1 Department of Woman, Child and General and Specialized Surgery, University of Campania “Luigi Vanvitelli”, 80138 Naples, Italy
2 Department of Neuroscience, Reproductive Sciences and Dentistry, School of Medicine, University of Naples, 80138 Naples, Italy
3 Pathology Unit, Department of Mental and Physical Health and Preventive Medicine, University of Campania “Luigi Vanvitelli”, 80138 Naples, Italy
Abstract
Lymphovascular space invasion (LVSI) is recognized as a significant prognostic factor in endometrial cancer. Systemic inflammation, as reflected by neutrophil-lymphocyte ratio (NLR), monocyte-lymphocyte ratio (MLR) and platelet-lymphocyte ratio (PLR) indices, may reflect or contribute to tumor progression. Increased endometrial thickness (ET) may also promote local invasion. Our study aims to discover the relationship between these parameters.
In this prospective study, we examined 161 patients with endometrial carcinoma treated at the Azienda Ospedaliera Universitaria Vanvitelli in Naples, Italy. The variables examined included inflammatory indices such as NLR, MLR, and PRL, as well as ultrasound-measured ET (mm). Statistical analysis was performed to compare the groups with and without LVSI and to evaluate the association between inflammatory indices, ET, and the presence of LVSI.
The LVSI-positive group showed statistically significantly higher average values for all analyzed parameters. Logistic regression analysis revealed an interdependence between NLR, MLR, PLR, ET, and LVSI. Multivariate logistic regression confirmed that ET is a significant predictor of LVSI.
The results of our analysis suggest an interaction between inflammation, ET, and LVSI in endometrial cancer. Logistic regression demonstrated that ET is a significant predictor of positive LVSI, while the other inflammatory indices showed a less defined correlation.
NCT05657483, https://clinicaltrials.gov/ct2/show/NCT05657483.
Keywords
- endometrial cancer
- LVSI
- inflammation index
- ultrasound
- cancer pathogenesis
Endometrial carcinoma is the most common gynecological oncological disease in Western countries [1]. Currently, there is increasing focus on the microscopic and molecular factors underlying its pathogenesis and progression [2]. In this context, the role of lymphovascular space invasion (LVSI) as an indicator of early microscopic tumor spread, and its prognostic impact is well established [3, 4, 5]. This progression can also be reflected in the immune response of the individual, leading to alterations in inflammatory indices, such as the neutrophil-lymphocyte ratio (NLR), monocyte-lymphocyte ratio (MLR), and platelet-lymphocyte ratio (PLR) [6, 7]. These are simple and easily accessible measures of systemic inflammation, and they have been studied in various solid tumors to assess their correlation with prognostic outcomes [8].
Studying the inflammatory indices and their association with LVSI is particularly relevant in this context. Their relationship can be interpreted in a bidirectional manner: the progression of the microscopic tumor may influence the subject’s inflammatory response, while the inflammatory state can also promote tumor progression [9].
It is also essential to consider other parameters that may reflect the development of the tumor microenvironment. One such parameter is tumor burden, which can be assessed by the ultrasound measurement of endometrial thickness (ET). ET may reflect changes in the local environment that facilitate LVSI [10, 11].
Understanding the interrelation between these parameters could provide a comprehensive view of the inflammatory response and its impact on disease progression and patient outcomes, as LVSI is an independent risk factor for endometrial cancer [4, 12, 13]. Although LVSI is not typically directly obtained from diagnostic biopsy, its association with other factors, such as the white blood cell-lymphocyte ratio, holds significant promise for improving our understanding of the tumor microenvironment and inflammatory response in endometrial carcinomas.
Our study prospectively evaluated the correlation between endometrial thickness (ET), indices of inflammation, and LVSI status in our population to identify the interactions between these parameters.
The research methods were established a priori and approved by the Ethics Committee of the participating centers (IRB 30661/2022, dated 31/03/2022). The study was subsequently registered on the clinicaltrials.gov platform under the identifier NCT05657483.
