1 Department of Maternity, Meizhou People’s Hospital, 514031 Meizhou, Guangdong, China
Abstract
Herein, we aimed to investigate the factors influencing abnormal gestational weight gain (GWG) during pregnancy and to develop a risk model for predicting deviations in GWG among pregnant women.
A retrospective analysis was conducted on the clinical data of 1200 pregnant women from May 2018 to May 2020, according to the standards recommended by the American Academy of Medicine in 2009. The pregnant women were divided into three groups: 186 cases in the weight gain below the recommended GWG (low GWG) group, 433 cases in the normal GWG group, and 581 cases in the weight gain above the recommended GWG (high GWG) group. Additionally, clinical data of 515 pregnant women who established perinatal records at our hospital and underwent regular antenatal examinations and deliveries from May 2020 to May 2022 were collected to serve as the validation group for external verification of the model. Single-factor and multi-factor logistic regression analyses were conducted to identify the factors influencing weight gain below or above the recommended GWG in pregnant women and to construct a risk model for predicting deviations in weight gain. The calibration curves and receiver operating characteristic (ROC) curves were plotted, and the area under the curve (AUC) was calculated to evaluate the performance of the risk prediction model.
Being underweight before pregnancy was identified as an independent risk factor for low GWG (p < 0.05), while primiparity and pregnancy occurring in spring and summer were found to be protective factors (p < 0.05). Obesity before pregnancy, a history of fetal macrosomia, and pregnancy occurring in spring and summer were identified as independent risk factors for high GWG (p < 0.05), whereas regular exercise during pregnancy was a protective factor (p < 0.05). The slope of the calibration curve for predicting weight gain deviations closely approached 1, with Hosmer-Lemeshow goodness-of-fit test values of Chi-square (χ2) = 8.388, 7.295, p = 0.397, 0.505; and AUCs of 0.753 and 0.761, respectively. External validation results indicated that the predicted probabilities closely matched the actual probabilities, demonstrating good consistency, with AUCs of 0.747 and 0.877, respectively.
The risk prediction model constructed in this study, incorporating pre-pregnancy body mass index (BMI) and the season of pregnancy, plays a crucial role in individually predicting weight gain deviations during pregnancy. This model is instrumental for the personalized management of body mass in pregnant women.
Keywords
- pregnancy
- body weight
- gestational weight gain
- influencing factors
- nomogram
With the improvement of living standards, abnormal gestational weight gain (GWG) among pregnant women has emerged as a significant health concern within the field of maternal and child health. Research has shown that weight gain below or above the recommended GWG in pregnant women can impact pregnancy outcomes, potentially leading to increased rates of cesarean delivery and macrosomia [1]. Existing studies have confirmed the association between GWG in pregnant women and the birth weight of newborns [2]. However, beyond the relatively well-established relationship with pre-pregnancy body mass index (BMI), research on other factors influencing of abnormal GWG is still limited and often inconsistent [3, 4]. Therefore, identifying the factors influencing abnormal GWG in pregnant women is crucial for developing effective measures for gestational weight management. Current research on the factors influencing weight gain below or above the recommended GWG primarily relies on multifactorial logistic regression analysis and lacks individualized risk prediction models. The nomogram model serves as a risk prediction tool, presenting the risk of a specific outcome graphically, thereby making the results easily understandable and highly practical [5]. This study aims to explore the factors influencing abnormal GWG in pregnant women and to construct a risk prediction model, offering a foundation for individualized gestational weight management.
A retrospective analysis was conducted on the clinical data of 1200 pregnant
women who established perinatal records and underwent regular prenatal check-ups
and deliveries at our hospital from May 2018 to May 2020. This group served as
the modeling cohort to develop a risk prediction model for abnormal GWG.
Additionally, clinical data from 515 pregnant women who established perinatal
records and underwent regular prenatal check-ups and deliveries at our hospital
from May 2020 to May 2022 were collected as the validation cohort to externally
validate the model. Inclusion criteria: (1) age
Pre-pregnancy BMI was calculated by dividing weight (kg) by the square of height
(m2), resulting in BMI (kg/m2). Underweight before pregnancy was
defined as BMI
| Pre-pregnancy BMI | Low GWG | Normal GWG | High GWG |
| BMI |
12.5~18.0 kg | ||
| 18.5 kg/m2 |
11.5~16.0 kg | ||
| 24.0 kg/m2 |
7.0~11.5 kg | ||
| BMI |
5.0~9.0 kg |
BMI, body mass index; GWG, gestational weight gain.
The collected data included various parameters such as the age of the pregnant women, pre-pregnancy BMI, primiparity status, number of miscarriages, history of cesarean delivery, history of macrosomia, pregnancy season (spring/summer or autumn/winter), exposure to passive smoking during pregnancy, and engagement in regular exercise during pregnancy (defined as at least 2 hours of exercise per week).
Data processing and analysis were performed using SPSS (IBM Corp. Released 2013.
IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY, USA) and R
Statistical Software (v3.6.3; R Core Team; R Foundation for Statistical
Computing, Vienna, Austria). Quantitative data (e.g., age), which followed a
normal distribution, were presented as mean
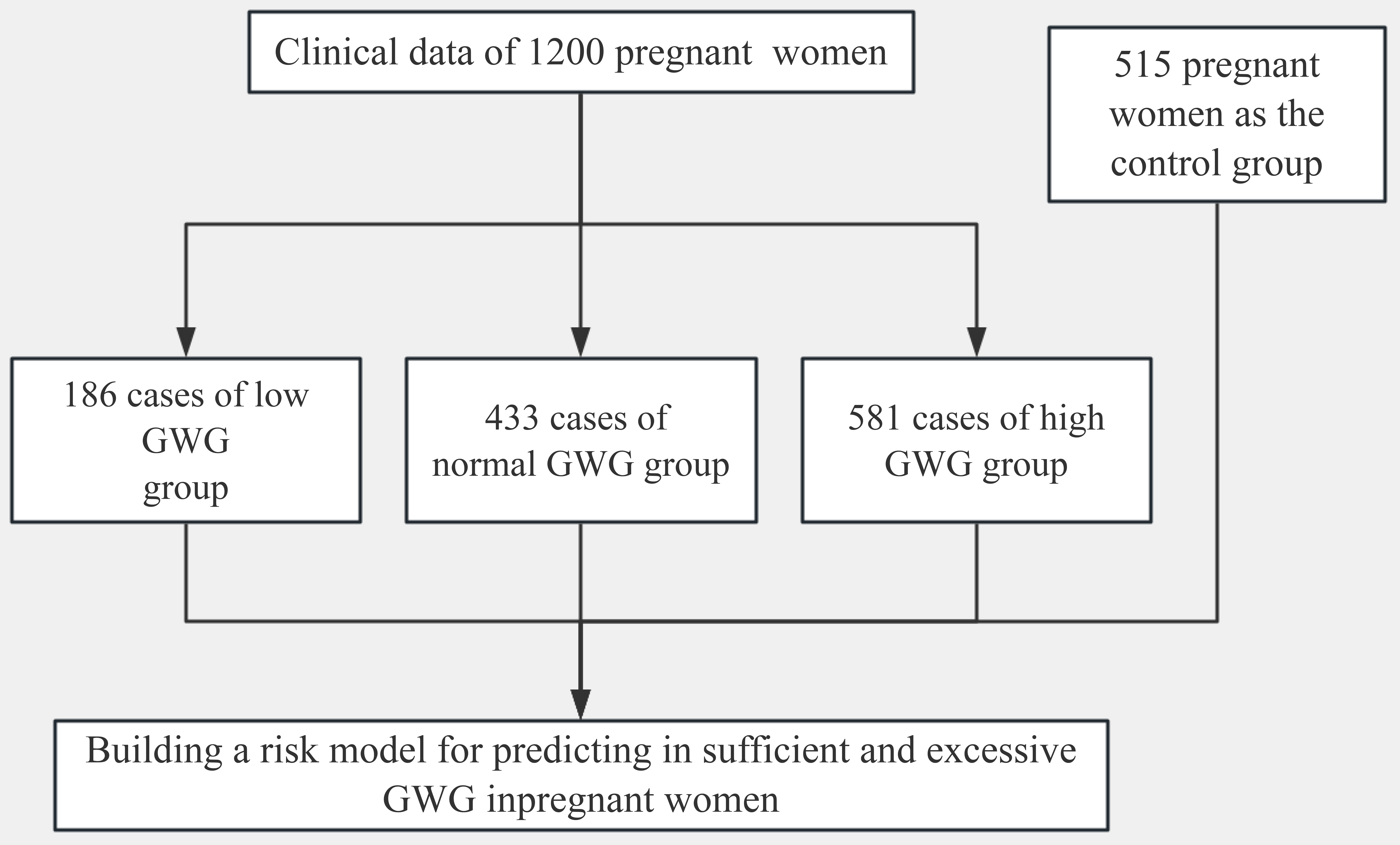
A total of 1200 pregnant women, aged 18–35 years, were included in the study,
with an average age of 27.11
 Fig. 1.
Fig. 1.
Flowchart of patients included in the study. GWG, gestational weight gain.
The GWG of pregnant women was not significantly associated with age, number of
miscarriages, history of cesarean delivery, or exposure to passive smoking during
pregnancy (p
| Parameter | Low GWG group (n = 186) | Normal GWG group (n = 433) | High GWG group (n = 581) | F/ |
p-value | |
| Age (years) | 27.63 |
27.09 |
26.54 |
2.068 | 0.127 | |
| Pre-pregnancy BMI (kg/m2) | 62.675 | 0.000 | ||||
| Thin | 26 (17.81) | 74 (50.68) | 46 (31.51) | |||
| Normal | 123 (15.69) | 290 (36.99) | 371 (47.32) | |||
| Overweight | 18 (8.91) | 44 (21.78) | 140 (69.31) | |||
| Obesity | 19 (27.94) | 25 (36.76) | 24 (35.29) | |||
| Primipara | 17.728 | 0.000 | ||||
| Yes | 101 (12.78) | 278 (35.19) | 411 (52.03) | |||
| No | 85 (20.73) | 155 (37.80) | 170 (41.46) | |||
| Pregnancy loss | 0.014 | 0.993 | ||||
| 7 (14.89) | 17 (36.17) | 23 (48.94) | ||||
| 179 (15.52) | 416 (36.08) | 558 (48.40) | ||||
| History of cesarean section | 0.139 | 0.933 | ||||
| Yes | 29 (14.80) | 70 (35.71) | 97 (49.49) | |||
| No | 157 (15.64) | 363 (36.16) | 484 (48.21) | |||
| History of macrosomia | - | 0.036* | ||||
| Yes | 1 (6.25) | 2 (12.50) | 13 (81.25) | |||
| No | 185 (15.63) | 431 (36.40) | 568 (47.97) | |||
| Pregnancy season | 29.216 | 0.000 | ||||
| Spring and Summer | 65 (11.15) | 193 (33.10) | 325 (55.75) | |||
| Autumn and Winter | 121 (19.61) | 240 (38.90) | 256 (41.49) | |||
| Passive smoking during pregnancy | 4.105 | 0.128 | ||||
| Yes | 49 (16.44) | 93 (31.21) | 156 (52.35) | |||
| No | 137 (15.19) | 340 (37.69) | 425 (47.12) | |||
| Regular exercise during pregnancy | 39.173 | 0.000 | ||||
| Yes | 74 (14.68) | 232 (46.03) | 198 (39.29) | |||
| No | 112 (16.09) | 201 (28.88) | 383 (55.03) | |||
BMI, body mass index; GWG, gestational weight gain; *representing Fisher’s exact probability test.
