1 Department of Pediatrics, The First Affiliated Hospital of Anhui Medical University, 230022 Hefei, Anhui, China
Abstract
This study is a prospective cohort study. It aims to investigate the relationship between blood gas parameters and neonatal respiratory outcomes and to develop a prognostic prediction model.
A total of 163 preterm newborns who satisfied the diagnostic criteria outlined in the European Guidelines for the Prevention and Treatment of NRDS-2010 from January 2022 to January 2025 were included. The baseline data of mothers and newborns were collected, and the blood gas parameters were dynamically monitored at 6, 12, 24, and 48 h after birth, including pH, oxygen partial pressure (PaO2), carbon dioxide partial pressure (PaCO2), lactic acid (Lac), and oxygenation index (OI), as well as PaO2/fraction of inspired oxygen (FiO2). Elastic net regression and the Boruta algorithm were used to screen predictive variables, and a multivariate Cox proportional hazards regression model was established. The performance of the model was evaluated using a time-dependent receiver operating characteristic (ROC) curve, Bootstrap resampling calibration curve, and decision curve analysis (DCA).
The poor prognosis group (n = 30) experienced a higher rate of maternal pregnancy comorbidities (50.0% vs. 26.3%; p = 0.011), had a smaller gestational age (29.4 weeks; p = 0.019), lower birth weight (1412.5 g; p < 0.001) and 5-minute Apgar score (p = 0.034), and a higher need for initial mechanical ventilation (53.3% vs. 27.1%; p = 0.005). Dynamic monitoring revealed significant acidosis in the early phase (6 hours), which remained at persistently low levels even at 48 hours. The OI progressively increased, oxygenation efficiency declined, and lactate clearance was markedly delayed. Elastic net regression (optimized λ = 0.1759 via 10-fold cross-validation) and Boruta algorithm screening identified core variables for inclusion in a multivariate Cox regression. Meanwhile, △OI_24 h (hazard ratio (HR) = 1.82, 95% confidence interval (CI) 1.51–2.21; p < 0.001) and Lac_48 h (HR = 1.95, 95% CI: 1.40–2.73; p < 0.001) were identified as independent risk factors. The model predicted a 7-day poor prognosis with an area under the ROC curve of 0.96 (95% CI 0.92–1.00). A 1000 Bootstrap validation model demonstrated high concordance between predicted and actual risks. The DCA indicated that the model provided a significant clinical net benefit compared to intervention or no intervention strategies when the risk threshold exceeded 0.15.
△OI_24 h and Lac_48 h serve as core early warning indicators for poor prognosis in NRDS. The model was constructed using elastic net regression and the Boruta algorithm, demonstrating robust predictive performance and clinical utility, and providing a basis for early risk stratification.
Keywords
- neonatal respiratory distress syndrome
- dynamic monitoring of blood gas
- oxygenation index
- prognosis
- elastic net regression
- Boruta algorithm
- Cox proportional risk model
Neonatal respiratory distress syndrome (NRDS), is a primary reason for morbidity and mortality among premature newborns, involving progressive alveolar degeneration and ventilation/blood flow disproportion due to deficient lung active substance [1]. Advancements in perinatal medicine technology, along with prenatal hormone prophylaxis and postnatal lung surfactant therapy, have notably increased the survival chances of newborns with NRDS, although the clinical prognosis remains highly heterogeneous [2, 3]. Some newborns rapidly encounter severe complications, including persistent hypoxemia and pneumothorax, and may die despite active efforts to intervene [4]. The morbidity and mortality rate for newborns suffering from severe NRDS can surpass 20%, as per statistics, and survivors are more likely to develop sequelae like bronchopulmonary dysplasia [5].
