1 Department of Obstetrics and Gynecology, Sakarya University Faculty of Medicine, 54100 Sakarya, Turkey
2 Department of Obstetrics and Gynecology, Sehit Prof. Dr. Ilhan Varank Sancaktepe Training and Research Hospital, University of Health Sciences, 34000 Istanbul, Turkey
3 Department of Public Health, Sakarya University Faculty of Medicine, 54100 Sakarya, Turkey
4 Department of Medical Biochemistry, Clinic of Medical Biochemistry, Sakarya Training and Research Hospital, 54100 Sakarya, Turkey
5 Department of Obstetrics and Gynecology, Cerrahpasa Faculty of Medicine, Istanbul University-Cerrahpasa, Kocamustafapasa, 34098 Istanbul, Turkey
Abstract
Insulin-regulated aminopeptidase (IRAP) is involved in insulin sensitivity and glucose metabolism and is important in the pathophysiology of type 2 diabetes. Serum IRAP levels are strongly associated with type 2 diabetes and insulin resistance. The aim of this study was to evaluate the IRAP level as a potential biomarker for the early diagnosis and management of insulin resistance in women with gestational diabetes mellitus (GDM).
This cohort study included 40 women with GDM and 40 women with healthy pregnancies. Maternal serum IRAP levels were measured with enzyme-linked immunosorbent assay (ELISA) and compared between the two groups.
The mean serum IRAP level was significantly lower in the GDM group (0.73 ± 0.12 ng/mL) compared to the controls (0.92 ± 0.10 ng/mL) (p = 0.001). Pairwise comparisons indicated, that diet modified and insulin treated GDM subgroups had significantly lower serum IRAP levels than the control group (p < 0.017 and p < 0.017, respectively). Serum IRAP levels showed significant negative correlations with fasting glucose, insulin, homeostatic model assessment of insulin resistance (HOMA-IR) levels and hemoglobin A1c (HbA1c) (r = –0.541, p = 0.001; r = –0.447, p = 0.001; r = –0.584, p = 0.001; r = –0.361, p = 0.001). The optimum serum IRAP cut-off value was calculated to be 0.857 ng/mL, with a sensitivity of 85% and a specificity of 80% for the prediction of GDM (p = 0.001).
The serum IRAP level in pregnant women diagnosed with GDM was significantly lower than in healthy pregnant women. Moreover, the serum IRAP level was negatively correlated with the levels of insulin, HbA1c, and HOMA-IR. These findings suggest that low serum IRAP level could be a novel biomarker for the prediction of GDM.
The study has been registered on https://classic.clinicaltrials.gov/ (registration number: NCT06716320).
Keywords
- insulin-regulated aminopeptidase
- gestational diabetes mellitus
- glucose transporter 4
- insulin resistance
- hemoglobin A1c
Gestational diabetes mellitus (GDM) describes a condition of glucose intolerance that first appears during the second or third trimester of pregnancy in women with no prior diagnosis of diabetes [1]. GDM typically resolves after childbirth. In Europe, GDM affects approximately 11% of pregnant women, with a reported prevalence of up to 31.5% in Eastern European countries [2]. The International Association of the Diabetes and Pregnancy Study Groups (IADPSG) criteria for GDM diagnosis offers a standardized approach to screening and treatment and has led to a 75% increase in prevalence rates [3]. Since GDM is associated with adverse pregnancy outcomes for both the mother and fetus, it is essential to achieve glycemic control during pregnancy [4].
The primary characteristics of GDM include insulin resistance and insulin deficiency. These occur due to elevated levels of anti-insulin hormones such as prolactin, progesterone, estradiol, and placental growth hormone, all of which are secreted by the placenta as pregnancy progresses [5, 6]. Normally, an adaptive increase in
Insulin-regulated aminopeptidase (IRAP) is a zinc-dependent metallopeptidase with two main functional domains: an extracellular/intraluminal C-terminal domain that regulates type II transmembrane peptide hormones, and a cytosolic N-terminal domain that affects the intracellular distribution of IRAP [8, 9]. IRAP plays a major role in glucose regulation by interacting with glucose transporter 4 (GLUT4) in skeletal muscle and adipose tissue [10, 11]. Although early studies on IRAP were mostly conducted in adipocytes, Keller et al. [12] reported it was expressed in several tissues, including the heart, brain, spleen, lung, muscles, and kidney. The role of IRAP in GLUT4 translocation to the plasma membrane is critical for insulin signaling and the reduction of insulin resistance [11, 13].
