1 Department of Obstetrics and Gynecology, Prof. Dr. Cemil Taşcıoğlu City Hospital, 34384 Istanbul, Turkey
2 Department of Obstetrics and Gynecology, Istinye University, 34250 Istanbul, Turkey
3 Department of Physiology, Demiroğlu Bilim University Medical School, 34256 Istanbul, Turkey
Abstract
Hyperglycemia is associated with ovarian dysfunction. Advanced glycation end products (AGE) may affect ovarian function by binding to particular AGE receptors (RAGE). Hematopoiesis and immunological conditioning are both controlled by the Janus kinase/signal transducer and activator of transcription (JAK/STAT) signaling pathway. Many JAK-STAT signaling inhibitors, including ruxolitinib, have been approved to treat inflammatory disorders. We aimed to examine the potential protective effect of ruxolitinib, on ovarian dysfunction by comparing biochemical, pro-inflammatory, and histological abnormalities in a diabetic rat model.
24 female Wistar albino rats were included in the study. Diabetes was induced by streptozotocin (STZ) in 16 rats. Group 1: control (no diabetes mellitus, n = 8), Group 2 (diabetic = 8, 1 mL/kg/day saline, 4 weeks), and Group 3 (diabetic, n = 8, 2 mg/kg/day ruxolitinib, 4 weeks). The animals were euthanized, and bilateral hysterectomy and ovariectomy were performed for histopathological examination. The levels of signal transducer and activator of transcription 3 (STAT3) in tissue supernatants were measured.
Endometrial gland, ovarian stromal, and ovarian follicle degeneration scores were higher in group 2 compared with group 3 at p < 0.001, whereas ovarian STAT3 level was significantly higher in group 2 compared with group 3 at p < 0.001.
Ruxolitinib can be a promising candidate for providing endometrial and ovarian structure continuity by JAK-STAT inhibition in diabetes.
Keywords
- diabetes
- endometrium
- experimental
- ovary
- ruxolitinib
The prevalence of diabetes mellitus (DM) is projected to increase to 700 million individuals by 2045, compared to 465 million in 2019 [1]. Diabetes impacts various physiological systems, including the reproductive system [2]. Ovarian dysfunction has been linked to insulin resistance and hyperglycemia [2, 3]. Insulin imitates the actions of follicular stimulating hormone (FSH) and luteinizing hormone (LH) by binding to insulin receptors and insulin-like growth factor 1 (IGF-1) receptors [4]. Insulin promotes the secretion of androgen, estrogen, and progesterone by granulosa and theca cells [5, 6]. Research has indicated that administering insulin to prepubescent females with type 1 diabetes can enhance the growth and multiplication of granulosa cells. This is evidenced by the presence of increased levels of anti-mullerian hormone. When amino groups in proteins and lipids combine with reducing sugars (advanced glycation end products, AGEs) without the involvement of enzymes, they create cross-linking reactive compounds called advanced glycation end products. They can induce damage to tissues by attaching to special receptors called AGE receptors (RAGE) on several types of cells, including endothelial cells, mononuclear phagocytes, neurons, and smooth muscle cells [7]. Individuals who have hyperglycemia/diabetes, as well as conditions associated with higher levels of oxidative stress and inflammation, such as rheumatoid arthritis, renal failure, and cigarette smoking, have elevated levels of AGEs [7, 8]. According to a study, both AGE and RAGE were found to negatively affect ovarian function and the development of follicles [9]. The Janus kinase/signal transducer and activator of transcription (JAK/STAT) signaling system is an essential part of cellular communication. The JAK/STAT signaling system is linked to more than 50 cytokines and growth factors, which encompass hormones, interferons (IFN), interleukins (ILs), and colony-stimulating factors [10]. The JAK/STAT pathway is involved in other subsequent biological processes, such as immunological conditioning and hematopoiesis [11]. The JAK/STAT pathway has been associated with multiple metabolic disorders [12]. Diabetic human kidney tissues were found to exhibit an increased expression of JAK-STAT family members [13]. Ruxolitinib, tofacitinib, and baricitinib, which are inhibitors of JAK-STAT signaling, have received clinical approval for the treatment of various autoimmune and inflammatory diseases [14]. The objective of our study was to investigate the potential of ruxolitinib, a JAK1-JAK2 inhibitor, to provide protection against diabetes. We accomplished this by analyzing biochemical, proinflammatory, and histological changes in a rat model of diabetes generated by streptozotocin.