The prognostic assessment system for NRDS is facing a paradigm shift from static anatomic evaluation to dynamic functional monitoring. In modern medical practice, despite the continued use of traditional markers such as gestational age, birth weight, Apgar score, and chest radiograph grading for initial risk classification, their ability to predict outcomes declines significantly during disease progression [6, 7]. This limitation stems from the underlying spatiotemporal heterogeneity of the pathophysiological processes in NRDS [8, 9]. Decreased lung compliance triggered by alveolar surface-active substance deficiency is only one part of the disease phenotype, and the resulting cascade of responses, including ventilation/blood flow dysregulation due to pulmonary vasoconstriction, pulmonary edema triggered by altered alveolar-capillary barrier permeability, deterioration of oxygenation due to increased right-to-left shunting, and secondary respiratory muscle fatigue and increased respiratory work, constitute a multidimensional dynamic regulatory network.
Blood gas analysis is being reconsidered for its clinical significance in reflecting respiratory-metabolic coupling [10]. Traditional monitoring focuses on the instantaneous values of isolated parameters such as partial pressure of O2 (PaO2), partial pressure of CO2 (PaCO2), and pH, but ignores the temporal evolutionary characteristics of these indicators. For example, simply maintaining PaO2 in the normal range may mask the progression of lung injury due to alveolar hyperexpansion under the support of mechanical ventilation, whereas compensatory stabilization of pH may delay the detection of respiratory failure thresholds [11]. Recent studies have revealed that the dynamic coupling relationship between lactate clearance (Lac clearance) and oxygenation index (OI) can more precisely indicate the alignment of tissue perfusion and oxygen use [12, 13]. The pathophysiologic process of NRDS is dynamic and continuous, involving multidimensional interactions of impaired oxygenation, ventilation failure, acidosis and compensatory metabolic changes. Especially for newborns who have been on respiratory support, identifying high-risk newborns who seem stable but are on the verge of collapse through ongoing, multidimensional blood gas parameter monitoring is a significant hurdle. However, existing studies have focused on OI or traditional blood gas indices at a single time point, and there is a lack of systematic exploration of the synergistic patterns of changes in blood gas indices (including basal parameters, such as pH, PaO2, and derived parameters, such as OI and PaO2/fraction of inspired oxygen (FiO2)) at multiple time points and in multiple dimensions in the critical postnatal window (e.g., within the first 48 h after birth) of newborns with NRDS. There is no systematic exploration of constructing a prognostic warning model based on such dynamic changes. Prognostic and early warning modeling.
Therefore, there is an urgent clinical need to fill the research gap on the association between dynamic monitoring of blood gas and NRDS prognosis and to establish an early risk stratification tool. This research gathered dynamic blood gas data in multiple dimensions at 6, 12, 24, and 48 h after birth from newborns with NRDS, along with the birth characteristics of the mothers and the newborns. The aim is to reveal the characteristic blood gas evolution patterns of newborns with poor prognosis, identify key early warning indicators such as OI and metabolic compensation patterns, and construct a Cox proportional risk early warning model integrating dynamic blood gas parameters and clinical variables, which will ultimately provide a quantitative tool to realize early risk identification and precise intervention to improve the prognosis of newborns with NRDS.
This study was a prospective cohort study. Patients were newborns enrolled in
diagnosed cases of NRDS meeting criteria from January 2022 to January 2025 at The
First Affiliated Hospital of Anhui Medical University. Inclusion criteria: (1)
compliance with the European Consensus Guidelines on the Management of
Respiratory Distress Syndrome: 2022 Update [14], regarding clinical symptoms and
imaging data; (2) single fetus; (3) gestational age
Maternal information: age, gestational comorbidities (e.g., gestational diabetes mellitus or hypertension), prenatal hormone use, and mode of delivery (eutocia or cesarean section) were recorded.
Neonatal information: gestational age, birth weight, gender, Apgar scores at 1 and 5 minutes (1-min Apgar and 5-min Apgar), initial respiratory support (non-invasive continuous positive airway pressure [CPAP] or mechanical ventilation, initial respiratory support equipment including Weikang V60 (Shenzhen Weikang Biological Technology Co., Ltd., Shenzhen, Guangdong, China) non-invasive ventilator 25A (Delgado Medical Systems, Frankfurt, Germany) for non-invasive CPAP treatment, Delgado Evita XL invasive ventilator for mechanical ventilation treatment), and chest X-ray classification (grade I–IV based on the severity of NRDS). Chest X-ray examination was performed using Philips DigitalDiagnost DR chest X-ray machine (C90, Philips Healthcare, Amsterdam, The Netherlands) [14].