IRAP activity has been investigated as an indicator of insulin resistance in patients with type 2 diabetes. By promoting GLUT4 translocation and reducing oxidative stress and inflammation, IRAP inhibitors have been shown to improve glucose tolerance and metabolic functions, as evidenced by study using diabetic and obese rat models [14].
The aim of this study was to compare IRAP levels between pregnant women diagnosed with GDM, and women with a healthy pregnancy. This should deepen our understanding of the role of IRAP in insulin regulation and its potential as a therapeutic target in the management of GDM.
This prospective cohort study was conducted between 30 April 2021 and 30 April 2022 at the Gynecology and Obstetrics Clinic of Sakarya University Training and Research Hospital. The study received approval from the Ethics Committee of the Sakarya University School of Medicine (date: 21/04/2021, number: 89). All pregnant women who volunteered to participate in this study provided written informed consent in accordance with the principles outlined in the Declaration of Helsinki. This study was initiated prior to the requirement to register prospective cohort studies. The study has been registered on https://classic.clinicaltrials.gov/ (registration number: NCT06716320).
A total of 80 pregnant women were evaluated in this study. The study group (GDM group) consisted of 40 pregnant women diagnosed with GDM between 24 and 28 weeks of gestation through a 2 h, 75 g oral glucose tolerance test (OGTT). GDM was diagnosed based on the criteria of the IADPSG. This defines GDM using one or more abnormal plasma glucose values: fasting glucose level
Serum insulin, fasting plasma glucose, hemoglobin A1c (HbA1c) and IRAP levels were measured for all participants after at least 8 h of fasting. Body mass index (BMI, kg/m2) measurements were taken for each patient, and homeostatic model assessment of insulin resistance (HOMA-IR) values were calculated. To evaluate insulin resistance, the study utilized the HOMA-IR formula: fasting glucose (mmol/L)
In the GDM group, 11 women required insulin treatment to achieve blood sugar control, while the remaining 29 managed to regulate their blood sugar levels through dietary changes alone. For those requiring insulin, blood sugar threshold values were set as follows: fasting blood sugar of 95 mg/dL, 1 h postprandial blood sugar of 140 mg/dL, and 2 h postprandial blood sugar of 120 mg/dL [1].
The control groupcomprised 40 pregnant women who matched the GDM group in terms of age, BMI, and gestational week. These women were normoglycemic according to the 75 g OGTT results, and no complications were observed during their pregnancies.
• Patients who had an OGTT test between 24–28 weeks of gestation and were diagnosed with GDM according to IADPSG criteria. These were evaluated as the patient group at the end of the study.
• Patients who showed normoglycemia as a result of the OGTT test. These were evaluated as the control group.
Pregnant women were excluded from the study if they met any of the following criteria:
• History of preeclampsia or chronic hypertension (blood pressure
• Pregestational diabetes mellitus (HbA1c
• Having any acute or chronic illness (autoimmune disease, active infection, or systemic disease diagnosed by clinical evaluation that impairs glucose metabolism).
• Risk of preterm labor (cervical length
• Premature rupture of membranes (PPROM) (observation of amniotic fluid leakage during sterile speculum examination, or confirmation by a positive nitrazine/fern test).
• Presence of fetal structural or chromosomal anomalies (diagnosis by ultrasound or genetic testing, such as amniocentesis or chorionic villus sampling).
• Multiple pregnancies (confirmation of the presence of more than one fetus by ultrasound).
• Fetal growth restriction (fetal weight measured by ultrasound below 10% of the estimated fetal weight according to gestational age, and presence of Doppler abnormalities).