The experiments performed in this study have been carried out according to the rules in the Guide for the Care and Use of Laboratory Animals adopted by National Institutes of Health (U.S.A). The Animal Ethics Committee approved the present study (Demiroglu Science University, Ethical number: 26220121). The rats used in the experiment were obtained from the Experimental Animal Laboratory of Bilgi University. Informed consent was not applicable (N/A).
In this study, 24 female Wistar albino rats at weighing 150–200 g and 10–12
weeks old were used. Rats were fed ad libitum and housed in pairs in steel cages
having a temperature-controlled environment (22
Hematoxylin and Eosine were used to stain formalin-fixed uterine and ovarian sections. Slides stained with hematoxylin for 1 minute and washed with 4–5 changes of Tap water or until blue stops coming off slides. Then slides were counterstained in Alcoholic-Eosin for 1 minute. Then we dehydrated through 3 changes of 95% Ethanol (EtOH) and 2 changes of 100% EtOH 1 minute each. Later they were cleared in 3 changes of Xylene 1 minute each.
All sections were captured using an Olympus BX43F microscope (Serial Number: 7E48677, Tokyo, Japan) and an Olympus LC30 digital camera (Serial Number: 65000668, Hamburg, Germany). Endometrial gland degeneration and stromal fibrosis were graded on a scale of 0 to 3, with 0 representing no pathologic findings and 1, 2, and 3 representing pathologic findings in less than 33%, 33% to 66%, and more than 66% of the uterine section, respectively [18]. Follicular degeneration, stromal degeneration, and stromal fibrosis were rated from 0 to 3, with 0 representing no pathologic findings and 1, 2, and 3 representing pathologic abnormalities in less than 33%, 33% to 66%, and more than 66% of the ovarian section, respectively [19].
Plasma transforming growth factor beta (TGF-
In accordance with the manufacturer’s instructions, each animal’s samples were measured in triplicate. Using a microplate reader, the absorbances were analyzed (MultiscanGo, Thermo Fisher Scientific Laboratory Equipment, Newington, NH, USA).
SPSS version 15.0 (IBM-SPSS Statistics, Chicago, IL, USA) for Windows was used to analyze the data. Since the number of groups was less than 50, the normality distribution was checked with the Kolmogorov-Smirnov test. The parametric variable groups were compared using the student’s t-test and analysis of variance (ANOVA). The Mann-Whitney U test was used to compare nonparametric variable groupings. The results were presented as the mean standard error of the mean (SEM). A p-value of 0.05 was considered statistically significant.
