt-3.5 in order to check spell and grammar. After using this tool, the authors reviewed and edited the content as needed and took full responsibility for the content of the publication.
All data points generated or analyzed during this study are included in this article and there are no further underlying data necessary to reproduce the results. The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
SS, EC and İK designed the research study. SS and EC performed the research. FA and EKC acquisition of data, EC and İK analyzed and interpretation of data, SS, EC and EKC participated in drafting the manuscript or revising it critically for important intellectual content. All authors contributed to editorial changes, read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
A written informed consent for participating in this treatment was obtained from all patients. Patients gave their informed agreement to have their data published. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the İstanbul Yeni Yuzyıl Hospital Ethics Committee (approval number: 2024/609).
The authors express their gratitude to the Yuzyıl embryology laboratory members for their invaluable contributions to this research. Thanks to all the peer reviewers for their opinions and suggestions.
This research received no external funding.
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.