Radial artery or dorsalis pedis artery (preferred arterial catheter) was
selected and sterilized with 75% alcohol twice. Blood samples (0.3–0.5 mL) were
collected by puncture using a heparinized syringe, gently mixed for
anticoagulation, and sent for testing within 15 min. Testing was performed using
the Siemens Rapidpoint 500 blood gas analyzer (Siemens Healthineers, Erlangen,
Germany). Blood collection time, site and newborn status were recorded to avoid
hemolysis or air bubble interference. The device was calibrated daily, with
abnormal results (e.g., pH
Blood gas levels were dynamically monitored at several time points: within 6 h
of birth (baseline), and at 12, 24, and 48 h after birth, as well as during any
condition worsening, like a sudden decrease in the oxygenation index or the need
to adjust the ventilator parameters. In terms of monitoring indicators, pH,
PaO2, PaCO2, HCO3–, base excess (BE), and Lac were monitored. The
derived indicators, i.e., OI (FiO2
The endpoint metrics consisted of primary and secondary outcomes. The primary
outcomes were a poor prognostic composite endpoint that encompassed death in the
first week after birth, pneumothorax or mediastinal emphysema requiring closed
chest drainage, and severe respiratory failure requiring high-frequency
oscillatory ventilation support for
The required sample size (N) must satisfy the following criteria: limiting
overfitting: N
Based on preliminary studies and literature, k = 4 was set. Substituting into the formula yields a required event count of 40. This study ultimately enrolled 163 pediatric patients, with 30 events. While the total sample size did not fully meet the theoretical calculation, it reached a common scale for this type of research.
Statistical analyses were performed using SPSS 25.0 software (IBM Corp., Armonk,
NY, USA). To evaluate the normality of the data, Shapiro-Wilk tests were
conducted. Continuous variables conforming to normal distribution were expressed
as mean
This study included 163 newborns with NRDS who were categorized into a good
prognosis group (n = 133) and a poor prognosis group (n = 30) based on the