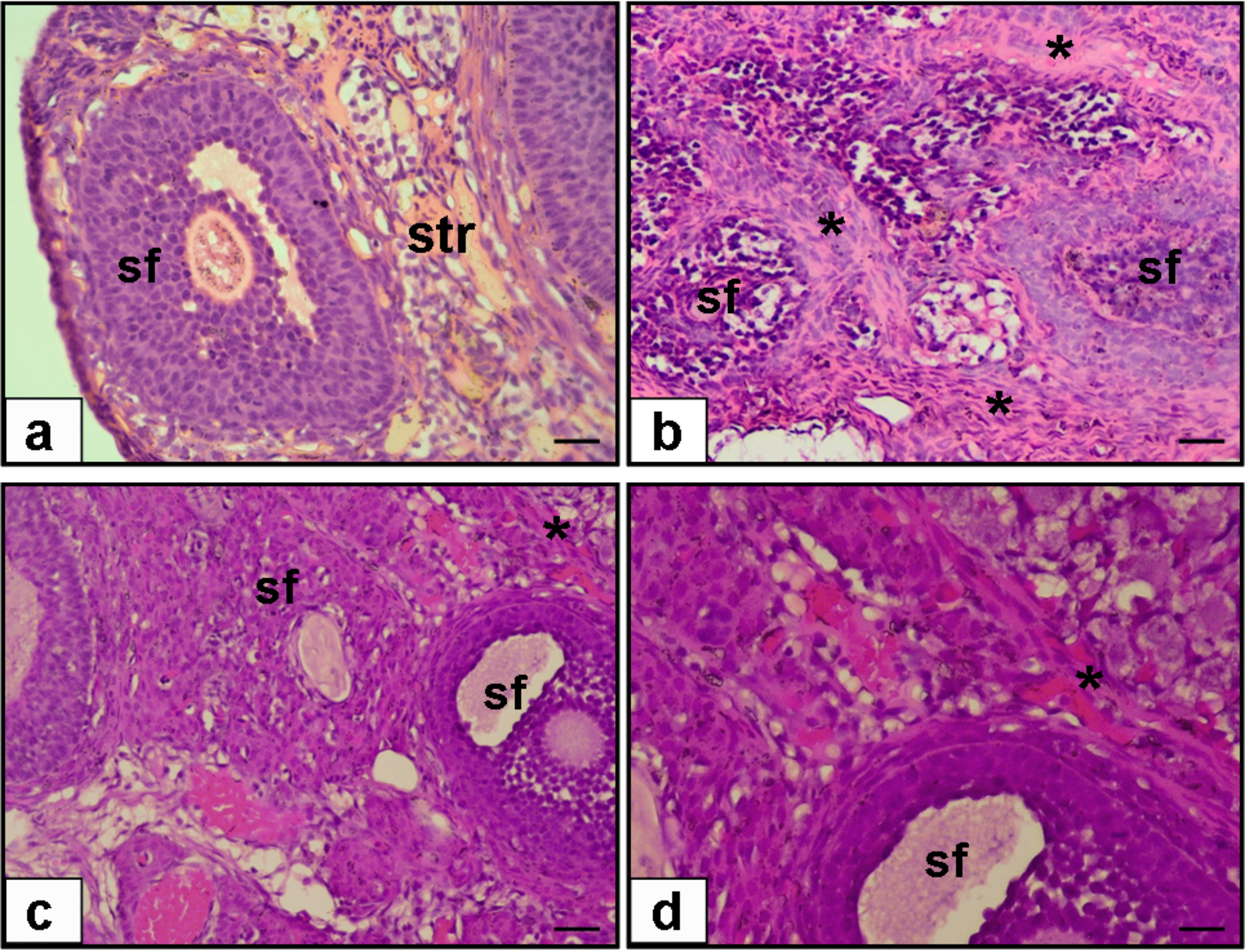
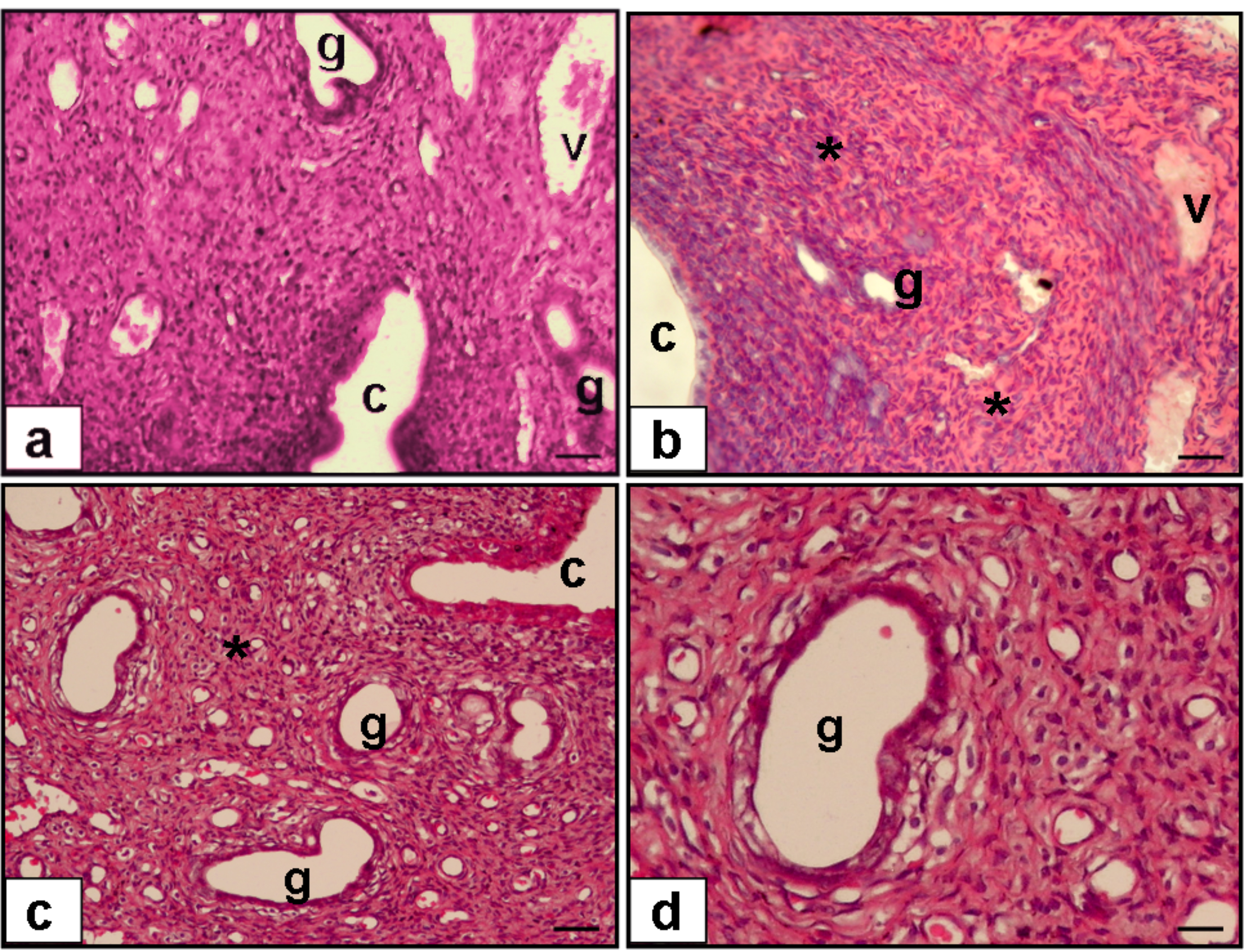
Fig. 1 outlines the ovarian histopathology of the groups that were looked into. The experimental condition of saline treatment (Group 2) resulted in ovarian sections with fibrotic ovarian stroma, whereas ruxolitinib treatment in diabetic rats (Group 3) resulted in decreased ovarian stromal fibrosis. Fig. 2 outlines the endometrial histopathology of the same groups. While uterine sections of saline treatment in diabetic rats showed fibrotic uterine stroma and decreased endometrial gland, ruxolitinib treatment in diabetic rats resulted in decreased uterine fibrosis and increased endometrial gland.
 Fig. 1.
Fig. 1.
Hematoxylin & Eosine (H & E) staining of sections
from rat ovary (a–c, 20
 Fig. 2.
Fig. 2.