Analyzing with GWG status as the dependent variable (normal GWG = 0, low GWG =
1), and considering the statistically significant indicators—pre-pregnancy BMI
(normal = 0, underweight = 1, overweight = 2, obese = 3), primipara (no = 0, yes
= 1), history of macrosomia (no = 0, yes = 1), pregnancy season (autumn/winter =
0, spring/summer = 1), and regular exercise during pregnancy (yes = 0, no = 1)—as
independent variables for analysis, the following results were obtained: being
underweight before pregnancy (odds ratio (OR) = 1.920, 95% confidence interval
(CI) = 1.205~3.061) is an independent risk factor for low GWG in
pregnant women (p
| Variable | SE value | Wald value | p-value | OR value | 95% CI | |
| Pre-pregnancy lean | 0.652 | 0.238 | 7.522 | 0.006 | 1.920 | 1.205~3.061 |
| Pre-pregnancy overweight | –0.249 | 0.332 | 0.562 | 0.454 | 0.780 | 0.407~1.494 |
| Pre-pregnancy obesity | 0.088 | 0.353 | 0.063 | 0.802 | 1.092 | 0.547~2.180 |
| Primipara | –1.512 | 0.373 | 16.397 | 0.000 | 0.220 | 0.106~0.458 |
| Having a huge history of childhood | –0.060 | 0.873 | 0.005 | 0.945 | 0.942 | 0.170~5.215 |
| Pregnancy in spring and summer | –0.460 | 0.174 | 7.003 | 0.008 | 0.631 | 0.449~0.887 |