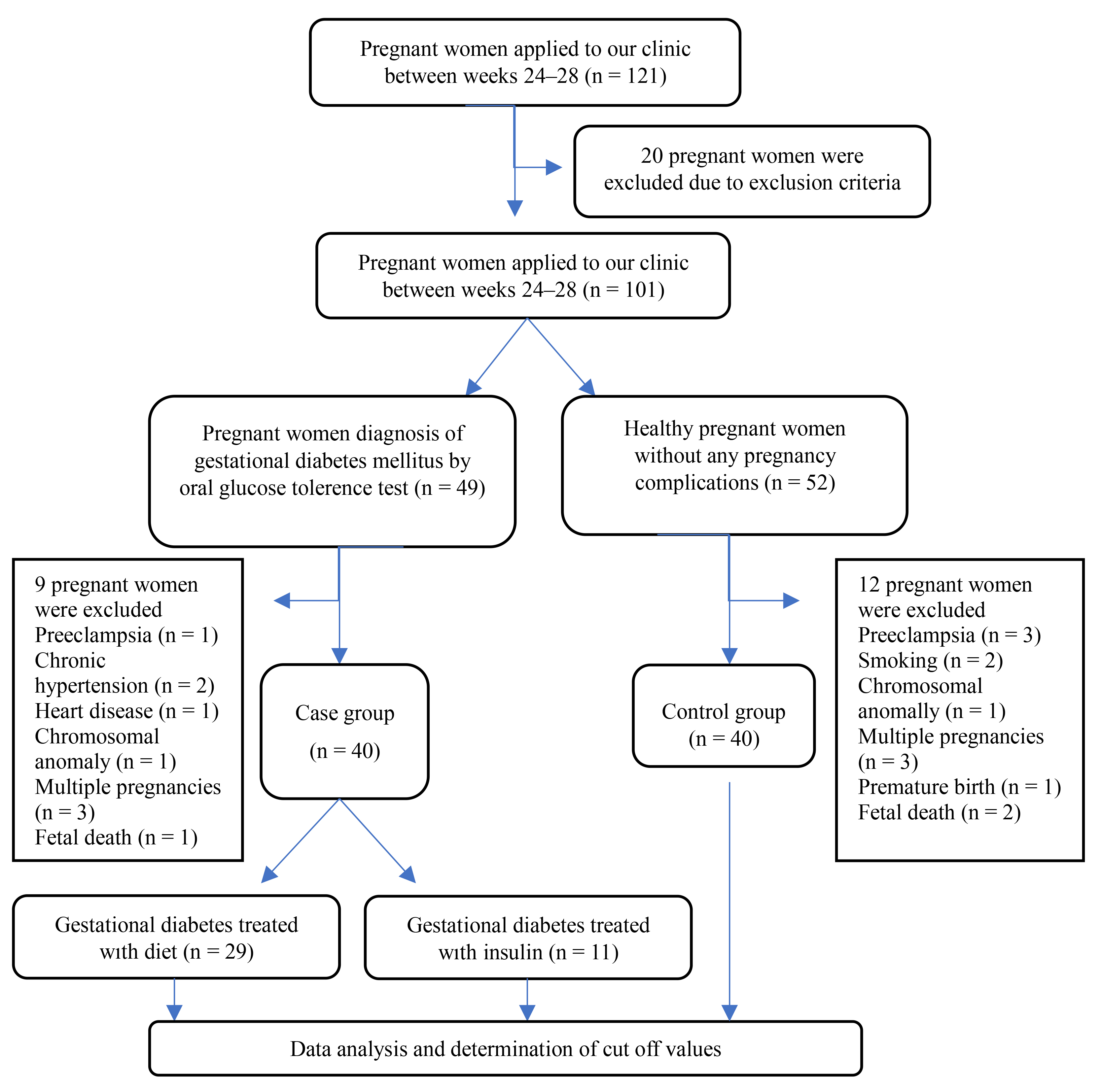
The study flow chart is shown in Fig. 1.