composite endpoints. As for maternal baseline characteristics, there were no
significant differences between the two groups in terms of age (p =
0.076) and prenatal hormone use (p = 0.659) and cesarean section rate
(p = 0.322), but there was a significantly higher incidence of pregnancy
comorbidities in the poor prognosis group (50.0% vs. 26.3%, p
= 0.011). Based on neonatal characteristics, gestational age (p =
0.019), birth weight (p
| Variables | Total (n = 163) | Poor prognosis group (n = 30) | Good prognosis group (n = 133) | p value | |
| Maternal information | |||||
| Age, years | 32.7 (29.6, 35.2) | 33.5 (32.8, 36.6) | 32.10 (29.4, 34.8) | 0.076 | |
| Gestational comorbidities | 50 (30.7) | 15 (50.0) | 35 (26.3) | 0.011 | |
| Prenatal hormone use | 82 (50.3) | 14 (46.7) | 68 (51.1) | 0.659 | |
| Mode of delivery (cesarean section) | 90 (55.2) | 19 (63.3) | 71 (53.4) | 0.322 | |
| Neonatal information | |||||
| Gestational age, weeks | 30.2 (28.7, 31.8) | 29.4 (28.0, 30.5) | 30.6 (28.9, 31.9) | 0.019 | |
| Birth weight, g | 1598.0 (1400.0, 1820.0) | 1412.5 (1353.8, 1536.5) | 1650.0 (1426.0, 1920.0) | ||
| Gender (male) | 85 (52.2) | 17 (56.7) | 68 (51.1) | 0.583 | |
| 1-min Apgar | 6 [4, 7] | 5 [4, 7] | 6 [4, 7] | 0.056 | |
| 5-min Apgar | 8 [6, 9] | 6 [6, 8] | 8 [6, 9] | 0.034 | |
| Chest radiograph classification (II and above) | 60 (36.8%) | 12 (40.0%) | 38 (28.6%) | 0.220 | |
| Initial respiratory support | 0.005 | ||||
| CPAP | 111 (68.1%) | 14 (46.7%) | 97 (72.9%) | ||
| Mechanical ventilation | 52 (31.9%) | 16 (53.3%) | 36 (27.1%) | ||
CPAP, continuous positive airway pressure; categorical data expressed as n (%)
were compared using the chi-square test; continuous values expressed as mean
Neonatal blood gas indices in the poor and good prognosis groups were
dynamically monitored (Table 2), and significant differences were found between
the two groups in some parameters (Table 2). The poor prognosis group showed a
triad of evolution of blood gas indicators in comparison with the good prognosis
group. A significant acidosis was observed in the early stage (6 h) (pH 7.21