Between August 2023 and March 2024, all women with endometrial cancer who met all the inclusion criteria and none of the exclusion criteria were prospectively enrolled in the study at Azienda Ospedaliera Universitaria (AOU) Vanvitelli in Naples, Italy, without randomization. All patients voluntarily participated in the study and provided written informed consent.
The inclusion criteria were as follows: age
All patients enrolled in the study underwent transvaginal pelvic ultrasound and blood sampling on the day before surgery.
The data collected preoperatively included blood counts of neutrophils, leukocytes, monocytes, eosinophils, basophils, and platelets, as well as ultrasound-measured ET (mm). The hematological parameters were subsequently expressed as NLR, MLR, and PLR. These ratios were calculated by dividing the absolute cell count by the absolute lymphocyte count. All patients then underwent surgery in accordance with international standards of care [14, 15].
We also collected histopathological data on myometrial infiltration, tumor grade, histotype, lymph node positivity (including micrometastases and macrometastases) [16, 17, 18], and microsatellite stability. The primary outcome of interest, LVSI, was assessed using a semiquantitative evaluation [4, 5, 19]. Negative or focal involvement was considered as negative. Only diffuse infiltration was considered positive. All collected parameters were collected in unique Clinical Registration Forms (CRF) and digitized into an anonymous electronic database using Excel software, under the responsibility of the Principal Investigator. The data will be destroyed after the results are published.
The sample was analyzed based on the LVSI outcome.
Nominal variables were expressed as absolute frequencies and percentages, and compared using Fisher’s exact test [20] and Chi-square test [21], based on expected counts. Continuous variables were expressed as median and interquartile range and compared using the Wilcoxon test [22] due to non-normal distribution.
The null hypothesis of our study was that there was no difference in the mean values of NLR, MLR, PLR, and ET between patients with negative or focal LVSI (negative) or those that with positive LVSI (H0: µ1 = µ2; H1: µ1–µ2
The weights of the individual values on the dichotomous dependent variable LVSI were calculated using logistic regression [23]. The significance of the model was assessed using the maximum likelihood method [24]. The distribution of continuous variables for the individual parameters of the reference outcome was visualized using boxplots. All statistical analyses were performed using R software and R Studio version 2023.12.1 + 402 (PBC, Boston, MA, USA). The statistical significance level was set at 0.05 (p
Our prospective observational cohort study was conducted at our institution between August 2023 and March 2024. It examined the inflammatory indices and ultrasound-measured ET in 161 patients undergoing surgery for endometrial cancer. The sample was stratified according to the presence or absence of LVSI: 109 individuals with negative LVSI and 52 with positive LVSI.
The demographic variables analyzed included age and body mass index (BMI). Clinical and laboratory variables included microsatellite instability (MSI) status, degree of myometrial invasion, histological grade, endometrioid histology, and presence of lymph node metastasis. Analyses revealed significant differences between the two groups in characteristics related to myometrial invasion (
The demographic characteristics of the cohort are shown in Table 1.
| LVSI | ||||
| Characteristic | Negative, N = 1091 | Positive, N = 521 | p-value2 | |
| Age (years) | 61 (56, 70) | 64 (57, 68) | 0.448 | |
| BMI (kg/m2) | 29 (21, 32) | 30 (23, 36) | 0.401 | |
| MSI | 0.317 | |||
| MSI | 18 (17%) | 12 (23%) | ||
| MSS | 91 (83%) | 40 (77%) | ||
| Myometrial Invasion | ||||
| 99 (91%) | 28 (55%) | |||
| 10 (9.2%) | 23 (45%) | |||
| Missing | 0 | 1 | ||
| Grading | ||||
| 1 | 53 (49%) | 8 (15%) | ||
| 2 | 32 (29%) | 15 (29%) | ||
| 3 | 24 (22%) | 29 (56%) | ||
| Histology | 0.002 | |||
| Endometrioid | 103 (94%) | 43 (83%) | ||
| Serous | 6 (5.5%) | 3 (5.8%) | ||
| Other | 0 (0%) | 6 (12%) | ||
| Lymphnodes | ||||
| Negative | 103 (98%) | 39 (80%) | ||
| Positive | 2 (1.9%) | 10 (20%) | ||
| Missing | 4 | 3 | ||
| Risk Class | ||||
| Low | 17 (61%) | 2 (7.4%) | ||
| Intermediate | 6 (21%) | 0 (0%) | ||
| High-Intermediate | 5 (18%) | 11 (41%) | ||
| High | 0 (0%) | 11 (41%) | ||
| Metastatic | 0 (0%) | 3 (11%) | ||
| Missing | 81 | 25 | ||
| Neutrophils | 4.60 (3.88, 6.20) | 5.36 (3.66, 7.25) | 0.203 | |
| Lymphocytis | 2.20 (1.78, 2.69) | 1.72 (1.20, 2.20) | 0.001 | |
| Monocytis | 0.46 (0.37, 0.58) | 0.46 (0.36, 0.61) | 0.977 | |
| Eosinophils | 0.12 (0.06, 0.20) | 0.10 (0.05, 0.13) | 0.075 | |
1Median, (Q1, Q3); 2Wilcoxon rank sum test; Fisher’s exact test. LVSI, lymphovascular space invasion; BMI, body mass index; MSI, microsatellite instability; MSS, microsatellite stability.