| Regular exercise during pregnancy | –0.098 | 0.182 | 0.291 | 0.589 | 0.907 | 0.635~1.294 |
GWG, gestational weight gain; SE, standard error; OR, odds ratio; 95% CI, 95% confidence interval.
Analyzing with GWG status as the dependent variable (normal GWG = 0, high GWG =
1) and considering the same independent variables and assignments as above, the
following results were obtained: being obese before pregnancy (OR = 2.064, 95%
CI = 1.069~3.986), having a history of macrosomia (OR = 2.669,
95% CI = 1.130~6.304), and experiencing pregnancy during
spring/summer (OR = 1.342, 95% CI = 1.041~1.731) are identified
as independent risk factors for high GWG in pregnant women (p
| Variable | SE value | Wald value | p-value | OR value | 95% CI | |
| Pre-pregnancy lean | –0.076 | 0.202 | 0.140 | 0.708 | 0.927 | 0.624~1.378 |
| Pre-pregnancy overweight | 0.091 | 0.171 | 0.284 | 0.594 | 1.096 | 0.783~1.533 |
| Pre-pregnancy obesity | 0.725 | 0.336 | 4.657 | 0.031 | 2.064 | 1.069~3.986 |
| Primipara | 0.076 | 0.138 | 0.298 | 0.585 | 1.079 | 0.822~1.415 |
| History of macrosomia | 0.982 | 0.439 | 5.007 | 0.025 | 2.669 | 1.130~6.304 |
| Pregnancy in spring and summer | 0.294 | 0.130 | 5.148 | 0.023 | 1.342 | 1.041~1.731 |