| Variables | Poor prognosis group (n = 30) | Good prognosis group (n = 133) | p value | |
| pH value | ||||
| Baseline (6 h) | 7.21 |
7.28 |
0.004 | |
| 12 h | 7.28 |
7.30 |
0.102 | |
| 24 h | 7.36 (7.28, 7.40) | 7.36 (7.31, 7.40) | 0.382 | |
| 48 h | 7.34 |
7.38 |
0.031 | |
| PaO2, mmHg | ||||
| Baseline (6 h) | 54 |
54 |
0.921 | |
| 12 h | 57 |
57 |
0.685 | |
| 24 h | 58 |
60 |
0.306 | |
| 48 h | 61 |
63 |
0.299 | |
| PaCO2, mmHg | ||||
| Baseline (6 h) | 49 |
47 |
0.162 | |
| 12 h | 45 |
43 |
0.374 | |
| 24 h | 42 |
41 |
0.466 | |
| 48 h | 41.26 |
38.43 |
0.076 | |
| HCO3–, mmol/L | ||||
| Baseline (6 h) | 19.45 |
20.05 |
0.331 | |
| 12 h | 20.74 |
21.57 |
0.183 | |
| 24 h | 21.89 |
23.06 |
0.060 | |
| 48 h | 23.45 |
25.01 |
0.015 | |
| BE, mmol/L | ||||
| Baseline (6 h) | –4.75 (–5.60, –3.50) | –3.90 (–4.60, –3.50) | 0.214 | |
| 12 h | –2.85 (–3.68, –2.05) | –3.06 |
1.000 | |
| 24 h | –1.65 (–2.17, –0.90) | –1.10 (–2.40, –0.20) | 0.297 | |
| 48 h | –0.10 (–0.80, 0.95) | 0.60 (–0.60, 1.50) | 0.157 | |
| Lac, mmol/L | ||||
| Baseline (6 h) | 4.16 |
3.93 |
0.347 | |
| 12 h | 3.51 |
3.08 |
0.085 | |
| 24 h | 2.86 |
2.15 |
0.004 | |
| 48 h | 2.60 (1.72, 3.67) | 1.60 (1.00, 2.20) | ||
| OI | ||||
| Baseline (6 h) | 21.05 |
21.35 |
0.691 | |
| 12 h | 24.10 |
23.42 |
0.319 | |
| 24 h | 27.35 |
24.03 |
||
| 48 h | 24.10 |
19.88 |
||
| PaO2/FiO2 | ||||
| Baseline (6 h) | 169.50 |
180.15 |
0.219 | |
| 12 h | 198.05 |
208.10 (183.30, 234.60) | 0.200 | |
| 24 h | 225.65 |
241.05 |
0.098 | |
| 48 h | 243.00 |
265.35 |
0.017 | |
Continuous data were expressed as mean
Subsequently, the dynamic characteristics of key blood gas indicators in
newborns in the poor prognosis group were compared with those in the good
prognosis group, and significant differences were found between the two groups in
the magnitude of changes in several indicators (Table 3). In terms of acid-base
balance, the increase in pH was greater in the poor prognosis group during the
first 24 h after birth (p = 0.016), whereas the change in pH tended to
slow down during the period of 24–48 h (p