The primary outcome of the study was to compare the mean values of the inflammation indices (NLR, MLR, and PLR) and ultrasonographic ET between the two patient groups. Significant differences were observed between the LVSI-negative and LVSI-positive groups in the values of NLR (2.08 vs 3.23, p
The results are summarized in Table 2.
| LVSI | |||
| Characteristic | Negative, N = 1091 | Positive, N = 521 | p-value2 |
| NLR | 2.08 (1.65, 2.81) | 3.23 (1.90, 5.71) | |
| MLR | 0.20 (0.17, 0.25) | 0.26 (0.19, 0.50) | 0.003 |
| PLR | 118 (90, 153) | 139 (109, 214) | 0.005 |
| ET (mm) | 14 (9, 22) | 20 (14, 28) | 0.004 |
1Median, (Q1, Q3); 2Wilcoxon rank sum test; ET, endometrial thickness; LVSI, lymphovascular space invasion; NLR, neutrophil-lymphocyte ratio; MLR, monocyte- lymphocyte ratio; PLR, platelet-lymphocyte ratio.
The distribution of the 4 analyzed parameters was visualized using boxplots, with values stratified by LVSI positivity or negativity, as shown in Fig. 1.
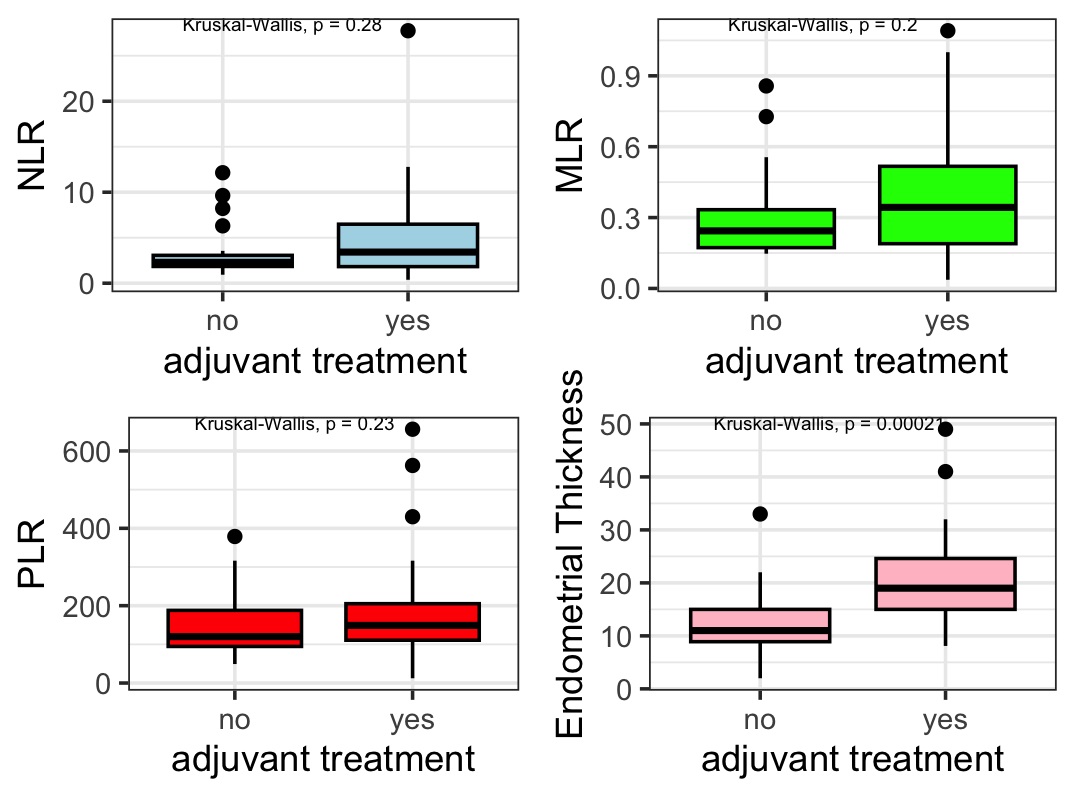 Fig. 1.