| Regular exercise during pregnancy | –0.311 | 0.142 | 4.785 | 0.029 | 0.733 | 0.555~0.968 |
GWG, gestational weight gain; SE, standard error; OR, odds ratio; 95% CI, 95% confidence interval.
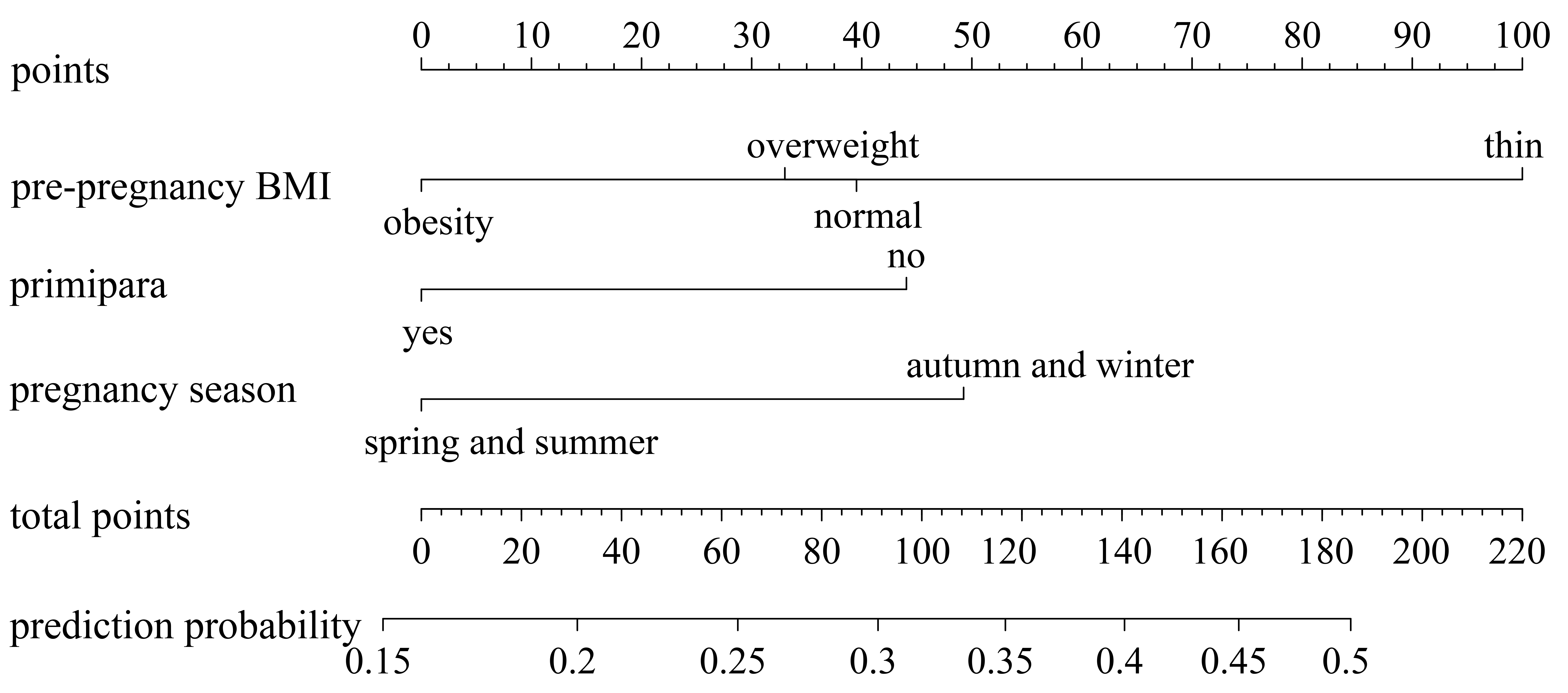
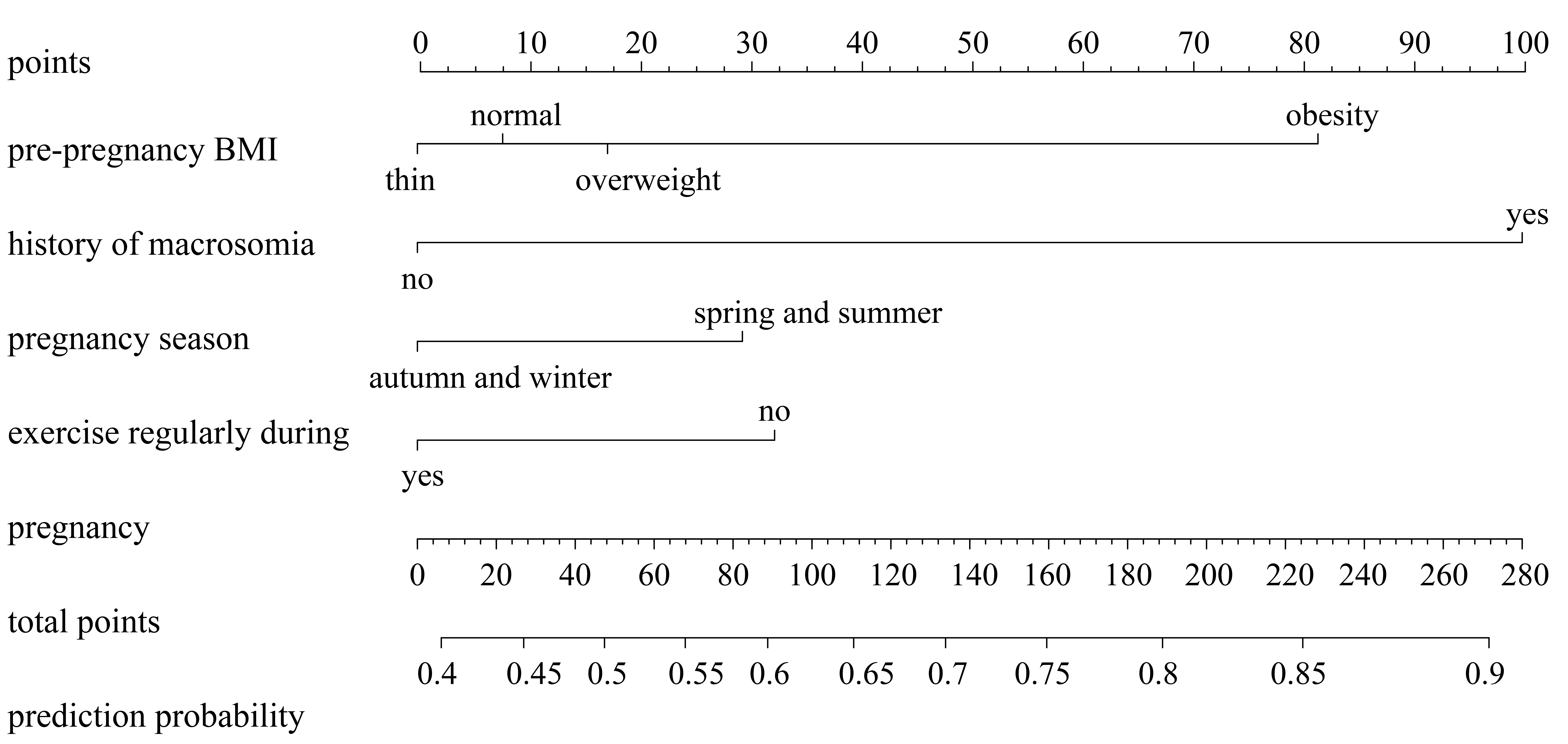
The nomogram results indicate that being underweight before pregnancy scores 100 points, not being a primipara scores 44.2 points, and getting pregnant during autumn/winter scores 49.3 points. See Fig. 2.
 Fig. 2.
Fig. 2.
Construction of a risk prediction model for pregnant women with low GWG. BMI, body mass index; GWG, gestational weight gain.
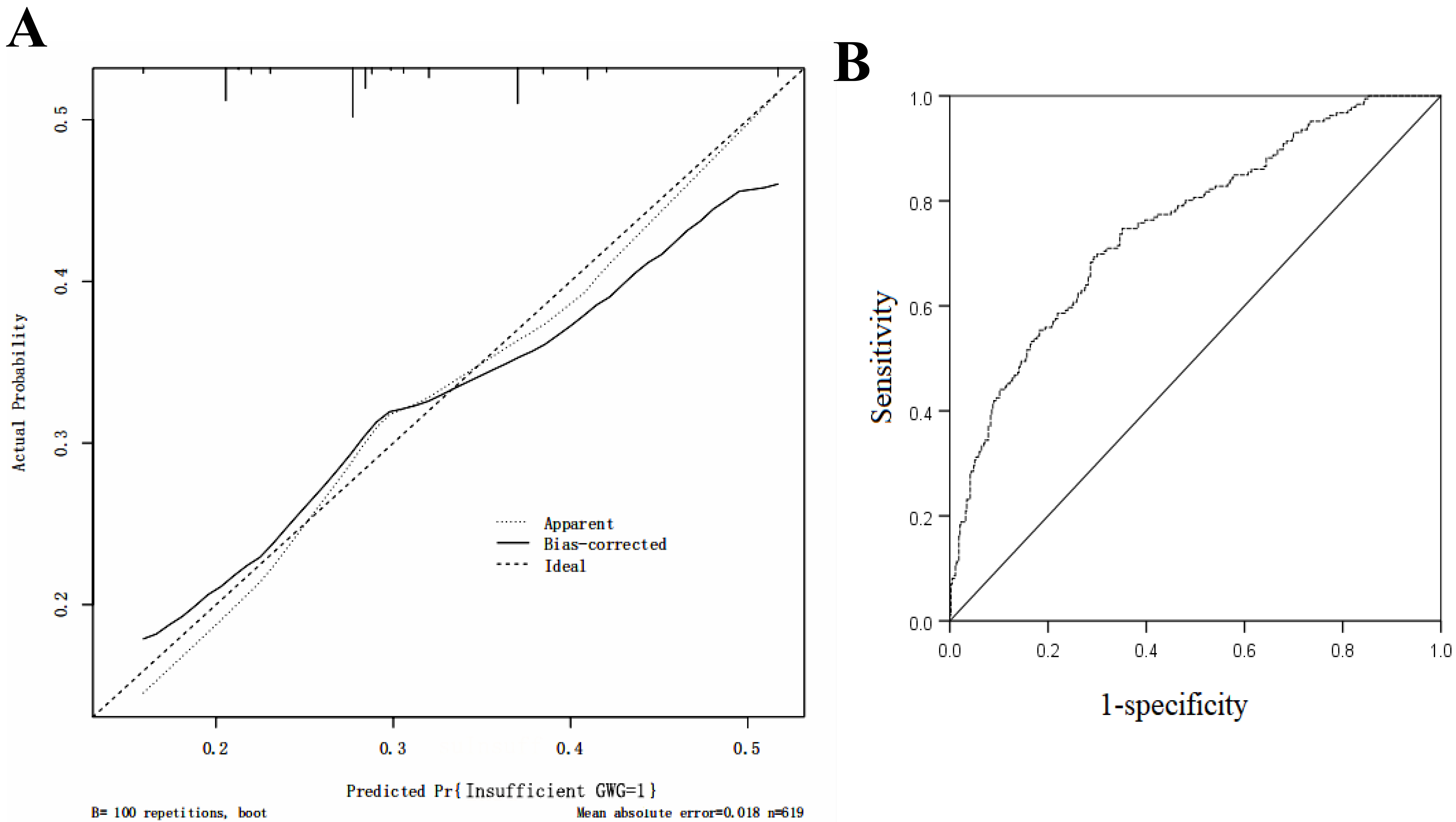
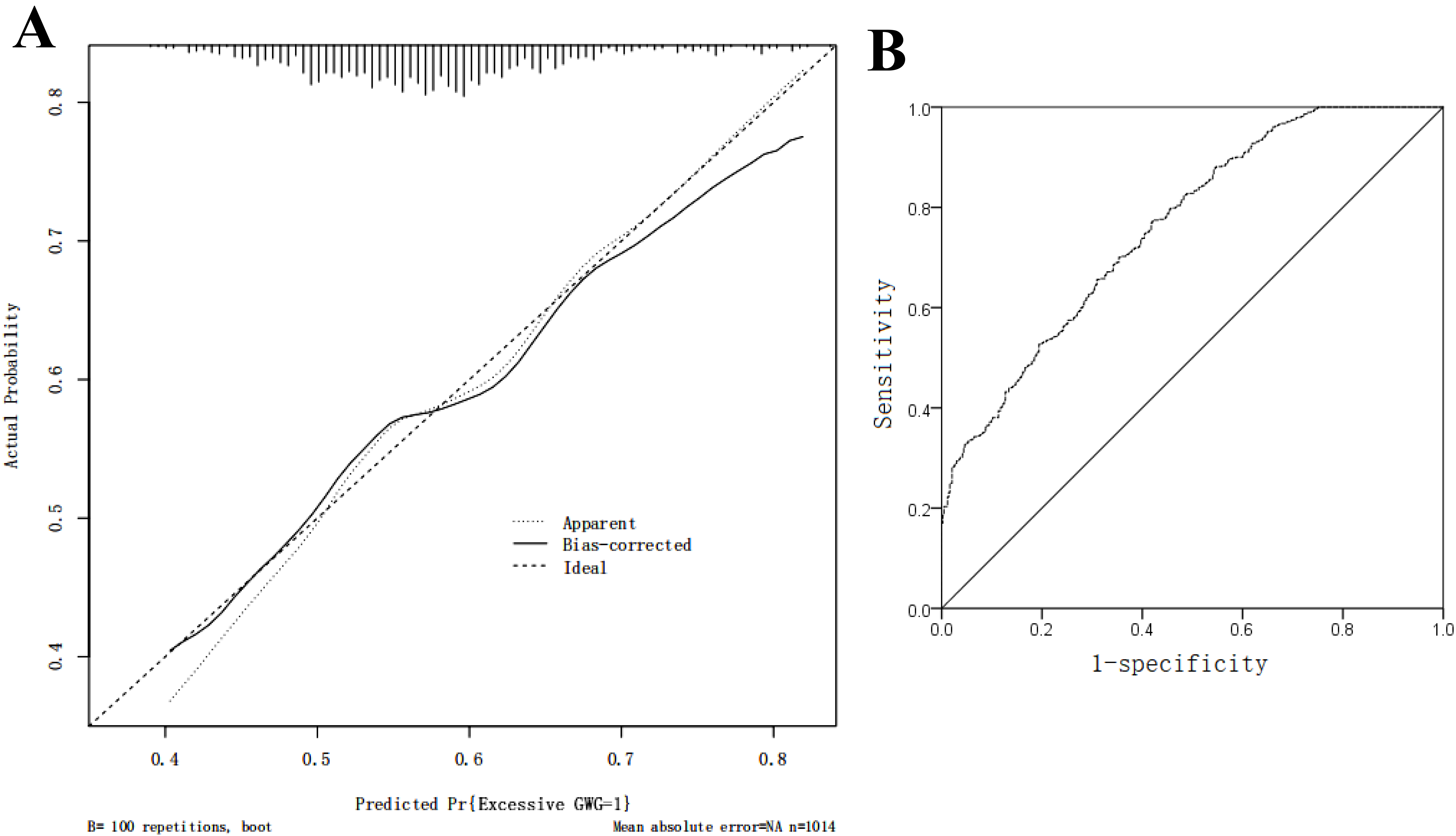
Internal validation of the nomogram prediction model with the validation population demonstrated that the predicted probabilities closely matched the actual probabilities. Indeed, Fig. 3A shows that the calibration curve slope for predicting low GWG in pregnant women is close to 1, with Hosmer-Lemeshow goodness-of-fit test = 8.388, p = 0.397. In Fig. 3B, the ROC curve shows that the AUC for predicting low GWG in pregnant women is 0.753 (95% CI = 0.712~0.795), demonstrating that the model exhibits good consistency and discriminative ability.