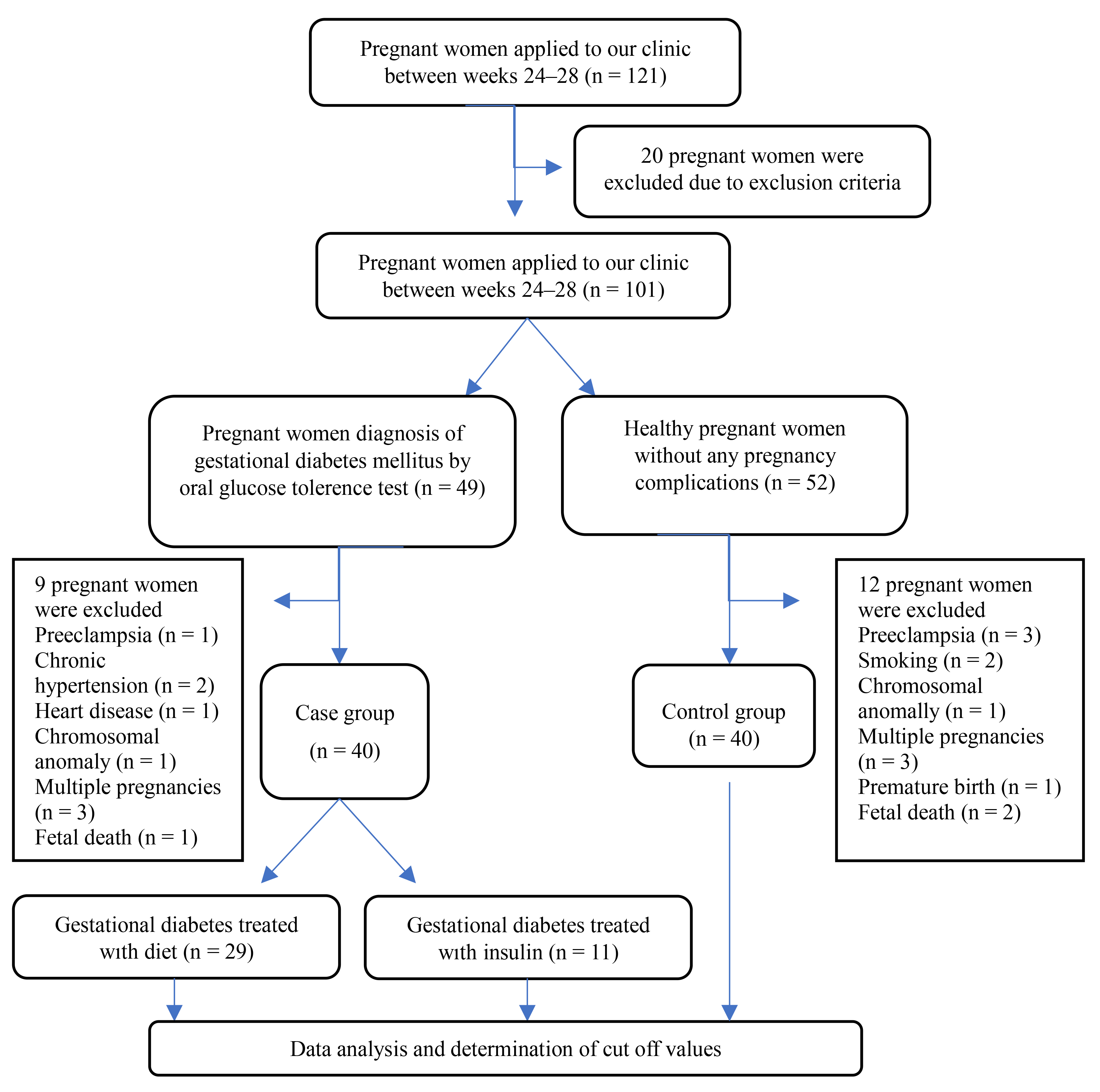 Fig. 1.
Fig. 1. Flow chart of the cohort study design.
In addition to the routine parameters evaluated at the initial admission of participants to our hospital, approximately 5 mL of blood was collected from each participant. The samples were centrifuged at 4000 rpm for 10 minutes to separate the serum, which was then stored at –80 °C until the day of the analysis.