Fig. 1. Inflammation index Boxplots showing the relationship between LVSI status and various parameters (ET, MLR, PLR, NLR). NLR, neutrophil-lymphocyte ratio; MLR, monocyte-lymphocyte ratio; PLR, platelet–lymphocyte ratio; LVSI, lymphovascular space invasion.
We initially conducted a univariate logistic regression for each parameter to assess their association with LVSI positivity. ET, NLR, MLR, and PRL were found to be significantly associated with LVSI. The association values from the univariate regression analysis are summarized in Table 3.
| Characteristic | OR | 95% CI | p-value |
| ET (mm) | 1.01 | 1.01, 1.02 | 0.016 |
| NLR | 1.04 | 1.02, 1.06 | 0.001 |
| MLR | 2.15 | 1.44, 3.21 | 0.001 |
| PLR | 1.01 | 1.01, 1.02 | 0.001 |
OR, odds ratio; CI, confidence interval; ET, endometrial thickness; NLR, neutrophil-lymphocyte ratio; MLR, monocyte-lymphocyte ratio; PLR, platelet-lymphocyte ratio.
Finally, we conducted a multivariate analysis by combining all 4 parameters. Of these, only ET retained statistical significance (p = 0.018).
The data from the multivariate logistic regression are summarized in Table 4.
| Characteristic | OR | 95% CI | p-value |
| ET (mm) | 1.01 | 1.01, 1.02 | 0.018 |
| NLR | 1.01 | 0.96, 1.04 | 0.89 |
| MLR | 1.64 | 0.96, 2.08 | 0.07 |
| PLR | 1.01 | 1.00, 1.01 | 0.21 |
OR, odds ratio; CI, confidence interval; ET, endometrial thickness; NLR, neutrophil-lymphocyte ratio; MLR, monocyte-lymphocyte ratio; PLR, platelet-lymphocyte ratio.
LVSI may represent the initial step in the metastasis of endometrial cancer cells [4, 5]. Invasion of vascular spaces may facilitate systemic spread and trigger a response in the host immune system. Conversely, the inflammatory state may also create a favorable microenvironment for tumor spread within the tumor microenvironment [7, 9]. Alternatively, tumor burden may increase the risk of lymphovascular dissemination by expanding the tissue mass, as hypothesized [25, 26]. Based on these hypotheses, we conduct our study, which demonstrated a relationship between the inflammatory response (measured by the inflammation indices NLR, MLR, and PLR), tumor size (expressed as ultrasound-measured ET), and LVSI. LVSI, as a gateway to dissemination, is a well-known risk factor for both recurrence and mortality in endometrial cancer. Therefore, the presence or absence of LVSI is a crucial factor to consider in determining the correct therapeutic approach and intensity of adjuvant treatments, according to Post Operative Radiation Therapy (PORTEC) group [13, 14, 15, 27, 28, 29]. However, this histopathological parameter is not always available at the diagnostic stage. It may be difficult to acquire when biopsy sampling of endometrial carcinoma is limited or when the sample is derived from dilatation and curettage [24]. In contrast, inflammation indices and ET are two parameters readily obtainable from routine clinical practice. To minimize potential biases, we selected the same pathologist for the analysis of all biopsies.