| Variables | Poor prognosis group (n = 30) | Good prognosis group (n = 133) | p value |
| 0.11 (0.04, 0.20) | 0.08 (0.07, 0.09) | 0.016 | |
| 0.00 (–0.01, 0.02) | 0.02 (0.01, 0.03) | ||
| 3.66 |
5.35 |
||
| 2.88 |
2.94 |
0.673 | |
| –6.95 |
–5.70 |
0.004 | |
| –1.30 (–1.50, –0.70) | –2.60 (–3.20, –2.10) | ||
| 2.45 (2.10, 2.85) | 3.00 (2.70, 3.40) | ||
| 1.56 |
1.95 |
0.013 | |
| 2.96 |
2.89 |
0.642 | |
| 1.62 |
1.71 |
0.314 | |
| –1.31 |
–1.78 |
||
| –0.20 (–0.20, –0.10) | –0.50 (–0.60, –0.30) | ||
| 6.20 (4.83, 7.33) | 2.70 (2.20, 3.20) | ||
| –3.25 |
–4.15 |
||
| 56 |
61 |
0.063 | |
| 17 |
24 |
Continuous data were expressed as mean
Elastic Net analysis (Supplementary Table 1) identified five non-zero
coefficient variables from all candidate variables under the optimal
regularization parameter (
Incorporating the above four variables into a multivariate Cox regression model
yielded the results shown in Table 4:
| Variables | S.E | Z | p value | HR (95% CI) | |
| –0.20 | 0.11 | –1.78 | 0.075 | 0.82 (0.66~1.02) | |
| 0.20 | 0.14 | 1.39 | 0.165 | 1.22 (0.92~1.62) | |
| Lac_48 h | 0.67 | 0.17 | 3.93 | 1.95 (1.40~2.73) | |
| 0.60 | 0.10 | 6.14 | 1.82 (1.51~2.21) |
HR, hazards ratio; CI, confidence interval.
As shown in the time-dependent ROC curve in Fig. 1, the model achieved an area under the curve (AUC) of 0.96 (95% CI: 0.92–1.00) for predicting 7-day adverse outcomes, indicating good discriminatory ability between poor and good outcomes. The adjusted curve obtained through 1000-time internal validation via Bootstrap resampling (Fig. 2A) demonstrates high consistency between predicted and observed risks, indicating excellent predictive accuracy with no significant bias after adjustment. DCA analysis indicated that when the risk threshold exceeded 0.15, the clinical net benefit derived from risk stratification using this predictive model consistently surpassed that of both intervention and no intervention strategies (Fig. 2B). This demonstrates the model’s strong clinical utility in supporting decision-making by clinicians.
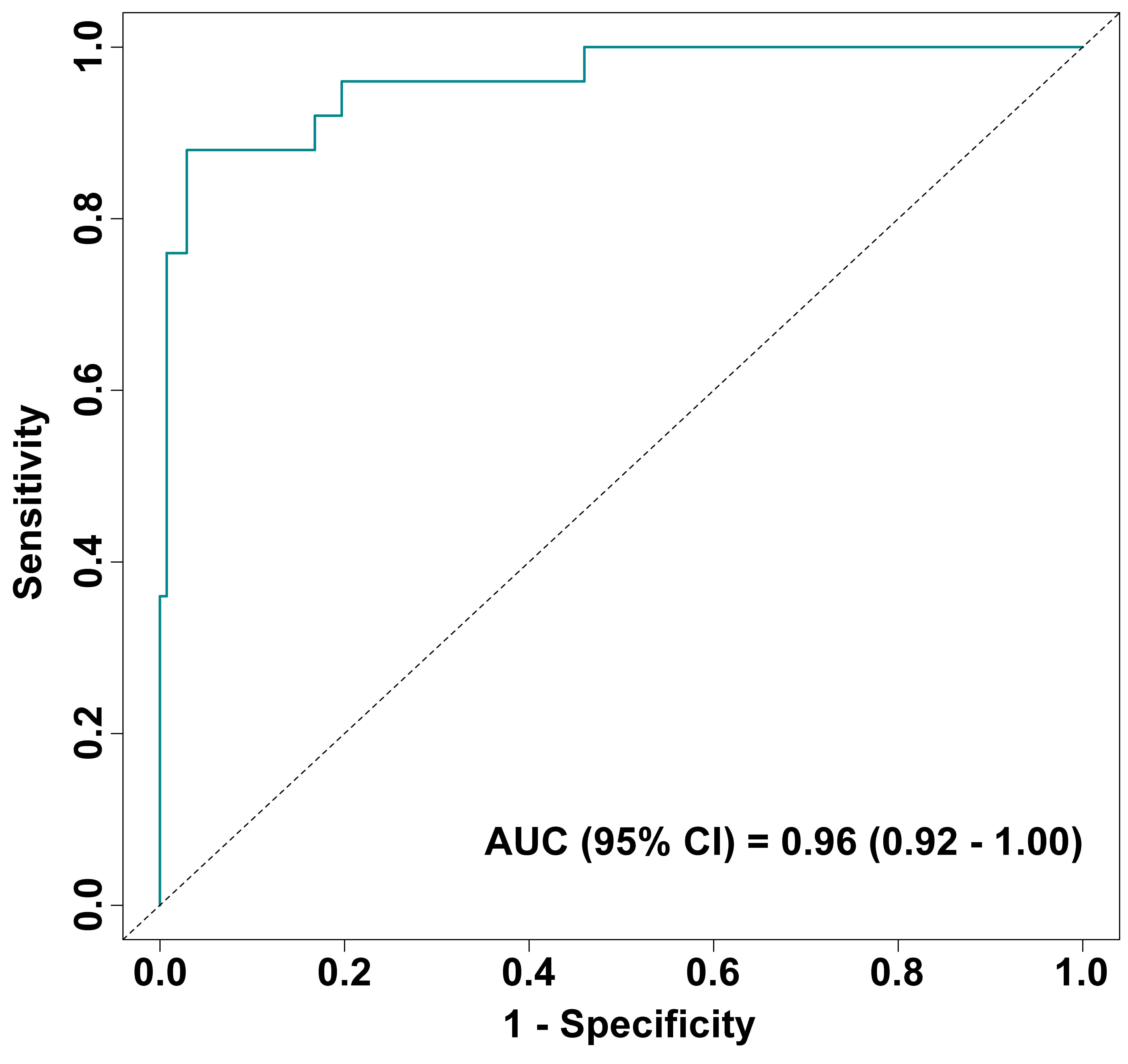 Fig. 1.
Fig. 1.