Hematoxylen & Eosine (H & E) staining of sections
from rat uterus (a–c, 20
Endometrial gland degeneration score was higher in Group 2 than in both Group 3
(p
| Group 1 (n = 8) | Group 2 (n = 8) | Group 3 (n = 8) | |
| Endometrial gland degeneration score | 0.22 |
2.11 |
0.8 |
| Endometrial stromal fibrosis score | 0.3 |
2.4 |
0.7 |
| Ovarian stromal degeneration score | 0.3 |
2.3 |
1.1 |
| Ovarian follicle degeneration score | 0.4 |
2.2 |
0.9 |
| Ovarian stromal fibrosis score | 0.11 |
1.7 |
1.0 |
Results were presented as mean
The plasma glucose levels before and after ruxolitinib were higher in both study
groups (Groups 2 and 3) than in the control group (both p
| Group 1 (n = 8) | Group 2 (n = 8) | Group 3 (n = 8) | |
| Glucose before treatment, mg/dL | 112.5 |
475.6 |
469.7 |
| Glucose after treatment, mg/dL | 101.8 |
432.1 |
451.6 |
| Plasma TGF- |
5.3 |
14.2 |
9.1 |
| Plasma AMH level, ng/mL | 2.5 |
0.9 |
1.5 |
| Over STAT3 level ng/mg | 0.65 |
1.13 |
0.81 |
Results were presented as mean
In this recent study, we found that the ovarian and endometrial tissues in a rodent model of diabetes were effectively protected by the administration of a JAK-STAT inhibitor called ruxolitinib, regardless of the blood glucose levels.
Insulin resistance and hyperglycemia can lead to ovarian dysfunction in individuals with diabetes [2, 3]. The granulosa, theca, and stroma of the ovary contain insulin receptors. Insulin, FSH, and LH bind to these receptors, as well as IGF-1 receptors, thereby mimicking ovarian stimulation and increasing the production of androgens, estrogens, and progesterone [5]. In women with diabetes, the levels of AHM increase, suggesting that insulin has an effect on granulosa cells in these individuals.
The action of AGEs and their receptor (RAGE) has been shown to significantly reduce ovarian function and reserves in the context of hyperglycemia [20]. The accumulation of AGE in the ovaries was hypothesized to cause oxidative stress and injury to the structure of the blood vessels by interacting with RAGE [21]. Research has shown that the accumulation of age-related changes may affect the conditions surrounding ovarian follicles [22]. Similar to ovarian follicles, the endometrial microenvironment is significantly impacted by AGEs and RAGE [23]. Diverse responses to modifications in the endometrium’s environment, including migration, proliferation, and inflammation, are demonstrated by epithelial, endothelial, and immune cells.
A series of signaling events through RAGE is initiated by the long-lasting elevated levels of glucose in the bloodstream that diabetes causes. The combination of AGEs and RAGEs activates a variety of downstream effectors, including p38, stress-activated protein kinase/c-Jun N-terminal kinase (SAPK/JNK), Ras-mediated extracellular signal-regulated kinase (ERK1/2), and JAK/STAT [24].
JAKs, STATs, and ligand-receptor subunit complexes comprise the JAK/STAT signaling pathway. The STAT family comprises seven members, while the JAK family comprises four. Non-receptor tyrosine protein kinases are included in the JAK family. Cytokines activate JAK tyrosine kinases, which subsequently transmit regulatory signals, upon interaction with their receptors [25, 26].
STAT proteins are transcription factors that are inactive and are situated in the cytoplasm. The signaling pathways of cytokines and growth factors are significantly influenced by STAT proteins. These effects are triggered by the phosphorylation of Janus kinase (JAK) [27] and encompass a wide range of gene expression targets. STAT molecules are essential for both adaptive and inflammatory immune responses. The activation of STAT3 is specifically induced by IL-10, resulting in a reduction in inflammatory reactions. As a result, the tolerance of antigen-specific T cells is improved by the enhancement of STAT3 [28]. JAK-STAT is an intracellular signaling mechanism that is unique to cytokines. This system is responsible for the regulation of critical biological processes, including hematopoiesis, immunological modulation, cell proliferation, differentiation, and cell death [26]. Receptor dimerization is facilitated by cytokines through the activation of JAK. The phosphorylation of STAT is a critical function of JAKs. STATs are activated and their dimerization is induced by phosphorylation. STATs are transported to the nucleus after dimerization, where they activate genes associated with cytokines [29].