Therefore, we believe it is valuable to understand the relationship between endometrial carcinoma and the subject’s inflammatory response, as this can help identify patients with an imbalance who may benefit from targeted interventions. In addition, systemic dissemination following LVSI positivity could also affect recurrence patterns by increasing the risk of distant recurrence, thereby representing a critical step for endometrial carcinoma to escape local control [13, 29, 30]. In light of this, it is not surprising that the two populations examined in our study were differently distributed with respect to significant prognostic factors (myometrial invasion, grading, histotype, lymph node positivity, and risk class) [2, 14, 31]. However, this imbalance may lead to a different prognostic outcome between the two groups. In our opinion, this imbalance did not affect the validity of the observations, as we focused solely on the association between the parameters under investigation and LVSI, rather than their prognostic significance. Our data show that invasion of vascular spaces produces a change in the subject’s inflammation response, as evidenced by all three parameters under consideration (NLR, MLR, PLR). Univariate analysis also suggests a correlation between all the parameters (both inflammatory and ultrasound) and LVSI. Finally, it is reasonable to assume that there is a correlation between the inflammation indices and ET, as both represent key aspects of tumor microenvironment progression. Therefore, we conducted a multivariate logistic regression, which revealed that only changes in ET presented a statistically significant impact on the probability of positive LVSI. The limitations of our study are related to the lack of prognostic information, which will only be available after extended follow-up of our cohort. The strengths of our study lie in its prospective nature and strict inclusive criteria, which limited the risk of bias, particularly the risk of observing a population with an altered inflammatory response linked to other systemic conditions [32, 33]. Our study could be further building block in explaining the pathogenic mechanisms of endometrial carcinoma progression. While hematological parameters are not independently associated with LVSI, ET is significantly associated with LVSI in the current study. Incorporating additional ultrasound and/or biopsy grading criteria prior to surgery could be valuable for predicting LVSI [34].
The findings of our study suggest an interaction between inflammatory response, ET, and LVSI in endometrial cancer. Logistic regression confirmed that ET is a significant predictor of the presence of LVSI, while the other inflammatory indices show a less pronounced correlation. These results suggest that the immune response may contribute to the microscopic progression of endometrial carcinoma, highlighting new avenues for research to better characterize these mechanisms.
LVSI, lymphovascular space invasion; NLR, neutrophil-lymphocyte ratio; MLR, monocyte-lymphocyte ratio; PLR, platelet-lymphocyte ratio; ET, ultrasound endometrial thickness; BMI, body mass index; MS (I/S), microsatellite (is-) stability.
The authors declare that no AI was used to write the original draft. A grammar correction tools (Grammarly, inc), were used to improve the quality of English and readability. The technology has been used under human oversight and control.
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
CR did Conceptualization; Formal analysis; Methodology; Project administration; Software; Supervision; Validation; Writing — original draft; and Writing — Review & editing, II performed Data curation; Investigation; Project administration; Software; Writing — Review & editing, LDC did Data curation; Investigation; Project administration; Software; Supervision, RC performed the Data curation and Investigation, GA performed the Data curation and Formal analysis, ELM did both Investigation and Methodology, PDF Supervised; moreover, he performed Validation and Visualization, GB performed Vlidation and Visualization, LC performed Conceptualization: Supervision; Validation; Visualization. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
All subjects gave their informed consent for inclusion before they participated in the study. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee No. 30661/2022 of 31/03/2022.
We would like to express our gratitude to all those who helped us during the writing of this manuscript.
This research received no external funding.
The authors declare no conflict of interest. Carlo Ronsini and Luigi Della Corte are serving as Guest Editors of this journal. We declare that Carlo Ronsini and Luigi Della Corte had no involvement in the peer review of this article and have no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to Yasuhiko Ebina.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.