All stored serum samples were thawed simultaneously in preparation for the analysis. Serum IRAP levels were measured using enzyme-linked immunosorbent assay (ELISA) kits from MyBioSource (San Diego, CA, USA; Catalog no: MBS260922). The Biotek ELX800 (BioTek Instruments, Winooski, VT, USA) ELISA reader was used to calculate the results.
The intra-assay coefficient of variation (CV) for the kit was
Statistical analysis was performed using SPSS 24.0 software (IBM Corp., Chicago, IL, USA). The Kolmogorov-Smirnov test was applied to assess the normality of data distribution. Variables with normal distribution were reported as the mean
For variables with parametric distribution, including gestational age, HbA1c, and IRAP, comparisons between groups with and without GDM were conducted using the independent two-sample t-test. Non-parametric variables such as age, BMI, fasting glucose, insulin, and HOMA-IR were compared between groups using the Mann-Whitney U test.
The Kruskal-Wallis test was used to compare multiple groups, followed by Bonferroni post hoc correction. Spearman’s correlation coefficient was utilized to assess correlations. Additionally, a receiver operating characteristic (ROC) curve was generated to determine the optimal cut-off point of IRAP for diagnosing GDM, with the cut-off value identified using the Youden index formula. Statistical significance was defined as a p-value
Of the 80 pregnant women in this study, 40 constituted the study group diagnosed with GDM, and 40 constituted the healthy control group. The demographic, clinical, and biochemical characteristics of both groups are shown in Table 1. No statistically significant differences were observed between the two groups in terms of age, BMI, and gestational age at which blood samples were taken (p
| GDM (n = 40), median (IQR)/mean | Control group (n = 40), median (IQR)/mean | p-value | Z, t | |
| Age (years) | 31.00 (9.00) | 31.00 (8.00) | 0.813 | 0.236 |
| BMI (kg/m2) | 28.80 (3.42) | 28.90 (5.45) | 0.791 | 0.265 |
| Gestational age at sampling (weeks) | 26.40 | 26.28 | 0.264 | 1.127 |
| Fasting glucose (mg/dL) | 97.00 (91.00) | 82.00 (7.00) | 0.001 | 6.361 |
| Insulin (µU/mL) | 7.90 (1.48) | 6.75 (1.00) | 0.001 | 5.107 |
| HOMA-IR | 1.87 (0.48) | 1.38 (0.30) | 0.001 | 7.001 |
| HbA1c | 5.60 | 5.20 | 0.001 | 3.392 |
| IRAP (ng/mL) | 0.73 | 0.92 | 0.001 | 7.607 |
Data are summarized as mean
GDM patients were divided into two subgroups according to the type of routine treatment: diet-modified patients (n = 29) and insulin-treated patients (n = 11). These subgroups were then compared to the control group. Kruskal-Wallis test revealed that IRAP levels were significantly different between the control group, diet-regulated GDM, and insulin-treated GDM (p
| Variable | Diet-modified, GDM (n = 29), median (IQR) | Insulin-treated, GDM (n = 11), median (IQR) | Controls, (n = 40), median (IQR) | p-value | H (df = 2) |
| IRAP (ng/mL) | 0.71 (0.14) | 0.80 (0.21) | 0.92 (0.09) | 37.661 | |
| Fasting glucose (mg/dL) | 95.00 (12.00) | 109.00 (27.00) | 82.00 (7.00) | 41.048 | |
| Insulin (µU/mL) | 7.80 (1.40) | 8.00 (1.60) | 6.75 (1.00) | 26.729 | |
| HOMA-IR | 1.82 (0.39) | 2.03 (0.66) | 1.38 (0.30) | 49.530 | |
| HbA1c | 5.30 (0.70) | 6.00 (1.10) | 5.20 (0.67) | 0.001ac | 14.452 |
HOMA-IR, homeostasis model assessment of insulin resistance; HbA1c, hemoglobin A1c; IRAP, insulin-regulated aminopeptidase; H, Hypothesis Test Statistic; df, degrees of freedom. Pairwise comparisons with Bonferroni correction consider p
When the correlation of the values with statistically significant differences between the groups with IRAP was examined, it was found that the IRAP level showed a significant negative correlation with fasting glucose, insulin, HOMA-IR levels and HbA1c (r = –0.541, p = 0.001; r = –0.447, p = 0.001; r = –0.584, p = 0.001; r = –0.361, p = 0.001) (Table 3).