Time-dependent ROC curve of the predictive model (7 days). AUC, area under the curve.
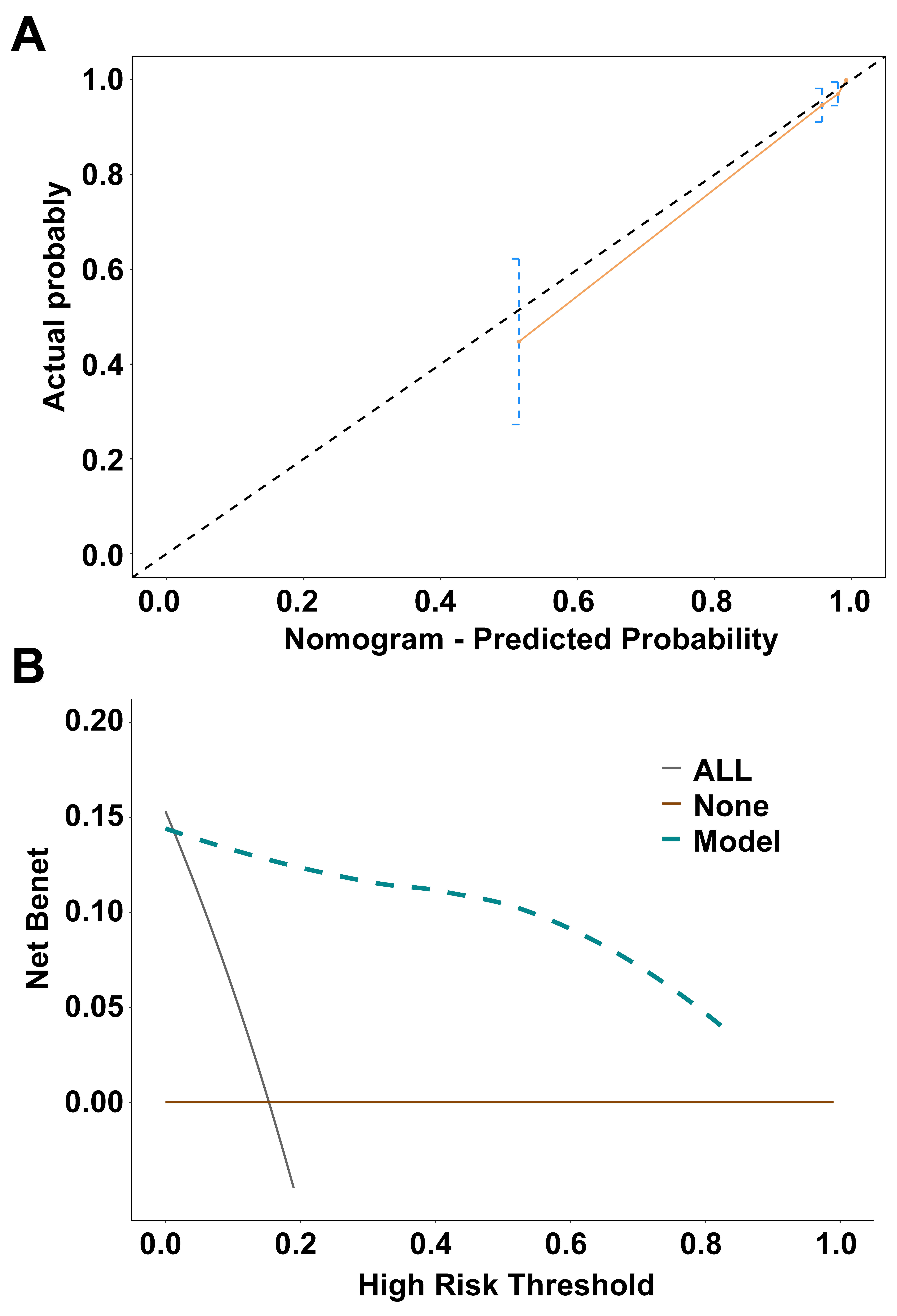 Fig. 2.
Fig. 2.
Internal validation of the model. (A) Calibration curve from internal validation via 1000 Bootstrap resamples. (B) DCA evaluating the model’s clinical net benefit at different risk thresholds. DCA, decision curve analysis.
This study enrolled 163 preterm infants with NRDS. By integrating dynamic blood
gas monitoring data with clinical characteristics, it innovatively employed
elastic net regression combined with the Boruta algorithm to optimize variable
selection. A prognostic early warning system was constructed based on a
multivariable Cox proportional hazards model. The study not only further
validated the association between traditional high-risk factors—such as
maternal pregnancy complications and low gestational age—and NRDS prognosis,
but also identified the independent predictive value of the 24-hour change in
oxygenation index (
This study first confirmed the association between maternal pregnancy
complications, low gestational age, low birth weight, and initial mechanical
ventilation requirements with poor prognosis in preterm infants, consistent with
previous research findings [15]. Specifically, the incidence of maternal
pregnancy complications was significantly higher in the poor prognosis group than
in the good prognosis group, suggesting that pathological conditions during
pregnancy (such as gestational diabetes and hypertension) may increase the
severity of NRDS by disrupting the fetal lung development microenvironment. Low
gestational age and low birth weight reflect the core pathophysiological basis of
immature lung tissue development in preterm infants. This finding corroborates
previous reports indicating that birth weight
Compared with previous studies, this study newly identified an association
between the 5-min Apgar score and NRDS prognosis. The 5-min Apgar score was
significantly lower in the poor prognosis group than in the good prognosis group,
whereas the difference in 1-min Apgar scores was not statistically significant.