 Fig. 3.
Fig. 3.
Internal validation of the risk prediction model for low GWG in pregnant women. (A) Calibration curve for low GWG in pregnant women. (B) ROC curve of low GWG in pregnant women. GWG, gestational weight gain; ROC, receiver operating characteristic.
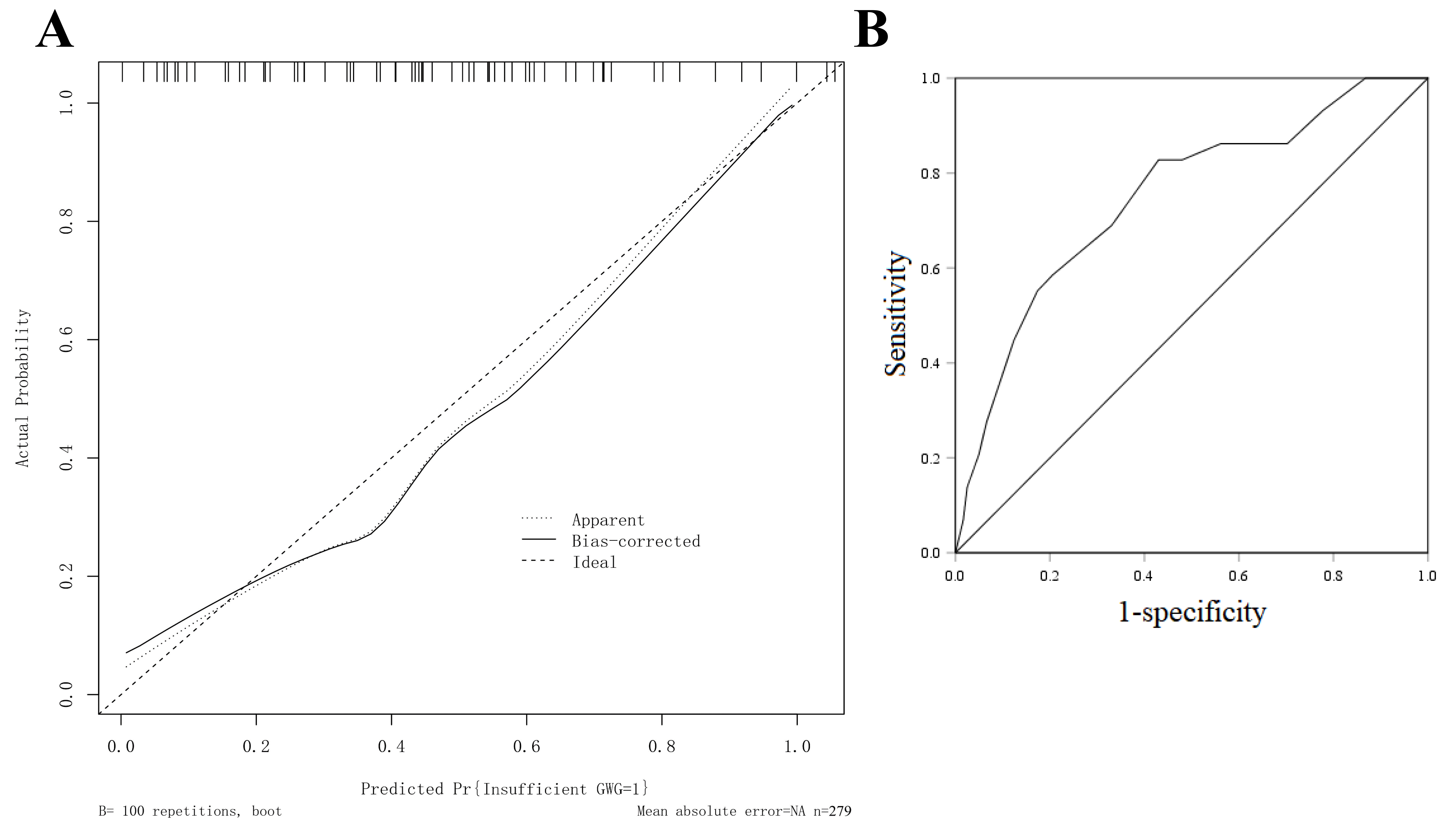
Among the 515 pregnant women in the control group, 77 (14.95%) exhibited low
GWG, and 202 (39.22%) had normal GWG. Compared to the normal GWG group, the low
GWG group had a higher proportion of underweight women before pregnancy, lower
proportions of primiparas, and fewer instances of pregnancy during spring/summer
(p
 Fig. 4.
Fig. 4.
External validation of the risk prediction model for low GWG in pregnant women. (A) Calibration curve. (B) ROC curve. GWG, gestational weight gain; ROC, receiver operating characteristic.
| Parameter | Low GWG group (n = 77) | Normal GWG group (n = 202) | t/ |
p-value | |
| Pre-pregnancy BMI (kg/m2) | - | 0.006* | |||
| Thin | 27 (35.06) | 32 (15.84) | |||
| Normal | 41 (53.25) | 138 (68.32) | |||
| Overweight | 7 (9.09) | 20 (9.90) | |||
| Obesity | 2 (2.60) | 12 (5.94) | |||
| Primipara | 15.386 | 0.000 | |||
| Yes | 21 (27.27) | 108 (53.47) | |||
| No | 56 (72.73) | 94 (46.53) | |||
| Pregnancy season | 11.606 | 0.001 | |||
| Spring and Summer | 20 (25.97) | 98 (48.51) | |||
| Autumn and winter | 57 (74.03) | 104 (51.49) | |||
BMI, body mass index; GWG, gestational weight gain; *representing Fisher’s exact probability test.
The nomogram results reveal the following scores: being obese before pregnancy scores 81.7 points, having a history of macrosomia scores 100 points, getting pregnant during spring/summer scores 29.6 points, and not exercising regularly during pregnancy scores 32.5 points. Please refer to Fig. 5.
 Fig. 5.
Fig. 5.