| Variables | IRAP | |
| r | p-value | |
| Fasting glucose (mg/dL) | –0.541 | 0.001 |
| Insulin (µU/mL) | –0.447 | 0.001 |
| HOMA-IR | –0.584 | 0.001 |
| HbA1c | –0.361 | 0.001 |
IRAP, insulin-regulated aminopeptidase; HOMA-IR, homeostasis model assessment of insulin resistance; HbA1c, hemoglobin A1c.
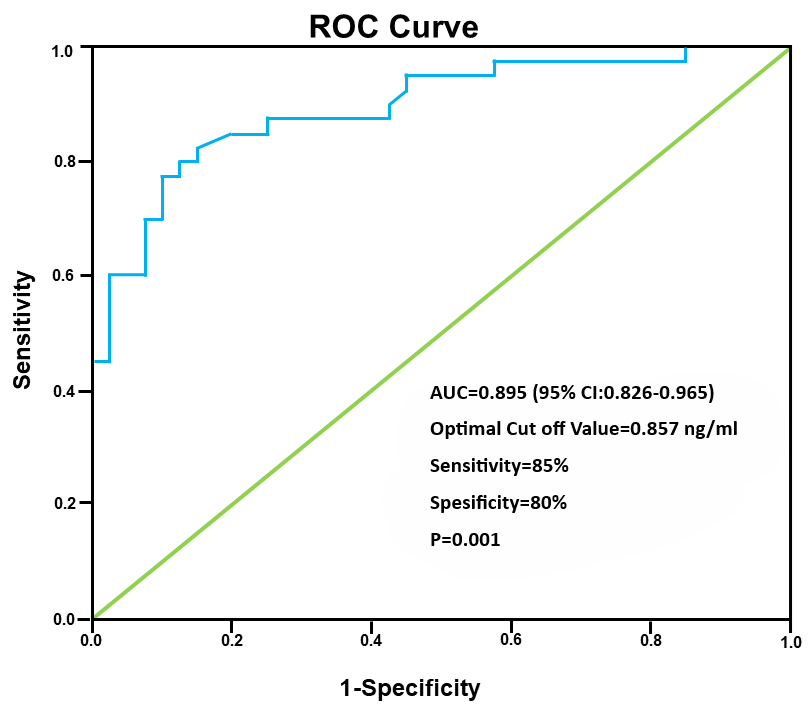
ROC curve for IRAP concentrations in the GDM group calculated the area under the curve as 0.895 (95% confidence interval (CI): 0.826–0.965) (Fig. 2). The optimum threshold value was 0.857 ng/mL, giving a sensitivity of 85% and specificity of 80% for the detection of GDM (p = 0.001).
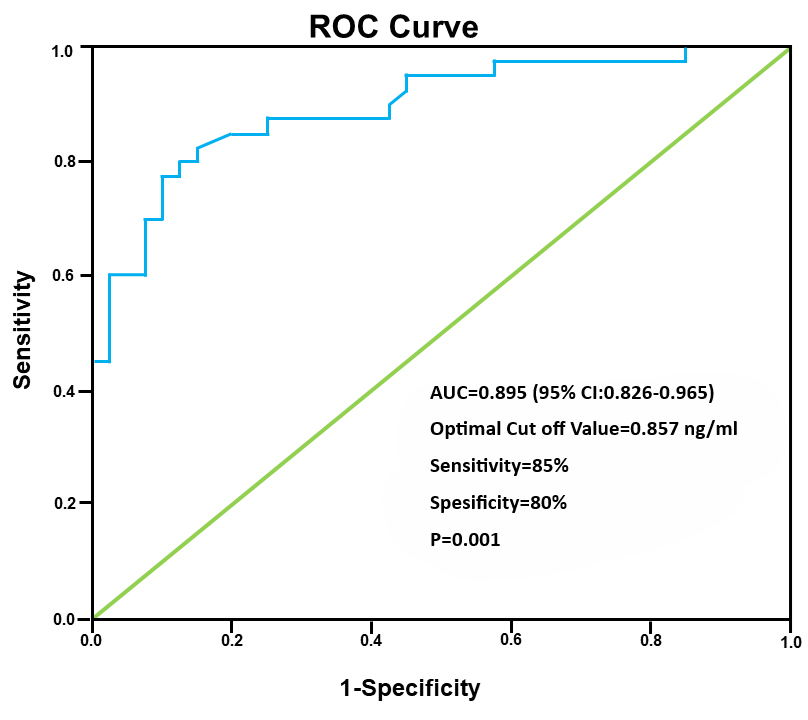 Fig. 2.