JAK/STAT inhibitors are employed to treat a variety of conditions, including rheumatoid arthritis and systemic lupus erythematosus (SLE). The JAK/STAT pathway is activated to a greater extent in individuals with diabetes due to the elevated levels of proinflammatory cytokines. STAT3 proteins that have been activated are involved in the stimulation of cytokine production in a variety of malignancies. Ruxolitinib, a medication that inhibits the activity of JAK1 and JAK2, can be administered orally or topically. Ruxolitinib has been utilized to treat malignant tumors, acute graft-versus-host disease (aGVHD), multiple myeloma (MF), polycythemia vera, alopecia areata, vitiligo, essential thrombocythemia, and coronavirus disease 2019 (COVID-19) [30, 31].
Brosius and He [27] discovered that the renal tissues of individuals with diabetic kidney disease (DKD) exhibit elevated expression of several members of the JAK-STAT family. The ligand induces dimerization as a result of binding to JAK membrane receptors, which in turn recruits JAK protein partners to intracellular receptor sites. This, in turn, induces receptor activation through autophosphorylation. As a result, active JAKs phosphorylate STAT proteins, which subsequently translocate to the nucleus and stimulate the transcription of target genes that are associated with a variety of cytokines, adhesion molecules, growth factors, extracellular matrix proteins, pro-oxidant enzymes, and scavenger receptors [32]. This pathway results in lipotoxicity, fibrosis, oxidative stress, and inflammation. After 8 weeks of treatment, the JAK inhibitor ruxolitinib substantially improved kidney function and decreased proinflammatory indicators of DKD in mice with diabetes caused by STZ in a preclinical trial [33].
Ruxolitinib’s therapeutic benefits are thought to be dose-dependent, with higher concentrations producing more significant decreases in inflammatory markers and greater improvements in tissue function. However, it is critical to take caution while choosing the optimal therapeutic window in order to strike a balance between efficacy and probable side effects [34].
Diabetic rats that were not administered ruxolitinib exhibited elevated levels of STAT3 in comparison to diabetic rats that received ruxolitinib treatment and control rats in the current study. Our investigation demonstrated that the adverse effects of diabetes on the ovary and endometrium were alleviated by the administration of ruxolitinib, a JAK-STAT inhibitor. We assert that these therapeutic benefits are contingent upon the STAT3 pathway.
This investigation underscores the numerous detrimental consequences of diabetes mellitus on the endometrium and follicles. Ruxolitinib is a viable treatment option for the preservation of the endometrial cycle, endometrial structure, ovarian follicular expansion, oocyte maturation, and normal ovarian function. This is due to its anti-inflammatory properties and functioning as a JAK-STAT inhibitor. Additional clinical and experimental research investigations are necessary to verify our preliminary findings.
The significance of the JAK-STAT pathway in mediating the adverse effects of diabetes on the reproductive system has been demonstrated by this study. Ruxolitinib, which inhibits this pathway, has shown potential in terms of reducing these consequences and enhancing reproductive health in diabetes patients. Additional research is required to optimize dosage regimens and gain a comprehensive understanding of the long-term implications of JAK-STAT inhibition on reproductive outcomes in diabetes.
All data points generated or analyzed during this study are included in this article, and no further underlying data is necessary to reproduce the results.
SÖ designed the research study. FŞ, OE, SÖ performed the research and analyzed the data. All authors are credited with the editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
The experiments performed in this study have been carried out according to the rules in the Guide for the Care and Use of Laboratory Animals adopted by National Institutes of Health (U.S.A). The present study was approved by the Animal Ethics Committee’s consent (Demiroglu Science University, 03.06.2022 Ethical number: 26220121). The rats used in the experiment were obtained from the Experimental Animal Laboratory of Bilgi University. Informed consent was N/A. The study was conducted in accordance with the Principles of the Declaration of Helsinki.
We would like to express our gratitude to all those who helped us while writing this manuscript. Thanks to all the peer reviewers for their opinions and suggestions.
This research received no external funding.
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.