Construction of a risk prediction model for high GWG in pregnant women. BMI, body mass index; GWG, gestational weight gain.
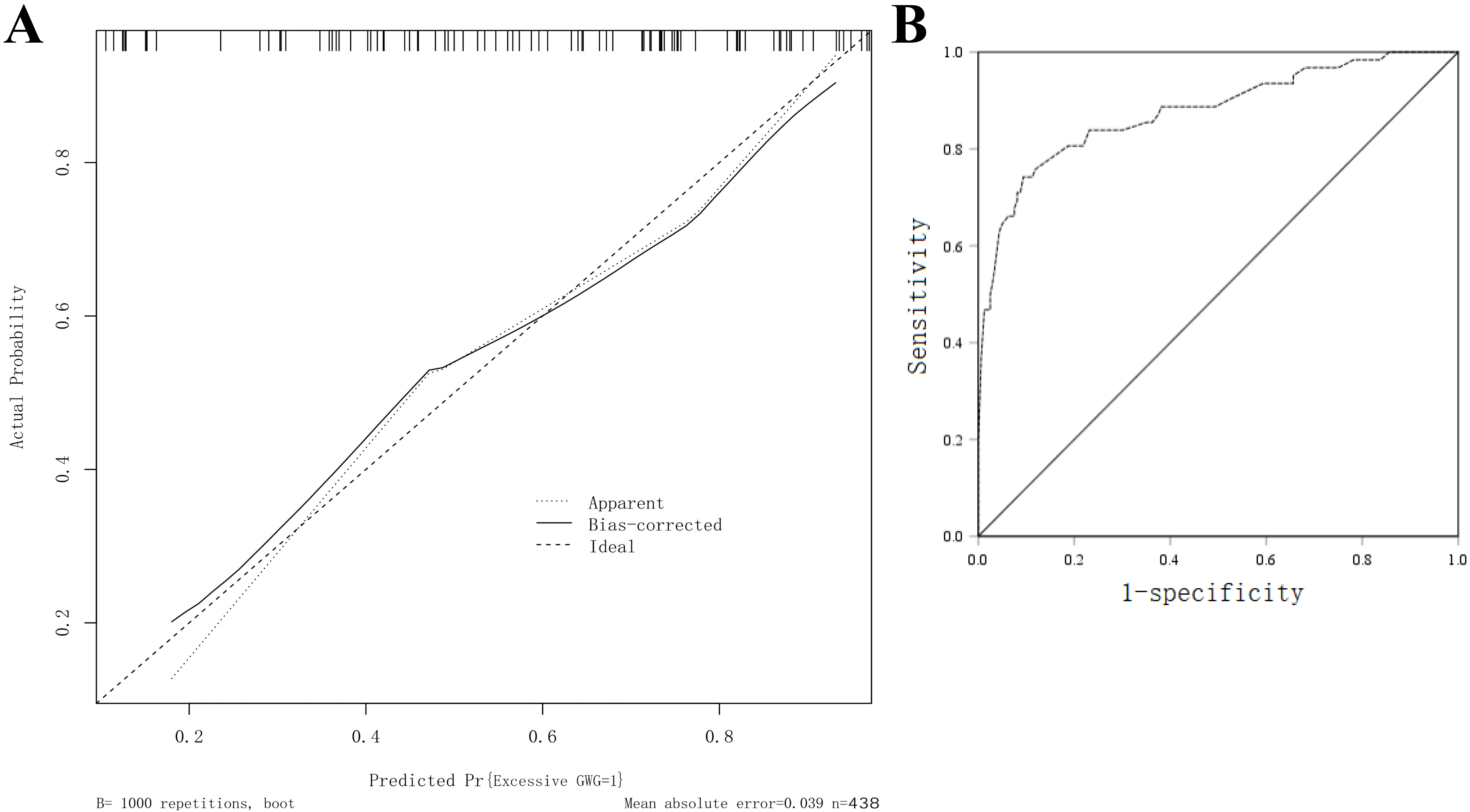
Internal validation of the nomogram prediction model with the validation population demonstrated that the predicted probabilities closely matched the actual probabilities. In fact, Fig. 6A shows that the calibration curve slope for predicting high GWG in pregnant women is close to 1, with Hosmer-Lemeshow goodness-of-fit test = 7.295, p = 0.505. In Fig. 6B, the ROC curve shows that the AUC for predicting high GWG in pregnant women is 0.761 (95% CI = 0.732~0.790), demonstrating that the model exhibits good consistency and discriminative ability.
 Fig. 6.
Fig. 6.
Internal validation of the risk prediction model for high GWG in pregnant women. (A) Calibration curve of high GWG in pregnant women. (B) ROC curve of high GWG in pregnant women. GWG, gestational weight gain; ROC, receiver operating characteristic.
Among the 515 pregnant women in the control group, 236 (45.83%) exhibited high
GWG. Compared to the normal GWG group, the high GWG group had higher proportions
of women who were obese before pregnancy, had a history of macrosomia, got
pregnant during spring/summer, and had lower proportions of regular exercise
during pregnancy (p
 Fig. 7.
Fig. 7.
External validation of the risk prediction model for high GWG in pregnant women. (A) Calibration curve. (B) ROC curve. GWG, gestational weight gain; ROC, receiver operating characteristic.
| Parameter | Normal GWG group (n = 202) | High GWG group (n = 236) | t/ |
p-value | |
| Pre-pregnancy BMI (kg/m2) | 69.285 | 0.000 | |||
| Thin | 32 (15.84) | 6 (2.54) | |||
| Normal | 138 (68.32) | 112 (47.46) | |||
| Overweight | 20 (9.90) | 55 (23.31) | |||
| Obesity | 12 (5.94) | 63 (26.69) | |||
| History of macrosomia | 4.307 | 0.038 | |||
| Yes | 2 (0.99) | 10 (4.24) | |||
| No | 200 (99.01) | 226 (95.76) | |||
| Pregnancy season | 6.881 | 0.009 | |||
| Spring and Summer | 98 (48.51) | 144 (61.02) | |||
| Autumn and winter | 104 (51.49) | 92 (38.98) | |||
| Regular exercise during pregnancy | 18.774 | 0.000 | |||
| Yes | 98 (48.51) | 67 (28.39) | |||
| No | 104 (51.49) | 169 (71.61) | |||
BMI, body mass index; GWG, gestational weight gain.