This finding suggests that the persistence of neonatal resuscitation outcomes
(5-min score) better reflects the severity of tissue hypoxia and ischemia than
the immediacy (1-minute score). A low 5-min Apgar score typically indicates
delayed recovery of respiratory and circulatory function after birth, potentially
compounded by NRDS-related ventilatory/oxygenation impairment, thereby
exacerbating prognostic risk [17]. Additionally, the initial requirement for
mechanical ventilation was significantly higher in the poor prognosis group
(53.33%) compared to the good prognosis group (27.07%, p = 0.005).
This disparity essentially reflects the severity of lung injury: children
requiring mechanical ventilation often exhibit more pronounced alveolar collapse
and reduced lung compliance, while increased respiratory support intensity
indirectly indicates a higher risk of disease progression [18, 19]. Notably, the
proportion of chest radiographs graded
Through dynamic monitoring of blood gas parameters at 6, 12, 24, and 48 h, this
study identified a triad evolution pattern of “early acidosis-progressive
oxygenation impairment-delayed metabolic clearance” in the poor prognosis group.
Furthermore, analysis of parameter change magnitudes (e.g.,
Compared to traditional single-time-point OI (e.g., 48-h OI),
This study did not rely on subjective selection variables but instead adopted a
data-driven strategy combining elastic net regression (for handling
multicollinearity and variable screening) with the Boruta algorithm (a feature
importance confirmation algorithm based on random forests) to objectively
identify the most predictive variables. The combined approach identified four
core dynamic parameters:
This study has several limitations that warrant caution in interpreting the
findings. It is a single-center study with a limited sample size (particularly in
the poor prognosis group, n = 30), which may have restricted the power of certain
statistical tests and increased the risk of model overfitting. The findings
require further validation in prospective, multicenter, large-scale cohort
studies. Although the model adjusted for several important variables, unmeasured
confounders may still exist. These include detailed ventilator settings
strategies, fluid management practices, patent ductus arteriosus and its
hemodynamic impact, as well as the specific timing and frequency of surfactant
administration—all of which could influence outcomes. The primary outcome of
this study was defined as a composite endpoint within 7 days postnatal. These
dynamic indicators were not evaluated for their association with longer-term
outcomes such as bronchopulmonary dysplasia. Future studies may extend follow-up
periods. Based on this, a standardized dynamic monitoring pathway was established
using the 6/24/48 h blood gas monitoring points and incorporating
This study has several limitations that warrant caution in interpreting
conclusions and generalizing findings. It is a single-center observational study
with a limited sample size, particularly in the poor prognosis group (n = 30).
This limits statistical power, and the generalizability of results requires
validation. While the primary composite outcome encompassed severe events,
follow-up duration was short (within 7 days). Therefore, the findings cannot
infer associations between these dynamic indicators and critical long-term
neonatal outcomes (e.g., bronchopulmonary dysplasia, neurodevelopmental
prognosis), which are equally vital for assessing the overall burden of NRDS.
Although we conducted internal validation using Bootstrap and demonstrated good
performance, this remains an internal validation. Whether the model maintains the
same predictive efficacy in other populations or medical centers with different
clinical practice standards must be confirmed through rigorous external
validation. Future studies should involve multicenter collaboration to establish
an external validation cohort of
The blood gas parameters identified through elastic net regression and the
Boruta algorithm—
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
MYZ and YW designed the research study. MYZ performed the research. YW provided help and advice on the experiments. MYZ analyzed the data. Both authors contributed to editorial changes in the manuscript. Both authors read and approved the final manuscript. Both authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
The guardians of the newborns voluntarily signed an informed consent form. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of The First Affiliated Hospital of Anhui Medical University (approval number: No. 2021AMU-0532).
We would like to express our gratitude to all those who helped us during the writing of this manuscript. Thanks to all the peer reviewers for their opinions and suggestions.
This research received no external funding.
The authors declare no conflict of interest.
Supplementary material associated with this article can be found, in the online version, at https://doi.org/10.31083/CEOG44812.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.