Fig. 2. ROC curve for the serum IRAP level in the detection of GDM. ROC, receiver operating characteristic; IRAP, insulin-regulated aminopeptidase; GDM, gestational diabetes mellitus; AUC, area under the curve; 95% CI, 95% confidence interval.
This study found that serum IRAP levels were significantly lower in women with GDM compared to the control group. A notable finding was the negative correlation between serum IRAP levels and markers of insulin resistance, such as insulin, HbA1c, and HOMA-IR levels. These results suggest the serum IRAP level could be a promising biomarker for GDM in future clinical applications.
Under insulin stimulation, IRAP translocates to the cell surface along with GLUT4, where it plays a critical role in facilitating glucose uptake into cells. IRAP is a primary cargo protein in GLUT4 storage vesicles (GSVs) found in adipocytes and myocytes. It is integral to the glucose transport process and relocates to the plasma membrane in response to insulin [17, 18]. This function is important in meeting the increased insulin requirements during pregnancy. When the IRAP level decreases, the glucose uptake process may be disrupted, leading to insulin resistance. The interaction between IRAP and GLUT4 enhances the effect of insulin on glucose uptake, as demonstrated by study showing the role of IRAP in the translocation of GLUT4 to the cell surface when stimulated by insulin [19].
In individuals with type 2 diabetes, disruption of IRAP translocation impairs the glucose transport system, thereby contributing to insulin resistance [20]. Specifically, as cells become resistant to insulin, the movement of GLUT4 and IRAP to the plasma membrane is reduced, thus decreasing IRAP levels in the bloodstream. This phenomenon is observed in both type 2 diabetes and GDM [21]. Furthermore, it aligns with findings from our study, where the serum IRAP level was inversely correlated with insulin resistance markers, suggesting that IRAP is an indicator of insulin resistance [13].
Mostafa et al. [13] added insulin to serum samples from both euglycemic individuals and diabetic patients in order to assess the IRAP response. Their findings showed the IRAP level increased in euglycemic patients post-injection, whereas no such increase was observed in diabetic patients. Individuals with type 2 diabetes had notably lower IRAP levels compared to healthy individuals, suggesting IRAP could potentially serve as a direct marker for detecting insulin resistance in diabetic populations.
To date, only two studies have investigated serum IRAP levels in gestational diabetes. In the first study, Tian et al. [22] compared IRAP levels between hypertensive pregnant women, women with GDM, cases of fetal death, and healthy controls. These authors found significantly lower IRAP levels in the GDM and hypertensive groups compared to the controls, with the lowest level observed in the fetal death group. Later, Guleroglu et al. [23] measured the IRAP level in 19 GDM patients and 90 patients with normal glucose tolerance at 20–22 weeks of gestation. They concluded that serum IRAP levels at this stage of pregnancy were not predictive for GDM.
The present study differed from previous research in terms of its sample size. We observed a negative correlation between the IRAP level and various indicators of insulin resistance, including the levels of insulin, HOMA-IR, and HbA1c. By performing ROC curve analysis, we calculated an IRAP threshold of
These findings support the clinical relevance of decreased IRAP levels in managing GDM. The monitoring of IRAP levels in clinical practice could guide patient follow-up and treatment planning, potentially improving the outcome from interventions such as dietary modification. The integration of IRAP measurements may contribute to a more personalized approach, thereby enhancing the efficacy of treatment.