In this study, it was observed that 15.50% pregnant women had low GWG, 36.08% had normal GWG, and 48.42% had high GWG. These findings underscore a high proportion of pregnant women experiencing abnormal GWG [8]. GWG serves as an indicator of the nutritional status of pregnant women during pregnancy, which is a crucial factor affecting pregnancy outcomes. Abnormal GWG can contribute to gestational diabetes, gestational hypertension, and an elevated risk of adverse pregnancy outcomes such as premature delivery and cesarean section. Furthermore, it may also lead to long-term metabolic problems for the offspring. Therefore, it is crucial to explore the factors affecting abnormal GWG. Our findings suggest that GWG in pregnant women is associated with pre-pregnancy BMI, primiparity status, history of macrosomia, pregnancy season, and regular exercise during pregnancy. Multivariate analysis uncovered pre-pregnancy underweight stands as an independent risk factor for low GWG. Conversely, being a primipara and getting pregnant during spring/summer emerged as protective factors against low GWG. On the contrary, pre-pregnancy, a history of macrosomia, and getting pregnant during spring/summer emerged as independent risk factors for high GWG, whereas consistent exercise during pregnancy acts as a protective factor against high GWG. However, the aforementioned results alone cannot predict low or high GWG in pregnant women. Therefore, this study proceeded to develop a risk prediction model integrating factors affecting low or high GWG in pregnant women, aiming for individualized management of gestational weight.
Prior studies have validated the effectiveness of nomogram models in predicting various risks, including urinary retention after pelvic floor reconstruction surgery in women [9], postpartum hemorrhage in women with scarred uteri after cesarean delivery [10], and preeclampsia in pregnant women [11]. Our nomogram results demonstrated that the absence of primiparity contributed to 44.2 point increase in the model score for predicting low GWG in pregnant women, in comparison to primiparity. This observation may be associated with experienced pregnant women potentially overlooking the importance of maintaining balanced nutrition [8]. Additionally, our nomogram results indicated that getting pregnant during autumn/winter led to 49.3 points increase in model score for predicting low GWG compared to spring/autumn. Conversely, getting pregnant during spring/summer resulted in a 29.6 points increase in the model score for predicting high GWG. This could be attributed to pregnant women conceiving in autumn/winter reaching the mid-to-late stage of pregnancy during spring/summer, a period characterized by potentially lower energy intake and increased engagement in outdoor activities due to favorable weather conditions. Since the mid-to-late stage of pregnancy is a critical period for weight gain, this may result in low GWG. Conversely, pregnant women who conceive in spring/summer may be more prone to high GWG [12]. Therefore, it is recommended that pregnant women who conceive in autumn/winter to prioritize maintaining a balanced diet with adequate energy intake, while those conceiving in spring/summer should carefully manage their diet appropriately and increase exercise within their health constraints to avoid or prevent the occurrence of low or high GWG.
Ornaghi et al. [13] reported that high pre-pregnancy BMI and abnormal GWG could increase the risk of pregnancy complications in women with chronic hypertension. Zhao et al. [14] observed that abnormal pre-pregnancy BMI and abnormal GWG can increase the risk of macrosomia and large-for-gestational-age infants. According to our nomogram results, pre-pregnancy underweight status was associated with a model score of 100 points for predicting low GWG in pregnant women, while pre-pregnancy obesity was linked to a model score of 81.7 points for predicting high GWG. Therefore, it becomes imperative to strengthen nutritional education efforts for women during the preconception stage, enabling then to understand the risks associated with both insufficient and excessive GWG for both maternal and infant health. Providing guidance on adopting appropriate exercise regimens and adhering to balanced diets to control BMI within the normal range prior to pregnancy can significantly improve maternal and infant outcomes. Uchinuma et al. [15] found a certain degree of association between neonatal birth weight, pre-pregnancy BMI, and GWG. Our study identified a history of macrosomia as having a model score of 100 points for predicting high GWG in pregnant women. Hence, it is recommended that women in the preconception stage actively manage their weight through scientific validated methods to ensure neonatal birth weight falls within the ideal range. Vargas-Terrones et al. [16] found that engaging in physical exercise during pregnancy is beneficial for maintaining appropriate GWG, thereby helping to control neonatal birth weight. Our study discovered that not exercising regularly during pregnancy increased the model score by 32.5 points compared to regular exercise during pregnancy. Therefore, appropriate exercise during pregnancy may play a role in preventing or controlling weight gain beyond the recommended GWG.
The risk models for predicting weight gain below or above GWG in pregnant women, constructed in this study, showed strong validation results. The calibration curve slopes were close to 1, with Hosmer-Lemeshow goodness-of-fit tests yielding = 8.388, 7.295, p = 0.397, 0.505, and AUCs of 0.753 and 0.761, respectively. These results indicate that the risk models can effectively predict low or high GWG in pregnant women, thereby aiding in the individualized management of gestational weight. However, the data from the internal validation might have exhibited overfitting. Therefore, further external validation using recently collected data from pregnant women was conducted, which also indicated good consistency and discriminative ability of the prediction model. Many factors, such as diet and psychological state, influence abnormal gestational weight. However, due to the retrospective nature of this study and incomplete information on gestational diet, these factors were not included in the analysis. Future studies should be prospective and incorporate a broader range of factors to validate the results of this study.
The risk prediction model based on pre-pregnancy BMI, pregnancy season, and other factors constructed in this study plays a significant role in the individualized prediction of low or high GWG in pregnant women. This model contributes to the personalized management of gestational weight.
The original contributions presented in the study are included in the article.
XC and FL designed the research study. LL, QZ, YHe, MZ and YHu collected data. XC, LL and FL analyzed the data. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of Meizhou People’s Hospital (ethics number: 2018-C-17, approval date: 04/25/2018). Informed consent was waived due to the retrospective nature of the study.
We would like to express our gratitude to all those who helped us during the writing of this manuscript. Thanks to all the peer reviewers for their opinions and suggestions.
This research received no external funding.
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.