IRAP inhibitors have shown potential for ameliorating insulin resistance by reducing oxidative stress and inflammation, suggesting their therapeutic application in GDM management [22]. Monitoring of the serum IRAP biomarker could provide more effective glycemic control in GDM management by helping to predict insulin resistance [11].
In the present study, no significant difference in the serum IRAP level was observed between dietary-modified and insulin-treated GDM patients. The lack of notable improvement in the IRAP level following insulin treatment may be due to the relatively small number of insulin-treated patients, and the need for a longer duration to observe significant changes.
Further exploration of the molecular mechanism of IRAP in GDM could deepen our understanding of insulin resistance at the cellular level and facilitate more effective interventions [24]. The study of potential variation in IRAP levels across different ethnic groups and the resulting clinical implications could also provide additional insights into population-specific responses and outcomes. The development of new therapeutic strategies should focus on modulating IRAP levels to address possible ethnic differences.
When evaluating IRAP levels in GDM patients, factors such as BMI, age, and comorbid conditions should also be considered, as they could influence IRAP expression and insulin sensitivity. Higher BMI is particularly relevant, as it is associated with increased insulin resistance which potentially leads to lower IRAP levels. Similarly, advanced maternal age and the presence of comorbidities may also affect IRAP levels, making it essential to consider these factors when interpreting IRAP as a biomarker for GDM [25].
The use of IRAP as a biomarker in the diagnosis and management of GDM, particularly in cases with poor glycemic control, could support the development of individualized treatment strategies aimed at preserving maternal and fetal health. Further validation of IRAP in larger and more diverse patient groups would strengthen its potential as a routine screening tool in clinical practice.
Limitations of this study include the relatively small sample size and the limited number of cases within GDM subgroups. Additionally, serum IRAP levels were not measured in the postpartum period, when insulin resistance typically resolves. We measured IRAP levels using ELISA, a widely accepted and sensitive method for serum protein quantification. However, there are some limitations when relying solely on ELISA. Alternative techniques such as immunohistochemistry could provide complementary insights by allowing visualization of IRAP localization within tissues, albeit with a more qualitative focus. Mass spectrometry offers precise quantitative analysis and can detect IRAP at the peptide level, although this technique requires specialized equipment and expertise. Flow cytometry can assess IRAP expression on the cell surface, particularly in live cells, which may better reflect dynamic IRAP activity. Each of these methods has unique advantages that could enrich our understanding of IRAP beyond what ELISA alone can provide.
Despite these limitations, our findings offer a foundation for further research into the role of IRAP in GDM. In particular, they could help to guide more in-depth studies on IRAP metabolism in GDM patients. Longitudinal studies that examine the association between decreased IRAP levels and the subsequent risk of developing type 2 diabetes could provide valuable insights into the clinical relevance of IRAP.
In conclusion, the serum IRAP level is significantly lower in pregnant women with GDM compared to healthy pregnant women. Strong negative correlations occur between the IRAP level and the levels of HOMA-IR, insulin and HbA1c. These findings suggest that serum IRAP may serve as a novel biomarker for GDM, offering potential clinical utility for early diagnosis and monitoring. Reduced IRAP levels could provide an effective means of identifying and managing GDM, supporting the application of IRAP as a valuable marker in clinical settings of GDM.
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request. The data are not publicly available due to privacy or ethical restrictions.
OK: conceptualization, data curation, funding acquisition, investigation, validation, writing — original draft, writing — review & editing. KG: conceptualization, data curation, investigation, writing — review & editing. SIG: conceptualization, methodology, supervision, writing — review & editing. AT: conceptualization, methodology, project administration, writing — review & editing. MSB: conceptualization, funding acquisition, investigation, project administration, writing — original draft, writing — review & editing. EK: conceptualization, methodology, project administration, supervision, writing — review & editing. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Local Ethics Committee of Sakarya University Faculty of Medicine (date: 21/04/2021, number: 89). Informed consent forms were signed by all patients who participated in the study.
We would like to express our sincere gratitude to everyone who contributed to this study. We are also grateful to all the peer reviewers for their insightful comments and suggestions, which greatly helped improve the quality of this manuscript.
This research received no external funding.
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.